Alignment Disorders
Overview
Malalignment of bony components of the extremities may be congenital or acquired. A defect that results in malalignment may be primary to bone or secondary to soft tissue or neuromuscular disorders. Malalignment is often seen with congenital deformities characterized by embryologic failure of development, discussed in Chapter 128. One of the most common alignment disorders is developmental dysplasia of the hip, discussed in Chapter 133.
Upper Extremities
Etiologies, Pathophysiology, and Clinical Presentation: Erb palsy is caused by birth trauma to the C5 and C6 nerve roots. It is associated with prolonged, difficult delivery (dystocia) and with larger infants. Infants present soon after birth with decreased motion of the involved extremity. Children with a persistent defect will hold their arms in internal rotation with pronation of the forearm and flexion of the wrist. The brachial plexus injury is the primary insult leading to secondary glenohumeral dysplasia.
Imaging: At birth, radiographs serve to exclude fractures of the clavicle and humerus. Ultrasonography and magnetic resonance imaging (MRI) have been used to directly evaluate the brachial plexus;1,2 however, the primary goal of advanced imaging is to evaluate orthopedic anatomy to determine function and orthopedic treatment since primary repair of the brachial plexus injury is difficult.
Progressive deformity with secondary glenohumeral dysplasia manifests as a small, flattened humeral head, which may sublux, usually in the posterior direction relative to a small, shallow, abnormally retroverted glenoid (Fig. 134-1).3 In normal shoulders, the glenoid is mildly retroverted by approximately 5 degrees posteriorly relative to a line perpendicular to the axis of the scapular body.4 With Erb palsy, glenoid retroversion averages 25 degrees. The scapula is hypoplastic and elevated; the acromion is tapered and inferiorly directed, as is the coracoid; the clavicle is shortened.
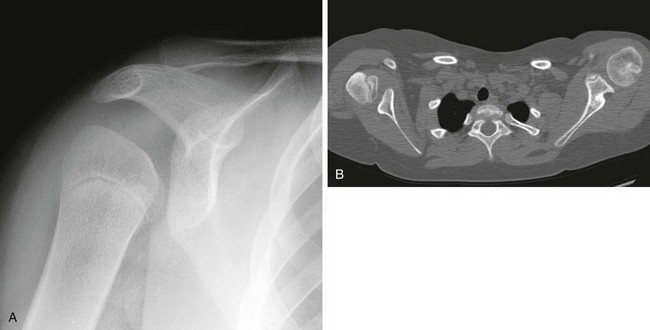
Figure 134-1 Erb palsy in a 9-year-old girl.
A, On radiography, the humeral head appears small and abnormally contoured, the glenoid margins are indistinct, and the acromion is curved. B, Axial computed tomography shows the right glenoid to be small and retroverted. The right humeral head is small and squared in contour but appears normally located relative to the glenoid. Note the asymmetric muscle volume of the right shoulder girdle compared with the left shoulder girdle.
Many of these findings are seen on radiography; however, computed tomography (CT) or MRI can be used to quantify the degree of glenoid retroversion, deformity of the glenoid and humeral head, glenoid–humeral head congruence, and relative muscle volume and quality of the affected shoulder.5–9 The glenoid normally has a concave shape. With glenohumeral dysplasia, the glenoid becomes progressively flat, convex, or biconvex with a pseudoarticulation with the humeral head. MRI is preferred in young children (<5 years old) (e-Fig. 134-2) and CT in older children. To determine glenoid version, the articular cartilage should be used when MRI is performed; the glenoid cortical bone should be used when CT is performed. In infants, ultrasonography may be used to assess instability of the glenohumeral joint.10–12
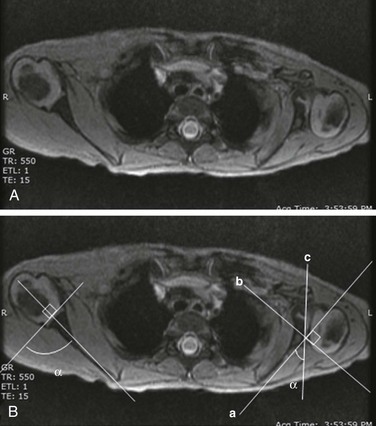
e-Figure 134-2 Erb’s palsy in a 2-year-old girl.
A, Gradient recalled echo axial sequence demonstrates severe retroversion and dysplastic convex left glenoid and posteriorly subluxed humeral head as well as global left shoulder girdle muscle atrophy. B, Same sequence with glenoid version measurements. Version is calculated by first (a) a line drawn along scapula, (b) a perpendicular line drawn with respect to line a, and (c) a line drawn along articular surface of glenoid. The glenoid angle is determined between line a and c. If the glenoid is directed posteriorly with respect to scapula, the glenoid is in retroversion. If the glenoid is directed anteriorly, the glenoid is in anteversion.
Treatment: Microsurgery techniques may be attempted to address the underlying traumatic neural injury.13 Controversy exists as to their role and proper timing. The main focus of treatment is on the reconstructive surgical techniques of the shoulder, elbow and forearm, and hand and wrist, aimed at preserving joint integrity and maximizing function.13,14 Therefore, imaging optimization should be tailored for orthopedic anatomy rather than the brachial plexus.
Madelung Deformity
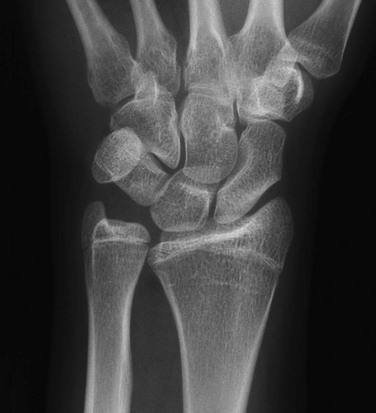
Etiologies, Pathophysiology, and Clinical Presentation: In Madelung deformity, the radius is short and its distal articular surface tilted toward to ulna.15 In most cases, the cause of Madelung deformity is unknown. Madelung deformity occurs more often in girls.16 Patients may have pain; however, treatment is more often sought because of deformity or limited range of motion. With an underlying syndrome, the deformity is more likely to be bilateral. Madelung deformity is occasionally seen with Turner syndrome and is a characteristic in dyschondrosteosis (Léri-Weill syndrome) (Fig. 134-3).15 Among these cases, 10% to 15% are familial. A Madelung-like deformity may also be seen in patients with hereditary osteochondromatosis or enchondromatosis, also suggesting a defect in normal distal radial maturation. Madelung deformity may occur as a complication of infection or trauma that results in medial and volar radial physeal growth disturbance.17
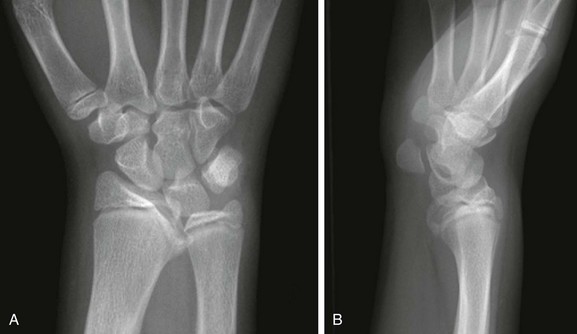
Figure 134-3 Madelung deformity in a 10-year-old girl who presented with right wrist pain.
A, Posteroanterior radiograph shows medial tilt of the distal radial articular surface and physis with thinning of the medial aspect of the distal radial epiphysis. B, Lateral radiograph shows anterior tilt of the distal radial articular surface with mild anterior displacement of the carpus.
Imaging: The distal articular surface of the radius is tilted in an ulnar and volar direction.18,19 The radius is short and bowed dorsally and laterally (“bayonet deformity”). Secondary distortion of the carpus is observed with an abnormally narrow carpal angle and proximal lunate migration.18,19 The distal ulna is subluxed. The distal radial growth plate may prematurely fuse along its ulnar aspect. CT or MRI may be used to assess the extent of distal radial physeal fusion.20
Ulnar Variance
Etiologies, Pathophysiology, and Clinical Presentation: Trauma, infection, inflammatory disease (juvenile idiopathic arthritis) or other causes of premature fusion may lead to shortening of the ulna relative to the radius (negative ulnar variance) or shortening of the radius relative to the ulna (positive ulnar variance) (Fig. 134-4).21 Most cases are idiopathic. Processes associated with hyperemia, for example, fracture healing, infection and vascular lesions, may lead to over growth of one of the bones.
Imaging: At skeletal maturity, the distal radial and ulnar articular surfaces are nearly at the same level, and the radial styloid projects 9 to 12 mm distal to the ulnar articular surface.21,22 With negative ulnar variance, the ulna ends more proximally, and with positive ulnar variance, the ulna ends more distally (e-Fig. 134-5).21,23 Ulnar variance may be exaggerated with forearm pronation and decreased with forearm supination.
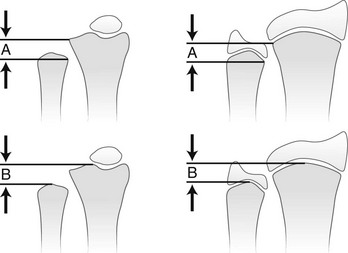
e-Figure 134-5 Method of measuring ulnar variance.
A, Distance from the most proximal point of the ulnar metaphysis to the most proximal point of the radial metaphyses. B, Distance from the most distal point of the ulnar metaphysis to the most distal point of the radial metaphysis. (Modified from Hafner F, Poznanski AK, Donavan JM. Ulnar variance in children—standard measurements for evaluation of ulnar shortening in juvenile rheumatoid arthritis, hereditary multiple exostosis and other bone and joint disorders of childhood. Skeletal Radiol. 1989;18:513-516.)
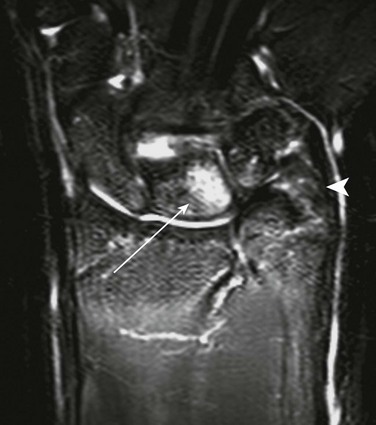
Treatment: Osteotomy with ulnar or radial shortening may be indicated to reduce symptoms and avoid sequelae. Negative ulnar variance is associated with the development of avascular necrosis of the lunate (Kienböck disease).24 Positive ulnar variance is associated with ulnolunate impaction syndrome (Fig. 134-6) and triangular fibrocartilage complex degeneration.
Lower Extremities
Etiologies, Pathophysiology, and Clinical Presentation: The normal neck-shaft angle of the proximal femur is approximately 150 degrees at birth and decreases to 120 to 130 degrees in adulthood.25 External or internal rotation of the hip or femoral anteversion may affect measurement.26
Functional coxa vara occurs with disorders that result in femoral neck shortening, as in trauma, infection, or epiphyseal osteonecrosis.27 True coxa vara occurs as a congenital anomaly that is caused by bone softening (e.g., rickets, osteogenesis imperfecta, fibrous dysplasia) or to abnormal growth (e.g., spondyloepiphyseal dysplasia congenita, spondyloepimetaphyseal dysplasia, cleidocranial dysplasia).28,29 Children with developmental coxa vara present with a limp (unilateral deformity) or a waddling gate (bilateral deformities). Coxa vara may occur as the result of abnormal growth at the proximal femoral physis that results in abnormal angulation of the physis. Congenital coxa vara occurs with a congenital short femur (i.e., proximal focal femoral deficiency) and does not spontaneously resolve.28 With infantile or developmental coxa vara, the hip is normal at birth, and deformity is noted when the child begins to walk.28,30 Infantile coxa vara may be self-limited. Acquired coxa vara is caused by another process such as trauma.28
Imaging: In coxa vara, the femoral neck-shaft angle is decreased from normal. A measurement below 110 degrees is considered coxa vara.31 Fragmentation and sclerosis may be seen at the medial margin of the proximal femoral metaphysis (Fig. 134-7).30 The Hilgenreiner epiphyseal angle is the angle between the Hilgenreiner line and a line drawn through the physis (e-Fig. 134-8).28,31 If it is less than 45 degrees, progression is unlikely. If over 60 degrees, progression is likely. If 45 to 60 degrees, prognosis is less predictable.
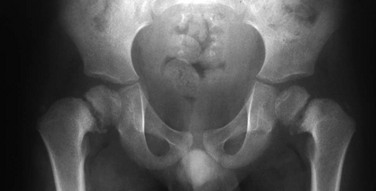
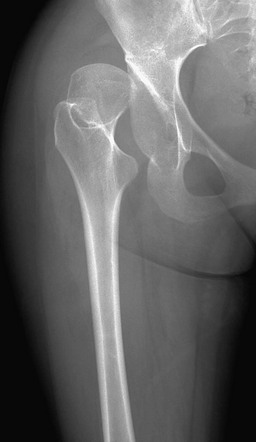
Figure 134-7 Bilateral congenital coxa vara in a 4-year-old boy.
Proximal femoral growth plates are tilted, medial side down. Slight fragmentation is noted at the medial aspect of each proximal femoral physis.
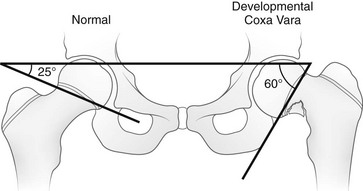
e-Figure 134-8 Hilgenreiner epiphyseal angle.
This angle is created by a single line through the triradiate cartilage and its intersection with another line through the physis. The normal angle is about 25 degrees. (From Beals RK. Coxa vara in childhood: evaluation and management. J Am Acad Orthop Surg. 1998;6:93-99.)
Treatment: Surgical management may be warranted for progressive disease, especially if asymmetric or associated with pain, leg length discrepancy, or both.28 Valgus osteotomy is performed, and physeal fixation or tendon transfers may also be performed to deter progression and improve mechanical function.
Coxa Valga
Etiologies, Pathophysiology, and Clinical Presentation: With coxa valga, the neck-shaft angle of the proximal femur is increased.32 Coxa valga is most often seen in patients who are nonambulatory and nonerect, such as those with cerebral palsy and other neuromuscular disorders (Fig. 134-9).26,33
Imaging: The femoral neck-shaft angle is measured on radiographs. External rotation may mimic coxa valga and can be differentiated through the positioning of the greater trochanter.26 With external rotation, the greater trochanter projects through the femur, whereas with true coxa valga, it is located laterally. Increased femoral anteversion may cause the femoral neck-shaft angle to be overestimated.32 Acetabular dysplasia and femoral subluxation are frequently concomitant findings.32
Femoral Anteversion
Etiologies, Pathophysiology, and Clinical Presentation: Increased femoral anteversion may hinder proper localization of the femoral head relative to the acetabulum. Increased femoral anteversion is seen in hip deformity caused by developmental dysplasia of the hip, Legg-Calvé-Perthes disease, and cerebral palsy.32 With increased anteversion, in-toeing of feet is noted.
Femoral version is the angulation of the femoral neck in the transverse plane measured relative to the femoral condyles distally (Fig. 134-10). If the femoral neck is anteriorly angulated with respect to the femoral condyles, the femur is anteverted. If the femoral neck is posterior with respect to the femoral condyles, the femur is retroverted. Normal femoral anteversion is 35 to 50 degrees at birth, decreasing steadily to 10 to 15 degrees in adulthood (Fig. 134-11).27
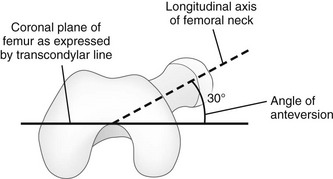
Figure 134-10 Schematic diagram of the right femur as viewed from below.
The femoral neck is angulated anterior (anteversion) relative to the transcondylar axis of the distal femur. (Modified from Greenspan A. Orthopedic imaging: a practical approach. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2004.)
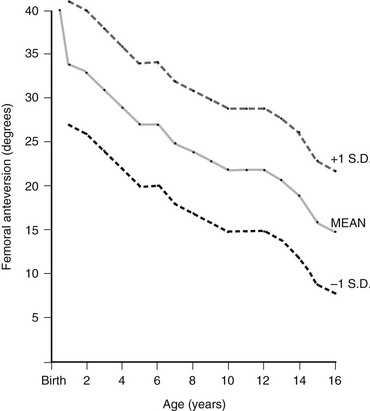
Figure 134-11 Normal femoral anteversion.
The mean value is an average of those reported by Shands and Steele (1958), Crane (1959), and Fabry and associates (1973). Standard deviations are only an approximation and are based on the range of those determined by Fabry and associates. (From Ozonoff MB: Pediatric orthopedic radiology. 2nd ed. Philadelphia: WB Saunders; 1992.)
Imaging: On CT, femoral anteversion is the angle between the axis of the femoral neck and the transcondylar axis at the distal femur (Fig. 134-12).34–36 Limited low-dose axial images are usually performed; however, axial oblique or three-dimensional images may improve measurement.37,38
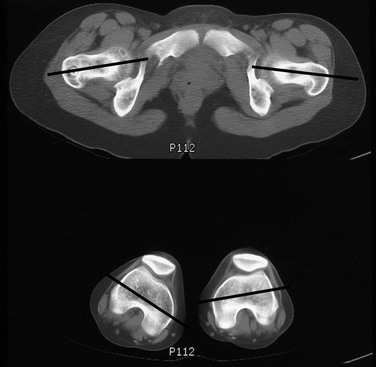
Figure 134-12 Computed tomography method for assessing femoral anteversion in a 17-year-old girl with a history of right femoral fracture.
Lines denote the axes of the femoral necks and the transcondylar axes of the distal femora. An angle of 49 degrees of anteversion was measured on the right and an angle of 14 degrees was measured on the left.
Leg
Etiologies, Pathophysiology, and Clinical Presentation: Tibial torsion is the degree of rotation of the distal end of the tibia relative to the upper end of the tibia. Newborns have internal tibial torsion relative to older children and adults. Lack of normal progression results in in-toeing. Internal tibial torsion is the most common cause of in-toeing in the preschool age group.
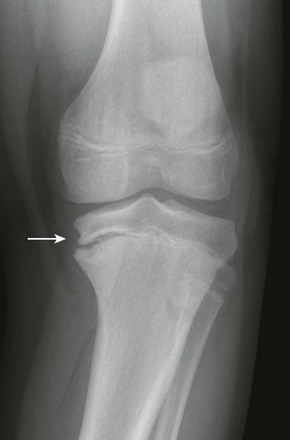
Imaging: Assessment of tibial torsion is often performed with assessment of femoral version. Limited axial low-dose CT images are obtained through the proximal and distal tibias.41 Tibial torsion is best measured as the angle between the posterior epiphyseal cortical margin of the tibia proximally and a bimalleolar line distally. Normal values are 5 degrees of external rotation in a newborn, which progresses to 15 to 20 degrees of external rotation in an adult.40,42
Bowleg (Genu Varum)
Etiologies, Pathophysiology, and Clinical Presentation: Bowleg deformity manifests as separation of the knees, with the legs in anatomic position. Pathologic causes include rickets, osteogenesis imperfecta, neurofibromatosis, skeletal dysplasias (i.e., campomelic dysplasia, achondroplasia), focal fibrocartilaginous dysplasia, congenital bowing, Blount disease, and, occasionally, growth plate trauma.43–46 Recently, greater prevalence of bowleg and tibia vara has been noted in some adolescent athletes, most notably soccer players.47–49 Repetitive stress on the proximal tibial physis may play a role in this.48 Most lateral bowing in otherwise normal infants and children younger than 2 years of age is normal and developmental (“physiologic”) and resolves without treatment.44,50–52 Similar to Blount disease, exaggerated physiologic bowing is seen in early walkers, African Americans, and heavier children.44
Imaging: Ozonoff described the following findings as characteristic of physiologic lower extremity bowing: (1) The tibia is abducted relative to the femur, and both bones are intrinsically bowed laterally; relative tibial torsion produces external rotation of the upper tibia relative to the distal tibia; (2) margins of the distal femoral and proximal tibial metaphyses are mildly accentuated with small beaks; (3) medial cortices of the tibia and femur are thickened; (4) distal femoral and proximal tibial epiphyses are not well ossified medially and are wedge shaped; (5) the distal tibial growth plate may be tilted lateral.53
Radiographically, the femur and the tibia are also mildly bowed anteriorly, with beaking occurring posteriorly (Fig. 134-13). Physiologic bowing is usually more marked in the tibias. Occasionally, lateral bowing may almost exclusively be seen in the distal femur (e-Fig. 134-14). The varus deformity is common in normal infants and converts to valgus between 18 and 36 months of age. Degree of valgus reduces spontaneously by 6 to 7 years of age to a mild degree that remains throughout life. Approximate normal angles are 17 degrees varus in a newborn, 9 degrees varus at 1 year, 2 degrees valgus at 2 years, 11 degrees valgus at 3 years, and 5 to 6 degrees valgus at 13 years (e-Fig. 134-15).52,54,55
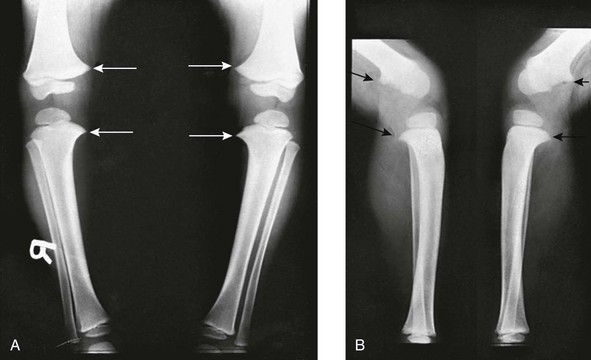
Figure 134-13 Bilateral idiopathic bowed legs in a 22-month-old boy.
A, Frontal projection. Arrows point to the medial beaking of the femoral and tibial metaphyses at the knees. The increased stress of weightbearing has thickened the medial and dorsal cortical walls of the tibias inward. The medial femoral epiphyseal ossification centers are too small, which are under greater stress of weightbearing when the legs are bowed. B, Lateral projection. Arrows point to dorsal beaking of the femoral and tibial metaphyses. After correction of bowed legs, these “stress” phenomena disappear after several months.
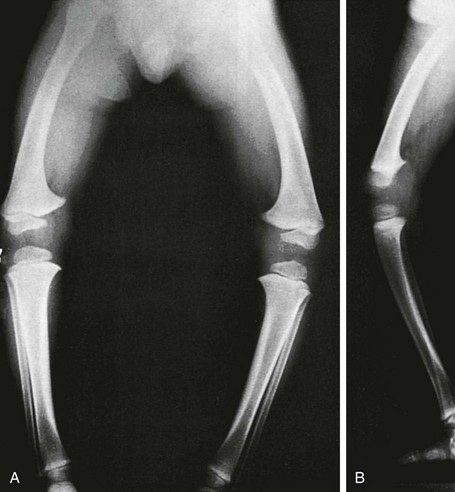
e-Figure 134-14 Femoral bowed legs (femora vara): Endogenous stress trauma at the knees without weightbearing.
Anteroposterior projection shows marked bowlegs, in which the tibias are straight and both femora are bowed abruptly lateral near their distal ends (femora vara). Femoral epiphyseal ossification centers are small and flattened on their medial halves.
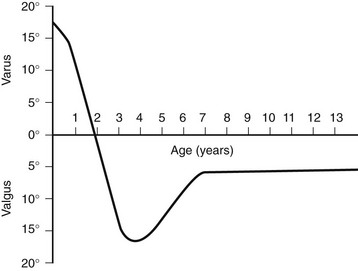
e-Figure 134-15 Development of the tibiofemoral angle during growth.
This graph shows the normal physiologic progression of bowlegs to knock-knees and then to normal during the early years of growth. (From Bruce RW Jr. Torsional and angular deformities. Pediatr Clin North Am. 1996;43:867-881.)
Radiographs should be taken with the patient bearing weight as soon as he or she is able to stand (Fig. 134-16). The radiographs may suggest an underlying disorder such as rickets or a dysplasia.
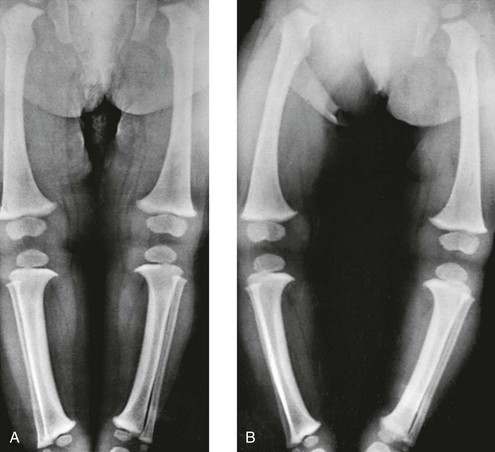
Figure 134-16 Bowed legs of a 12-month-old girl who started to walk at 8 months.
A, In recumbent position, the tibias and femora are bowed, but the legs are not bowed because the knees and ankles are in apposition. B, In erect position, during weightbearing and with ankles in apposition, the legs are bowed, with a gap of 12 cm at the knees. The real clinical deformity of bowed legs is shown most accurately when the patient is erect and is bearing weight.
Treatment: Persistent varus with delayed conversion to valgus may indicate a higher likelihood of Blount disease (tibia vara). In the second year, it may be difficult to distinguish normal physiologic bowing from Blount disease.56 Exaggerated varus during the second year is likely developmental or physiologic and does not require treatment.50,51,54 Any varus at the knee after 2 years of age should raise concern. Such patients must be monitored to exclude progression to Blount disease.55,57 Realignment surgery is rarely needed in isolated bowleg without underlying dysplasia, metabolic bone disease, or Blount disease.
Blount Disease (Tibia Vara)
Etiologies, Pathophysiology, and Clinical Presentation: Blount disease (tibia vara; osteochondrosis deformans tibiae) is a progressive deformity affecting the proximal tibia (Fig. 134-17).58–60 It is theorized that stress on the posteromedial proximal tibial physis causes growth suppression.
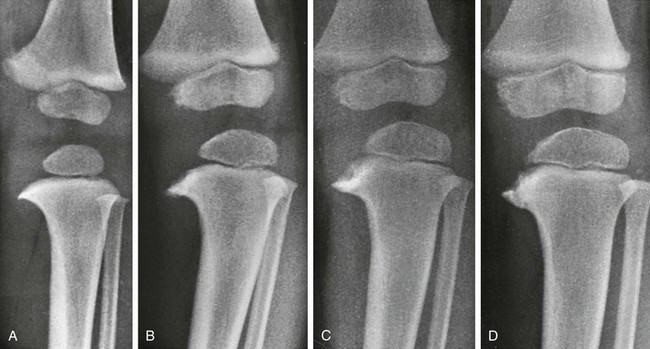
Figure 134-17 Progressive changes in Blount tibia vara.
A, At 17 months, the medial segment of the tibial metaphysis is widened and sharpened into a short beak or spur that is bent slightly caudad. B, At 26 months, the spur is longer, sharper, and more bent; a radiolucent strip on its upper edge represents noncalcified cartilage. C, At 32 months, the amount of cartilage is increased and the spur thickened. D, At 38 months, the beak of the spur is displaced caudad, possibly owing to trauma; the medial edge of the ossification center is flattened, and the femur has shifted mediad in relation to the tibia.
The infantile form of Blount disease, which develops in children between 1 and 3 years of age, must be differentiated from developmental bowing.61 Infantile Blount disease may represent normal developmental bowing that fails to correct and progresses. The diagnosis is made when progressive clinical bowing is seen in the presence of characteristic radiographic changes in the proximal tibia.61 The disease is typically bilateral (60% to 80%) but often asymmetric and occasionally unilateral (Fig. 134-18). A family history is often reported. The disorder is more common in early walkers, African Americans, and obese children.44,60,62
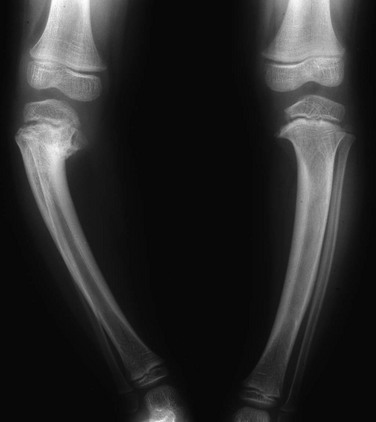
Figure 134-18 Bilateral Blount disease in a 3-year-old girl.
Abnormality is greater on the right, where more sclerosis and irregularity of the proximal tibial metaphysis are evident.
Adolescent or late-onset tibia vara is a separate entity from the infantile form. It occurs in children 8 to 14 years of age; obese African American males of normal height are at particular risk (Fig. 134-19).43 Adolescent tibia vara is commonly unilateral but may be bilateral. Adolescent Blount disease is slowly progressive and probably results from the repetitive trauma of weight bearing on the medial physis of the proximal tibia.43 Patients often present with knee pain.
Imaging: The characteristic radiographic feature of infantile Blount disease is deformity of the medial metaphysis of the proximal tibia (Fig. 134-20).44 Irregularity with a more vertically oriented growth plate creates a beaked appearance. The severity of radiographic changes has been described by the six-stage Langenskiöld classification (e-Fig. 134-21).63 Higher stage denotes greater abnormality; bone bridging between the diaphysis and the metaphysis is seen with stage IV and higher. The medial portion of the epiphyseal ossification center is often smaller than the lateral portion. The tibia may subluxate laterally.
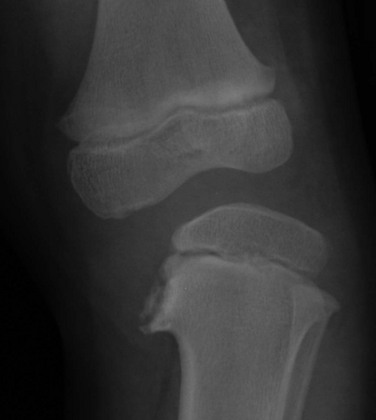
Figure 134-20 Infantile Blount disease in a 4-year-old girl.
Unlike adolescent Blount disease, sharp metaphyseal angulation, beaking, and markedly diminished and near-absent medial epiphyseal ossification of the proximal tibia are seen.
The metaphyseal–diaphyseal angle is measured by drawing a single line through the widest portion of the proximal tibial metaphysis (between the medial and lateral beaks) and another line perpendicular to the long axis of the tibia.51 With physiologic bowing, this angle measures approximately 5 degrees, whereas with Blount disease, average angle measurement is 16 degrees (e-Fig. 134-22). A metaphyseal–diaphyseal angle greater than 11 degrees suggests Blount disease.44–51 However, several studies have questioned the validity of the metaphyseal–diaphyseal angle, and measurement may be affected by tibial rotation.
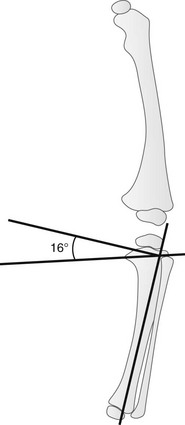
e-Figure 134-22 Metaphyseal-diaphyseal angle.
This angle is defined as the angle between a line perpendicular to the axis of the tibia and a line through the most distal ossified beak of the medial and lateral beak of the tibial metaphysis. (From Eggert P, Viemann M. Physiological bowlegs or infantile Blount’s disease: some new aspects to an old problem. Pediatr Radiol. 1996;26:349-352.)
Changes related to infantile Blount disease should be distinguished from those of adolescent Blount disease (see Figure 134-19). The metaphyseal angular deformities, diminished medial epiphyseal height, and degree of tibia vara are less severe with adolescent Blount disease.
CT or MRI can assess the degree of proximal tibial growth plate fusion and epiphyseal cartilage abnormalities (e-Fig. 134-23).64,65 Cartilage-sensitive MRI sequences display the cartilage model of the proximal tibial epiphysis.65
Treatment: Although spontaneous resolution of infantile Blount disease is reported in some instances, treatment with bracing is begun usually between ages 18 and 24 months and is continued during waking hours for an average of 2 years. If conservative management fails, the patient may undergo a realignment osteotomy, which is most effective when performed before 5 years of age. For adolescent Blount disease, a lateral tibial epiphyseodesis may be performed if sufficient growth still remains. If the proximal tibla physis are near fused, a proximal tibial valgus osteotomy to produce normal alignment may be performed.60
Knock-Knee (Genu Valgum)
Etiologies, Pathophysiology, and Clinical Presentation: Genu valgum is a normal developmental phase that lasts from 2 to 12 years of age and is most apparent at 3 to 4 years of age in normal children.52,54,55,66 Persistent knock-knee can be related to pathologic causes such as trauma, skeletal dysplasia, obesity, metabolic disease, or laxity of muscles and ligaments.
Leg Length Discrepancy
Overview: A leg length discrepancy of less than 1 cm is considered within normal limits; however, in most normal individuals, the legs are within 1 mm of each other in length. The presence of a leg length discrepancy may reflect overgrowth of the long limb or decreased size or growth of the short limb. Leg length discrepancy is often of clinical significance because of associated alteration of gait and resultant pelvic tilt. With significant pelvic tilt, secondary compensatory scoliosis may develop.
Etiologies, Pathophysiology, and Clinical Presentation: The differential diagnoses for overgrowth and undergrowth is lengthy. Overgrowth is associated with a number of syndromes and vascular abnormalities. Mild overgrowth may occur as the result of fracture healing. In many cases, the cause is unknown. Undergrowth may be caused by hypoplasia or aplasia of a segment of the limb. Acquired shortening most often occurs as a consequence of premature growth plate fusion due to trauma, infection, or vascular insufficiency.
Imaging: Radiographic techniques seek to determine bone lengths in a manner that minimizes magnification and other technical factors. Orthoroentgenography (“scanogram”) uses three separate exposures collimated to the hip, knee, and ankle with a radiopaque ruler.67 CT digital scout images may be used to measure bone length.68,69 It is preferable to quantify length and alignment abnormalities with standing radiographs.
Treatment: Mild leg length discrepancy does not warrant treatment. The type of treatment varies with age, amount of projected growth remaining, site of abnormality, and degree of leg length discrepancy. Treatment may be aimed at halting growth on the longer side (i.e., epiphyseodesis) or lengthening the shorter side (i.e., lengthening osteotomy procedure).70 With a lengthening osteotomy procedure, bone on either side of a diaphyseal corticotomy is distracted slowly utilizing an external frame. New bone forms within the gap to add length.
Feet
Overview: Alignment disorders of the feet may be idiopathic or caused by an underlying disorder. The standard method of radiologic evaluation of the foot involves weight bearing or simulated weight bearing anteroposterior (dorsoventral) and lateral views. Diagnosing alignment abnormalities of the feet should be approached with caution when non-weightbearing radiographs are obtained.
The talus is more proximal and is considered fixed at the ankle because it has no musculotendinous attachments of its own. The calcaneus is linked to the midfoot and forefoot and moves as a unit with these structures relative to the talus. In a normal foot, on the anteroposterior view, the axis of the talus extends through the base of the first metatarsal (Fig. 134-25). With hindfoot varus, the more distal calcaneus is angulated inward, and the axis of the talus passes lateral to the base of the first metatarsal. With hindfoot valgus, the more distal calcaneus is angulated outward, and the axis of the talus passes medial to the base of the first metatarsal.81 The normal lateral talocalcaneal angle is approximately 45 degrees, decreasing to 30 degrees in older children and adults.71–73 With hindfoot varus, the lateral talocalcaneal angle is decreased, whereas with hindfoot valgus, the angle is increased.
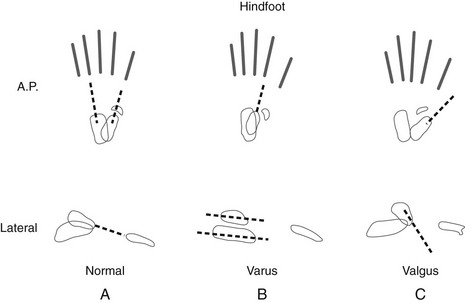
Figure 134-25 Hindfoot and forefoot relationships in the anteroposterior and lateral projections.
A, Normal hindfoot. The talar axial line intersects or points slightly medial to the first metatarsal base. The navicular is situated directly opposite the head of the talus. The calcaneus points toward the fourth metatarsal base, forming a definable angle with the talus. On the lateral projection, the anterior portion of the talus is slightly plantarflexed, and the calcaneus is slightly dorsiflexed. The talar axial line points down the shaft of the first metatarsal. B, Hindfoot varus. The talocalcaneal angle is decreased, with these two bones more parallel to each other and actually superimposed. The navicular is medially displaced, and the axial talar line points lateral to the first metatarsal base. On lateral projection, the calcaneus and the talus are more horizontal and parallel to each other. C, Hindfoot valgus. The talocalcaneal angle is increased, with the navicular and other midfoot bones displaced lateral to the talus. The talar axial line passes medial to the first metatarsal base. On lateral projection, the talus is more vertical than normal. (From Ozonoff MB. Pediatric orthopedic radiology. 2nd ed. Philadelphia: WB Saunders; 1992.)
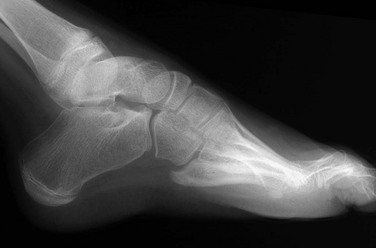
Etiologies, Pathophysiology, and Clinical Presentation: Talipes (“talus” = ankle; “pes” = foot) equinovarus, or clubfoot, is a common congenital anomaly that is clinically obvious at birth. The principal components of clubfoot deformity include plantar flexion of the ankle (equinus), inversion of the heel (varus), and adduction of the forefoot (varus) (Fig. 134-26). Abnormal intrauterine pressures contribute to the development of clubfoot.74 Genetics also appear to play a role.74
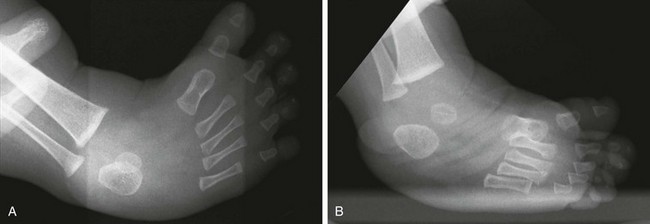
Figure 134-26 Radiographs of congenital clubfoot obtained with simulated weightbearing.
A, Note the inversion and forefoot adduction in the anteroposterior view. B, Inversion on the lateral view shows “laddering” of the metatarsals. Equinus of the hindfoot and parallelism of the talus and calcaneus are evident.
Imaging: Radiographs are obtained with true or simulated weight bearing, as this is the position of best correction.74 An anteroposterior radiograph shows superimposition and parallelism of the talus on the calcaneus with the talar axis directed lateral to the first metatarsal (hindfoot varus). Parallelism of the talus and calcaneus is also seen on a lateral view. The lateral view shows plantar flexion of the calcaneus (equinus) and a step-ladder arrangement of the metatarsals, with the first metatarsal highest and the fifth metatarsal at the weight-bearing surface of the foot. Ultrasonography has been used in select centers to assess the flexibility of clubfoot and to guide therapeutic decision making (e-Fig. 134-27).75–78
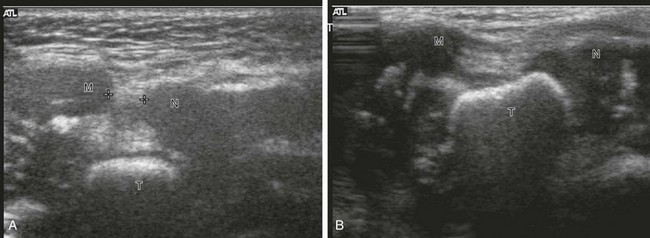
e-Figure 134-27 Ultrasonography of clubfoot in a 6-month-old girl: longitudinal scans at the medial aspect of the feet.
A, In clubfoot, the cartilaginous navicular (N) and the medial malleolus (M) are closely spaced (cursors). These structures overlie the talus (T), which is ossified. B, In the normal foot, the navicular and the medial malleolus are widely spaced, and the talus is not covered. The distance between the navicular and the medial malleolus is an indicator of the severity of deformity and can be monitored over time. Dynamic assessment reveals the flexibility of the deformed clubfoot. (Courtesy Dr. M. A. DiPietro, Ann Arbor, MI.)
Treatment: Approaches to treatment include serial casting for supple clubfoot deformity, and casting followed by surgical correction for rigid clubfoot deformity.74,79,80 Anatomic deformities persist after treatment of clubfoot and should be recognized as children grow. Many treated clubfeet have small, squared tali with flattened heads, decreased talocalcaneal angles, subtalar joint changes, and medial displacement of the navicular. Valgus deformity may result from overcorrection.
Congenital Vertical Talus
Etiologies, Pathophysiology, and Clinical Presentation: Congenital vertical talus (congenital rigid flatfoot) may be an isolated condition or may be seen in association with various syndromes (e.g., trisomies) or other systemic abnormalities (i.e., central nervous system defects, arthrogryposis, and neurofibromatosis).81,82
Imaging: The talus is almost completely vertical in this condition (parallel with the longitudinal axis of the tibia), and the calcaneus is fixed in plantar flexion (equinus) (Fig. 134-28). In the anteroposterior projection, the talar axis is medial to the base of the first metatarsal (valgus). The navicular dislocates dorsally, and clinically, a pronated rocker-bottom foot is present. After the navicular ossifies, its abnormal position helps to distinguish congenital vertical talus from severe planovalgus or flatfoot deformity. Prior to ossification, the position of the navicular can be determined by ultrasonography.83
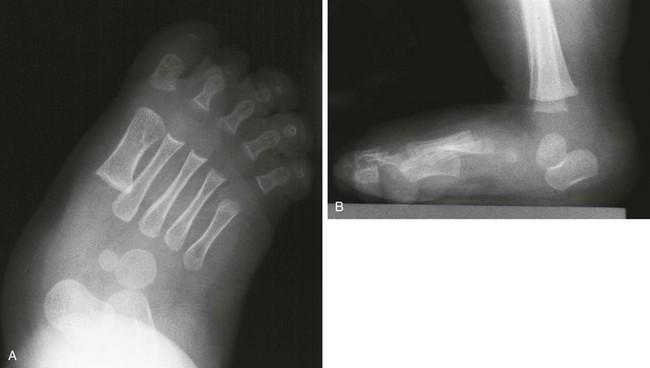
Figure 134-28 Congenital vertical talus in 7-month-old boy.
A, In the anteroposterior view, the axis of the talus projects well medial to the base of the first metatarsal, consistent with hindfoot valgus. B, In the lateral view, the talus is nearly vertical in orientation, and the calcaneus is plantarflexed.
Rocker-Bottom Foot
Metatarsus Varus (Adductus)
Etiologies, Pathophysiology, and Clinical Presentation: Metatarsus adductus is a cause of in-toeing that is usually seen in children younger than 5 years of age. Forefoot adduction differs from clubfoot in that midfoot and hindfoot relationships are normally maintained. The affected foot has a C-shaped contour on examination. Forefoot adduction simulating metatarsus adductus may occur as a result of excessive internal tibial torsion or increased femoral neck anteversion.
Skewfoot
Etiologies, Pathophysiology, and Clinical Presentation: Skewfoot (Z-foot; serpentine metatarsus adductus) is considered a severe form of metatarsus adductus. It is not a congenital deformity but develops in the young child idiopathically or may result from the treatment of other congenital foot deformities such as treated clubfoot or vertical talus. Skewfoot occurs in children with cerebral palsy and with some dysplasias and in otherwise normal children as well.85
Flatfoot (Pes Planus)
Etiologies, Pathophysiology, and Clinical Presentation: Flatfoot (pes planus) is a descriptive term. The differential diagnosis of pes planus includes flexible planovalgus foot, peroneal spastic (rigid) flatfoot related to tarsal coalition, congenital vertical talus (congenital rigid flatfoot), and congenital calcaneovalgus (congenital flexible flatfoot).81,86–88 Tarsal coalition is discussed in Chapter 128.
The common flatfoot is a painless, flexible planovalgus foot.91 Variable degrees of hindfoot valgus, plantar arch flattening, and forefoot pronation occur. Pathology is thought to involve excess ligamentous laxity, which allows the calcaneus to shift into a valgus position under the talus. Abduction and eversion result from loss of calcaneal support. Patients may develop peroneal muscle spasm and pain caused by irritation.
Imaging: With flexible planovalgus foot, the anteroposterior projection shows hindfoot valgus with an increased talocalcaneal angle and the midtalar line passing medial to the first metatarsal. On the lateral projection, hindfoot valgus causes the talus to be more vertical than normal. The navicular subluxes dorsally and laterally with respect to the talar head. The calcaneus and metatarsals are horizontal longitudinally, and loss of the plantar arch is noted.81 Similar deformity may occur in some children with cerebral palsy (e-Fig. 134-30). With a rigid or painful flatfoot (peroneal spastic flatfoot), CT may be performed to detect an underlying subtalar coalition.
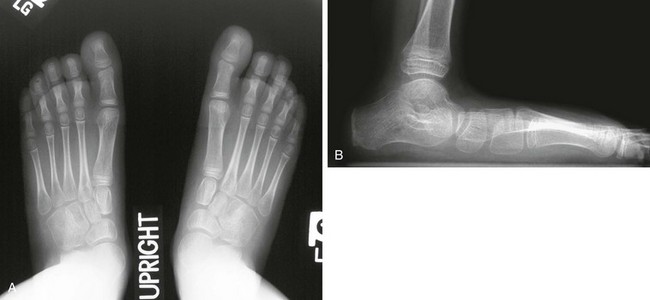
e-Figure 134-30 Bilateral planovalgus feet in a 9-year-old with cerebral palsy.
A, In the anteroposterior view, hindfoot valgus is symmetric. B, Lateral view of the right foot shows pes planus with a mild increase in the lateral talocalcaneal angle, also consistent with hindfoot valgus. The calcaneus is horizontal in orientation.
Calcaneovalgus
Etiologies, Pathophysiology, and Clinical Presentation: Calcaneovalgus feet are reflective of intrauterine positioning. A total of 30% to 50% of newborns have mild calcaneovalgus deformity.91 In severe congenital calcaneovalgus, the foot is dorsiflexed with respect to the talus. The defect is flexible and correctable.
Cavus Foot
Etiologies, Pathophysiology, and Clinical Presentation: An idiopathic congenital form of cavus foot exists, but more commonly, the finding is related to neuromuscular abnormalities, including peroneal muscular atrophy (Charcot-Marie-Tooth disease), Friedreich ataxia, myelomeningocele, poliomyelitis, and other paralytic conditions.89,90 A cavus foot may also result from treated clubfoot deformity.
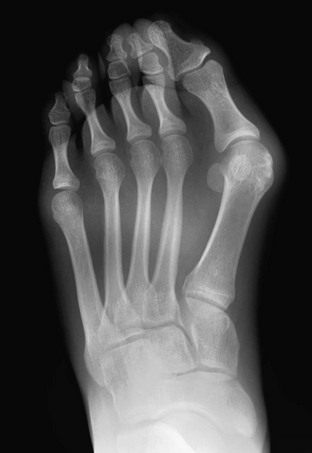
Hallux Valgus
Etiologies, Pathophysiology, and Clinical Presentation: Hallux valgus deformity begins to develop and may occasionally present in childhood.91 Hallux valgus affects females ten times as frequently as males. Patients present with pain and deformity which may interfere with proper shoe fitting.
Imaging: The upper normal limit of medial angulation of the proximal phalanx of the great toe to the first metatarsal is 14 to 16 degrees. Higher angles are considered hallux valgus (e-Fig. 134-32). With increasing angulation, the medial margin of the first metatarsal head may become more uncovered and protuberant.
Generalized Disorders
Overview: Children with cerebral palsy are afflicted with multiple orthopedic disorders requiring radiographic investigation and surveillance.102,103 Variable manifestations of cerebral palsy are caused by variation in severity and location of the original insult.102 In most cases, cerebral palsy is the consequence of fetal or perinatal insult to the brain, usually hypoxic–ischemic or hemorrhagic in nature. Cerebral palsy also occurs as the result of injury to the brain matter that occurs during early childhood.
Etiologies, Pathophysiology, and Clinical Presentation: The orthopedic disorders of cerebral palsy are caused by imbalanced muscular forces. Although cerebral palsy is a static encephalopathy, the resultant musculoskeletal disease may be progressive, even after skeletal maturity.
Imaging: About 25% of cerebral palsy patients have scoliosis.32,104 Scoliosis is more prevalent and severe with greater neurologic deficit. Scoliosis occurs as the result of imbalanced forces on the two sides of the spinal column. As opposed to the S-shaped curves of idiopathic scoliosis, curves in cerebral palsy often have a long C-shaped contour, and the curves may progress after skeletal maturation.32,94 Associated findings may include accentuated thoracic kyphosis or lumbar lordosis, spondylolysis or spondylolisthesis, and pelvic obliquity. Nonambulatory patients also show caninization of the vertebral bodies (narrow anteroposterior diameter) caused by lack of weightbearing forces during development.
Upper extremity defects include radial head dislocation (Fig. 134-33) and contractures at the elbow, wrist, and fingers.95,96 Kienböck disease and negative ulnar variance are of increased incidence in cerebral palsy, but a link between the two findings has not been shown in these patients.97 Accelerated degenerative changes in the elbow and wrist are common in older patients with cerebral palsy.95
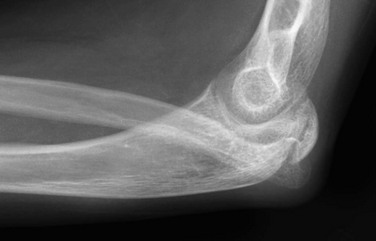
Figure 134-33 Chronic radial head dislocation in a 14-year-old boy with cerebral palsy.
The radial head is displaced posteriorly and has a rounded contour. The radius is bowed anteriorly. Similar findings were present on the opposite side.
Abnormalities at the hip include coxa valga caused by lack of weightbearing, increased femoral anteversion, prominence of the lesser trochanter caused by external rotation forces by the iliopsoas tendon, femoral head subluxation or dislocation, acetabular hypoplasia and dysplasia, and flattening of the femoral head (see Fig. 134-9).32,98 Posterior and superior acetabular deficiency is seen. Patients may experience pain with progressive hip subluxation, which progresses to dislocation. The “windswept” pelvis is seen when one hip has an adductor contracture and the other hip has an abductor contracture, reflecting the asymmetric neuromuscular defects often seen in nonambulatory patients with severe spasticity. Older patients with cerebral palsy show evidence of superimposed degenerative disease at the hips.
At the knee, flexion contractures may be present. The patella is often high (patella alta) and elongated.32,99 Tug lesions are common at the lower pole of the patella, producing a fragmented appearance.32,100 The tibial tuberosity may appear elevated and irregular. Genu recurvatum may be seen with rectus femoris contracture. Valgus is seen at the ankle, and equinus and hindfoot valgus are common in the foot (see e-Fig. 134-30).32,101 Osteopenia predisposes patients with cerebral palsy to fracture.102
Myelomeningocele
Overview: Children with myelomeningocele and related spinal disorders may be afflicted with multiple orthopedic disorders of the spine and lower extremity requiring radiographic investigation and surveillance.103,104 Variable manifestations of myelomeningocele relate to the level of the defect. Children with other spinal pathologies, including those suffering injury to the spinal cord at a young age, may develop orthopedic disorders similar to those with myelomeningocele.
Etiologies, Pathophysiology, and Clinical Presentation: Orthopedic disorders in myelomeningocele patients are caused by lack of normal muscle development and function. This leads to scoliosis and joint malalignments.
Imaging: Scoliosis in patients with myelomeningocele may be congenital or developmental and progressive.104 In 20% of patients with myelomeningocele, congenital scoliosis is often caused by vertebral segmentation errors. Congenital kyphosis is most common at L1-L2. Kyphosis may also be acquired.104 Lumbar lordosis is commonly accentuated.
One third to one half of patients with myelomeningocele have hip dysplasia. The hips are occasionally dislocated at birth; however, more often, dysplasia develops over time as the result of paralysis of the hip extensors and abductors with unopposed hip flexors and adductors (e-Fig. 134-34).104 Coxa valga and increased femoral anteversion are common.104 Excessive external tibial torsion with out-toeing or internal tibial torsion with in-toeing may be seen. In all, 80% to 95% of patients have foot deformities. Equinovarus (clubfoot), congenital vertical talus, and other foot deformities may also be seen in these patients.104
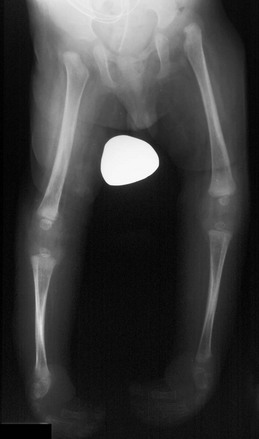
e-Figure 134-34 A 7-month-old boy with myelomeningocele.
Both hips are dislocated, and clubfeet deformities are present. The musculature is atrophic. A ventriculoperitoneal shunt is present.
Infants with myelomeningocele have an increased incidence of fracture of a lower extremity during birth.105 A higher incidence of fracture is seen with contractures and higher levels of spinal defect. Patients with myelomeningocele may develop neuropathic injuries as the result of osteoporosis and lack of sensation.104,106,107 Fractures are most commonly metaphyseal or diaphyseal, but they may occur through a physis. Periosteal new bone and callous may be exuberant because of delayed diagnosis without immobilization and abundant subperiosteal hemorrhage.104,106 Such injuries may be mistaken for tumor or infection.
Driscoll, SW, Skinner, J. Musculoskeletal complications of neuromuscular disease in children. Phys Med Rehabil Clin North Am. 2008;19:163–194.
Kling, TF. Angular deformities of the lower limbs in children. Orthop Clin North Am. 1987;18:513–527.
Morrell, DS, Pearson, JM, Sauser, DD. Progressive bone and joint abnormalities of the spine and lower extremities in cerebral palsy. Radiographics. 2002;22:257–268.
Ozonoff, MB. Pediatric orthopedic radiology, 2nd ed. Philadelphia: WB Saunders; 1992.
Oestreich, AE. How to measure angles from foot radiographs: a primer, 1st ed. New York: Springer-Verlag; 1990.
References
1. Martinoli, C, Bianchi, S, Santacroce, E, et al. Brachial plexus sonography: a technique for assessing the root level. AJR Am J Roentgenol. 2002;179:699–702.
2. Martinoli, C, Valle, M, Malattia, C, et al. Paediatric musculoskeletal US beyond the hip joint. Pediatr Radiol. 2011;41(suppl 1):S113–S124.
3. Pollock, AN, Reed, MH. Shoulder deformities from obstetrical brachial plexus paralysis. Skeletal Radiol. 1989;18:295–297.
4. Mintzer, CM, Waters, PM, Brown, DJ. Glenoid version in children. J Pediatr Orthop. 1996;16:563–566.
5. Pöyhiä, TH, Nietosvaara, YA, Remes, VM, et al. MRI of rotator cuff muscle atrophy in relation to glenohumeral joint incongruence in brachial plexus birth injury. Pediatr Radiol. 2005;35:402–409.
6. Waters, PM, Smith, GR, Jaramillo, D. Glenohumeral deformity secondary to brachial plexus birth palsy. J Bone Joint Surg Am. 1998;80:668–677.
7. Hernandez, RJ, Dias, L. CT evaluation of the shoulder in children with Erb’s palsy. Pediatr Radiol. 1988;18:333–336.
8. Gudinchet, F, Maeder, P, Oberson, JC, et al. Magnetic resonance imaging of the shoulder in children with brachial plexus birth palsy. Pediatr Radiol. 1995;25:S125–S128.
9. Pöyhiä, TH, Nietosvaara, YA, Remes, VM, et al. MRI of rotator cuff muscle atrophy in relation to glenohumeral joint incongruence in brachial plexus birth injury. Pediatr Radiol. 2005;35:402–409.
10. Hunter, JD, Franklin, K, Hughes, PM. The ultrasound diagnosis of posterior shoulder dislocation associated with Erb’s palsy. Pediatr Radiol. 1998;28:510–511.
11. Saifuddin, A, Heffernan, G, Birch, R. Ultrasound diagnosis of shoulder congruity in chronic obstetric brachial plexus palsy. J Bone Joint Surg Br. 2002;84:100–103.
12. Pöyhiä, TH, Lamminen, AE, Peltonen, JI, et al. Brachial plexus birth injury: US screening for glenohumeral joint instability. Radiology. 2010;254:253–260.
13. Waters, PM. Update on management of pediatric brachial plexus palsy. J Pediatr Orthop. 2005;25:116–126.
14. Pearl, ML. Shoulder problems in children with brachial plexus birth palsy: evaluation and management. J Am Acad Orthop Surg. 2009;17:242–254.
15. Zebala, LP, Manske, PR, Goldfarb, CA. Madelung’s deformity: a spectrum of presentation. J Hand Surg Am. 2007;32:1393–1401.
16. Arora, AS, Chung KC. Otto, W. Madelung and the recognition of Madelung’s deformity. J Hand Surg Am. 2006;31:177–182.
17. Schmidt-Rohlfing, B, Schwöbel, B, Pauschert, R, et al. Madelung deformity: clinical features, therapy and results. J Pediatr Orthop B. 2001;10:344–348.
18. Tuder, D, Frome, B, Green, DP. Radiographic spectrum of severity in Madelung’s deformity. J Hand Surg Am. 2008;33:900–904.
19. McCarroll, HR, Jr., James, MA, Newmeyer, WL, 3rd., et al. Madelung’s deformity: quantitative assessment of x-ray deformity. J Hand Surg Am. 2005;30:1211–1220.
20. Cook, PA, Yu, JS, Wiand, W, et al. Madelung deformity in skeletally immature patients: morphologic assessment using radiography, CT, and MRI. J Comput Assist Tomogr. 1996;20:505–511.
21. Hafner, R, Poznanski, AK, Donovan, JM. Ulnar variance in children—standard measurements for evaluation of ulnar shortening in juvenile rheumatoid arthritis, hereditary multiple exostosis and other bone or joint disorders in childhood. Skeletal Radiol. 1989;18:513–516.
22. Goldfarb, CA, Strauss, NL, Wall, LB, et al. Defining ulnar variance in the adolescent wrist: measurement technique and interobserver reliability. J Hand Surg Am. 2011;36:272–277.
23. Palmer, AK, Glisson, RR, Werner, FW. Ulnar variance determination. J Hand Surg Am. 1982;7:376–379.
24. Goeminne, S, Degreef, I, De Smet, L. Negative ulnar variance has prognostic value in progression of Kienböck’s disease. Acta Orthop Bel. 2010;76:38–41.
25. Zippel, H. Normal development of the structural elements of the hip joint in adolescence. Beitr Orthop Traumatol. 1971;18:255–270.
26. Robin, J, Kerr Graham, H, Selber, P, et al. Proximal femoral geometry in cerebral palsy: a population-based cross-sectional study. J Bone Joint Surg Br. 2008;90:1372–1379.
27. Fabry, G, MacEwen, GD, Shands, AR, Jr. Torsion of the femur. A follow-up study in normal and abnormal conditions. J Bone Joint Surg Am. 1973;55:1726–1738.
28. Beals, RK. Coxa vara in childhood: evaluation and management. J Am Acad Orthop Surg. 1998;6:93–99.
29. Aarabi, M, Rauch, F, Hamdy, RC, et al. High prevalence of coxa vara in patients with severe osteogenesis imperfecta. J Pediatr Orthop. 2006;26:24–28.
30. Pavlov, H, Goldman, AB, Freiberger, RH. Infantile coxa vara. Radiology. 1980;135:631–640.
31. Oh, C-W, Thacker, MM, Mackenzie, WG, et al. Coxa vara: a novel measurement technique in skeletal dysplasias. Clin Orthop Relat Res. 2006;447:125–134.
32. Morrell, DS, Pearson, JM, Sauser, DD. Progressive bone and joint abnormalities of the spine and lower extremities in cerebral palsy. Radiographics. 2002;22:257–268.
33. Bobroff, ED, Chambers, HG, Sartoris, DJ, et al. Femoral anteversion and neck-shaft angle in children with cerebral palsy. Clin Orthop Relat Res. 1999;364:194–204.
34. Gose, S, Sakai, T, Shibata, T, et al. Morphometric analysis of the femur in cerebral palsy: 3-dimensional CT study. J Pediatr Orthop. 2010;30:568–574.
35. Davids, JR, Marshall, AD, Blocker, ER, et al. Femoral anteversion in children with cerebral palsy: assessment with two and three-dimensional computed tomography scans. J Bone Joint Surg Am. 2003;85:481–488.
36. Hernandez, RJ, Tachdjian, MO, Poznanski, AK, et al. CT determination of femoral torsion. AJR Am J Roentgenol. 1981;137:97–101.
37. Jarrett, DY, Oliveira, AM, Zou, KH, et al. Axial oblique CT to assess femoral anteversion. AJR Am J Roentgenol. 2010;194:1230–1233.
38. Sugano, N, Noble, PC, Kamaric, E. A comparison of alternative methods of measuring femoral anteversion. J Comput Assist Tomogr. 1998;22:610–614.
39. Reference deleted in proofs.
40. Staheli, LT, Corbett, M, Wyss, C, et al. Lower-extremity rotational problems in children. Normal values to guide management. J Bone Joint Surg Am. 1985;67:39–47.
41. Lee, SH, Chung, CY, Park, MS, et al. Tibial torsion in cerebral palsy: validity and reliability of measurement. Clin Orthop Relat Res. 2009;467:2098–2104.
42. Dietz, FR. Intoeing—fact, fiction and opinion. Am Fam Physician. 1994;50:1249–1259. [1262-1264].
43. Do, TT. Clinical and radiographic evaluation of bowlegs. Curr Opin Pediatr. 2001;13:42–46.
44. Cheema, JI, Grissom, LE, Harcke, HT. Radiographic characteristics of lower-extremity bowing in children. Radiographics. 2003;23:871–880.
45. Jouve, JL, Kohler, R, Mubarak, SJ, et al. Focal fibrocartilaginous dysplasia (“fibrous periosteal inclusion”): an additional series of eleven cases and literature review. J Pediatr Orthop. 2007;27:75–84.
46. Bell, SN, Campbell, PE, Cole, WG, et al. Tibia vara caused by focal fibrocartilaginous dysplasia: three case reports. J Bone Joint Surg Br. 1985;67:780–784.
47. Yaniv, M, Becker, T, Goldwirt, M, et al. Prevalence of bowlegs among child and adolescent soccer players. Clin J Sport Med. 2006;16:392–396.
48. Laor, T, Wall, EJ, Vu, LP. Physeal widening in the knee due to stress injury in child athletes. AJR Am J Roentgenol. 2006;186:1260–1264.
49. Witvrouw, E, Danneels, L, Thijs, Y, et al. Does soccer participation lead to genu varum? Knee Surg Sports Traumatol Arthrosc. 2009;17:422–427.
50. Holt, JF, Latourette, HB, Watson, EH. Physiological bowing of the legs in young children. J Am Med Assoc. 1954;154:390–394.
51. Levine, AM, Drennan, JC. Physiological bowing and tibia vara: the metaphyseal-diaphyseal angle in the measurement of bowleg deformities. J Bone Joint Surg Am. 1982;64:1158–1163.
52. Salenius, P, Vankka, E. The development of the tibiofemoral angle in children. J Bone Joint Surg Am. 1975;57:259–261.
53. Ozonoff, MB. The lower extremity. In Pediatric orthopedic radiology, 2nd ed, Philadelphia, PA: WB Saunders; 1992:304–396.
54. Heath, CH, Staheli, LT. Normal limits of knee angle in white children—genu varum and genu valgum. J Pediatr Orthop. 1993;13:259–262.
55. Shopfner, CE, Coin, CG. Genu varus and valgus in children. Radiology. 1969;92:723–732.
56. Eggert, P, Viemann, M. Physiological bowlegs or infantile Blount’s disease. Some new aspects on an old problem. Pediatr Radiol. 1996;26:349–352.
57. Davids, JR, Blackhurst, DW, Allen, BL, Jr. Radiographic evaluation of bowed legs in children. J Pediatr Orthop. 2001;21:257–263.
58. Blount, WP. Tibia vara. J Bone Surg. 1937;19:1–29.
59. Blount, WP. Tibia vara, osteochondrosis deformans tibiae. Curr Pract Orthop Surg. 1966;3:141–156.
60. Sabharwal, S. Blount disease. J Bone Joint Surg Am. 2009;91:1758–1776.
61. Loder, RT, Johnston, CE, 2nd. Infantile tibia vara. J Pediatr Orthop. 1987;7:639–646.
62. Gettys, FK, Jackson, JB, Frick, SL. Obesity in pediatric orthopaedics. Orthop Clin North Am. 2011;42:95–105. [vii].
63. Langenskiöld, A. Tibia vara: a critical review. Clin Orthop Relat Res. 1989:195–207.
64. Iwasawa, T, Inaba, Y, Nishimura, G, et al. MR findings of bowlegs in toddlers. Pediatr Radiol. 1999;29:826–834.
65. Craig, JG, van Holsbeeck, M, Zaltz, I. The utility of MR in assessing Blount disease. Skeletal Radiol. 2002;31:208–213.
66. Lin, C-J, Lin, S-C, Huang, W, et al. Physiological knock-knee in preschool children: prevalence, correlating factors, gait analysis, and clinical significance. J Pediatr Orthop. 1999;19:650–654.
67. Green, WT, Wyatt, GM, Anderson, M. Orthoroentgenography as a method of measuring the bones of the lower extremity. J Bone Joint Surg Am. 1946;28:60–65.
68. Aaron, A, Weinstein, D, Thickman, D, et al. Comparison of orthoroentgenography and computed tomography in the measurement of limb-length discrepancy. J Bone Joint Surg Am. 1992;74:897–902.
69. Poutawera, V, Stott, NS. The reliability of computed tomography scanograms in the measurement of limb length discrepancy. J Pediatr Orthop B. 2010;19:42–46.
70. Friend, L, Widmann, RF. Advances in management of limb length discrepancy and lower limb deformity. Curr Opin Pediatr. 2008;20:46–51.
71. Ozonoff, MB. The foot. In Pediatric orthopedic radiology, 2nd ed, Philadelphia, PA: WB Saunders; 1992:397–460.
72. Vanderwilde, R, Staheli, LT, Chew, DE, et al. Measurements on radiographs of the foot in normal infants and children. J Bone Joint Surg Am. 1988;70:407–415.
73. Gentili, A, Masih, S, Yao, L, et al. Pictoral review: foot axes and angles. Br J Radiol. 69, 1996. [9688-974].
74. Cummings, RJ, Davidson, RS, Armstrong, PF, et al. Congenital clubfoot. J Bone Joint Surg Am. 2002;84:290–308.
75. Coley, BD, Shiels, WE, Kean, J, et al. Age-dependent dynamic sonographic measurement of pediatric clubfoot. Pediatr Radiol. 2007;37:1125–1129.
76. Shiels, WE, Coley, BD, Kean, J, et al. Focused dynamic sonographic examination of the congenital clubfoot. Pediatr Radiol. 2007;37:1118–1124.
77. Aurell, Y, Johansson, A, Hansson, G, et al. Ultrasound anatomy in the normal neonatal and infant foot: an anatomic introduction to ultrasound assessment of foot deformities. Eur Radiol. 2002;12:2306–2312.
78. Aurell, Y, Johansson, A, Hansson, G, et al. Ultrasound anatomy in the neonatal clubfoot. Eur Radiol. 2002;12:2509–2517.
79. Gore, AI, Spencer, JP. The newborn foot. Am Fam Physician. 2004;69:865–872.
80. Fridman, MW, de Almeida Fialho, HS. The role of radiographic measurements in the evaluation of congenital clubfoot surgical results. Skeletal Radiol. 2007;36:129–138.
81. Sullivan, JA. Pediatric flatfoot: evaluation and management. J Am Acad Orthop Surg. 1999;7:44–53.
82. McKie, J, Radomisli, T. Congenital vertical talus: a review. Clin Podiatr Med Surg. 2010;27:145–156.
83. Schlesinger, AE, Deeney, VF, Caskey, PF. Sonography of the nonossified tarsal navicular cartilage in an infant with congenital vertical talus. Pediatr Radiol. 1989;20:134–135.
84. Hutchinson, B. Pediatric metatarsus adductus and skewfoot deformity. Clin Podiatr Med Surg. 2010;27:93–104.
85. Napiontek, M. Skewfoot. J Pediatr Orthop. 2002;22:130–133.
86. Harris, EJ, Vanore, JV, Thomas, JL, et al. Diagnosis and treatment of pediatric flatfoot. J Foot Ankle Surg. 2004;43:341–373.
87. Rodriguez, N, Choung, DJ, Dobbs, MB. Rigid pediatric pes planovalgus: conservative and surgical treatment options. Clin Podiatr Med Surg. 2010;27:79–92.
88. Blitz, NM, Stabile, RJ, Giorgini, RJ, et al. Flexible pediatric and adolescent pes planovalgus: conservative and surgical treatment options. Clin Podiatr Med Surg. 2010;27:59–77.
89. Schwend, RM, Drennan, JC. Cavus foot deformity in children. J Am Acad Orthop Surg. 2003;11:201–211.
90. Allard, P, Sirois, JP, Thiry, PS, et al. Roentgenographic study of cavus foot deformity in Friedreich ataxia patients: preliminary report. Can J Neurol Sci. 1982;9:113–117.
91. Talab, YA. Hallux valgus in children: a 5-14-year follow-up study of 30 feet treated with a modified Mitchell osteotomy. Acta Orthop Scand. 2002;73:195–198.
92. Kerr Graham, H, Selber, P. Musculoskeletal aspects of cerebral palsy. J Bone Joint Surg Br. 2003;85:157–166.
93. Morrell, DS, Pearson, JM, Sauser, DD. Progressive bone and joint abnormalities of the spine and lower extremities in cerebral palsy. Radiographics. 2002;22:257–268.
94. Imrie, MN, Yaszay, B. Management of spinal deformity in cerebral palsy. Orthop Clin North Am. 2010;41:531–547.
95. Nishioka, E, Yoshida, K, Yamanaka, K, et al. Radiographic studies of the wrist and elbow in cerebral palsy. J Orthop Sci. 2000;5:268–274.
96. Abu-Sneineh, AK, Gabos, PG, Miller, F. Radial head dislocation in children with cerebral palsy. J Pediatr Orthop. 2003;23:155–158.
97. Leclercq, C, Xarchas, C. Kienbock’s disease in cerebral palsy. J Hand Surg Br. 1998;23:746–748.
98. Sauser, DD, Hewes, RC, Root, L. Hip changes in spastic cerebral palsy. AJR Am J Roentgenol. 1986;146:1219–1222.
99. Topoleski, TA, Kurtz, CA, Grogan, DP. Radiographic abnormalities and clinical symptoms associated with patella alta in ambulatory children with cerebral palsy. J Pediatr Orthop. 2000;20:636–639.
100. Kaye, JJ, Freiberger, RH. Fragmentation of the lower pole of the patella in spastic lower extremities. Radiology. 1971;101:97–100.
101. Davids, JR. The foot and ankle in cerebral palsy. Orthop Clin North Am. 2010;41:579–593.
102. Leet, AI, Mesfin, A, Pichard, C, et al. Fractures in children with cerebral palsy. J Pediatr Orthop. 2006;26:624–627.
103. Karol, LA. Orthopedic management in myelomeningocele. Neurosurg Clin North Am. 1995;6:259–268.
104. Westcott, MA, Dynes, MC, Remer, EM, et al. Congenital and acquired orthopedic abnormalities in patients with myelomeningocele. Radiographics. 1992;12:1155–1173.
105. Boytim, MJ, Davidson, RS, Charney, E, et al. Neonatal fractures in myelomeningocele patients. J Pediatr Orthop. 1991;11:28–30.
106. Kumar, SJ, Cowell, HR, Townsend, P. Physeal, metaphyseal, and diaphyseal injuries of the lower extremities in children with myelomeningocele. J Pediatr Orthop. 1984;4:25–27.
107. Lock, TR, Aronson, DD. Fractures in patients who have myelomeningocele. J Bone Joint Surg Am. 1989;71:1153–1157.