Algorithmic Approach to Diagnosis of Inflammatory Disorders of the Gastrointestinal Tract
Amitabh Srivastava
Gregory Y. Lauwers
Robert D. Odze
Introduction
The conventional approach to teaching inflammatory disorders of the gastrointestinal (GI) tract is to describe clinical and histologic features of individual disease entities. Unfortunately, mucosal biopsies of the GI tract are not normally given a specific diagnosis. Therefore, it is incumbent on the pathologist to evaluate all of the clinical, endoscopic, and histologic features in each case to establish a specific diagnosis. One of the biggest challenges in mucosal biopsy pathology is to recognize a specific morphologic pattern of injury. Use of standardized terminology is critical because each discrete morphologic pattern of injury has well-defined etiologic associations and therapeutic implications. For instance, a diagnosis of “Barrett’s esophagus” or “inflammatory bowel disease” leads to lifelong endoscopic surveillance of the patient and imposes a significant burden on patients and on the health care system. Use of nonstandardized terms such as “nonspecific esophagitis” or “nonspecific colitis” is misleading and, therefore, not helpful for patient management.
The purpose of this chapter is to outline a simple algorithmic approach to the diagnosis of common inflammatory disorders of the GI tract in mucosal biopsies. The process begins by dividing biopsies into one of several broad categories (e.g., normal, inflammatory, neoplastic) and is followed by a systematic evaluation of morphologic features to determine a specific morphologic pattern of injury. This approach often implies a fairly standard etiologic differential diagnosis.
Esophagus
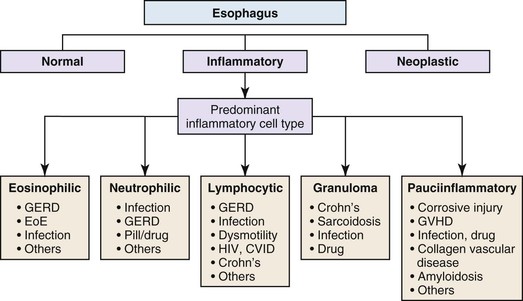
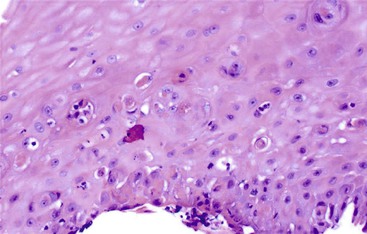
Esophageal biopsies can be broadly categorized as normal, inflammatory, or neoplastic (Fig. 13.1). Normal esophageal mucosa is composed of nonkeratinizing squamous epithelium with a basal zone that is one to three cell layers thick, papillae that are confined to the lower half of the mucosa, and lamina propria that is composed of loose connective tissue without inflammatory cells (Fig. 13.2). The presence of surface keratinization, parakeratosis, spongiosis, or inflammatory cells in the epithelium is a sign of mucosal injury and should lead to further evaluation of the biopsy specimen for a specific morphologic pattern of injury.
If a biopsy is considered inflammatory, the next step is to evaluate the dominant inflammatory cell type. Inflammation in the esophagus may be dominated by eosinophils, neutrophils, lymphocytes, granulomas, or a combination of these cells, and each of these categories has well-defined etiologic associations (see Fig. 13.1). Disorders with prominent eosinophilic and/or neutrophilic patterns of inflammation are classified as active esophagitis and are further graded as mild, moderate, or severe based on the severity of inflammation. As illustrated in Figure 13.1, gastroesophageal reflux disease (GERD) and infections may be associated with more than one pattern of inflammation; for that reason, they are always in the differential diagnosis of inflamed (or even near-normal) esophageal biopsies. However, endoscopic or histologic evidence of esophagitis is present in only approximately 40% of patients with well-established GERD.
Eosinophil-Predominant Esophagitis
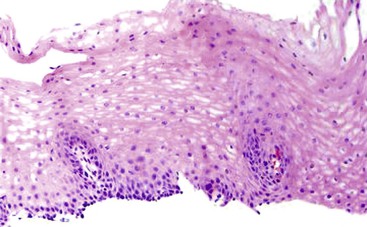
Intraepithelial eosinophilic infiltration is the most common pattern of inflammatory injury in esophageal biopsies. GERD and eosinophilic esophagitis (EoE) are the diseases most commonly associated with this pattern of injury. The presence of eosinophils in esophageal mucosa is not necessarily diagnostic of primary EoE, a well-defined clinicopathologic disorder which is discussed in detail in Chapter 14. No single clinical, endoscopic, histologic, or treatment parameter allows for absolute distinction of GERD from EoE. EoE is a clinicopathologic diagnosis and requires correlation with patient symptoms; endoscopic features; the density, pattern, and gradient of eosinophilic infiltration in mucosal biopsies; and treatment response. Some of the salient features that help distinguish GERD from EoE are summarized in Table 13.1 and illustrated in Fig. 13.3. Eosinophilic infiltration in esophageal mucosa may also be present in disorders other than GERD and EoE. A list of these disorders and the clinical or morphologic features that are helpful in making these diagnoses is presented in Table 13.2.
Table 13.1
Eosinophil-Predominant Inflammatory Pattern
| Features | Reflux Esophagitis | Eosinophilic Esophagitis |
| Clinical History | ||
| Allergy/asthma | Low incidence | High incidence |
| Food impaction | Uncommon | Common |
| Symptoms | ||
| GERD symptoms | Typical | May be present |
| Dysphagia | May be present | Common |
| Endoscopy | ||
| Rings | Uncommon | Typical |
| Linear furrows | Uncommon | Typical |
| Erosions/ulcers | Typical | Uncommon |
| pH/impedance | Abnormal | Normal |
| Pathology | ||
| Inflammation gradient | Distal >> Mid/upper | Mid/upper >> Distal |
| Eosinophilic infiltrate | ||
| Maximum no. | <10/HPF | >15/HPF |
| Clusters | No | Yes |
| Superficial aggregates | No | Yes |
| Microabscesses | No | Yes |
| BCH/spongiosis | Mild | Severe |
| Therapy | ||
| Response to PPIs | Yes | No |
| Response to steroids | No | Yes |
BCH, Basal cell hyperplasia; GERD, gastroesophageal reflux disease; HPF, high-power field; PPIs, proton pump inhibitors.
Table 13.2
Other Causes of Esophageal Eosinophilia
| Diagnosis | Diagnostic Features |
| Infections | Viral inclusions/parasites on biopsy |
| Eosinophilic gastroenteritis | Gastric/SI involvement |
| Achalasia | Motility studies |
| Hypereosinophilic syndrome | Peripheral blood hypereosinophilia |
| Crohn’s disease | Granulomas, colon/SI involvement |
| GVHD | Apoptosis, history of BMT |
| Drug hypersensitivity | Clinical history |
BMT, Bone marrow transplantation; GVHD, graft-versus-host disease; SI, small intestine.
Neutrophil-Predominant Esophagitis
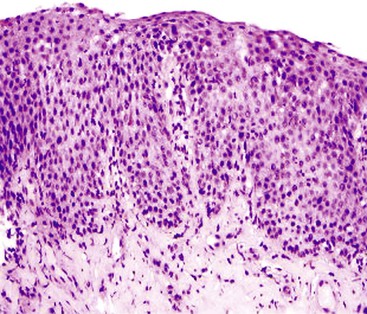
A neutrophil-predominant inflammatory pattern of injury may be associated with infections, GERD, pill esophagitis, or corrosive mucosal injury (Fig. 13.4), among other causes. The presence of surface hyperkeratosis in association with a neutrophilic inflammation should prompt a search for fungi, such as Candida. Yeast and pseudohyphae may, at times, be confined to surface keratinous debris and may be associated with either minimal or no inflammation in the epithelium or lamina propria. Cytomegalovirus (CMV) and herpes esophagitis may also be associated with a neutrophil-predominant pattern of inflammation, and these infections may be diagnosed when typical viral inclusions are demonstrated in the biopsy. A diagnosis of pill esophagitis should be considered when the inflammation, or ulcer, is present in the upper and middle levels of the esophagus. This diagnosis requires correlation with the patient’s medication history. Similarly, the presence of active esophagitis containing scattered atypical cells with large, hyperchromatic epithelial and/or stromal nuclei may be indicative of radiation esophagitis. Corrosive mucosal injury often leads to necrosis of the upper half of the squamous epithelium, and a band of neutrophils may be present between the superficial necrotic and deeper viable mucosa.
Lymphocyte-Predominant Esophagitis
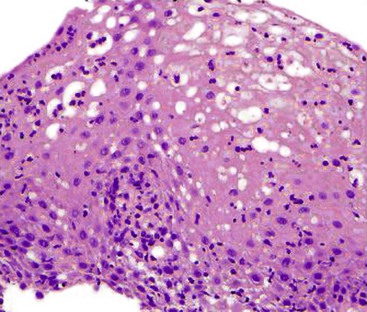
Intraepithelial lymphocytosis of variable severity is not an uncommon finding in esophageal biopsies. When it is associated with basal cell hyperplasia and scattered eosinophils and/or neutrophils, it is most likely a result of GERD. The presence of hyperkeratosis and scattered neutrophils should always raise suspicion for Candida esophagitis. The presence of a severe (and pure) lymphocytic infiltrate, particularly in the peripapillary epithelium, is diagnostic for lymphocytic esophagitis (Fig. 13.5). Lymphocytic esophagitis is a poorly defined entity with myriad etiologic associations; the most notable are motility disorders and Crohn’s disease. Crohn’s disease manifests in the esophagus with a lymphocytic esophagitis pattern of injury more often in pediatric patients than in adults. Other disorders that cause intraepithelial lymphocytosis in the esophagus are listed in Box 13.1. When the lymphocytic infiltrate is distributed predominantly at the epithelial lamina propria interface and scattered necrotic keratinocytes are present, the possibility of a dermatologic disorder such as lichen planus should be considered.
Granulomas
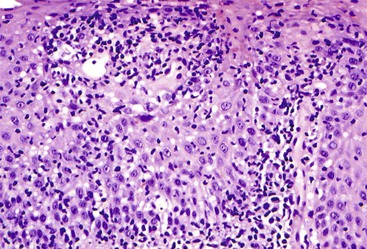
Granulomatous esophagitis (Fig. 13.6) is associated with Crohn’s disease or sarcoidosis in almost all cases. Rare causes of granulomatous esophagitis include mycobacterial or fungal infection, vasculitis, and drug-induced mucosal injury.
Pauciinflammatory Esophagitis
A list of pauciinflammatory disorders is shown in Table 13.3. Graft-versus-host disease (GVHD) is the disorder that most frequently manifests with this pattern of mucosal injury (Fig. 13.7). Apoptosis and necrosis in the absence of inflammation, characteristic of GVHD, overlaps with mycophenolate-induced mucosal injury. Infections in immunocompromised patients also may lack an inflammatory response. Amyloidosis is another disorder that may be easily missed because the mucosa is typically completely normal and the diagnostic changes are subtle, usually confined to the blood vessels in the lamina propria and superficial submucosa.
Table 13.3
Pauciinflammatory Pattern of Mucosal Injury in the Esophagus
| Diagnosis | Features |
| CMV, HSV | Viral inclusions |
| GVHD, mycophenolate | Increased apoptosis |
| Corrosive injury | Superficial necrosis |
| Taxol, colchicine toxicity | Mitotic arrest |
| Skin disorders (pemphigus) | Vesicles, bullae |
| Scleroderma | Submucosal fibrosis |
| Amyloidosis | Perivascular deposits |
CMV, Cytomegalovirus; GVHD, graft-versus-host disease; HSV, herpes simplex virus.
Ulcers
In some instances, one may see ulcerated granulation tissue, without viable mucosa, in a biopsy of the esophagus. A specific diagnosis cannot be rendered in most of these cases. However, biopsies with this pattern of injury should be evaluated for viral inclusions, pill fragments, and neoplastic cells before they are dismissed as “nonspecific.”
Gastroesophageal Junction
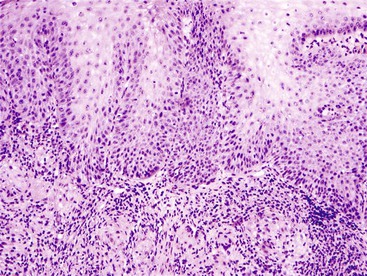
The anatomic gastroesophageal junction (GEJ) is defined, endoscopically, as the proximal limit of the gastric rugal folds. The squamocolumnar junction (SCJ) represents the point of transition from pearly-gray squamous mucosa of the esophagus to salmon-red columnar mucosa of the proximal stomach (or the distal esophagus in cases of Barrett’s esophagus). In healthy individuals, the SCJ is located at the GEJ, whereas in patients with Barrett’s esophagus (BE), the SCJ is located proximal to the anatomic GEJ. When an endoscopic biopsy is submitted with a question of BE, it is imperative for the pathologist to know the precise location of the biopsy, even before histologic evaluation is performed. Biopsies labeled as “GEJ,” “SCJ,” or “Z-line” cannot be definitely labeled as BE, even when goblet cells are identified in the biopsy, unless there are morphologic features in the biopsy specimen that help determine that the columnar epithelium is located in the anatomic esophagus. These features include multilayered epithelium, mucosal or submucosal glands or ducts (Fig. 13.8), subsquamous (“buried”) glands, or hybrid epithelium. A definite diagnosis of BE can be rendered only in biopsies obtained from (and confined to) the distal esophagus and in which goblet cells are identified in the columnar epithelium.
Ultimately, the differential diagnosis of goblet cells in a GEJ biopsy includes BE and carditis with intestinal metaplasia. Distinction between these two conditions is important, because patients with BE require lifelong surveillance with biopsies for early detection of dysplasia or cancer. Carditis with intestinal metaplasia is most often caused by Helicobacter pylori infection, whereas BE is a complication of long-standing GERD. The epidemiologic, clinical, and pathologic differences between these two conditions and the methods of distinguishing them in mucosal biopsies from the GEJ region are discussed in Chapter 14.
Stomach
General Comments
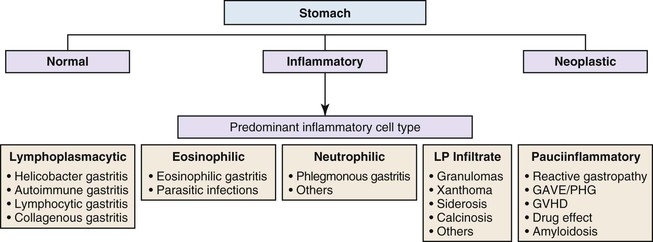
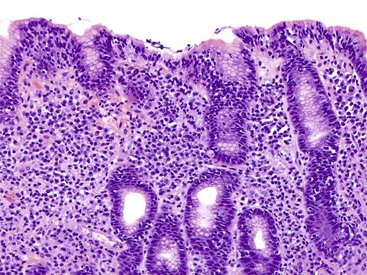
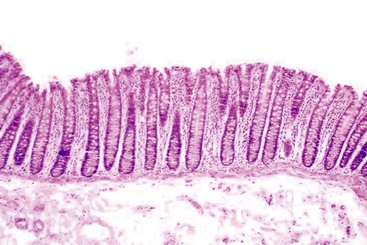
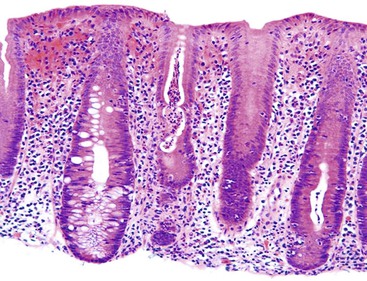
Gastric mucosal biopsies may be broadly categorized on morphologic evaluation as either normal, inflammatory, or neoplastic. Another category consists of lamina propria infiltrates or pigment deposition in the absence of significant lymphoplasmacytic, neutrophilic, or eosinophilic inflammation (Fig. 13.9). Normal gastric corpus mucosa is composed of closely packed oxyntic glands, whereas the antrum is composed of pure mucus glands (Fig. 13.10). In older children and young adults, both compartments show minimal intervening stroma and few or no scattered plasma cells in the lamina propria. However, with advancing patient age, a mild lymphocytic or plasma cell infiltrate is considered “normal” if not associated with epithelial injury or repair. Primary lymphoid follicles without active germinal centers are normally present at the base of gastric corpus mucosa and should not be considered a manifestation of chronic gastritis. However, lymphoid aggregates in the antrum, lymphoid aggregates with active germinal centers, and the presence of a band-like lymphoplasmacytic infiltrate beneath the surface epithelium are all features of chronic gastritis.
Gastritis
Determination of the predominant inflammatory cell type in the lamina propria is the first step in diagnosing gastritis (see Fig. 13.9). A lymphoplasmacytic infiltrate is by far the most common type of inflammatory reaction seen in gastric biopsies in routine practice. This pattern (also broadly referred to as chronic gastritis) includes several well-defined clinicopathologic entities. This pattern of inflammation should be evaluated for pattern of injury (focal versus diffuse), region of the stomach predominantly involved (antrum versus corpus or both), location in the mucosa (superficial versus deep), degree of active inflammation, presence or absence of microorganisms, atrophy of oxyntic glands, endocrine cell hyperplasia, intraepithelial lymphocytes (IELs), and subepithelial collagen layer, among others features (Table 13.4).
Table 13.4
Differential Diagnosis of Diffuse Chronic Gastritis Pattern of Injury
| Feature | Helicobacter | Other Infectious | Autoimmune | Lymphocytic | Collagenous | Eosinophilic |
| Lymphoplasmacytic infiltrate | +++ | ++ | ++ | ++ | ++ | + |
| Antral predominance | +++ | − | − | ± | ± | − |
| Superficial bandlike infiltrate | +++ | ± | − | ± | ± | − |
| Microorganisms or viral inclusions | + | + | − | − | − | − |
| Oxyntic gland atrophy | ± | ± | +++ | − | − | − |
| Endocrine cell hyperplasia | − | − | +++ | − | − | − |
| Increased IELs | ± | − | − | +++ | ± | − |
| Thick subepithelial collagen layer | − | − | − | − | +++ | − |
| Eosinophil clusters, aggregates, and epithelial damage | − | ± | − | − | ± | +++ |
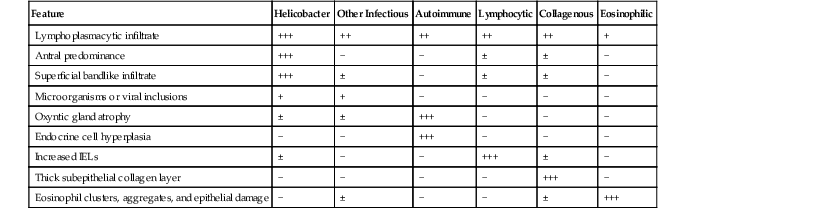
IELs, Intraepithelial lymphocytes; −, absent; ±, may be present; +, mild; ++, moderate; +++, marked.
Focal Gastritis
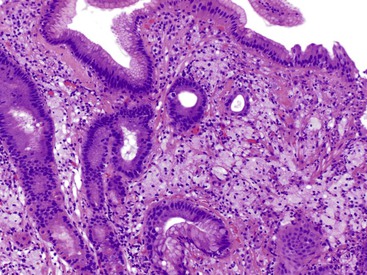
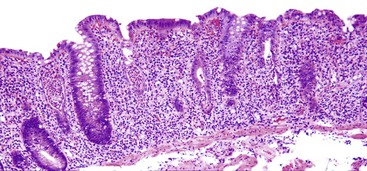
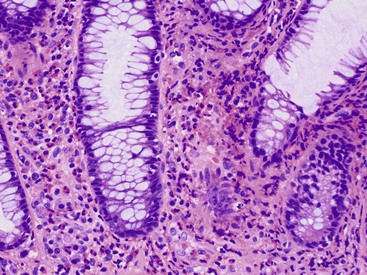
Focal active gastritis may be a gastric manifestation of inflammatory bowel disease (IBD), particularly in pediatric patients (Fig. 13.11). It may also be seen in children with autism. The etiologic associations of focal active gastritis in adults are diverse, and the association with IBD is weak. Infections such as CMV and adenovirus, Helicobacter heilmannii, and drug reactions can also cause focal active gastritis.
Diffuse Gastritis
Lymphoplasmacytic
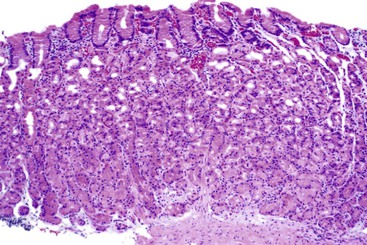
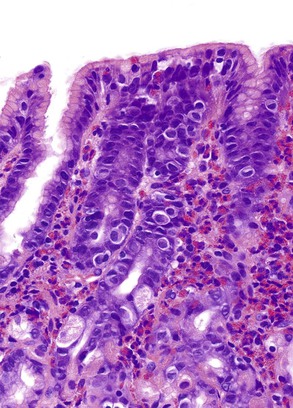
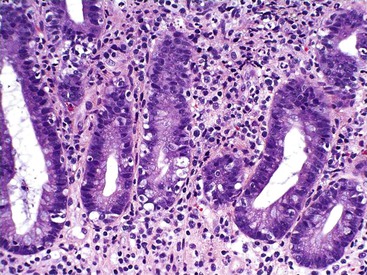
The two diseases most commonly encountered in routine clinical practice in association with diffuse chronic gastritis with lymphoplasmacytic inflammation are H. pylori infection and autoimmune gastritis (Fig. 13.12). The presence of a neutrophilic infiltrate should always raise suspicion of H. pylori gastritis, but an active inflammatory component may be present in almost any type of chronic gastritis, such as autoimmune, environmental, drug-induced, lymphocytic, or collagenous gastritis. Helicobacter gastritis is an antral-predominant gastritis with a superficial bandlike inflammatory infiltrate beneath the surface foveolar epithelium. It is associated with a variable active (neutrophilic) component. The latter finding may be mild in cases of H. heilmannii gastritis. Autoimmune gastritis involves the corpus, causing atrophy of the oxyntic glands, and leads to intestinal metaplasia, endocrine cell hyperplasia, and eventually gastric carcinoids.
Intraepithelial Lymphocytes
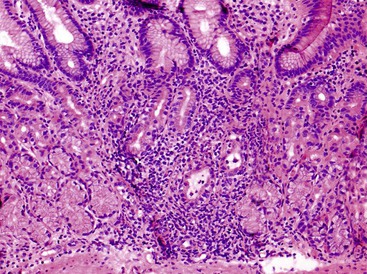
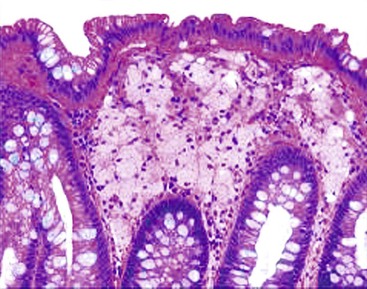
Increased IELs (>25 per 100 epithelial cells) in the absence of other significant pathologic changes is diagnostic of lymphocytic gastritis (Fig. 13.13). This condition usually manifests as pangastritis, but the disease may be patchy in some cases. Common etiologic associations of this pattern of mucosal injury include celiac disease, hypersensitivity response to H. pylori infection, human immunodeficiency virus (HIV) infection, and drugs (e.g., ticlopidine).
Thick Subepithelial Collagen
Thickening of the subepithelial collagen layer is rarely seen in gastric biopsies, but this pattern of injury encompasses two distinct clinical disorders. Collagenous gastritis in pediatric patients usually involves only the stomach, whereas in adults it typically occurs in association with involvement of the small intestine (collagenous sprue) or colon (collagenous colitis) or both. The inflammatory component in collagenous gastritis may be dominated by IELs or eosinophils in the lamina propria and some cases may be misdiagnosed as “lymphocytic” or “eosinophilic” gastritis if attention is not paid to the character of the subepithelial collagen layer.
Increased Eosinophils
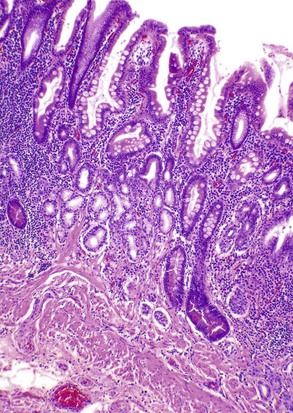
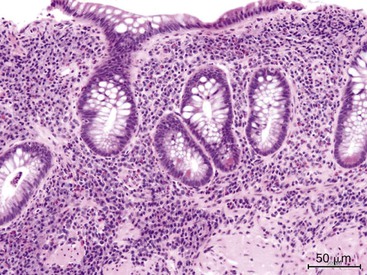
Eosinophilic gastritis is characterized by an eosinophil-predominant infiltrate (Fig. 13.14). It is composed of large clusters and aggregates of eosinophils in the lamina propria and epithelium. A clinical history of asthma, food allergies, connective tissue disorder, or drug intake is typically present. Rarely, parasitic infection may lead to eosinophilic gastritis.
Increased Neutrophils
A pure neutrophilic pattern of inflammation (without lymphocytes or plasma cells) is rare but is characteristic of phlegmonous gastritis. In this condition, severe active inflammation is centered in the submucosa, whereas the overlying mucosa is typically completely normal. This disease has a high mortality rate unless prompt clinical intervention, often in the form of gastrectomy, is instituted.
Pauciinflammatory Gastritis (“Reactive Gastritis”)
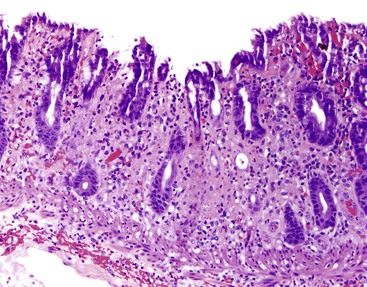
A pauciinflammatory pattern of gastric mucosal damage is typically associated with injury from noxious stimuli, such as nonsteroidal antiinflammatory drugs (NSAIDs), steroids, alcohol, or bile reflux (Fig. 13.15). It is also a common pattern of repair and regeneration after gastric injury from many causes (e.g., ulcers). The characteristic morphologic features include prominent regenerative epithelial changes in the absence of a significant inflammatory infiltrate. Surface erosions or ulcers and prominent lamina propria hemorrhage are features of “acute erosive” and “acute hemorrhagic” gastritis, respectively, but these are simply severe forms of reactive gastropathy. Use of one or more drugs or bile reflux may lead to erosive or hemorrhagic gastritis. The presence of marked foveolar hyperplasia composed of corkscrew-shaped (regenerative) gastric foveolar epithelium is typical of reactive gastropathy in general.
The morphologic clues to the correct diagnosis in diseases that share a pauciinflammatory pattern of injury are summarized in Table 13.5. Prominent apoptotic activity, particularly in the antrum, is typical of GVHD. Blood vessels should always be evaluated for amyloidosis before a biopsy is diagnosed as “normal.” Infections in immunocompromised hosts can produce a pauciinflammatory pattern of injury. These include CMV, adenovirus, and even severe fungal infections such as Aspergillus.
Table 13.5
Differential Diagnosis of Pauciinflammatory Pattern of Injury in the Stomach
| Feature | Reactive Gastropathy | GVHD | GAVE | PHG | Amyloidosis |
| Foveolar hyperplasia | +++ | − | ± | − | − |
| Mucin depletion | +++ | − | − | − | − |
| Vascular ectasia | + | − | +++ | +++ | − |
| Distribution of injury | Antrum > Corpus | Antrum > Corpus | Antrum | Corpus | Antrum or corpus |
| Fibrin thrombi | − | − | +++ | − | − |
| Prominent submucosal vessels on endoscopy | − | − | − | +++ | − |
| Increased apoptosis | − | +++ | − | − | − |
| Perivascular deposits | − | − | − | − | +++ |
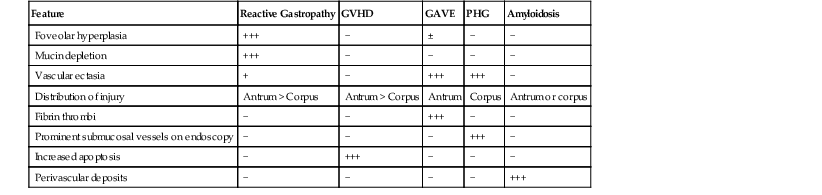
GAVE, Gastric antral vascular ectasia; GVHD, graft-versus-host disease; PHG, portal hypertensive gastropathy; −, absent; ±, may be present; +, mild; +++, marked.
Infiltrates
The lamina propria should always be inspected carefully for infiltrates, and in females, metastatic breast cancer should always be ruled out. Granulomas in the stomach are a manifestation of Crohn’s disease or sarcoidosis in most instances. Other infectious and noninfectious causes of granulomatous gastritis are summarized in Table 13.6. Foamy histiocytes with abundant cytoplasm and small, grooved, pale nuclei are typical of mucosal xanthoma (Fig. 13.16). At endoscopy, this may appear as small, white or yellow nodules or plaques. Iron and calcium deposits are the two most common types of pigment deposition in gastric mucosal biopsies. Iron deposition may result from iron pill gastritis or hemochromatosis, among other causes. Gastric mucosal calcinosis may occur in organ transplant recipients, or in patients with end-stage renal failure who are consuming aluminum hydroxide–containing antacids or sucralfate.
Table 13.6
Granulomatous Gastritis
| Infectious | Noninfectious |
| Mycobacterial | Crohn’s disease |
| Candida, aspergillosis | Sarcoidosis |
| Mucormycosis | Foreign body |
| Histoplasmosis | Cancer |
| Blastomycosis | Chronic granulomatous disease |
| Anisakis, Strongyloides stercoralis | Idiopathic |
| Schistosoma mansoni |
Small Intestine
General Comments
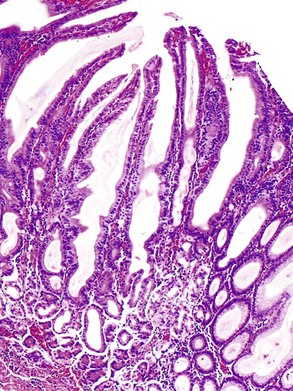
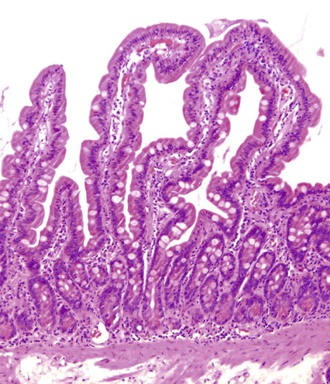
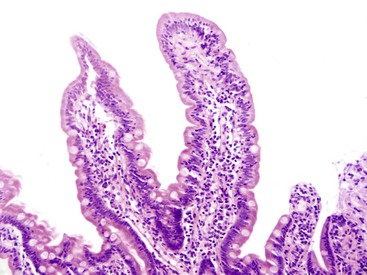
Small intestine biopsies may be broadly categorized as normal, inflamed, or neoplastic (Fig 13.17). Normal small-intestinal mucosa shows a crypt-to-villus ratio between 1 : 3 and 1 : 5. The normal mucosal immune system of the small intestine is composed of lymphoid aggregates and scattered IELs, particularly in epithelium overlying lymphoid follicles, and an admixture of inflammatory cells including lymphocytes, plasma cells, eosinophils, and mast cells (Fig. 13.18). Therefore, suspicion of an inflammatory disorder is based on an expansion of the lamina propria with inflammatory cells, the presence of an increased number of IELs, or both. Inflammatory disorders of the small intestine may also show a variable degree of villous atrophy. As a result, inflammatory disorders are also broadly grouped into those that often cause complete loss of normal villous architecture and those in which there is partial or no loss of villous architecture.
Complete Villous Atrophy
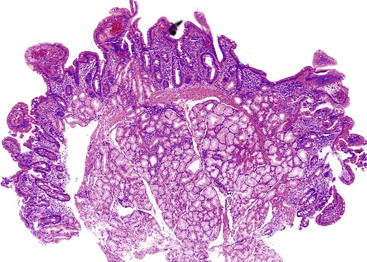
Celiac disease is, by far, the most common disorder associated with complete villous atrophy (Fig. 13.19). A systematic approach to small intestine disorders with complete villous atrophy also requires evaluation for the presence and degree of intraepithelial lymphocytosis, atypical IELs, subepithelial collagen thickness, active (neutrophilic) inflammation, eosinophils in the lamina propria, goblet cells, and apoptosis. The specific disorders that manifest with these features are summarized in Table 13.7.
Table 13.7
Differential Diagnosis of Duodenal Mucosa with Complete Villous Atrophy and Increased Intraepithelial Lymphocytes
| Feature | Celiac Disease | Refractory Sprue | Collagenous Sprue | Inflammatory Bowel Disease | Autoimmune Enteropathy |
| Ileal and colonic involvement | ± | − | − | +++ | ± |
| Active inflammation | ± | ± | ± | +++ | ± |
| Granulomas | − | − | − | +++ | − |
| Absent goblet cells | − | − | − | − | +++ |
| Increased apoptosis | − | − | − | − | +++ |
| Abnormal T cells | − | +++ | − | − | − |
| Thick subepithelial collagen | − | − | +++ | − | − |
| Antibodies | Anti-tTG | Anti-tTG | Anti-tTG | − | Anti-enterocyte; anti-goblet cell |
| Response to gluten withdrawal | +++ | − | − | − | − |
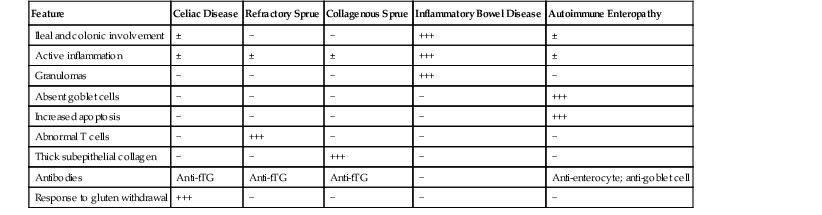
tTG, Tissue transglutaminase; −, absent; ±, may be present; +, mild; +++, marked.
Celiac disease is typically associated with a marked increase in IELs (>30 per 100 epithelial cells). In patients with a known history or serologic suspicion of celiac disease, the possibility of collagenous sprue or refractory celiac disease should always be considered. Collagenous sprue is characterized by the presence of an irregular, thickened subepithelial collagen layer. Establishing a diagnosis of refractory celiac disease requires additional tests, such as immunohistochemical evaluation of the type of intraepithelial lymphocytic infiltrate, flow cytometry (for loss of surface T-cell antigens), and, in some cases, polymerase chain reaction (for assessment of clonality of lymphocytes). Typically, celiac disease and type 1 refractory celiac disease show increased CD3- and CD8-positive IELs, whereas type 2 refractory celiac disease shows loss of CD8 in a significant proportion of infiltrating T lymphocytes. A diagnosis of type 2 refractory celiac disease is clinically significant because of the high risk for enteropathy-associated T-cell lymphoma (EATL). The salient clinical and pathologic differences between type 1 and type 2 refractory celiac disease are summarized in Table 13.8.
Table 13.8
Type 1 versus Type 2 Refractory Celiac Disease
| Feature | Type 1 | Type 2 |
| Aberrant T cells | − | + |
| Clonal TCR | − | + |
| HLA DQ2 homozygous | ± | + |
| Associated UJ/EATL | − | + |
| Immunosuppression response | + | − |
| EATL risk | Low | As much as 60% at 5 yr |
| Mortality | Slight increase | <50% at 5 yr |
EATL, Enteropathy-associated T-cell lymphoma; HLA, human leukocyte antigen; TCR, T-cell receptor; UJ, ulcerative jejunitis; −, absent; ±, may be present; +, present.
Complete villous atrophy with increased IELs may also be seen in other autoimmune or immunodeficiency disorders, including HIV infection, common variable immunodeficiency (CVID), and autoimmune enteropathy (AIE). Patients with AIE reveal circulating anti-enterocyte and anti-goblet cell antibodies and an absence of goblet cells in the mucosa, either with or without increased apoptotic activity. IELs are also increased, which may lead to a misdiagnosis of celiac disease. In patients with a history of celiac disease unresponsive to gluten withdrawal, the possibility of AIE should be considered strongly, particularly if the symptoms are severe. A similar pattern of mucosal injury may also be seen in some patients with bacterial overgrowth. The nitrogen breath test and evaluation of the patient’s response to antibiotics are helpful in diagnosing this condition.
Active inflammation (neutrophils or eosinophils), is not uncommon in patients with celiac disease. However, the presence of a significant increase in IELs is etiologically more important than active crypt inflammation in diagnosing celiac disease. Nevertheless, the presence of neutrophils in the lamina propria or in crypt epithelium should always raise suspicion of an infectious or other inflammatory process. Increased eosinophils in the epithelium and lamina propria, when associated with crypt damage, are suspicious for eosinophilic gastroenteritis.
Increased apoptosis in patients with complete villous atrophy should prompt an additional workup for AIE. Loss of goblet cells in mucosal biopsies is seen in only approximately 50% of cases of AIE, so workup for anti-enterocyte antibodies is an important step in establishing this diagnosis. If the serologic tests are not available, the patient’s response to a trial of steroids is also helpful in diagnosing AIE. Increased apoptotic activity may be present in patients with HIV infection, mycophenolate toxicity, or GVHD as well.
Partial Villous Atrophy
The differential diagnosis of inflammatory disorders of the small intestine associated with partial villous atrophy is extensive. Broadly, they are divided into those conditions that are associated with increased IELs and those that are associated with an increased inflammatory infiltrate in the lamina propria but without an increase in IELs. The differential diagnosis and approach to patients with partial villous atrophy and increased IELs is similar to that used for patients with complete villous atrophy and increased IELs (discussed earlier). Biopsies showing increased inflammation but without any increase in IELs can be further evaluated according to the type of inflammatory infiltrate.
Lymphoplasmacytic
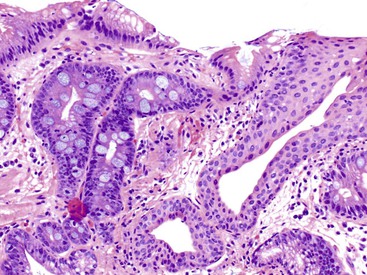
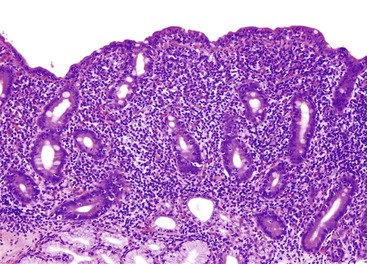
Increased lymphocytes and plasma cells, associated with partial villous atrophy and an absence of increased IELs, is commonly seen in patients with peptic duodenitis or IBD (Crohn’s disease, and, rarely, ulcerative colitis). The presence of mucinous metaplasia is a sign of chronic injury, and this feature can be seen in these disorders and many others (Fig. 13.20). Involvement with IBD is characterized by a more severe inflammatory infiltrate, a greater degree of active inflammation, granulomas, and concurrent involvement of the ileum or colon or both. H. pylori gastritis and NSAIDs may be associated with peptic duodenitis.
Eosinophils
Increased eosinophils may be seen in parasitic infections, eosinophilic gastroenteritis, drug hypersensitivity reactions, and mastocytosis (Fig. 13.21). Eosinophilic gastroenteritis occurs in mucosal, mural, and serosal forms. However, only the mucosal form can be excluded with certainty in biopsy specimens.
Lamina Propria Infiltrates
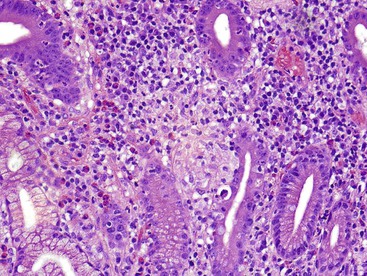
Granulomatous inflammation of the small intestine is most commonly a manifestation of Crohn’s disease or sarcoidosis. The presence of a diffuse histiocytic infiltrate in the lamina propria (Fig. 13.22) should raise concern for atypical mycobacterial infection or Whipple disease. Histochemical stains demonstrate acid-fast bacilli in the former case, and periodic acid–Schiff stain with diastase digestion (d-PAS) demonstrates typical positive macrophages in Whipple disease.
Normal Villous Architecture
Many disorders of the small intestine are associated with normal villous architecture. For instance, infection with Giardia or Cryptosporidium usually does not incite a significant inflammatory response but may cause increased IELs. Other conditions include abetalipoproteinemia (cytoplasmic vacuolation in enterocytes), CVID (absence of plasma cells in lamina propria), and amyloidosis.
The differential diagnosis of small-intestinal biopsies with normal villous architecture and increased IELs (Fig. 13.23) is broad and includes latent celiac disease, nongluten food hypersensitivity, tropical sprue, bacterial overgrowth, immunodeficiency disorders, and, in rare instances, IBD.
Colon
General Comments
The diagnostic approach to interpretation of colon biopsies is similar to that recommended in other portions of the GI tract, with a few exceptions. On initial review, it is helpful to place the colon biopsy in one of four broad categories: normal, inflammatory, lamina propria infiltrate, or neoplasia. In contrast to biopsies obtained from upper GI endoscopy, those obtained by colonoscopy often show mild pathologic abnormalities and artifacts as a result of the bowel preparation procedure.
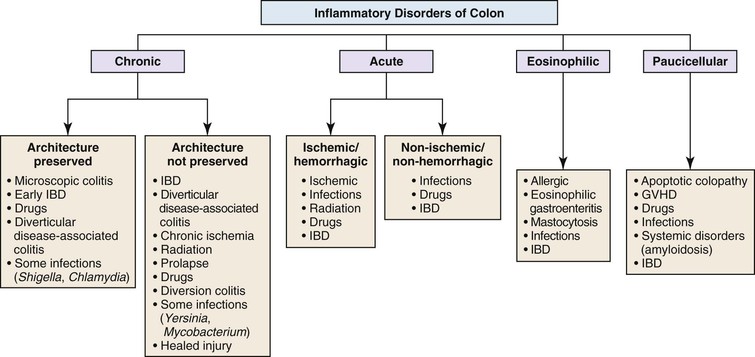
On initial evaluation of mucosal biopsies of the colon, inflammatory disorders may be separated into four general categories: chronic, acute, eosinophilic, and paucicellular (Fig. 13.24). In contrast to noncolonic biopsies, the degree of preservation or disturbance of crypt architecture is an important discriminatory feature that helps pathologists distinguish certain categories of inflammatory disorders. Interpretation of colon biopsies should incorporate all clinical (particularly endoscopic) information about the patient, with particular emphasis on the anatomic distribution of endoscopic abnormalities. Clinical information such as the timing and sequence of colonic symptoms and signs, medication history, prior illnesses of the patient and family members, and, most importantly, the type and quality of diarrhea (e.g., bloody versus nonbloody) is helpful when evaluating colon biopsies. For instance, almost all patients with ulcerative colitis have bouts of bloody diarrhea, whereas patients with microscopic colitis or an infiltrative disorder have mainly nonbloody (“watery”) diarrhea.
In some instances, it is perfectly acceptable to use a broad pattern of colitis as a diagnostic term in pathology reports, as is often done with upper GI biopsies. In these instances, it is frequently helpful to offer clinicians a differential diagnosis of common causes of the particular pattern of colitis recognized by the pathologist. Ultimately, it is incumbent on the physician to interpret the diagnostic pattern of colitis in conjunction with the clinical and endoscopic findings to formulate a priority diagnosis. Diagnoses such as “chronic active colitis” or “acute hemorrhagic colitis” are usually sufficient to guide patient management.
Normal versus Abnormal
As mentioned earlier, colon biopsies that do not have an obvious inflammatory component, lamina propria infiltrate, or neoplastic lesion should be evaluated initially in regard to whether they appear within normal limits given the effects of bowel preparation (Fig. 13.25 and Box 13.2; see also Chapter 1). In terms of normal variations, the distal several centimeters of rectum is an area often subject to chronic and repeated injury. As a result, with advancing age, it often shows a slight degree of crypt atrophy and distortion. This finding should not be overinterpreted as evidence of chronic colitis. Because of the normal undulation of the mucosa in the colon, superficial or tangential biopsies may show branching crypts. If this is present in the superficial portion of the mucosa, it is considered normal.
The quantity of lamina propria inflammation, and particularly the number of eosinophils, varies throughout the colon and among people in different geographic regions of the world. For instance, the right colon often shows a slightly greater degree of lamina propria mononuclear inflammation compared with the left colon. In addition, lamina propria eosinophils are typically more numerous in the right colon, but this varies significantly according to geographic region and climate. The osmotic effect of most bowel preparatory agents can result in clumping of lymphocytes and plasma cells within the lamina propria, edema, and even congestion and hemorrhage, the latter possibly related to endoscope trauma as well. Other effects of bowel preparatory procedures include a mild degree of surface epithelial degeneration, crypt regeneration, and apoptosis. In some instances, apoptoses are quite prominent and may lead to a false diagnosis of apoptotic colopathy. Infiltration of the superficial aspects of lymphoid aggregates by neutrophils may also develop as a result of bowel preparation. Insufflation of air at the time of endoscopy can lead to the acquisition of air vacuoles within the mucosa and submucosa, termed “pseudolipomatosis.” However, in contrast to a true lipoma, air vacuoles are not lined by lipocytes; instead, they are bordered by lamina propria or submucosal tissue, and they exhibit substantial variation in size and shape.
Inflammatory Disorders
Chronic Colitis
Biopsies of the colon that show increased inflammation (colitis) may be initially classified as either chronic, acute, eosinophilic, or paucicellular for the purpose of determining the most likely pattern of colitis and, ultimately, the etiology of the inflammatory disorder. Separation into acute or chronic types is performed by identifying histologic features of chronicity (see Chapter 17). Table 13.9 provides a list of features of chronicity and activity in the colon. The finding of one or more features of chronicity is sufficient to diagnose colitis as chronic, whereas the presence of one or more features of activity indicates an active (acute) phase of disease. Therefore, biopsies that show chronicity are best categorized as either chronic inactive or chronic active (the latter graded as mild, moderate, or severe). Biopsies that show features of activity without features of chronicity are interpreted as active or acute colitis.
Table 13.9
Inflammatory Disorders of the Colon
| Features of Chronicity (“Chronic Colitis”) |
Features of Activity (“Active Colitis”) |
| Crypt distortion/atrophy | Epithelial degeneration |
| Basal plasmacytosis | Epithelial regeneration |
| Diffuse mixed inflammation | Edema |
| Basal lymphoid aggregates | Hemorrhage |
| Metaplasia (Paneth, pyloric, other) | Necrosis |
| Paneth hyperplasia | Erosion/ulcer |
| Fibrosis/collagen | Neutrophils |
| Granulomas | Eosinophils (intraepithelial) |
| Apoptosis |
Chronic Colitis with Well-Preserved Architecture
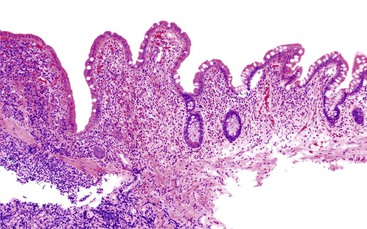
The next decision point in evaluated chronic (or active) colitides is determination of the status of the crypt architecture (normal versus abnormal). Common disorders that show preserved crypt architecture but also reveal other well-established features of chronicity (Fig. 13.26) are summarized in Figure 13.27. These include microscopic colitis (both lymphocytic and collagenous colitis), early IBD (both ulcerative colitis and Crohn’s disease), posttreatment IBD, drug reactions, diverticulosis, and some forms of infectious colitis. Shigella or Chlamydia infection can result in the development of features of chronicity, such as basal lymphoplasmacytosis, basal lymphoid aggregates, mild crypt distortion, and granulomas. Biopsies demonstrating chronic colitis but preserved crypt architecture may be subclassified as predominantly neutrophilic, predominantly lymphoplasmacytic, or pauciinflammatory for the purpose of narrowing the differential. The most common forms of chronic colitis with preserved crypt architecture and prominent neutrophilic inflammation are early-onset IBD, infectious colitis, and drug reactions. Table 13.10 summarizes some of the discriminating features of infectious colitis that may mimic Crohn’s disease. This topic is covered in more detail in Chapter 17.
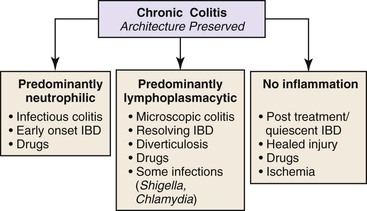
Table 13.10
Chronic Infection versus Crohn’s Disease
| Feature | Mycobacterium tuberculosis | Yersinia | Crohn’s Disease |
| Caseating granulomas | Frequent | Rare | Absent |
| Confluent granulomas | Frequent | Frequent | Absent |
| Few granulomas | Rare | Rare | Common |
| Prominent lymphoid cuff | Frequent | Frequent | Uncommon |
| Ulcers (both aphthous and deep) | Common | Common | Common |
| Architectural distortion | Common | Common | Common |
| Changes of chronicity unassociated with sites of granulomatous inflammation | Absent | Absent | Common |
| Multiple sites of involvement | Common | Rare | Common |
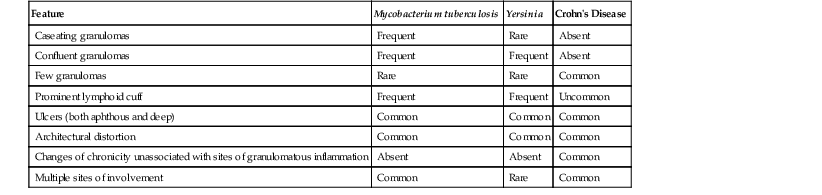
Determination of the anatomic distribution of disease helps one arrive at a more specific etiology. For instance, many types of infectious colitis are segmental rather than diffuse in distribution, or involve the right colon more often than the left. In contrast, IBD is either diffuse and continuous and always involves the rectum (ulcerative colitis) or the ileum and colon (Crohn’s disease). In general, infectious colitis is characterized by the presence of neutrophils in the lamina propria and superficial crypt epithelium; in contrast, in IBD, full-thickness mucosal involvement by neutrophils is more typical.
Biopsies that show chronic colitis with preserved crypt architecture and a predominantly lymphoplasmacytic inflammatory infiltrate occur often in patients with microscopic colitis, early or resolving IBD, drug reactions, diverticulosis, or even some forms of (resolving) infectious colitis. One of the major difficulties in this category of inflammatory disorders involves differentiating microscopic colitis from IBD (Table 13.11). Both the clinical/endoscopic and microscopic features of the biopsies are helpful to distinguish these disorders. For instance, patients with microscopic colitis usually have watery (nonbloody) diarrhea and either normal or only mildly abnormal (erythematous) endoscopic abnormalities. In contrast, patients with IBD usually have recurrent bouts of bloody diarrhea and show more significant endoscopic abnormalities, such as erythema, friability, and ulceration. Biopsies from a small proportion of patients with microscopic colitis may show features of chronicity such as basal plasmacytosis, crypt distortion, basal lymphoid aggregates, and Paneth cell metaplasia; however, in contrast to IBD, these features are typically mild and focal. Rarely, patients with lymphocytic or collagenous colitis develop a bandlike histiocytic infiltrate in the subepithelial lamina propria, which on occasion includes the development of giant cells and granulomas.
Table 13.11
Microscopic Colitis versus Inflammatory Bowel Disease
| Feature | Microscopic Colitis | Inflammatory Bowel Disease |
| Clinical presentation | “Watery” diarrhea | Malabsorptive or bloody diarrhea ± systemic signs |
| Endoscopic appearance | Normal or mildly abnormal (erythema); rare ulcers | Segmental or diffuse erythema; friability, ulcers common |
Chronic Colitis with Abnormal Crypt Architecture
The most common diseases in this category are IBD, diverticular disease–associated colitis (sometimes referred to as “segmental colitis”), chronic ischemia, chronic radiation colitis, and mucosal prolapse. To narrow the differential diagnosis when evaluating biopsies that reveal chronic colitis and abnormal crypt architecture (Fig. 13.28), it is helpful to distinguish those that show fibrosis or hyaline-like material in the lamina propria from those that do not. Lamina propria fibrosis and deposition of hyaline-like material in the lamina propria are common features in patients with ischemia, radiation injury, or mucosal prolapse. Table 13.12 summarizes some of the characteristics that differentiate these three disorders.
Table 13.12
Differential Diagnosis of Chronic Colitis with Abnormal Crypt Architecture and Lamina Propria Fibrosis or Hyalinelike Material
| Feature | Chronic Ischemia (Primary) | Radiation | Prolapse |
| Polyp/mass formation | Rare | Rare | + |
| Primarily rectal involvement | Rare | ++ | ++ |
| IBD-like mucosal inflammation | ++ | ± | − |
| Withering crypts | ± | ± | ± |
| Pseudomembrane | ± | − | − |
| Hyalinization | ++ | ++ | − |
| Fibromuscular hyperplasia | ± | − | ++ |
| Vascular dilatation/proliferation | − | ++ | ± |
| Atypical stromal/epithelial cells | − | ++ | − |
| Hyperplastic changes | − | − | ++ |
| Thrombi | ++ | ± | ± |
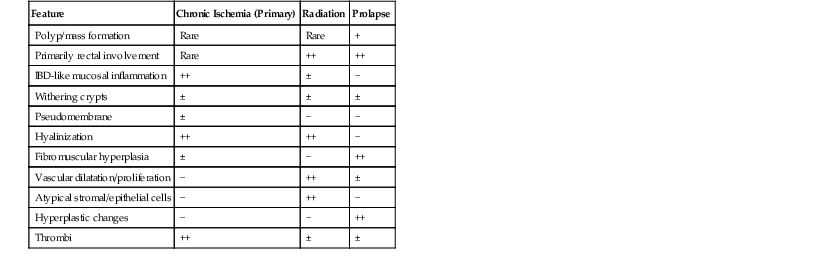
IBD, Inflammatory bowel disease.
In contrast, ulcerative colitis, Crohn’s disease, diverticular disease–associated colitis, and diversion colitis are the most frequent disorders that show chronic colitis with crypt architectural distortion but without lamina propria fibrosis or hyaline-like material. Biopsies from patients with ulcerative colitis or Crohn’s disease are categorized as chronic inactive or chronic active. The latter may be subdivided into mild, moderate, or severe depending on the severity of inflammation and the degree of epithelial injury. Distinguishing ulcerative colitis from Crohn’s disease in mucosal biopsies is usually not possible, particularly in patients who have had prior medical treatment, in which case portions of the mucosa may show reversion of architectural changes and a normal or near-normal appearance (Table 13.13). Therefore, in posttreatment IBD, the principal role of the pathologist is to determine the extent and severity of disease and the presence or absence of dysplasia. Distinguishing ulcerative colitis from Crohn’s disease is usually not possible unless the biopsies show granulomas unrelated to ruptured crypts, there is chronic or chronic active ileal disease (unrelated to backwash ileitis, bowel preparation, or drug reaction) or the patient has Crohn’s disease–associated perianal disease. However, in IBD patients whose biopsies are obtained before treatment, ulcerative colitis can be distinguished from Crohn’s disease if the inflammatory changes are segmental or patchy, there is absence of rectal involvement, granulomas unrelated to ruptured crypts are present, the patient has Crohn’s disease–associated perianal disease, or there is upper GI or ileal disease. In some cases of ulcerative colitis, there is relative or absolute rectal sparing (initial presentation in children) and/or segmental disease (left-sided colitis with a cecal patch). This subject is discussed in more detail in Chapter 17.
Table 13.13
Differential Diagnosis of Inflammatory Bowel Disease
| Feature | UC Treatment | UC after Treatment | Crohn’s Disease |
| Diffuse disease | Always | ± | ± |
| Rectal involvement | Always (except children) | ± | ± |
| Segmental involvement | Rare (cecal patch, appendix) | ± | ± |
| Ileal involvement | Mild | Distal 1-5 cm | Mild → severe, >5 cm |
| UGI involvement | Rare | Rare | ± |
| Granulomas | Mucin-related | Mucin-related | Non–mucin-related ± |
| Anal disease | − | − | ± |
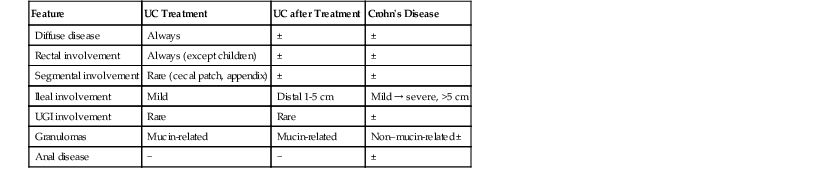
UC, Ulcerative colitis; UGI, upper gastrointestinal tract; −, absent; ±, may be present; +, mild; +++, marked.
Mucosal biopsies in areas of involvement of diverticular disease–associated colitis are identical to ulcerative colitis. Therefore, these two disorders cannot be distinguished by analysis of mucosal biopsies. Absence of rectal involvement and absence of involvement of mucosa proximal to areas of diverticulosis favor diverticular disease–associated colitis.
Many types of drugs, such as mycophenolate and ipilimumab, can mimic IBD. Knowledge of the timing and sequence of symptoms and signs in relation to drug intake is essential for establishing a particular drug as the cause of a patient’s colitis.
As mentioned earlier, some types of infectious colitis can develop histologic features of chronicity, mimicking IBD. For instance, infection with either Mycobacterium tuberculosis or Yersinia enterocolitica can lead to the development of granulomas, segmental and patchy ileal and colonic disease, multiple sites of involvement, and biopsies with features of chronic active colitis, including crypt distortion. Therefore, all patients with granulomatous ileitis or colitis should be evaluated for possible tuberculosis or Yersinia infection.
Acute Colitis
Biopsies that show features of activity, but without features of chronicity, are best categorized as “active colitis.” The term acute colitis is reserved for patients who have no history of IBD. It is helpful to separate biopsies with active colitis into those that have an ischemic/hemorrhagic appearance and those that do not. The former may be diagnosed as “acute hemorrhagic colitis.”
Acute Hemorrhagic Colitis
The most common causes of an acute ischemic/hemorrhagic colitis pattern of injury are ischemia, infections, radiation injury, certain drug reactions, and some phases of IBD (Fig. 13.29). Escherichia coli and Clostridium difficile are two of the more common infections that cause acute hemorrhagic colitis. Injury to the bowel mucosa is mediated by toxins released by these organisms that lead to intestinal ischemia, resulting in an ischemic-like acute hemorrhagic colitis. Ultimately, the most useful way to differentiate these two disorders is by analysis of stool for C. difficile toxin and special cultures for growth of E. coli O157:H7. The discriminating features of these disorders are discussed in more detail in Chapters 4 and 17.
Table 13.14 summarizes some of the characteristics that distinguish primary acute ischemia from infectious colitis (e.g., E, coli O157:H7) and acute radiation injury. For example, ischemia often involves watershed areas of the bowel such as the splenic flexure and shows prominent withering crypts and lamina propria hyalinization, whereas infectious colitis more often involves the right colon, shows more prominent edema and hemorrhage and neutrophils, and has less prominent withering crypts. Radiation colitis should be suspected in patients who have had a prior pelvic carcinoma. The changes of acute radiation injury are typically limited to the left colon. Common features of radiation injury include dilated and proliferating lamina propria and submucosal blood vessels and atypical epithelial and/or stromal cells. In some cases, lamina propria fibrosis or hyalinization is prominent, mimicking collagenous colitis (see Chapters 4 and 17).
Table 13.14
Active Colitis, Ischemic/Hemorrhagic Type
| Feature | Ischemia | Infection (E. coli) | Radiation |
| Anatomic distribution | Watershed | Right > Left | Left > Right |
| Edema/hemorrhage | + | ++ | + |
| Withered crypts | ++ | ± | ± |
| Fibrin thrombi | ± | + | ± |
| Neutrophils | ± | ++ | ± |
| Atypical cells | − | − | ± |
| Dilated blood vessels | ± | − | ++ |
| LP hyalinization | ++ | ± | ± |

LP, Lamina propria; −, absent; ±, may be present; +, mild; ++, moderate-marked.
Acute (Nonhemorrhagic) Colitis
Biopsies that show active (acute) colitis but without a hemorrhagic or ischemic appearance (Fig. 13.30) are most often caused by infections, drugs, or IBD. Acute infectious colitis is often referred to as “self-limited colitis,” because the symptoms and signs typically improve without treatment. The most common causes of acute self-limited (infectious) colitis in the colon are Salmonella, Shigella, Campylobacter, Aeromonas, and E. coli (see Chapters 4 and 17). Although there is substantial overlap in histologic features among these infectious colitides, some organisms are associated with certain characteristics that help identify them (see Chapter 17). Most infectious colitides are characterized by predominantly neutrophilic inflammation in the lamina propria and crypts, often involving the upper half of the mucosa more significantly than the lower half. Features of chronicity, such as basal plasmacytosis and crypt distortion, are typically absent. Infectious colitides are often segmental or patchy in distribution and may involve the right colon more often than the left. The pattern of involvement may help suggest one type of organism over another. However, the precise diagnosis is ultimately established by stool cultures.
In some cases, patients with IBD, particularly if it is mild, show features of infectious colitis (without well-established features of chronicity). Posttreatment biopsies from patients with IBD may show similar features. Finally, many types of drug reactions can mimic infectious colitis (see Chapter 12 for a more detailed discussion of drug-induced colitis). NSAIDs use is one of the most common causes of colitis in the general population. The development of colitis is increased with use of sustained-release NSAIDs (e.g., fenamates, diclofenac). NSAIDs-induced colonic injury can mimic almost any type or pattern of colitis, including infectious colitis, IBD, ischemic colitis, microscopic colitis, and even eosinophilic colitis. Therefore, a drug reaction should be considered in the differential diagnosis of all inflammatory disorders of the GI tract, including the colon.
Eosinophil-Predominant Colitis
Eosinophils are a common component of inflammatory infiltrates in many different types of disorders of the colon. However, their occurrence as the predominant or only type of inflammatory cell elicits a specific differential diagnosis that includes allergic colitis, eosinophilic gastroenteritis, parasitic infection, and certain mast cell disorders. Eosinophilic colitis also (known as allergic colitis), is mainly a disorder of young infants and neonates and usually involves the rectum (Fig. 13.31). Eosinophilic gastroenteritis is a poorly understood condition that often affects children and young adults. However, this condition involves mainly the esophagus, stomach, and/or small intestine. The colon is rarely involved in isolation from these other organs.
Parasitic infections often elicit a prominent eosinophilic infiltrate, but the eosinophils are usually in the form of microabscesses, and they do not typically involve the epithelium or cause epithelial injury. Mast cell disorders such as systemic mastocytosis can elicit a prominent eosinophilic response in some cases, but these patients usually have a known history of systemic mastocytosis.
In some circumstances, eosinophils can be a prominent inflammatory component of other disorders, such as IBD, microscopic colitis (particularly collagenous colitis), many different types of drug reactions, and vasculitis. These other conditions usually reveal other, more typical histologic features and other types of inflammatory cells that are characteristic of the specific disorder that is causing the patient’s illness.
Paucicellular Colitis
Colitis associated with a minimal inflammatory component, or none at all, is a common reaction pattern in many different types of (resolving) inflammatory disorders of the colon. However, certain inflammatory conditions are more typically associated with paucicellular lamina propria inflammation at the peak of the patient’s illness (Fig. 13.32). These include drug reactions, certain infections, and systemic disorders that involve the colon (e.g., amyloidosis). A common pattern in this group is apoptotic colopathy, defined as colitis associated with apoptosis as a prominent histologic feature of the disorder. Apoptoses may be associated with other types of inflammatory cells, or it may be present in isolation.
The differential diagnosis of apoptotic colopathy is outlined in Box 13.3. Some bowel preparation solutions are associated with the development of prominent apoptoses in the bases of the crypts. The right colon is often affected more severely than the left colon. In this condition, there is usually no evidence of colitis other than the presence of apoptosis. Chemotherapeutic drugs and other immunosuppressants such as mycophenolate are associated with prominent apoptosis and apoptotic microabscesses. GVHD, transplant rejection, certain infections (CMV or HIV), and radiation injury can also cause prominent apoptotic injury to the epithelium. Certain phases of IBD are also associated with prominent apoptosis, but in that condition, other features of IBD usually provide clues to the correct diagnosis.
Mycophenolate-induced colitis may mimic GVHD, but the former condition often shows more prominent eosinophils and neutrophils in the inflammatory infiltrate, and only rare endocrine cell aggregates, compared with GVHD. CMV infection can also be difficult to differentiate from GVHD, and both conditions can be present in the same patient. Considering that differential diagnosis, crypt distortion, hypereosinophilic crypts, and prominent crypt necrosis and dropout are more characteristic of GVHD than CMV infection, particularly in patients who have severe disease. In bone marrow transplant recipients in whom GVHD-like changes in the colon develop and in whom viral inclusions are identified, it is probably best to consider the latter a superimposed infection, particularly if the patient has evidence of GVHD in other organs of the body (e.g., skin, liver, upper GI tract).
Lamina Propria Infiltrates
The causes and differential diagnosis of lamina propria infiltrates of the colon are covered extensively in Chapter 6. Figure 13.33 shows an example of xanthomatous infiltrates. Table 13.15 separates infiltrates into pigmented and nonpigmented types and provide a summary of the histologic, histochemical, and immunohistochemical methods for separating these two disorders. Most infiltrative disorders do not cause colitis but instead cause expansion of the lamina propria and distortion of the crypts by the infiltrate. Therefore, although technically not a form of “colitis,” lamina propria infiltrates may cause an endoscopic appearance of colitis and a diarrheal illness.
Table 13.15
Lamina Propria Infiltrates
| Diagnosis | Histology | Histochemical | Immunohistochemical |
| Pigmented | |||
| Melanosis | Dark brown, granular macrophages | PAS and acid-fast positive | CD68 positive (must bleach pigment) |
| Pseudomelanosis | Black, subepithelial macrophages | Iron positive | CD68 positive |
| Brown bowel syndrome | Brown smooth muscle cells | PAS, acid-fast positive Yellow autofluorescence |
N/A |
| Barium granuloma | Gray, finely granular refractile pigment | PAS negative | N/A |
| Chronic granulomatous disease | Golden-brown macrophages | Fat stain and PAS positive | N/A |
| Nonpigmented | |||
| Xanthoma | Clear, foamy macrophages | Fat stain positive on unfixed tissue | α1-Antitripsin, monocyte chemotactic and activating factors |
| Muciphages | Clear, foamy macrophages | d-PAS, Alcian blue positive | CD68 lysozyme positive |
| Mycobacterium avium | Pink, foamy macrophages | Acid-fast or Fite positive d-PAS diffuse positive |
CD68 positive |
| Malakoplakia | Pink, foamy macrophages with nuclear grooves Michaelis-Gutmann bodies (MGB) |
d-PAS positive MGB positive for calcium and iron |
CD68 positive |
| Granular cell tumor | Pink, granular histiocytic cells | d-PAS positive | S100 positive |
| Signet ring cell carcinoma | Clear cytoplasm with displaced nucleus | d-PAS, Alcian blue, and mucin positive globules | Cytokeratin positive |
| Clear cell carcinoid tumor | Clear cells with foamy cytoplasm | d-PAS and mucin negative | Chromogranin positive |
| Malignant histiocytosis | Granular Langerhans cells with irregular elongate nuclei and nuclear grooves | d-PAS and mucin negative | S100 and CD1a positive |
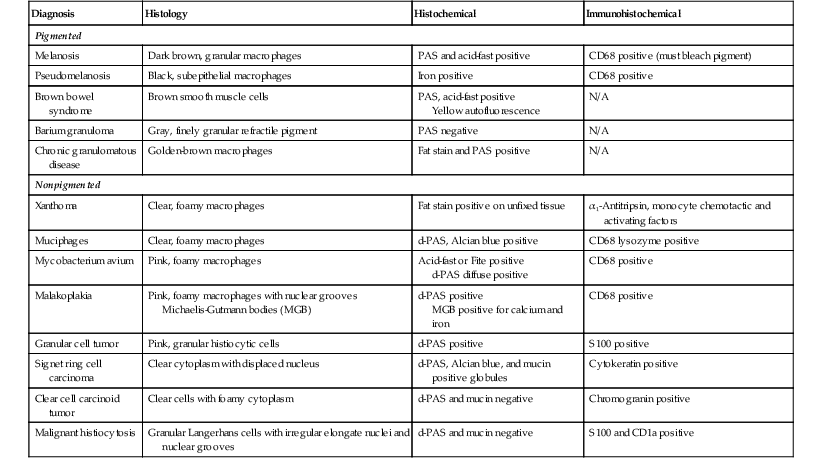
d-PAS, Periodic acid–Schiff stain with diastase digestion.
Neoplasia
Neoplastic conditions of the colon are covered in detail in Chapters 17, 22, and 27. Some neoplastic conditions, such as dysplasia, arise in patients with chronic inflammatory diseases of the colon (e.g., ulcerative colitis, Crohn’s disease). Distinguishing regenerative changes from dysplasia in IBD is also covered in Chapter 17.