CHAPTER 16 Allograft Osteochondral Transplantation
The treatment of chondral defects presents a formidable challenge. A variety of treatment methods are available, but all have shortcomings.1 Osteochondral allografts have been used for the treatment of chondral lesions for more than 20 years.2 They have the advantage of providing true articular cartilage rather than hyaline-like cartilage or fibrocartilage. They are particularly valuable when treating large defects and those with bone loss because they are not limited by size, depth, or shape of the lesion.
BASIC SCIENCE
Although the efficacy of osteochondral allografts has been shown for many years, disease transmission continues to be the area of greatest concern.3 Unfortunately, methods used to sterilize tissue have significant detrimental effects on osteochondral allografts.4 Sterilization methods not only devitalize all the chondrocytes, but also have negative effects on the material properties of the graft. Fortunately, the risk of disease transmission is slight if the tissue is retrieved, handled, and processed in strict accordance with standardized guidelines of the American Association of Tissue Banks (AATB).5
The initial clinical series reported using grafts within 1 week from procurement because it was found that the sooner the graft is implanted, the greater the chance of chondrocyte survival.6,7 Although the minimum chondrocyte viability for graft success is unknown, it is clear that this play a vital role in graft integrity.8 However, disease testing and safety precautions have resulted in tissue banks generally not releasing grafts for use until about 3 weeks. Fortunately, current storage methods are able to maintain 80% cell viability at 4 weeks.9,10 In addition, the biomechanical properties of the graft are not statistically affected at that time.11,12 However, more recent testing has shown that in commercially available grafts, a large percentage of the viable cells do not exhibit full function.13
Because of the limitations of fresh grafts with regard to storage and assurance of sterility, other methods of preservation, including freeze drying and fresh-frozen methods, have been evaluated.14 Unfortunately, fresh-frozen grafts have no viable chondrocytes. Freeze drying not only destroys all cells but also alters the graft’s material properties.15
Host immune response is another area of concern with allograft transplantation. It is well known that musculoskeletal allografts are capable of inducing cell-mediated and humeral immune responses in the host.16,17 The predominant mechanism is cell-mediated, and by reducing the number of allogenic cells, the immune response would therefore be reduced. The primary source of allograft cells is the blood and bone marrow elements. The immune load is significantly reduced by removing them during graft processing,.
Chondrocytes can also evoke an immune response and matching the surface antigens has been presented as one method to reduce the load further. However, the limited number of osteochondral allografts available would make it extremely difficult to match the donor to the recipient. Fortunately, any host sensitization has not precluded favorable results. It has been suggested that because chondrocytes are embedded in the dense matrix, which acts as a barrier, intact grafts are considered immunologically privileged. However, there is a consensus that patients with autoimmune disease or inflammatory arthropathies are not appropriate candidates for osteochondral allografts. Although it may be implemented, the morbidity associated with immunosuppression does not justify its use.
PATIENT EVALUATION
History and Physical Examination
Candidates for osteochondral allografts for focal defects typically fall into three categories—osteochondritis dissecans (OCD), post-traumatic lesions, and revision surgery.18–20
TREATMENT
Conservative Management
Focal chondral defects are a common finding and can have a significant effect on limiting a patient’s activities and quality of life.21,22 Symptomatic lesions can be treated nonoperatively with modification of activities, anti-inflammatories, viscosupplementation, and rehabilitation. Although these can be of benefit, many patients continue to be symptomatic.
Surgical Treatment
Several surgical options are available in addition to osteochondral allografts, but each has its advantages and disadvantages. The simplest is débridement-chondroplasty, but the benefits are commonly short-lived. Microfracture is technically easy to perform, with limited morbidity, but results in fibrocartilage filling the defect.23,24 As a result, the outcomes deteriorate after a few years, and lesions larger than a few centimeters do poorly. Autogenous osteochondral transfer has the benefit of improving normal articular cartilage and being able to fill bone deficiencies.25,26 However, donor availability is limited and it is recommended for lesions smaller than 2 to 2.5 cm2. Autologous chondrocyte implantation (ACI) is often discussed as an alternative to osteochondral allografts. It can also treat large lesions and is recommended for lesions up to 16 cm2.27 However, it results in hyaline-like cartilage and should not be used by itself for defects more than 6 to 8 mm deep. In addition, it is technically challenging, with many published studies having a reoperation rate of 30%.
Surgical Technique
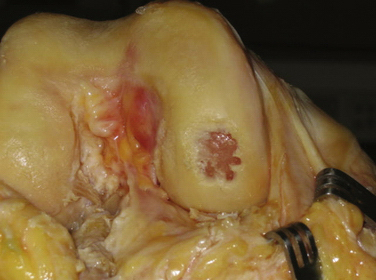
After the patient is found to meet the criteria for an osteochondral allograft, the next step is to obtain a graft (Fig. 16-1) The tissue bank used should be AATB certified to minimize the risk of infection and obtain a graft of high quality. The upper age limit of the donor of a graft is unclear. However, many surgeons recommend that the donor be younger than 35 to 40 years because of concern about tissue quality, with degenerative changes being present in older donors. Size and contour of the graft to match the recipient is another factor to be considered when selecting a graft.
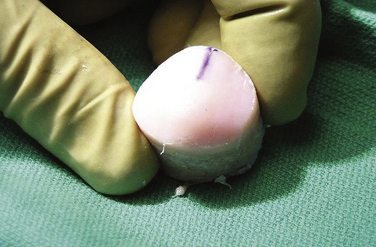
The graft can be prepared using a dowel or shell configuration.28,29 Dowel grafts are cylindrical plugs, often compared with the smaller autologous osteochondral autografts, and are prepared with commercially available instrumentation (Fig. 16-2). Shell grafts are manually prepared with osteotomes, saws, and rongeurs to match the defect (Fig. 16-3). Because dowel grafts are less time consuming to prepare, are easier to match, and are often press-fit, they are usually the method of choice for femoral and patellar defects. Shell grafts are generally reserved for massive grafts and those not amenable to dowel grafts. These would include tibial plateau grafts and posterior femoral condyle lesions, which cannot be reached at a 90-degree angle to the surface needed to prepare dowel grafts. In addition, if the meniscus is not intact, the tibial plateau allograft should include the meniscus. If the host meniscus is normal, the meniscus and its horn attachment are left intact.
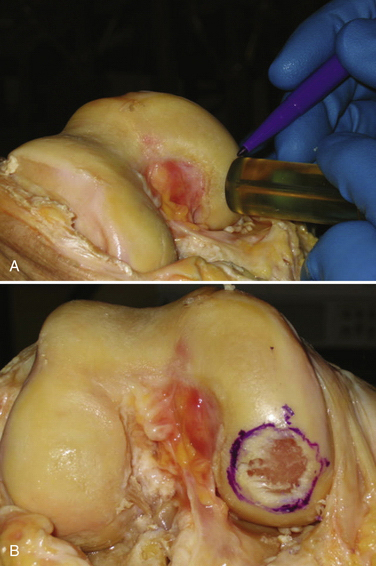
The initial step with the dowel method is to measure the defect using cannulated sizing cylinders (Fig. 16-4). Several systems are available, with most having cylinders in 5-mm increments ranging from 15 to 35 mm. The smallest cylinder that encompasses the defect should be selected. It is then stabilized perpendicular to the surface and a guide pin drilled through the cannulated cylinder and advanced until it is secure in the bone. Because the guide pin is used as a reference for subsequent drilling, it is crucial to have the pin at 90 degrees to the surface. Any tilt will result in the recipient site being oblique and increase the difficulty in matching the graft.
Before reaming, a scoring instrument of the same diameter is placed over the pin and manually twisted to cut through the outer border of the articular cartilage. Its purpose is to decrease the risk of the reamer damaging the normal articular cartilage. The reamer is advanced to a depth of 6 mm (Fig. 16-5) but, if needed, reaming can continue until a bleeding base is present. Irrigation should be performed during the use of any powered instrumentation to minimize the risk of thermal necrosis. A cannulated tamp is used to compress the bottom of the defect to ensure a firm base and limit the risk of collapse. The guide pin is removed and the four quadrants of the recipient site are measured to help determine the donor graft thickness.
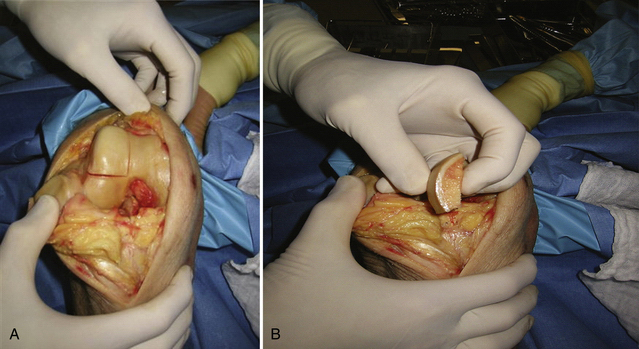
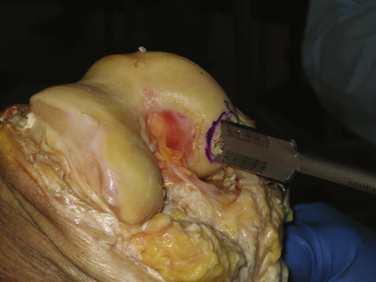
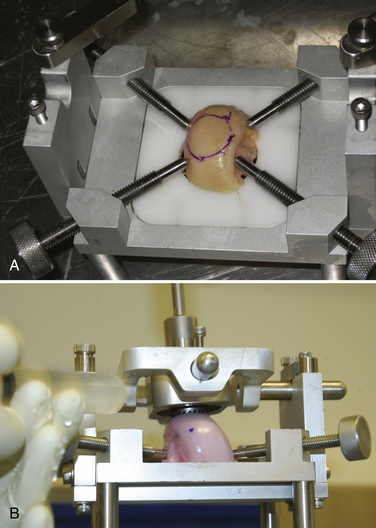
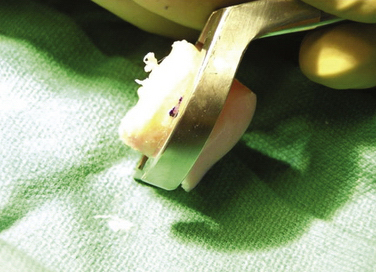
The allograft preparation starts by evaluation of the optimal harvest site (Fig. 16-6). The sizing cylinder is used to mark the area in the same manner as the defect. In addition, a marking pen is used to identify the 12 o’clock position of the graft to ensure correct orientation so it can be identified.30 The graft is then set in the cutting jig (Fig. 16-7). The entire graft may be set into the device, but it is prudent to take the time to cut the base of the graft so that it can be secured in a position that ensures that the coring device is 90 degrees to the articular cartilage. As noted, oblique cuts can result in a graft that may be difficult to place and could result in a plug with variable thickness of articular cartilage.
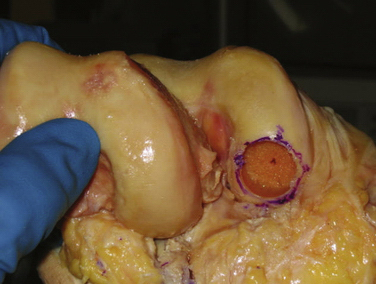
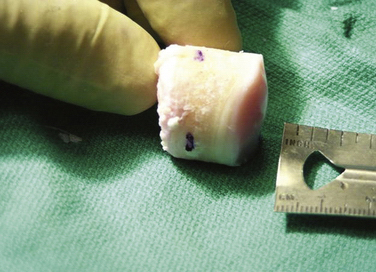
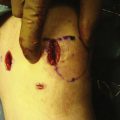
With the graft correctly positioned, the cylindrical core reamer, with its inserted extruding rod, is used to harvest the plug. The plug is removed and marked at its four quadrants to match the recipient site (Fig. 16-8). The articular side of the graft is held in the grasping clamp, with the four marks flush with the surface of the clamp (Fig. 16-9). An oscillating saw is then used to cut the graft to the measured length.
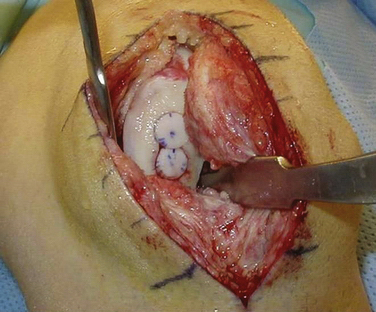
The bone tamp should again be used at the recipient site prior to inserting the graft. Occasionally, the borders of the site expand just enough to impede graft placement. If the initial positioning is difficult, an arthroscopic rasp can be used to round the bottom millimeter of the bone. Caution is needed so as not to remove excess bone, which can affect the stability of the graft. Optimally, the graft should be inserted with firm finger pressure. A tamp can be gently used to assist the insertion, but forceful pressure could damage the graft.31 If the graft is proud by as little as 1 mm, shear forces can result in articular damage and affect the stability of the graft.32,33 By contrast, if the graft is countersunk more than 1 mm, it will serve only as a bone filler. If there is insufficient bone, wafers of bone can be cut from the graft excess and used to make up the difference. If the graft is difficult to remove, one should not forcefully try to pry the graft out. A threaded K wire can be drilled in the graft for ease of removal.
If the graft is press-fit accurately, it will not require additional fixation (Fig. 16-10). If there is any question of stability, such as a graft that is not more than 75% to 80% contained, one should not hesitate to supplement fixation. Various methods have been used, but recently developed very small, compressive bioabsorbable screws are favored.
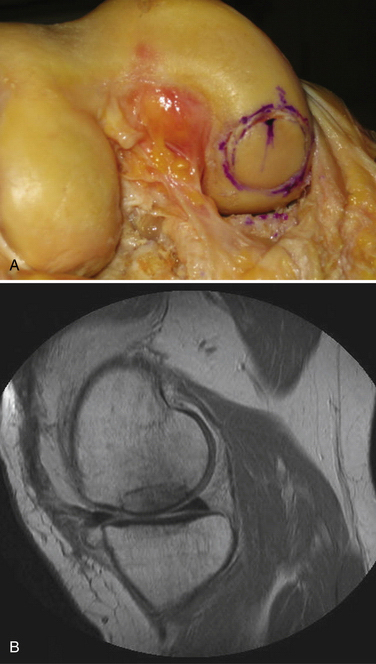
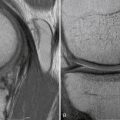
FIGURE 16-10 A, Final appearance of dowel graft secured with press fit. B, MRI scan of completed graft.
If a lesion is not circular or larger than 35 mm, multiple plugs can be used (Fig. 16-11). They are a placed in the same manner as multiple autogenous osteochondral grafts. The grafts abut each other, and attention to detail is needed to reproduce the nature curve of the surface.
PEARLS& PITFALLS
PEARLS
PITFALLS
OUTCOMES
The University of Toronto began its osteochondral allograft program in 1972 and reported the results of their first 100 patients in 1985.34 These patients encompassed a wide variety of pathology and had variable outcomes. However, it was found that using shell grafts for post-traumatic lesions resulted in a 75% success rate (36 of 48). They subsequently published their intermediate and long-term results using grafts for post-traumatic knee lesions. Of the 126 knees in 122 patients treated, 108 (86%) were successful.35 The average follow-up was 7.5 years (range, 2 to 22 years), with a survivorship of 95% at 5 years, 85% at 10 years, and 74% at 15 years for femoral condyle grafts. For tibial plateau grafts, the survivorship was 95% at 5 years, 80% at 10 years, and 65% at 15 years.36 In 2008, the University of Toronto reported their findings from a critical analysis of the 69 known failures treated at their institution.37 They found that the grafts that failed early (less than 1 year) lacked viable chondrocytes and cartilage matrix staining. The importance of proper graft handling and timely implantation to maintain chondrocyte viability and graft quality was stressed. Analyses also showed a trend toward better survivorship with adjunct meniscal allograft transplantation and realignment osteotomies. Finally, the importance of mechanical stability and fixation was evident. It was found that graft instability leads to nonunion and continued remodeling at the host-graft interface.
The University of California at San Diego (UCSD) instituted their use of osteochondral allografts in 1983. In 1999, they published the results of 211 patients treated for defects secondary to OCD, trauma, or AVN.38 The mean follow-up was 52 months (range, 12 to 186 months). Good to excellent results were reported in 177 of 211 patients (84%). Analyses of the treated knee lesions showed success in 116 of 125 (93%) femoral, 26 of 40 (65%) tibiofemoral, and 35 of 46 (76%) patellofemoral grafts. Uncorrected ligamentous instability, limb malalignment, and bipolar lesions were found to be associated with increased failure rates. In 2007, UCSD published their experience of treating OCD lesions with osteochondral allografts.39 In 64 patients, 66 knees were treated, with a mean allograft size of 7.5 cm2. The mean follow-up was 7.7 years (range, 2 to 22 years). Only one patient was lost to follow-up. Of the remaining 65 knees, 47 (72%) were rated good to excellent, 7 (11%) were rated fair, and 1 (2%) was rated poor. An additional 10 patients(15%) underwent reoperation.
CONCLUSIONS
Osteochondral allografts have proven application for treating chondral and osteochondral lesions. They provide true hyaline cartilage, are not limited by shape or size, and have the longest proven efficacy of any current treatment options. The disadvantages are possible disease transmission, immunogenicity, limited availability, and cost. At present, these allografts should be considered when treating chondral lesions larger than 2 cm2; they constitute one of the preferred methods for treating these lesions when bone loss is more than 4 to 5 mm.
1. Sgaglione NA, Miniaci A, Gillogly SD, et al. Update on advanced surgical techniques in the treatment of traumatic focal articular cartilage lesions in the knee. Arthroscopy. 2002;18:9-32.
2. Gortz S, Bugbee WD. Fresh osteochondral allograft processing and clinical application. J Knee Surg. 2006;19:231-240.
3. Tomford WW. Current concepts review. Transmission of disease through transplantation of musculoskeletal allografts. J Bone Joint Surg Am. 1995;11:1742-1754.
4. Asselmier MA, Caspari RB, Bottenfield S. A review of allograft processing and sterilization techniques and their role in transmission of human immunodeficiency virus. Am J Sports Med. 1993;21:170-175.
5. Pearson K, Dock N, Brubaker S, eds. Standards for Tissue Banking, 12 ed. McLean, Va: American Association of Tissue Banks, 2008.
6. Czitrom AA, Keating S, Gross AE. The viability of articular cartilage in fresh osteochondral allografts after clinical transplantation. J Bone Joint Surg Am. 1990;72:574-581.
7. Maury AC, Safir O, Las Heras F, et al. Twenty-five year chondrocyte viability in fresh osteochondral allograft. J Bone Joint Surg Am. 2007;89:159-165.
8. Oakeshott RD, Farine Pritzker KP, et al. A clinical and histologic analysis of failed fresh osteochondral allografts. Clin Orthop Relat Res. 1988;80:1795-1812.
9. Allen R, Robertson C, Pennock A, et al. Analysis of stored osteochondral allografts at the time of surgical transplantation. Am J Sports Med. 2005;33:1479-1484.
10. Ball ST, Amiel D, Wlliams SK, et al. The effects of storage on fresh human osteochondral allografts. Clin Orthop Relat Res. 2004;(418):246-252.
11. Pearsall A, Tucker J, Hester R, et al. Chondrocyte viability in refrigerated osteochondral allografts used for transplantation within the knee. Am J Sports Med. 2004;32:25-31.
12. Williams RJIII, Dreese JC, Chen CT. Chondrocyte survival and material properties of hypothermically stored cartilage. An evaluation of tissue used for osteochondral allograft transplantation. Am J Sports Med. 2004;32:132-139.
13. Lightfoot A, Martin J, Amendola A. Fluorescent viability stains overestimate chondrocyte viability in osteoarticular allografts. Am J Sports Med. 2007;35:1817-1823.
14. Gortz S, Bugbee WD. Allografts in articular cartilage repair. Instr Course Lect. 2007;56:469-480.
15. Malinin T, Temple HT, Buck BE. Transplantation of osteochondral allografts after cold storage. J Bone Joint Surg Am. 2006;88:762-770.
16. Phipatankul W, VandeVord P, Teitge R, et al. Immune response in patients receiving fresh osteochondral allografts. Am J Orthop (Belle Mead NJ). 2004;33:345-348.
17. Rodrigo JJ, Schnaser AM, Reynolds HMJr, et al. Inhibition of the immune response to experimental fresh osteochondral allografts. Clin Orthop Relat Res. 1989;(243):235-253.
18. Aubin PP, Cheah HK, Davis AM, et al. Long-term follow-up of fresh femoral osteochondral allografts for posttraumatic knee defects. Clin Orthop Relat Res. 2001;(391 suppl):S318-S327.
19. Garrett JC. Fresh osteochondral allografts for treatment of articular cartilage defects in osteochondritis dessicans of the lateral femoral condyle in adults. Clin Orthop Relat Res. 1994;(303):33-37.
20. Spak R, Teitge R. Fresh osteochondral allografts for patellofemoral arthritis. Long-term follow-up. Clin Orthop Relat Res. 2006;444:193-200.
21. Aroen A, Loken S, Heir S, et al. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32:211-215.
22. Curl WW, Krome J, Gordon E, et al. Cartilage injuries. A review of 31,516 knee arthroscopies. Arthroscopy. 1997;13:456-460.
23. Mithoefer K, Williams RJIII, Warren RF, et al. Chondral resurfacing of articular cartilage defects in the knee with the microfracture technique. Surgical technique. J Bone Joint Surg Am. 2006;88(suppl 1):294-304.
24. Steadman JR, Briggs KK, Rodrigo JJ, et al. Outcomes of microfracture for traumatic chondral defects of the knee. Average 11-year follow-up. Arthroscopy. 2003;19:477-484.
25. Bobic V, Carter T. Osteochondral autologous graft transfer. Oper Tech Sports Med. 2000;8:168-178.
26. Hangody L, Füles P. Autologous osteochondral mosaicplasty for the treatment of full thickness defects of weight bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85(suppl 2):25-32.
27. Peterson L, Brittberg M, Kiviranta I, et al. Autologous chondrocyte transplantation; biomechanics and long-term durability. Am J Sports Med. 2002;30:2-12.
28. Convery FR, Akeson WH, Meyers MH. The operative technique of fresh osteochondral allografting. Oper Tech Orthop. 1997;7:340-344.
29. Jamali AA, Emmerson BC, Chung C, et al. Fresh osteochondral allografts. Clin Orthop Relat Res. 2005;(437):176-185.
30. Below S, Arnocsky S, Dodds J, et al. The split-line pattern of the distal femur. A consideration in the orientation of autologous artilage grafts. Arthroscopy. 2002;18:613-617.
31. Bozazjani BH, Chen AC, Bae WC, et al. Effects of impact on chondrocyte viability during insertion of human osteochondral grafts. J Bone Joint Surg Am. 2006;88:1934-1943.
32. Huang FS, Simonian PT, Norman AG, et al. Effects of small incongruities in a sheep model of osteochondral autografts. Am J Sports Med. 2004;32:1842-1848.
33. Koh JL, Wirsing K, Lautenschlager E, et al. The effect of graft height mismatch on contact pressure following osteochondral grafting. A biomechanical study. Am J Sports Med. 2004;32:317-320.
34. McDermott AG, Langer F, Pritzker KP, et al. Fresh small-fragment osteochondral allografts. Long-term follow-up study on first 100 cases. Clin Orthop Relat Res. 1985;(197):96-102.
35. Gross AE, Shasha N, Aubin P. Long-term follow-up of the use of fresh osteochondral allografts for posttraumatic knee defects. Clin Orthop Relat Res. 2005;(435):79-87.
36. Shasha N, Krywulak S, Backstein D, et al. Long-term follow-up of fresh tibial osteochondral allografts for failed tibial plateau fractures. J Bone Joint Surg Am. 2003;85(suppl 2):33-39.
37. Gross AE, Ont O, Kim W, et al. Fresh osteochondral allografts for posttraumatic knee defects. Long-term follow-up. Clin Orthop Relat Res. 2008;(466):1863-1870.
38. Bugbee WD, Convery FR. Osteochondral allograft transplantation. Clin Sports Med. 1999;18:67-75.
39. Emmerson BC, Jamali A, Bugbee WD. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35:907-914.