Chapter 80 Air Pollution
History
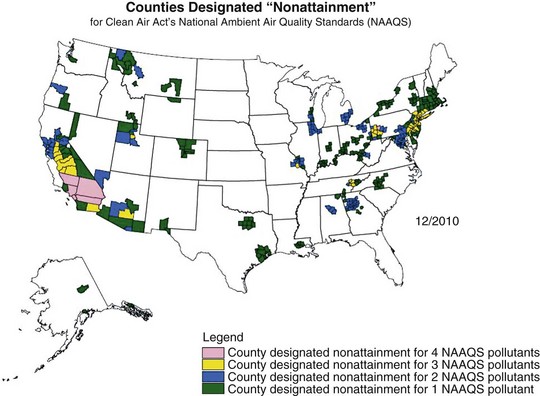
Air pollution has posed a threat to human health since the advent of fire, 500,000 years ago. Evidence of soot in prehistoric caves indicates that early humans were exposed to high levels of indoor air pollution. Outdoor pollution began to rise as populations grew and clustered in cities. During the Industrial Revolution, there was a dramatic increase in coal use by factories and households. The resulting smog caused significant morbidity and mortality, particularly when combined with stagnant atmospheric conditions. During the Great London Smog of 1952, heavy pollution for 5 days caused at least 4000 excess deaths (Figure 80-1). This episode highlighted the relationship between air pollution and human health and, together with other episodes, led to clean-air policies in Europe and in the United States. Over the next several decades, with improvements in the regulatory framework, air quality in the developed world steadily improved. Even as energy consumption and greenhouse gas emissions have increased, pollutants that are toxic to humans have been better controlled. Nevertheless, 186 million Americans, or 60% of the population, still live in counties with levels of air pollution higher than U.S. Environmental Protection Agency (EPA) standards (Figure 80-2). Air pollution also continues to be a growing problem in cities and households of the developing world.
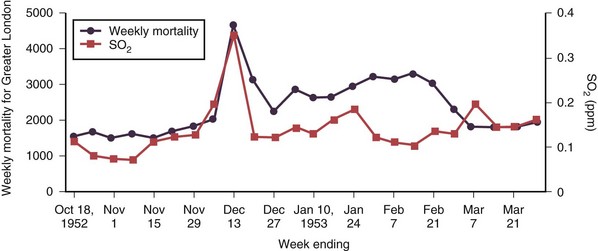
Figure 80-1 Weekly mortality in greater London, October 1952 to March 1953; SO2, oxygen saturation.
(Modified from Bell ML, Davis DL: Reassessment of the lethal London fog of 1952: novel indicators of acute and chronic consequences of acute exposure to air pollution, Environ Health Perspect 109(suppl 3):389–394, 2001.)
Composition of Air Pollution
Many different substances are considered “air pollutants,” which typically exist in combination in the environment; these include particulate matter (PM), carbon monoxide (CO), ozone (O3), nitrogen oxides (NOx), sulfur oxides (SOx), and volatile organic compounds (VOCs). The composition varies by season and regions, and the chemistry, state, and size of a pollutant influence the resulting health effects (Figure 80-3). Although CO has specific toxicity affecting oxygen transport, many pollutants appear to cause disease by triggering injury through inflammation and oxidative stress. Physiologic effects can be confirmed by experimental studies, but health impacts of air pollution on populations can be difficult to attribute to a single pollutant because they often share sources and vary together. A multipollutant approach to understanding health effects is becoming more common. Nevertheless, this section discusses some of the physiologic effects of individual pollutants before a description of the health outcomes seen with exposure to air pollution.
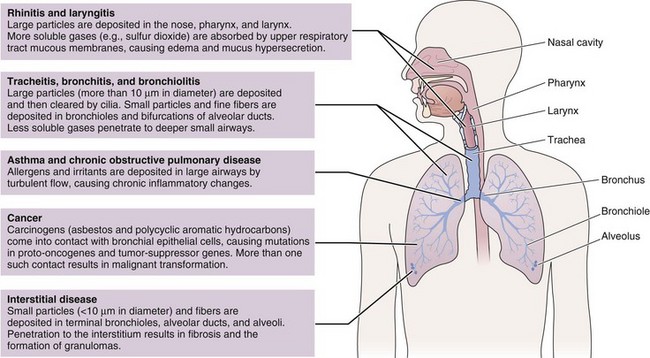
Figure 80-3 Physiologic effects of air pollutants on the respiratory tract.
(Modified from Beckett WS: Occupational respiratory diseases, N Engl J Med 342:406–413, 2000.)
Particulate Matter
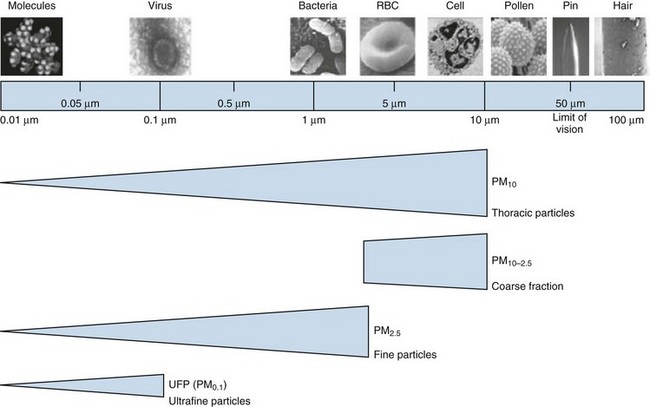
Among air pollutants, PM is most consistently associated with increased mortality effects at ambient exposure concentrations regularly encountered today. Although PM can be formed by a wide variety of natural and man-made processes, one of the largest sources of PM pollution is through combustion of biomass and fossil fuels. PM is composed of organic and inorganic compounds that vary greatly in size (Figure 80-4). Fine PM (<2.5 µm) has the most well-recognized health effects, and the ultrafine PM fraction (<0.1 µm) may be especially important. Larger PM is filtered and trapped in the proximal respiratory tract, but fine particles can penetrate deep into the lung, where they are efficiently deposited, cause local effects, and may be systemically absorbed.
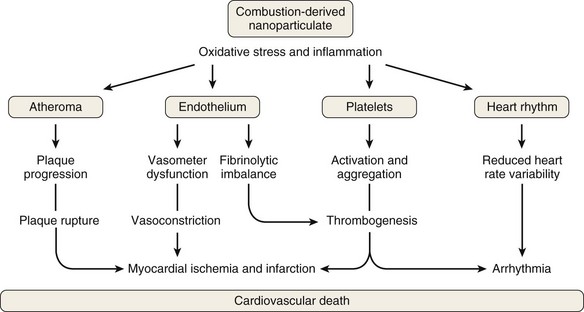
The composition of PM also influences its health effects. The mechanism of PM toxicity in the lung is still being elucidated but is likely multifactorial and can include impaired ciliary function, damage to epithelial cells, inflammation, and oxidative stress. Neutrophils and macrophages phagocytize the poorly soluble particles and initiate inflammatory cascades. PM also causes oxidative stress by generating reactive oxygen species (ROS) on the surface of particles that may then cause cellular effects. Inflammation and oxidative stress apparently provoke cardiovascular disease through atherosclerosis, thrombogenesis, vasoconstriction, and reduced heart rate variability (Figure 80-5).
Outdoor Air Pollution
Health Effects of Outdoor Air Pollution
Outdoor air pollution has many diverse health effects, ranging from mild respiratory irritation to hospitalization and death. The World Health Organization estimates that an excess 800,000 deaths occur annually because of exposure to outdoor air pollution. Daily fluctuation in levels of air pollution causes an acute increase in mortality, mostly from cardiovascular causes. Chronic air pollution exposure also contributes to morbidity and mortality through a variety of cardiopulmonary illnesses. Some of the first evidence for mortality of chronic exposure to PM was demonstrated by the Six Cities Study, in which citizens of six U.S. cities (representing a range of air pollution severity) were studied as a prospective cohort. Cities with higher levels of fine PM had an increased risk of all-cause mortality (risk ratio, 1.26 for most polluted vs. least polluted city; Figure 80-6). Many other cohort studies confirm this relationship, with improved determination of exposures and health outcomes.
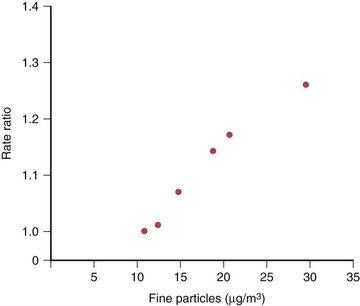
Figure 80-6 Association of fine particulate concentration and mortality from Harvard Six Cities Study.
(Modified from Dockery DW et al: N Engl J Med 329:1753–1759, 1993.)
Acute Respiratory Illnesses
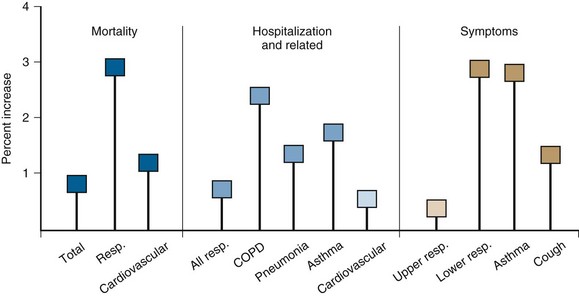
Exposure to air pollution produces acute changes in lung function in healthy adults. These changes usually go unnoticed by patients who are not predisposed to bronchospasm. Among patients with asthma and COPD, however, exposure to pollution can lead to serious effects. Asthmatic patients experience increased cough, wheezing, breathlessness, and medication needs on days with high air pollution. Hospitalizations for asthma, COPD, and pneumonia all increase as air pollution rises. There are also more respiratory symptoms and upper respiratory infections in the general population. Even small changes in PM concentration can lead to these effects (Figure 80-7). For every 10 µg/m3 rise in PM, there is approximately 1% increase in all respiratory diseases. Although this effect size seems small, it has a notable impact when applied to large populations.
Air Quality Index
Governmental agencies worldwide measure and report on the air quality in major cities and suburban areas. The U.S. EPA produces an Air Quality Index (AQI) for more than 100 U.S. cities and regions. The AQI is reported on a scale from 0 to 500, with higher values being more hazardous (Table 80-1). An AQI score more than 100 indicates unhealthy air quality, especially when compounded by high-risk weather conditions, such as hot and sunny days. Many newspapers, weather reporters, and radio stations report this score. On days with high AQI scores, it is recommended that people stay indoors as much as possible. Susceptible individuals should also limit physical activity, which increases ventilation and ultimately leads to five times the amount of PM deposition in lungs.
Table 80-1 Definitions of Air Quality Index (AQI) Levels by U.S. Environmental Protection Agency
| AQI Levels of Health Concern | Numeric Value | Description |
|---|---|---|
| Good | 0-50 | Air quality is considered satisfactory, and air pollution poses little or no risk. |
| Moderate | 51-100 | Air quality is acceptable; for some pollutants, however, there may be a moderate health concern for a small number of people unusually sensitive to air pollution. |
| Unhealthy for sensitive groups | 101-150 | Members of sensitive groups may experience health effects; the general public is not likely to be affected. |
| Unhealthy | 151-200 | Everyone may begin to experience health effects; members of sensitive groups may experience more serious health effects. |
| Very unhealthy | 201-300 | Health alert; everyone may experience more serious health effects. |
| Hazardous | 301-500 | Health warnings of emergency conditions. The entire population is more likely to be affected. |
Data from www.airnow.gov. Accessed March 2011.
Indoor Air Pollution
The vast majority of adverse health effects related to air pollution likely occur in developing countries, where there are higher levels of both outdoor and indoor air pollution. More than half of the world’s population depends on solid fuel as the primary source of domestic energy for cooking and heat. Most of this is in rural regions of the developing world where cooking is done over open fires in poorly ventilated homes (Figure 80-8). This creates intense exposure to indoor air pollution (IAP) with devastating health consequences.

Figure 80-8 Ecuadorian child sitting next to indoor cooking fire.
(From Rinne ST, Rodas EJ, Rinne ML, et al: Use of biomass fuel is associated with infant mortality and child health in trend analysis, Am J Trop Med Hyg 76:585–591, 2007.)
Among households that rely on solid fuels for energy, approximately 95% use biomass fuels (wood, charcoal, crop residues, dung) in open indoor fires. Household coal use is less common and is primarily used in parts of China and South Africa. Overall, solid fuels are less efficient and have greater emissions than costlier fuels such as gas and electricity. In fact, although developed countries use several times more energy per household, the fuels used are cleaner with less exposure to pollutants. These differences in domestic energy are referred to as the “energy ladder” (Figure 80-9).
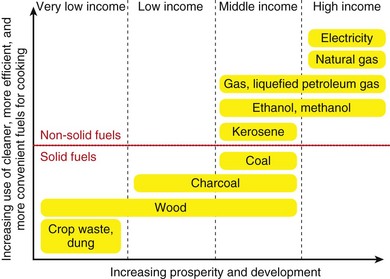
Figure 80-9 “Energy ladder” emphasizing the association of development with household energy.
(Modified from World Health Organization: Fuel for life: household energy and health, Geneva, 2006, WHO.)
Health Effects of Indoor Air Pollution
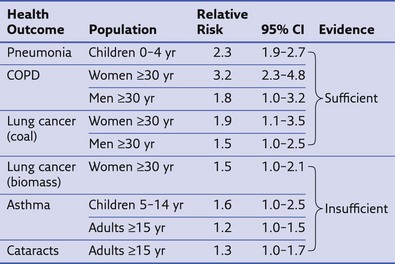
The World Health Organization estimates that exposure to solid fuel smoke causes 1.6 million deaths annually, primarily in the developing world (Figure 80-10). This includes deaths caused by pneumonia, COPD, and lung cancer (Table 80-2). Increasing evidence also is revealing the role of IAP in other infectious and noninfectious diseases. Most diseases associated with outdoor pollution are also linked to IAP exposure. Overall, IAP is responsible for 38.5 million disability-adjusted life years (DALYs), making it the 8th leading cause of DALYs and 11th cause of death worldwide.
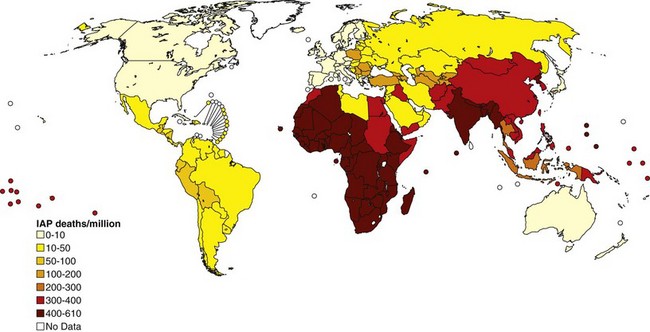
Figure 80-10 Distribution of mortality from indoor air pollution (IAP) throughout the world.
(Modified from World Health Organization: The World Health Report 2002: reducing risks, promoting healthy life, Geneva, 2002, WHO.)
Table 80-2 Results from Metaanalyses on Association of Indoor Air Pollution with Various Health Outcomes
Modified from World Health Organization: Fuel for life: household energy and health, Geneva, 2006, WHO.
CI, confidence interval; COPD, chronic obstructive pulmonary disease.
Chronic Respiratory Illnesses
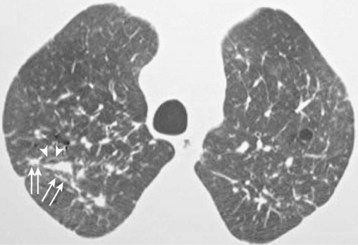
Chronic exposure to indoor smoke causes a broad range of chronic disorders referred to as domestic acquired particulate lung disease (DAPLD) or “hut lung.” DAPLD includes noninfectious, nonmalignant diseases such as COPD and pulmonary fibrosis. Women who cook with solid fuels are three times more likely to develop COPD than women who cook with cleaner fuels. Globally, IAP causes 22% of COPD cases. Although most people exposed to chronic indoor smoke develop obstructive pulmonary function, some also have restrictive patterns. Bronchoscopy typically reveals anthracotic plaques. Computed tomography (CT) may demonstrate a variety of patterns, including reticulation, peribronchial thickening, nodules, and ground-glass opacities (Figure 80-11). Although studies are still few and inconsistent, some reports find a higher incidence of asthma among children exposed to indoor smoke.
Brunekreef B, Holgate ST. Air pollution and health. Lancet. 2002;360:1233–1242.
Dockery DW, Pope CA, Xu X, et al. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329:1753–1759.
Fullerton DG, Bruce NN, Gordon SB. Indoor air pollution from biomass fuel smoke is a major health concern in the developing world. Trans R Soc Trop Med Hyg. 2008;102:843–851.
Laumbach RJ. Outdoor air pollutants and patient health. Am Fam Physician. 2010;81:175–180.
Minelli C, Wei I, Sagoo G, et al. Interactive effects of antioxidant genes and air pollution on respiratory function and airway disease: a HuGE review. Am J Epidemiol. 2011;173:603–620.
Perez-Padilla R, Schilmann A, Riojas-Rodriguez H. Respiratory health effects of indoor air pollution. Int J Tuberc Lung Dis. 2010;14:1079–1086.
Ren C, Tong S. Health effects of ambient air pollution: recent research development and contemporary methodological challenges. Environ Health. 2008;7:56.
Sun Q, Hong X, Wold LE. Cardiovascular effects of ambient particulate air pollution exposure. Circulation. 2010;121:2755–2765.
World Health Organization. Fuel for life: household energy and health. Geneva: WHO; 2006.
Yang IA, Fong KM, Zimmerman PV, et al. Genetic susceptibility to the respiratory effects of air pollution. Thorax. 2008;63:555–563.
Yang W, Omaye ST. Air pollutants, oxidative stress and human health. Mutat Res. 2009;674:45–54.