Chapter 273 Amebiasis
Laboratory Findings
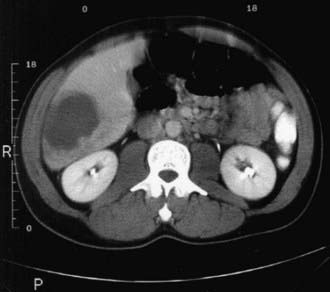
Laboratory examination findings are often unremarkable in uncomplicated amebic colitis, although mild anemia may be seen. Laboratory findings in amebic liver abscess are a slight leukocytosis, moderate anemia, high erythrocyte sedimentation rate, and elevations of hepatic enzyme (particularly alkaline phosphatase) levels. Stool examination for amebae is negative in more than half of patients with documented amebic liver abscess. Ultrasonography, CT, or MRI can localize and delineate the size of the abscess cavity (Fig. 273-1). The most common finding is a single abscess in the right hepatic lobe in about a half of these cases. Higher resolution ultrasound and CT studies have shown that left lobe abscess and multiple abscesses occur more often than previously recognized.
Treatment
Invasive amebiasis is treated with a nitroimidazole such as metronidazole or tinidazole and then a luminal amebicide (Table 273-1). Tinidazole has similar efficacy to metronidazole with shorter and simpler dosing and less frequent adverse effects. These adverse effects include nausea, abdominal discomfort, and a metallic taste that disappears after completion of therapy. Therapy with a nitroimidazole should be followed by treatment with a luminal agent, such as paromomycin (which is preferred) or iodoquinol. Diloxanide furoate can also be used in children >2 yr of age, but it is no longer available in the USA. Paromomycin should not be given concurrently with metronidazole or tinidazole, because diarrhea is a common side effect of paromomycin and may confuse the clinical picture. Asymptomatic intestinal infection with E. histolytica should be treated preferably with paromomycin or alternatively with either iodoquinol or diloxanide furoate. For fulminant cases of amebic colitis, some experts suggest adding dehydroemetine (1 mg/kg/day subcutaneously or IM, never IV), available only through the Centers for Disease Control and Prevention. Patients should be hospitalized for monitoring if dehydroemetine is administered. Dehydroemetine should be discontinued if tachycardia, T-wave depression, arrhythmia, or proteinuria develops.
| MEDICATION | ADULT DOSAGE (ORAL) | PEDIATRIC DOSAGE (ORAL)* |
|---|---|---|
| INVASIVE DISEASE | ||
| Metronidazole | Colitis or liver abscess: 750 mg tid for 7-10 days | Colitis or liver abscess: 35-50 mg/kg/day in 3 divided doses for 7-10 days |
| or | ||
| Tinidazole | Colitis: 2 g once daily for 3 days | Colitis: 50 mg/kg/day once daily for 3 days |
| Liver abscess: 2 g once daily for 3-5 days | Liver abscess: 50 mg/kg/day once daily for 3-5 days | |
| Followed by: | ||
| Paromomycin (preferred) | 500 mg tid for 7 days | 25-35 mg/kg/day in 3 divided doses for 7 days |
| or | ||
| Diloxanide furoate† or | 500 mg tid for 10 days | 20 mg/kg/day in 3 divided doses for 7 days |
| Iodoquinol | 650 mg tid for 20 days | 30-40 mg/kg/day in 3 divided doses for 20 days |
| ASYMPTOMATIC INTESTINAL COLONIZATION | ||
| Paromomycin (preferred) | As for invasive disease | As for invasive disease |
| or | ||
| Diloxanide furoate† | ||
| or | ||
| Iodoquinol | ||
* All pediatric dosages are up to a maximum of the adult dose.
Baxt LA, Singh U. New insights into Entamoeba histolytica pathogenesis. Curr Opin Infect Dis. 2008;21:489-494.
Bercu TE, Petri WA, Behm JW. Amebic colitis: new insights into pathogenesis and treatment. Curr Gastroenterol Rep. 2007;9:429-433.
Carrero JC, Cervantes-Rebolledo C, Aguilar-Díaz H, et al. The role of the secretory immune response in the infection by Entamoeba histolytica. Parasite Immunol. 2007;29:331-338.
Chavez-Tapia NC, Hernandez-Calleros J, Tellez-Avila FI, et al: Image-guided percutaneous procedure plus metronidazole versus metronidazole alone for uncomplicated amoebic liver abscess, Cochrane Database Syst Rev 1:CD004886, 2009.
Fotedar R, Stark D, Beebe N, et al. Laboratory diagnostic techniques for Entamoeba species. Clin Microbiol Rev. 2007;20:511-532.
Karp CL, Auwaerter PG. Coinfection with HIV and tropical infectious diseases. I. Protozoal pathogens. Clin Infect Dis. 2007;45:1208-1213.
Liang SY, Chan YH, Hsia KT, et al. Development of loop-mediated isothermal amplification assay for diagnosis of Entamoeba histolytica. J Clin Microbiol. 2009;47:1892-1895.
Santi-Rocca J, Rigothier MC, Guillén N. Host-microbe interactions and defense mechanisms in the development of amoebic liver abscesses. Clin Microbiol Rev. 2009;22:65-75.