Chapter 3 Airway evaluation in obstructive sleep apnea
1 METHODS OF AIRWAY EVALUATION
As the interest in sleep-disordered breathing (SDB) has increased, various attempts have been made to assess upper airway anatomy in patients with this relatively frequent disorder. From the very beginning, researchers and clinicians used a multitude of different techniques not only to reveal potential differences in upper airway anatomy to better understand the origin and the pathophysiology of the disease but also to improve patient management and treatment success. While the value of thorough clinical assessment remains indubitable, the value of the Mueller maneuver has been questioned from the beginning. Static radiologic imaging techniques such as x-ray cephalo-metry, computed tomography (CT) scanning and magnetic resonance imaging (MRI) have been used mostly to detect differences in airway anatomy. Dynamic scanning protocols (e.g. ultrafast CT or cine MRI) and multiple pressure recordings have been used to gain insights into the mechanism and level of airway obstruction. Upper airway endo-scopy has been inaugurated during sleep and sedated sleep to directly visualize airway obstruction, and the assessment of critical closing pressures has been used to quantify upper airway collapsibility.
2 CLINICAL EXAMINATION AND CLINICALSCORES
A clinical examination including an endoscopy of the upper airway during wakefulness still constitutes the basis of every airway evaluation in snorers and obstructive sleep apnea (OSA) patients. Anatomic and static clinical findings were the first parameters to be evaluated in order to improve treatment success. The impact of enlarged palatine tonsils became evident in the surgical experiences with children. If performed simultaneously, tonsillectomy was described by most authors as a positive predictive factor for a successful uvulopalatopharyngoplasty (UPPP). All the other anatomic parameters such as the size of the uvula, the existence of longitudinal pharyngeal folds and so forth did not show any relationship to the success rate of UPPP if evaluated separately. In contrast to the significant influence of enlarged tonsils in palatal obstruction, equivalent clinical finding for tongue base obstructions could not be detected. Woodson and Wooten only found hints that the oropharynx was normal in cases with retrolingual obstruction.1
Aware of this dilemma, Friedman et al. developed a clinical four degree staging system incorporating the tonsil size, the position of the soft palate, the tongue size, and the Body Mass Index (BMI).2 This anatomic staging system predicted the success rate better than OSA severity did.3 One may argue that the staging system merely reflects the clinical examination of an experienced sleep physician; nevertheless, such a system may be particularly helpful for less experienced observers.
3 THE MUELLER MANEUVER
3.1 TECHNIQUES OF THE MANEUVER
In order to be able to compare results between different investigators and patients as well as before and after an intervention, the Maneuver should be performed and documented in a standardized fashion. Due to its simplicity the classification according to Sher has been widely used to describe the finding obtained during the Maneuver.3 In this classification, four degrees of airway obstruction at the different levels are defined, ranging from minimal to complete occlusion. Furthermore, any visible obstruction linked to the epiglottis is described. The reproducibility and inter-rater reliability of the results remain problematic. Taking all the available data into account, the reliability of the Mueller Maneuver remains highly questionable and the evaluation of the Maneuver seems highly subjective and hard to reproduce.
3.2 PREDICTING AIRWAY OBSTRUCTION DURING SLEEP AND SURGICAL SUCCESS
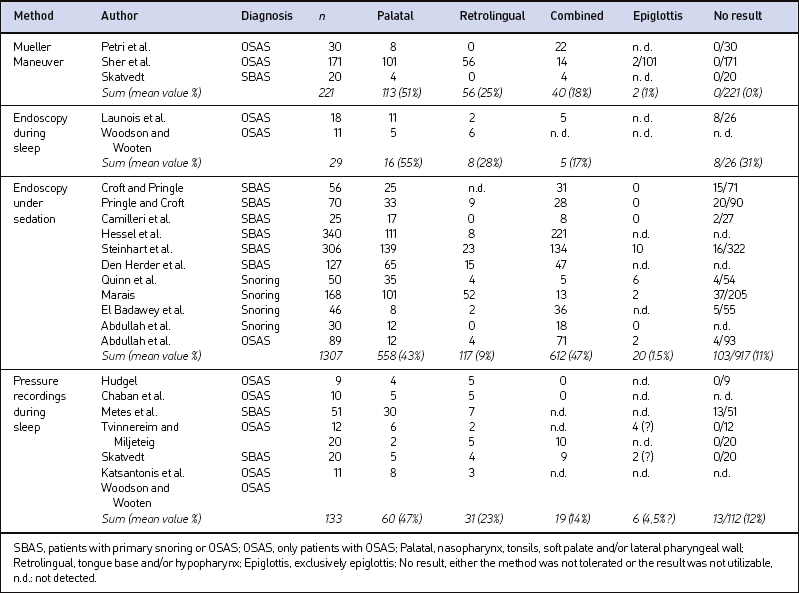
There is some evidence that the sites of obstruction detected with the Mueller Maneuver do not reliably reflect the sites of obstruction during sleep. This could be demonstrated through a comparison with videoendoscopy, multi-channel pressure recordings, and dynamic MRI during sleep. Table 3.1 shows the different sites of airway obstruction detected with the different methods of airway evaluation according to selected examples from the literature.
Table 3.1 Distribution of the sites of obstruction detected by different methods of airway evaluation (selected literature)
The impact of body position on the significance of the Mueller Maneuver remains unclear. During the Mueller Maneuver, healthy subjects may produce extreme negative pressures of −80 mbar without any signs of pharyngeal collapse.4 This clearly demonstrates the significant differences in upper airway collapsibility during wakefulness and sleep. All the data given do not support the idea that the results obtained by the Mueller Maneuver may be transferred to natural sleep.
Various research groups were not able to better predict the success rates obtained with UPPP when using the Mueller Maneuver. Some authors considered an additional retrolingual collapse during the Mueller Maneuver as an exclusion criterion for a UPPP or performed a partial resection of the epiglottis in UPPP failure patients with laryngeal obstruction during the Mueller Maneuver by partial resection of the epiglottis.
4 X-RAY CEPHALOMETRY
4.1 PROVIDING INSIGHTS INTO THEPATHOPHYSIOLOGY OF SDB
Extensive literature is available comparing upper airway anatomy and dentofacial structures using x-ray cephalo-metry between OSA patients and healthy controls. In siblings a significantly longer distance from the hyoid bone to the mandibular plane has been documented in those affected by SDB.5 Further differences were described by different working gropus. The concrete results are often difficult to compare, as the authors not only use different landmarks and parameters but also sometimes rather complex calculated indices and ratios to describe the differences they found. Therefore, the following findings in OSA patients can only be a selection: longer soft palates, reduced minimum palatal airway widths, increased thickness of the soft palate, differences in calculated craniofacial scores, increased pharyngeal lengths, retroposition of the mandible or the maxilla, micrognathia, increased mid-facial heights, and differences in hyoid bone position. In general, the differences are more pronounced in non-obese patients, suggesting that craniofacial changes play a dominant role in this subgroup. Furthermore, substantial differences in maxillofacial appearance of different ethnic groups need to be taken into account.
Various authors could demonstrate that the aberrations in craniofacial morphology they found in OSA patients were more pronounced in patients with severe OSA. Dempsey et al. demonstrated that in non-obese patients and in patients with narrow upper airway dimensions, four cephalometric dimensions were the dominant predictors of Apnea/Hypopnea Index (AHI) level, accounting for 50% of the variance.6 Rose et al. questioned the diagnostic relevance of x-ray cephalometry for OSA, as they found no direct correlation between skeletal cephalometric findings and OSA severity; nevertheless, they also reported a correlation with hyoid bone position.7
4.2 X-RAY CEPHALOMETRY AND THERAPEUTICINTERVENTIONS
One of the dominant indications for performing x-ray cephalometry has been treatment with oral appliances. Especially with regard to the evaluation of potential predictive parameters for treatment success and dental side effects, x-ray cephalometry has been the standard diagnostic tool. As early as 1995, Mayer and Meier-Ewert, two of the fathers of treatment with oral appliances in Europe, looked for cephalometric predictors of treatment success8 and reported that specific cephalometric variables were indeed predictive for the therapeutic effect. Other authors have confirmed the existence of predictive cephalometric parameters, especially in relation to hyoid bone position and oropharyngeal airway dimension. Nevertheless, the problems related to different nomenclature and selection of airway parameters described above remain.
X-ray cephalometry has also been evaluated with regard to potential predictive parameters for postoperative results of UPPP alone or in combination with other approaches. To date there is no convincing evidence that skeletal measurements obtained with x-ray cephalometry could predict the outcome of UPPP. Nevertheless, lateral x-ray cephalometry is the standard tool in the preoperative evaluation of the craniofacial skeletal anatomy before maxillomandibular advancement surgery. It can be regarded as a mandatory procedure and its value is not questioned.9
4.3 X-RAY CEPHALOMETRY IN PATIENTMANAGEMENT
X-ray cephalometry has provided substantial insights into the pathophysiology of OSA, demonstrating significant craniofacial characteristics associated with this disease. Although the results are not easy to compare, specific cephalometric characteristics have been repeatedly mentioned as a risk factor for OSA and correlate with the severity of the disease. Selected cephalometric parameters indicate favorable results of mandibular advancement by oral appliances. Nevertheless, no cephalometric parameter exists that would reliably rule out treatment success with an oral appliance and surgical outcome cannot be predicted. This may explain why x-ray cephalometry has not become a routine procedure in the diagnostic work-up of OSA as long as maxillomandibular surgery is not planned.
5 CT SCANNING
Providing insights into thepathophysiology of SDB
The majority of published data points to potential differences in upper airway structures and dimension between OSA patients and healthy controls or snorers. In general, the upper airway is described as smaller in apneic patients compared to controls, especially with regard to the retropalatal region. Cross-sectional areas were found to be significantly narrower in affected patients. Inversely, retropalatal tissue was described as being greater in OSA patients compared to controls and larger tongue and soft palates dimensions and volumes were found. Schwab et al. have pointed out the differences in upper airway configuration with an anterior–posterior configuration – a result that is in line with data obtained from magnetic resonance imaging.10
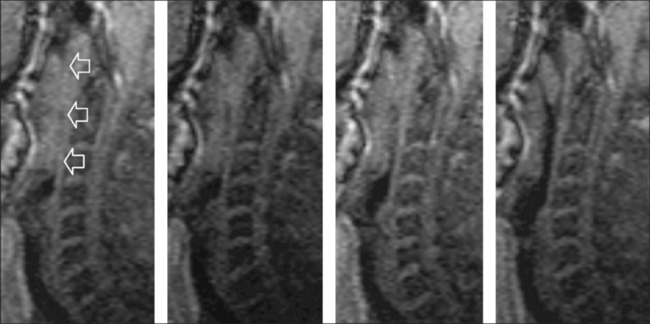
With the help of dynamic and ultrafast CT further insights into airway obstruction were gained. In addition to the fact that the naso- and oropharyngeal airways were smaller in OSA patients compared to weight-matched controls, an increased collapsibility in affected patients was found. During a respiratory cycle, substantial changes in cross-sectional areas were seen in patients with SDB, the velopharyngeal segment being the narrowest and most collapsible region.11 These results were essentially confirmed later, showing that patients with severe OSA have significantly narrower cross-sectional areas at the velopharyngeal level12 (see Figure 3.1).

Fig. 3.1 Upper airway narrowing during tidal breathing as assessed with CT scanning. A. Cross-sectional image of a patient at the level of uvula in tidal breathing. B. The significant narrowing at the same level in forced expiration is seen. The region of interest (white line) was used to assess total cross-sectional areas in each image (according to Yucel et al., 2005).12
5.3 EVALUATING THE EFFECTS OF THERAPEUTIC INTERVENTION
Effects of therapeutic intervention have been assessed, mostly with regard to treatment with oral appliances and surgical intervention. While a decrease in the diameters at the retropalatal and retroglossal level was seen during apnea, these cross-sectional areas were significantly enlarged with the help of the appliance. With regard to surgical treatment effects, it has been demonstrated that the upper airway increases after mandibular distraction osteogenesis in children and after maxillomandibular advancement in adults. Even more data are available for the effects of UPPP and its modifications, demonstrating that UPPP significantly increases the upper airway cross-sectional area and that the oropharyngeal enlargement seen in pharyngeal CT measures is associated with a good outcome in UPPP.
6 MR IMAGING
6.1 TECHNIQUES AND STANDARDS
Only a small number of authors have attempted to establish distinct protocols for MRI of the upper airway in SDB; the results of the measurements were either validated with a phantom or tested for variability in repeated measures over time and with different investigators.13 Validation and standardization of this imaging paradigm seem essential; nevertheless, in contrast to, for example, lateral x-ray cephalometry, hardly any consensual standards exist for this indication.
6.2 PROVIDING INSIGHTS INTO THEPATHOPHYSIOLOGY OF SDB
6.2.1 OSA IN CHILDREN
Especially in children, extensive research has been done with regard to the pathophysiology of OSA. Children with or without persistent OSA after tonsillectomy and adenoidectomy were compared and it was demonstrated that enlarged lingual tonsils were present in those children with persistent disease, especially in children with Down syndrome. Further findings were an airway restriction in the vicinity of both the adenoids and the tonsils and an enlarged soft palate in the affected group. Nevertheless, the airway restriction is not limited to these areas but seems to occur throughout the initial two thirds of the upper airway. Furthermore, a close dependency between the frequency of respiratory events and the size of the tonsils and the soft palate could be demonstrated and the upper airway narrowing was more pronounced in those children with a high number of respiratory events compared to the less affected group. With regard to dynamic airway evaluation, more pronounced fluctuations in airway area during tidal breathing14 and significant differences in airway motion15 were demonstrated in children with OSA compared to controls.
6.2.2 OSA IN ADULTS
As early as 1989, authors pointed out the significance of pharyngeal fat deposits in adult patients with OSA.16 In this group, more fat is present in the areas surrounding the collapsible segment of the pharynx and fat deposition has been described even in non-obese patients with OSA. Some authors could demonstrate a trend for larger tongues or a significantly higher tongue volume in relation to the oral cavity volume in patients with OSA compared to controls. Other anatomic conditions associated with SDB were an elliptic horizontal cross-sectional area of the pharynx and large volumes of the lateral pharyngeal walls and total soft tissue surrounding the upper airway. Nevertheless, other authors did not find any significant differences in tongue volume, soft palates or pharyngeal walls, but pointed out specific anatomic factors of the mandible in OSA patients.
Numerous authors have demonstrated that the mechanism and level of airway obstruction can be visualized by MRI, even under natural sleep17 (Figure 3.2). The fact that patients with OSA present multiple sites of pharyngeal abnormality was demonstrated by Suto et al. as early as 1993;18 nevertheless, the authors pointed out that the levels of airway obstruction during wakefulness did not match those levels found during sleep. Other trials have shown that no pharyngeal airway narrowing was seen in the healthy subjects but a significant narrowing was seen in the OSA patients during wakefulness, and even more so during sleep. Moreover, it was demonstrated that apneic patients have a more circular occlusion, underlining the relevance of the lateral pharyngeal walls in the pathogenesis of airway obstruction.
6.3 EVALUATING THE EFFECTS OF THERAPEUTIC INTERVENTION
With regard to surgical treatment, MRI has been used to assess potential effects of surgery on upper airway anatomy. In radiofrequency surgery, imaging has been used to visualize immediate postoperative effects on the soft palate and the tongue base, the latter leading to concrete recommendations for the technical settings of this technique.19 A standardized protocol has furthermore been used to study anatomic changes at the upper airway after radiofrequency surgery of the tongue base20 and after hyoid suspension.21
6.4 MRI IN PATIENT MANAGEMENT
Static and dynamic MRI has substantially improved our understanding of the pathophysiology of SDB. Significant differences in upper airway anatomy and structure have been detected between patients with SDB and healthy subjects, and relevant insights have been gained in terms of the mechanisms and levels of airway obstruction. Nevertheless, MRI has not become a standard procedure either in the diagnostic work-up for patients with SDB or in the management of the disease in terms of surgical or non-surgical treatment, as a number of issues remain unresolved. MRI during sleep (especially spontaneous sleep) is possible but not easy to perform and measurements during wakefulness or induced sleep are, to a certain extent, artificial or may simply not reflect clinical conditions. Furthermore, the results of MRI, even when performed during sleep, can only provide information concerning a short period of time and are limited to the supine position. For routine clinical application, the limited availability and the associated costs are additional limiting factors.
7 VIDEOENDOSCOPY DURING SPONTANEOUSSLEEP
As early as 1978 the first report about videoendoscopic recordings of the pharynx and larynx during sleep was published. Borowiecki and colleagues described a palatopharyngeal collapse at the end of expiration and directly before inspiration in patients with OSA.22 They described different degrees of airway obstruction, often associated with a medialization of the lateral pharyngeal walls. As there was no treatment other than tracheotomy available at this time for those patients, patient selection was not an issue.
8 VIDEOENDOSCOPY UNDER SEDATION
8.1 IMPACT OF VIDEOENDOSCOPY UNDERSEDATION ON SLEEP, BREATHING, ANDSNORING
Endoscopy under sedated sleep does not always succeed in inducing existing breathing disorders, and on the other hand, snoring may be provoked even in healthy patients. In a cohort study using propofol, Marais detected snoring sounds in 45% of 126 healthy, non-snoring controls.23 When titrating propofol with target-controlled infusion, all snorers did snore reliably whereas not a single control person did at the same plasma level, amounting to a sensitivity and specificity of 100%.24 Therefore, target-controlled infusion with propofol seems superior to manual titration.
8.2 DESCRIPTION OF FINDINGS
The patterns of snoring and airway obstruction that can be observed during videoendoscopy under sedation are multiform. Pringle and Croft were the first to standardize the findings according to their data obtained in a large series of patients.25 Currently, different classifications coexist and, ultimately, none of them are feasible. They distinguish either between an isolated or a multisegmental obstruction, or they are modifications of existing classifications comprising the epiglottis. The majority of authors do not classify their findings but enumerate the various mechanisms and anatomic sites of snoring and obstruction. The obstructive patterns are described as being circular, antero-posterior and latero-lateral at the level of the soft palate, the tonsils, the tongue base, and the epiglottis. A combination of as many as five different concomitant sites of obstruction in primary snorers and even six in sleep apnea patients was described and an isolated site of obstruction was found in only 15 % of OSA patients.
8.3 IMPACT ON CLINICAL DECISION MAKING
In clinical routine a large tongue – defined by a modified Mallampati score of 3 or 4 – is usually considered a negative predictive parameter for a successful UPPP. However, den Herder and co-workers could not demonstrate a correlation between videoendoscopy under sedation (retrolingual obstruction) and Mallampati index (tongue size).26 Pringle and Croft compared their results of the Mueller Maneuver to those obtained by videoendoscopy under sedation in a group of 50 patients and could demonstrate that treatment recommendations were not identical based on these two investigation to a significant extent.25 Other authors also reported substantial differences in treatment recommendation when adding sedated endoscopy to simply using the Mueller Maneuver. Taking the limited significance of the Mueller Maneuver into account, there seems to be a potential for an improvement in treatment selection based on sedated endoscopy.
8.4 IMPACT ON THE SUCCESS RATE
Surprisingly enough, no prospective data are available to date comparing success rates of surgical intervention with and without the use of videoendoscopy under sedation. This is particularly confusing as there have been numerous advocates of sedated endoscopy presenting data and videos on countless patients with SDB, but they have not yet been able to demonstrate its usefulness with regard to surgical outcome. For example, no superiority was seen with regard to success rates compared to historic controls, despite using sedated endoscopy.27 Yet an improved success rate was only found in those patients who did not even show the slightest involvement of structures other than the palate. Hessel and de Vries retrospectively reviewed snorers and sleep apnea patients after UPPP. In those patients where the soft palate was least involved in airway collapse during preoperative sedated videoendoscopy, the outcome was superior compared to the others.28 In another retrospective analysis of 55 sleep apnea patients after UPPP, the same investigators did not find significantly different success rates for different sites of obstruction as revealed by videoendoscopy under sedation.
9 MULTI-CHANNEL PRESSUREMEASUREMENTS
9.1 TOLERABILITY OF THE PRESSURE CATHETERS
Initially, pressure recordings in the field of SDB were performed with balloon and open catheters, but esophageal balloon catheters irritated the patients significantly. The reliability of the results of micro-tip catheters was shown during wakefulness and sleep for the esophagus and for the pharynx. Well-designed studies demonstrated that catheters with no more than 2 mm diameter did not alter the sleep structure of patients suspected of SDB and the results obtained with or without catheters in place did not differ significantly.29 In a large trial with 799 patients only 3% rejected the placement of the catheter and 1% refused further measurement during the night, while 96% tolerated the procedure.30
9.2 RELIABILITY OF MEASURING POINTS ANDASSESSMENT OF OBSTRUCTIVE EVENTS
Pressure catheters also can be used to measure nasal and pharyngeal airflow. This implies that they are suitable to assess increased respiratory effort as well as the AHI. Tvinnereim et al. demonstrated that the absolute number of obstructive and mixed apneas during sleep can be assessed with these catheters with minimal deviation from the results obtained by polysomnography.31 The overall sensitivity, specificity and negative predictive value for the detection of the different types of apneas and hypopneas may reach up to 100%. The data available indicate that multi-channel pressure recordings are suitable to assess the severity of SDB.
9.3 ASSESSING THE SITES OF AIRWAYOBSTRUCTION
Investigations concerning night-to-night variability of the distribution of the obstructive sites showed that the predominant site of obstruction can be reproduced during the second night in more than 70% of the cases. Rollheim et al. compared the patterns of obstruction as obtained in the hospital with a recording at home. Although the mean AHI was significantly higher in the hospital than at home, the occurrence of palatal obstructions did not differ between both recordings.32 In patients who had less than 40% or more than 60% palatal obstructions in the first recording, this relationship was reproduced in 90% of the cases during the second recording.
9.4 IMPACT ON THE SUCCESS RATE OF SURGERY FOR SDB
Metes et al. were the first to publish data concerning the impact of pharyngeal pressure measurements on the success rate of surgery.33 They used a catheter with one measuring point only which was pulled through the pharynx and placed at several sites along the upper airway in order to record several obstructive events at each site. The obstruction they found in this way persisted in eight of 12 patients after UPPP. Nevertheless, the success rate of UPPP did not differ between patients with predominant palatal or tongue base obstruction. Skatvedt et al. selected 16 patients with different degrees of SDB and predominant palatal obstruction detected by multi-channel pressure transducers for laser-assisted uvulopalatoplasty.34 While the rate of ‘upper’ obstructions dropped from 90% to 8.8% of all apneas, the number of upper hypopneas was reduced only minimally. Osnes et al. compared the efficacy of UPPP in patients with predominantly transpalatal and subpalatal obstructions.35 After UPPP, transpalatal apneas and hypopneas were reduced by 81% whereas subpalatal events only dropped by 42%. The success rate in patients with transpalatal obstruction was significantly higher than in those with subpalatal obstruction. Multi-channel pressure recordings seem to be superior to single-channel pull-through techniques in predicting surgical success of soft palate surgery.
10 CRITICAL CLOSING PRESSURE
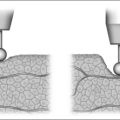
The severity of SDB is usually described by the AHI, representing the number of upper airway obstructions during sleep. Nevertheless, it has to be kept in mind that the AHI simply describes the frequency of upper airway obstructions, not the severity of the pharyngeal collapse itself. Furthermore, measuring the severity of upper airway collapse is believed to be important when estimating the forces needed to overcome these obstructions or to maintain upper airway stability. Schwartz et al.36 and Smith et al.37 first measured upper airway collapsibility. They assessed airflow and airway pressure using a special nasal continuous positive airway pressure (CPAP) device with integrated pneumotachograph and pressure sensor, being able to produce positive as well as negative pressure levels. A pressure flow diagram can be drawn at different levels of airway pressure and a regression line can be calculated. Smith defined the critical closing pressure (Pcrit) as being the upper airway pressure when the regression line is crossing the zero line, indicating that airflow completely stops (see Figure 3.3). Patients with obstructive apneas have a Pcrit clearly above zero, in simple snorers it drops to −3 to −12 mbar, whereas in normal controls Pcrit is on average below −8 mbar.
10.1 IMPACT OF TREATMENT ON PCRIT
Schwartz et al.38 showed that substantial weight loss resulted in an improvement of Pcrit and a concomitant reduction of the AHI. They investigated the effect of UPPP on Pcrit in 13 patients and found a significant decrease for the entire group.39 Even though a complete normalization of Pcrit could be demonstrated in the subgroup of responders in contrast to non-responders they could not find any preoperative predictor of response. An identical operation had individually varying effects on Pcrit.
1. Woodson BT, Wooten MR. Comparison of upper-airway evaluations during wakefulness and sleep. Laryngoscope. 1994;104:821-828.
2. Friedman M, Ibrahim H, Bass L. Clinical staging for sleep-disordered breathing. Otolaryngol Head Neck Surg. 2002;127:13-21.
3. Sher AE, Thorpy MJ, Shprintzen RJ, et al. Predictive value of Muller maneuver in selection of patients for uvulopalatopharyngoplasty. Laryngoscope. 1985;95:1483-1487.
4. Ritter CT, Trudo FJ, Goldberg AN, et al. Quantitative evaluation of the upper airway during nasopharyngoscopy with the Muller maneuver. Laryngoscope. 1999;109:954-963.
5. Riha RL, Brander P, Vennelle M, et al. A cephalometric comparison of patients with the sleep apnea/hypopnea syndrome and their siblings. Sleep. 2005;28:315-320.
6. Dempsey JA, Skatrud JB, Jacques AJ, et al. Anatomic determinants of sleep-disordered breathing across the spectrum of clinical and nonclinical male subjects. Chest. 2002;122:840-851.
7. Rose EC, Staats R, Lehner M, et al. Cephalometric analysis in patients with obstructive sleep apnea. Part I: diagnostic value. J Orofac Orthop. 2002;63:143-153.
8. Mayer G, Meier-Ewert K. Cephalometric predictors for orthopaedic mandibular advancement in obstructive sleep apnoea. Eur J Orthod. 1995;17:35-43.
9. Hochban W, Brandenburg U, Peter JH. Surgical treatment of obstructive sleep apnea by maxillomandibular advancement. Sleep. 1994;17:624-629.
10. Schwab RJ, Gefter WB, Hoffman EA, et al. Dynamic upper airway imaging during awake respiration in normal subjects and patients with sleep disordered breathing. Am Rev Respir Dis. 1993;148:1385-1400.
11. Shepard JWJr., Stanson AW, Sheedy PF, et al. Fast-CT evaluation of the upper airway during wakefulness in patients with obstructive sleep apnea. Prog Clin Biol Res. 1990;345:273-279.
12. Yucel A, Unlu M, Haktanir A, et al. Evaluation of the upper airway cross-sectional area changes in different degrees of severity of obstructive sleep apnea syndrome: cephalometric and dynamic CT study. AJNR Am J Neuroradiol. 2005;26:2624-2629.
13. Stuck BA, Kopke J, Maurer JT, et al. Evaluating the upper airway with standardized magnetic resonance imaging. Laryngoscope. 2002;112:552-558.
14. Arens R, Sin S, McDonough JM, et al. Changes in upper airway size during tidal breathing in children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2005;171:1298-1304.
15. Donnelly LF, Surdulescu V, Chini BA, et al. Upper airway motion depicted at cine MR imaging performed during sleep: comparison between young patients with and those without obstructive sleep apnea. Radiology. 2003;227:239-245.
16. Horner RL, Mohiaddin RH, Lowell DG, et al. Sites and sizes of fat deposits around the pharynx in obese patients with obstructive sleep apnoea and weight matched controls. Eur Respir J. 1989;2:613-622.
17. Schoenberg SO, Floemer F, Kroeger H, et al. Combined assessment of obstructive sleep apnea syndrome with dynamic MRI and parallel EEG registration: initial results. Invest Radiol. 2000;35:267-276.
18. Suto Y, Matsuo T, Kato T, et al. Evaluation of the pharyngeal airway in patients with sleep apnea: value of ultrafast MR imaging. AJR Am J Roentgenol. 1993;160:311-314.
19. Stuck BA, Kopke J, Maurer JT, et al. Lesion formation in radiofrequency surgery of the tongue base. Laryngoscope. 2003;113:1572-1576.
20. Stuck BA, Kopke J, Hormann K, et al. Volumetric tissue reduction in radiofrequency surgery of the tongue base. Otolaryngol Head Neck Surg. 2005;132:132-135.
21. Stuck BA, Neff W, Hormann K, et al. Anatomic changes after hyoid suspension for obstructive sleep apnea: an MRI study. Otolaryngol Head Neck Surg. 2005;133:397-402.
22. Borowiecki B, Pollak CP, Weitzman ED, et al. Fibro-optic study of pharyngeal airway during sleep in patients with hypersomnia obstructive sleep-apnea syndrome. Laryngoscope. 1978;88:1310-1313.
23. Marais J. The value of sedation nasendoscopy: a comparison between snoring and non-snoring patients. Clin Otolaryngol Allied Sci. 1998;23:74-76.
24. Berry S, Roblin G, Williams A, et al. Validity of sleep nasendoscopy in the investigation of sleep related breathing disorders. Laryngoscope. 2005;115:538-540.
25. Pringle MB, Croft CB. A grading system for patients with obstructive sleep apnoea, based on sleep nasendoscopy. Clin Otolaryngol Allied Sci. 1993;18:480-484.
26. den Herder C, van Tinteren H, de Vries N. Sleep endoscopy versus modified Mallampati score in OSA and snoring. Laryngoscope. 2005;115:735-739.
27. Camilleri AE, Ramamurthy L, Jones PH. Sleep nasendoscopy: what benefit to the management of snorers? J Laryngol Otol. 1995;109:1163-1165.
28. Hessel NS, de Vries N. Results of uvulopalatopharyngoplasty after diagnostic workup with polysomnography and sleep endoscopy: a report of 136 snoring patients. Eur Arch Otorhinolaryngol. 2003;260:91-95.
29. Skatvedt O, Akre H, Godtlibsen OB. Nocturnal polysomnography with and without continuous pharyngeal and esophageal pressure measurements. Sleep. 1996;19:485-490.
30. Oeverland B, Akre H, Kvaerner KJ, et al. Patient discomfort in polysomnography with esophageal pressure measurements. Eur Arch Otorhinolaryngol. 2005;262:241-245.
31. Tvinnereim M, Cole P, Haight JS, et al. Diagnostic airway pressure recording in sleep apnea syndrome. Acta Otolaryngol. 1995;115:449-454.
32. Rollheim J, Tvinnereim M, Sitek J, et al. Repeatability of sites of sleep-induced upper airway obstruction. A 2-night study based on recordings of airway pressure and flow. Eur Arch Otorhinolaryngol. 2001;258:259-264.
33. Metes A, Hoffstein V, Mateika S, et al. Site of airway obstruction in patients with obstructive sleep apnea before and after uvulopalatopharyngoplasty. Laryngoscope. 1991;101:1102-1108.
34. Skatvedt O, Akre H, Godtlibsen OB. Continuous pressure measurements in the evaluation of patients for laser assisted uvulopalatoplasty. Eur Arch Otorhinolaryngol. 1996;253:390-394.
35. Osnes T, Rollheim J, Hartmann E. Effect of UPPP with respect to site of pharyngeal obstruction in sleep apnoea: follow-up at 18 months by overnight recording of airway pressure and flow. Clin Otolaryngol Allied Sci. 2002;27:38-43.
36. Schwartz AR, Smith PL, Wise RA, et al. Induction of upper airway occlusion in sleeping individuals with subatmospheric nasal pressure. J Appl Physiol. 1988;64:535-542.
37. Smith PL, Wise RA, Gold AR, et al. Upper airway pressure-flow relationships in obstructive sleep apnea. J Appl Physiol. 1988;64:789-795.
38. Schwartz AR, Gold AR, Schubert N, et al. Effect of weight loss on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis. 1991;144:494-498.
39. Schwartz AR, Schubert N, Rothman W, et al. Effect of uvulopalatopharyngoplasty on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis. 1992;145:527-532.