Agglutination Methods
At the conclusion of this chapter, the reader should be able to:
• Describe the principles of agglutination.
• Identify and compare the characteristics of agglutination methods.
• Explain methods for enhancing agglutination.
• Describe the characteristics of graded agglutination reactions.
• Discuss the principles of pregnancy testing, including sources of error.
• Correctly answer case study related multiple choice questions.
• Be prepared to participate in a discussion of critical thinking questions.
• Explain agglutination reactions of the ABO blood group procedure.
• Describe the principle and sources of error of the ABO blood group procedure.
Principles of Agglutination
Precipitation and agglutination are the visible expression of the aggregation of antigens and antibodies through the formation of a framework in which antigen particles or molecules alternate with antibody molecules (Fig. 10-1). Precipitation is the term for the aggregation of soluble test antigens. Precipitation is the combination of soluble antigen with soluble antibody to produce a visible insoluble complex. Agglutination is the process whereby specific antigens (e.g., red blood cells) aggregate to form larger visible clumps when the corresponding specific antibody is present in the serum.
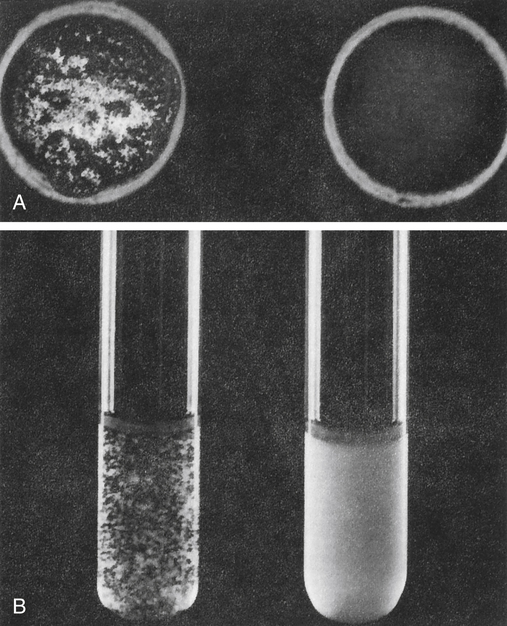
A, Slide agglutination of bacteria with known antisera or known bacteria. Left, Positive reaction; right, negative reaction. B, Tube agglutination. Left, Positive reaction; right, negative reaction. (From Barrett JT: Textbook of immunology, ed 5, St Louis, 1988, Mosby.)
Artificial carrier particles may be needed to indicate visibly that an antigen-antibody reaction has taken place; examples include latex particles and colloidal charcoal. Cells unrelated to the antigen, such as erythrocytes coated with antigen in a constant amount, can be used as biological carriers. Whole bacterial cells can contain an antigen that will bind with antibodies produced in response to that antigen when it is introduced into the host (Table 10-1).
Table 10-1
| Type (Reagent) | Type of Assay | Principle | Result |
| Latex particles | C-reactive protein (CRP) | A suspension of polystyrene latex particles of uniform size is coated with the IgG fraction of an antihuman CRP-specific serum. | If CRP is present in the serum, an antigen-antibody reaction takes place. This reaction causes a change in the uniform appearance of the latex suspension and a clear agglutination results. |
| Stabilized sheep erythrocytes sensitized with rabbit gamma globulin suspended in buffer solution | Rheumatoid factor (RF) | RF acts like antibodies against gamma globulin that acts as the antigen. | If gamma globulin is attached to a particular carrier (e.g., RBCs or latex particles), the reaction of RF with gamma globulin becomes a visible agglutination. |

The quality of test results depends on the following technical factors:
Latex Agglutination
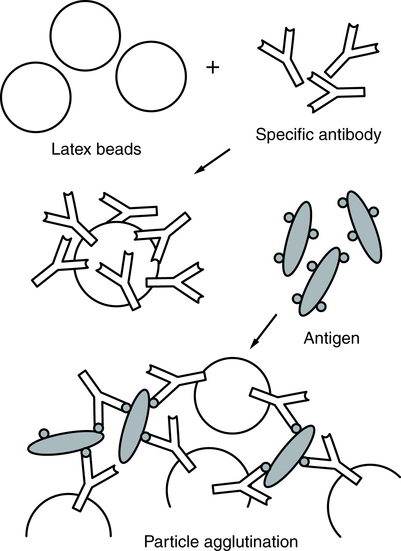
In latex agglutination procedures (Box 10-1), antibody molecules can be bound to the surface of latex beads. Many antibody molecules can be bound to each latex particle, increasing the potential number of exposed antigen-binding sites. If an antigen is present in a test specimen such as C-reactive protein, the antigen will bind to the combining sites of the antibody exposed on the surface of the latex beads, forming visible cross-linked aggregates of latex beads and antigen (Fig. 10-2). In some procedures (e.g., pregnancy testing, rubella antibody testing), latex particles can be coated with antigen. In the presence of serum antibodies, these particles agglutinate into large visible clumps.
Procedures based on latex agglutination must be performed under standardized conditions. The amount of antigen-antibody binding is influenced by factors such as pH, osmolarity, and ionic concentration of the solution. A variety of conditions can produce false-positive or false-negative reactions in agglutination testing (see Table 10-4).
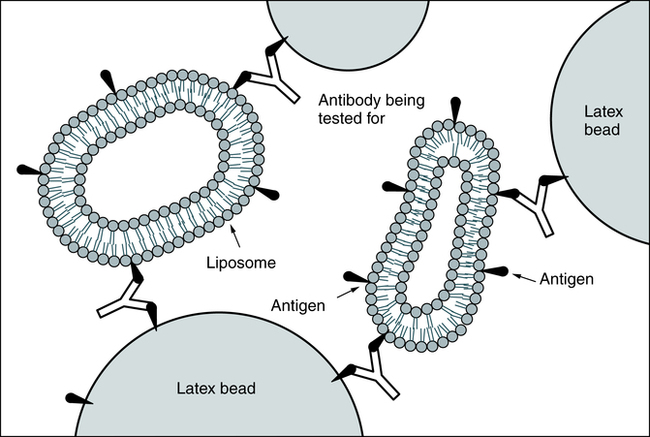
Coagglutination and liposome-enhanced testing are variations of latex agglutination (Fig. 10-3). Coagglutination uses antibodies bound to a particle to enhance the visibility of agglutination. It is a highly specific method but may not be as sensitive as latex agglutination for detecting small quantities of antigen.
 Pregnancy Latex Slide Agglutination
Pregnancy Latex Slide Agglutination
Principle
See ![]() for the procedural protocol.
for the procedural protocol.
Alternate Procedural Protocols
Latex agglutination slide tests have been replaced in many situations (e.g., home testing; see Chapter 9) by one-step chromatographic color-labeled immunoassays for the qualitative detection of hCG in urine (e.g., Clearview hCG II and Clearview hCG Easy, Wampole Laboratories, Princeton, NJ). Another variation is a one-step chromatographic color-labeled immunoassay for use with urine or serum (e.g., Wampole PreVue hCG Stick or Cassette, Status hCG).
Flocculation Tests
Flocculation testing can be used in syphilis serologic testing (see Chapter 18). These tests are the classic Venereal Disease Research Laboratories (VDRL) and rapid plasma reagin (RPR) tests. In the VDRL test, an antibody-like protein, reagin, binds to the test antigen, cardiolipin-lecithin–coated cholesterol particles, and produces the particles that flocculate. In the RPR test, the antigen, cardiolipin-lecithin–coated cholesterol with choline chloride, also contains charcoal particles that allow for macroscopically visible flocculation.
Hemagglutination
By binding different antigens to the RBC surface in indirect hemagglutination or passive hemagglutination (PHA), the hemagglutination technique can be extended to detect antibodies to antigens other than those present on the cells (Box 10-2). Chemicals such as chromic chloride, tannic acid, and glutaraldehyde can be used to cross-link antigens to the cells.
Some antibodies (e.g., immunoglobulin G [IgG]) do not directly agglutinate erythrocytes. This incomplete or blocking type of antibody may be detected by using an enhancement medium such as antihuman globulin (AHG) reagent (also known as Coombs reagent). If AHG reagent is added, this second antibody binds to the antibody present on the erythrocytes (see procedure in Chapter 26).
Mechanisms of Agglutination
Sensitization
• Electrolyte concentration and viscosity
• Physical conditions (e.g., pH, temperature, duration of incubation)
Particle Charge
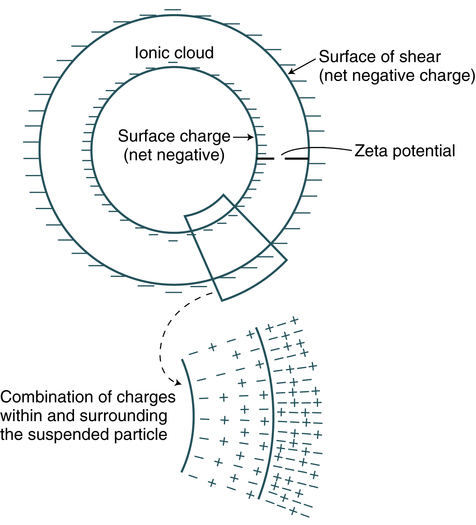
Inert particles such as latex, RBCs, and bacteria have a net negative surface charge called the zeta potential (Fig. 10-4). The concentration of salt in the reaction medium has an effect on antibody uptake by the membrane-bound erythrocyte antigens. Sodium (Na+) and chloride (Cl−) ions in a solution have a shielding effect. These ions cluster around and partially neutralize the opposite charges on antigen and antibody molecules, which hinders antibody-antigen association. By reducing the ionic strength of a reaction medium (e.g., using low ionic strength saline [LISS]), antibody uptake is enhanced. Charges can be overcome by centrifugation, addition of charged molecules (e.g., albumin, LISS), or enzyme pretreatment to permit the cross-linking that results in agglutination (Table 10-2).
Table 10-2
Techniques to Reduce Zeta Potential
| Technique | Action |
| Enzyme pretreatment of red blood cells | Removes negatively charged sialic acid residues from cell surface membrane |
| Addition of colloids (e.g., albumin) | Increases electrical conductivity of environment |
| Centrifugation | Mechanical process to force red blood cells closer together |
Adapted from Lehman CA: Saunders manual of clinical laboratory science, Philadelphia, 1998, WB Saunders, p 391.
Graded Agglutination Reactions
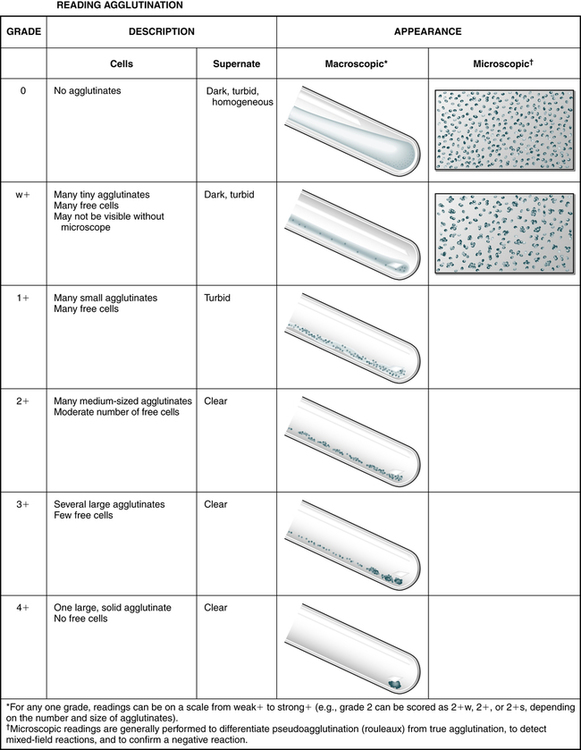
The strength of agglutination (Table 10-3; Fig. 10-5), called grading, uses a scale of 0, or negative (no agglutination), to 41 (all erythrocytes clumped). Table 10-4 describes false-positive and false-negative reactions. Pseudoagglutination, or the false appearance of clumping, may rarely occur because of rouleaux formation. Rouleaux formation can be encountered in patients with high or abnormal types of globulins in their blood, such as in multiple myeloma or after receiving dextran as a plasma expander. On microscopic examination, the erythrocytes appear as rolls resembling stacks of coins. To disperse the pseudoagglutination, a few drops of physiologic NaCl (saline) can be added to the reaction tube, remixed, and reexamined. This procedure, saline replacement, should be performed carefully after pseudoagglutination is suspected. It should never be done before the initial testing protocol is followed; a false-negative result may occur from the dilutional effect of the saline.
Table 10-3
Grading Agglutination Reactions
| Grade | Description |
| Negative | No aggregates |
| Mixed field | A few isolated aggregates; mostly free-floating cells; supernatant appears red |
| Weak (±) | Tiny aggregates barely visible macroscopically; many free erythrocytes; turbid and reddish supernatant |
| 1+ | A few small aggregates just visible macroscopically; many free erythrocytes; turbid and reddish supernatant |
| 2+ | Medium-sized aggregates; some free erythrocytes; clear supernatant |
| 3+ | Several large aggregates; some free erythrocytes; clear supernatant |
| 4+ | All erythrocytes are combined into one solid aggregate; clear supernatant. |
Table 10-4
Causes of False-Positive and False-Negative Agglutination Test Reactions
| Cause | Correction |
| False-Positive Reactions | |
| Contaminated equipment or reagents may cause particles to clump. | Store equipment and reagent in clean, dust-free environment, and handle with care. Use negative quality control (QC) steps. |
| Autoagglutination | Use a control with saline and no antibody as a negative control. If positive, patient’s result is invalid. |
| Delay in reading slide reactions results in drying out of mixture. | Follow procedural directions and read reactions exactly as specified. |
| Overcentrifugation causes cells or particles to clump too tightly. | Calibrate centrifuge to proper speed and time. |
| False-Negative Reactions | |
| Inadequate washing of red blood cells in antihuman globulin (AHG) testing∗ may result in unbound immunoglobulins neutralizing the reagent. | Wash cells according to directions. Use positive and negative QC steps. |
| Failure to add AHG reagent | Use positive QC steps. |
| Contaminated or expired reagents | Use positive and negative QC steps. |
| Improper incubation | Follow procedural protocol exactly. Use positive and negative QC steps. |
| Delay in reading slide reactions | Follow procedural protocol exactly. Use positive and negative control steps. |
| Undercentrifugation | Calibrate centrifuge to proper speed and time. |
| Prozone phenomenon | Dilute patient serum containing antibody, and repeat the procedure. |
 ABO Blood Grouping (Reverse Grouping)
ABO Blood Grouping (Reverse Grouping)
Principle
Reverse typing is a cross-check for forward typing (see Chapter 2 procedure). Because of the lack of synthesized immunoglobulins in newborn and very young infants, this procedure is not performed on specimens from these patients.
See ![]() for the procedural protocol.
for the procedural protocol.
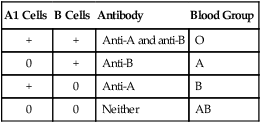
Reporting Results
Reactions of Patient Serum and Reagent Erythrocytes
| A1 Cells | B Cells | Antibody | Blood Group |
| + | + | Anti-A and anti-B | O |
| 0 | + | Anti-B | A |
| + | 0 | Anti-A | B |
| 0 | 0 | Neither | AB |
Biological Sources of Error
Incorrect typing can also result from additional antigens, caused by the following:
• Acquired B-like antigen; acquired A-like antigen
• Agents causing nonspecific erythrocyte agglutination
• Antibody-sensitized RBCs: effect of colloids and antiantibodies (e.g., hemolytic disease of the newborn, incompatible transfusion, autoimmune process)
Technical Sources of Error
Procedures that apply to all tests for ABO grouping include the following:
1. Do not rely on the color of dyes to identify reagent antisera. All tubes must be properly labeled.
2. Do not perform tests at temperatures higher than room temperature (20° C to 24° C [68° F to 75° F]).
3. Perform observations of agglutination with a well-lit background, not a warm view box.
4. Record results immediately after observation.
5. Remember that contaminated blood specimens, reagents, or supplies may interfere with the test results.
Chapter Highlights
• Agglutination of particles to which soluble antigen has been adsorbed is a serum method of demonstrating precipitins. Examples of artificial carriers include latex particles and colloidal charcoal. Cells unrelated to the antigen, such as erythrocytes coated with antigen in a constant amount, can be used as biological carriers.
• In latex agglutination procedures, antibody molecules can be bound to the surface of latex beads. If an antigen is present in a test specimen, the antigen will bind to the combining sites of the antibody exposed on the surface of the latex beads, forming visible cross-linked aggregates of latex beads and antigen.
• Flocculation tests for antibody detection are based on the interaction of soluble antigen with antibody, which results in the formation of a precipitate of fine particles.
• Direct bacterial agglutination can be used to detect antibodies directed against pathogens.