Chapter 10 Allograft Particulate Cartilage Transplantation:
DeNovo Natural Tissue (NT) Graft
Introduction: DeNovo NT Graft
The DeNovo Natural Tissue (NT) Graft is derived from articular cartilage allograft.1,2 The allograft articular cartilage is mechanically particulated into pieces of tissue and stored in a proprietary storage medium developed by ISTO Technologies, Inc. (St. Louis, Mo., United States).
As tissue comprising the DeNovo NT graft is analyzed in the same manner as fresh stored osteochondral allograft, per AATB standards and FDA regulations, it is released for use only after all viral screening and sterility tests are completed. Similar to other fresh allograft tissues such as fresh osteochondral allografts,3–6 the tissue has a limited shelf life.
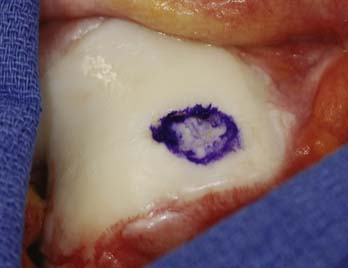
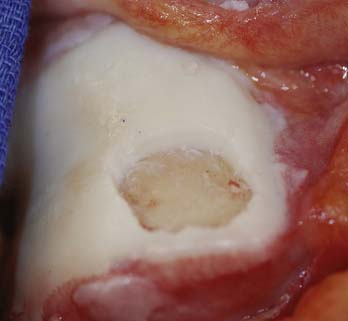
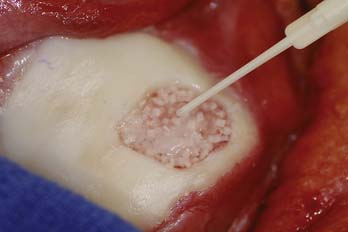
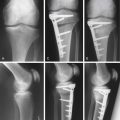
Preoperatively, the lesion sizes are documented. The lesion areas are calculated and used to order the separate packets of DeNovo NT graft. Each package contains particulated cartilage pieces to cover an area of up to 2.5 cm2.
Technique Overview
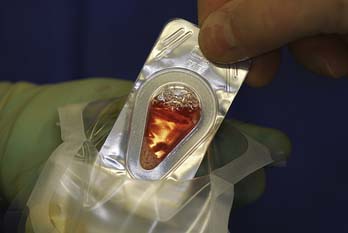
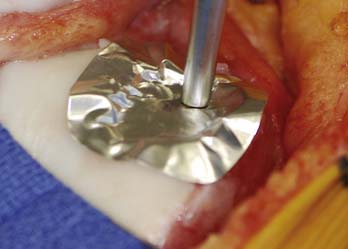
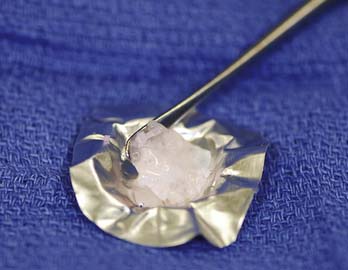
FIGURE 10-4 The DeNovo NT graft packet is removed from the protective sterile inside of the packaging.
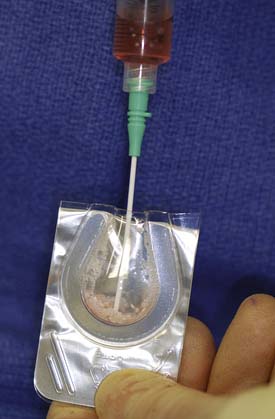
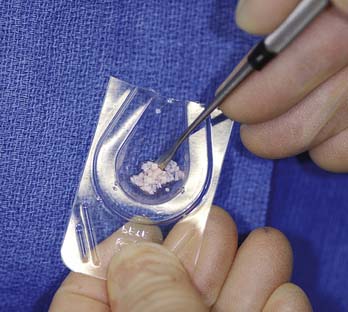
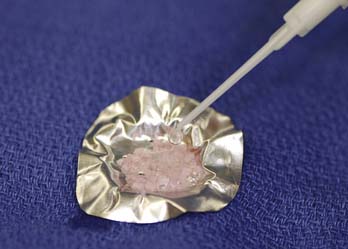
FIGURE 10-5 The storage medium is aspirated, leaving a few drops that improve handling of the DeNovo NT graft pieces.
Pearls and Pitfalls
1. Farr J. DE NOVO Natural Graft. Poster 182, ICRS meeting Miami, FL, 2009.
2. Gibson T., Davis W.B., Curran R.C. The long-term survival of cartilage homografts in man. British Journal of Plastic Surgery. 1958-1959;Volume 11:177-187.
3. Bugbee W.D. Fresh osteochondral allografts. J Knee Surg. 2002 Summer;15(3):191-195.
4. Convery F.R., Meyers M.H., Akeson W.H., Fresh osteochondral allografting of the femoral condyle Clin Orthop Relat Res, 1991(273) Dec:139-145.
5. Elves M.W., Zervas J. An investigation into the immunogenicity of various components of osteoarticular grafts. Br J Exp Pathol. 1974 Aug;55(4):344-351.
6. Gross A.E., Shasha N., Aubin P., Long-term followup of the use of fresh osteochondral allografts for posttraumatic knee defects Clin Orthop Relat Res 2005(435) Jun:79-87.