Chapter 3 Age-related macular degeneration (AMD) and cataracts
Introduction
AMD
Age-related macular degeneration (AMD) and cataracts are the 2 leading eye diseases of advanced age. AMD is the leading cause of blindness in the elderly worldwide, affecting 30–50 million people. The World Health Organization (WHO) statistics from the most recent WHO global eye disease surveys conducted in 2002 reported that 8.7% of worldwide blindness was due to AMD, the third leading cause of worldwide blindness after cataract and glaucoma.1 Conservatively, the WHO has estimated that 14 million persons worldwide could be blind or severely visually impaired because of AMD.2, 3 The majority of these impaired individuals and the millions more who are visually impaired from this condition live in developed countries.
The large population-based Beaver Dam Eye Study discovered that the prevalence of large extracellular ocular deposits known as drusen, a hallmark sign of AMD, was 2% in persons 43–54 years of age and 24% in persons over 75 years of age in the US.4 It has also been reported that late AMD is the most common cause of untreatable blindness in the Western world, with a prevalence that is 0.05% before the age of 50 years and that rises to 11.8% after 80 years of age.5
Numerous large population studies have identified important factors as to the epidemiology of AMD. The Age–Related Eye Disease Study (AREDS) found that 20.2% of individuals with an early stage diagnosis of AMD progressed to advanced disease over a 5–year period, a rate of 4.0% per year. Similar annual rates of progression, between 2.5% and 4.8%, were reported in the Rotterdam Study and Blue Mountains Eye Study (BMES) respectively.6, 7 Recently a report by Taylor and colleagues estimated that the progression rate for mild to moderate visual impairment was approximately 32%, and that the rate from moderate to severe visual impairment was 46%.8 The basis for this estimate was derived from the 2–3 year progression data that originated from the Macular Photocoagulation Study (MPS), the Treatment of AMD with Photodynamic Therapy (TAP) study and the Vertiporfin in Photodynamic (VIP) Therapy study. These reported rates are slightly higher than the rates of 16% and 17% per year reported by the Melbourne Visual Impairment Project (MVIP) study. Taylor and colleagues also showed that the incidence of mild visual impairment in individuals who were 40 years or older was 0.10% per year and, for moderate and severe visual impairment in this same group, it was 0.04% and 0.11% per year, respectively.9
However, as life expectancies are observed to increase in all parts of the world the prevalence of AMD is also expected to increase. It is likely that in the following decades, many millions of people worldwide will suffer blindness or severe visual impairment from AMD. 10–17
Cataracts
A recent review has reported that cataract, (opacification of the lens) is one of the commonest causes of loss of useful vision, with an estimated prevalence of 16 million people affected worldwide.18 Prevalence studies for cataract are made difficult by a lack of uniform grading scores for the clinically observed opacities of the lens. Several population studies have been reported though, that demonstrate an increasing risk for cataract development with increasing age.19, 20, 21,
The Framingham Eye Study from the US, reported in 1977,19 that the proportion of people with age–related cataracts causing loss of vision of 20/30 (6/9) or worse was 15·5% for all ages and 45·9% for those 75 years or older. In the 1992 Beaver Dam Eye Study,20, 21 which used a similar definition of loss of vision, the reported proportions were 38·8% of men and 45·9% of women older than 74 years.
Data from other studied populations demonstrate a wide range of rates, such as 82% of 75–85 year olds in India and 53% of 75–85 year olds in Tibet.22, 23
Lifestyle and lifetime risk factors
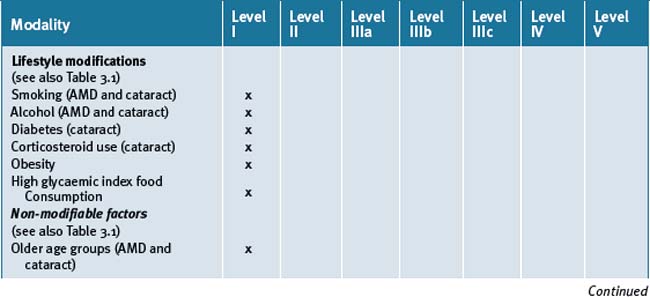
A recent report by the US Preventive Services Task Force has identified a number of risk factors for the prevention of AMD and cataracts (Table 3.1).24
Table 3.1 Scientifically proven robust risk factors for AMD and cataracts
| AMD | Cataracts |
|---|---|
| Older age groups | Older age groups |
| Family history | Female gender |
| White race | Black race |
| Smoking | Smoking |
| Alcohol use (heavy) | Alcohol use |
| Exposure to ultraviolet light B | |
| Diabetes (+associated pathologies) | |
| Corticosteroid use |
The reviewed epidemiological literature conclusively reports that the older ages are causal for the development of AMD and cataracts.22, 25
Genetic predispositions
Family history, white race, black race
Family history is a consistent risk factor identified in most epidemiological studies of AMD that have been conducted. Familial aggregation studies have shown that there is a genetic contribution that has been readily identified in up to 25% of AMD cases.26 Moreover, studies with twins also support a genetic basis for the disease, with the concordance of clinical features (i.e. drusen and pigmentary changes) for both early and late onset of the disease being approximately twice as high in identical (monozygotic) twins compared to non–identical (dizygotic) twins.27, 28
Numerous population studies have investigated the racial differences observed in the prevalence of AMD.29, 30, 31 Recently, in an eye disease prevalence investigation Bressler et. al.32 have described the differences in frequency of early fundus lesions associated with AMD by racial group in an extended study of the Salisbury Eye Evaluation population cohort. The study confirmed that white persons are generally more likely than black persons to have medium or large drusen, focal pigment abnormalities, and advanced AMD. Also racial differences were prominent for non–neovascular AMD features only when present in the central zone.
The Salisbury Eye Evaluation study has also reported racial differences in lens opacities, confirming that cataract was a highly significant problem in the African–American population more so than in the white population.33, 34
Smoking
There is robust evidence that causally associates AMD and cataract to smoking.22, 35, 36, 37
Alcohol
A recent systematic review and meta-analysis has concluded that heavy alcohol consumption that consists of more than 3 standard drinks per day is associated with an increased risk of early AMD.38 The link between alcohol and cataract is less robust — a relationship between alcohol consumption and an increased risk of cataract has been reported from cross–sectional studies, but with several prospective cohort studies failing to confirm this association.39, 40
Exposure to UVB
Over-exposure to ultraviolet B has also been concluded to be causally associated to lens opacifications as reported by numerous studies.41, 42, 43
Diabetes
The prevalence and causes of visual impairment in an epidemiologic study of aged, urban individuals in Denmark reported that diabetic retinopathy in that country has a prevalence of 5.6%.44 A recent study from the US has reported that the prevalence of diabetic retinopathy among persons with diagnosed diabetes was 9.9% and in that survey of self-reported age-related eye diseases there were approximately 1.3 million people in the US with diabetic retinopathy.45
Steroid and/or corticosteroid medications
The association of cataract with systemic corticosteroid therapy was first postulated in 1960.46 Later reviews of the scientific literature concluded that steroids remained a risk for the development of cataract.47, 48 Recently a report on steroid–induced posterior subcapsular cataracts concluded that it remains a serious concern even though some reports indicated that the evidence for glucocorticoid–protein adduct formation in the lens was inconclusive.49
Obesity
A prospective cohort study in a hospital–based retinal practice investigated and followed up patients that were 60 years or older, with some sign of non–advanced AMD and visual acuity of 20/200 or better in at least 1 eye.50 This study documented that overall and abdominal obesity increased the risk for progression to advanced AMD, and more physical activity tended to decrease risk.
Recently the association between changes in waist–hip ratio, a measure of abdominal obesity, and AMD in a total of 12 515 persons aged 45–64 years, from a population–based cohort study from 1987 to 1989, was followed up over 6 years.51
High glycaemic index foods (versus low glycaemic index foods)
As has been previously demonstrated in this section and within the framework of cellular metabolic dysfunction, dietary factors are known risk factors for AMD. The National Health and Nutrition Examination Survey (NHANES III) demonstrated that, in the population over 65 years of age, 18–20% have diabetes, with 40% having either diabetes or its precursor form of impaired glucose tolerance. Hyperglycaemia itself can promote the formation of advanced glycation end products. Hyperglycaemia damages blood vessels leading to various micro– and macro–vascular diseases such as diabetic retinopathy, which is the main microvascular complication of diabetes and the most common cause of blindness in working populations.52
The Blue Mountain Eye Study reported on the risk associated with high glycaemic index foods in its study population.53 The study concluded that a high glycaemic index diet was a risk factor for early AMD; the recognised precursor of sight-threatening late AMD. It was also noted that low glycaemic index foods, such as oatmeal, could protect against early AMD. This was consistent with an earlier study that advised that persons at risk of AMD progression, especially those at high risk of advanced AMD, could benefit from consuming smaller amounts of refined carbohydrates.54
A recent dietary/food composition and consumption study investigated a number of nutritional factors and the risk of AMD.55 It was reported that consuming diets that provided low glycaemic index foods and higher intakes of nutrients were associated with the greatest reduction in risk for prevalent drusen and advanced AMD.
Environmental
Sunlight/UVB exposure
There is no total accord on the role of light exposure in the development of AMD. A 10-year prospective study has demonstrated that prolonged exposure to sunlight may be associated with increased risk of earlier development of AMD but not AMD progression.56, 57 Furthermore, this study also found a protective effect from the use of a hat and sunglasses in reducing the incidence of early AMD. An earlier Australian case control study, however, had failed to find a link between sunlight exposure and AMD.58
Pesticide exposure
It has been reported that exposure to pesticides may increase the risk of macular degeneration. Studies from Japan have linked organophosphate exposure to Saku disease, which involves retinal degeneration and other adverse ocular effects.59 Moreover, in an early study from India the prevalence of macular degeneration was higher among pesticide workers exposed to the organophosphate fenthion than among unexposed controls.60
Physical activity
AMD
As part of the Beaver Dam Eye population-based study, a 15-year cumulative incidence of AMD was determined through 4 examination phases at 5-year intervals.61 The study showed that there was a protective effect of physical activity for incident exudative AMD, independent of body mass index and other confounders. Moreover the study also suggested a possible modifiable behaviour that might be protective against developing AMD.
A recent study tested whether the risk of AMD could be decreased with vigorous physical activity.62 This was a prospective study of self–reported clinically diagnosed macular degeneration in male (n = 29 532) and female (n = 12 176) runners followed prospectively for 7.7 years. The study reported that higher doses of vigorous exercise (running — risk for AMD decreased 10% per km per day increment in running distance) was associated with a lower incident AMD risk independent of weight, cardiorespiratory fitness, and cigarette use. Hence, compared to men and women running < 2 km per day, those averaging 2 to 4 km per day had 19% lower adjusted risk, and those averaging ≥ 4 km per day had 42% to 54% lower adjusted AMD risk.
Cataracts
Similarly, a prospective study investigated physical activity and incident cataract.63 The risk for incident cataract increased with BMI, such that the risk in men > 27.5 kg/m2 was 88% larger than in men < 20 kg/m2. Men’s cataract risk declined significantly in relation to running distance, even when adjusted for BMI. Men who ran ≥64 km/week had 35% lower risk for cataract than those reporting < 16 km/week (28% lower risk when adjusted for BMI). Furthermore, men with greater cardiorespiratory fitness were at significantly less risk for development of cataract than were the least fit men.
Nutrition
Diets
Vegetables
Early descriptive reports64 hypothesised that there was an inverse relationship between increased consumption of foods rich in certain carotenoids (an important constituent of the retinal pigments), in particular dark green, leafy vegetables, and a decreased risk of developing advanced or exudative AMD.
A recent descriptive study that measured the regular dietary intake of antioxidants and the associated lowered risk of incident AMD reported that an above-median intake of all 4 nutrients — beta–carotene, vitamin C, vitamin E, and zinc — from foods was associated with a 35% reduced risk for incident AMD in elderly persons.65
In a further prospective observational study of plant food consumption from a large cohort of female health professionals, higher dietary intakes of lutein and/or zeaxanthin (refer also to later sections this chapter) and vitamin E from food and supplements were associated with significantly decreased risks of cataract.66
Meats
A recent epidemiological study has shown that higher consumption of fresh and processed red meat intake was positively associated with early AMD.67 Specifically the study reported that consumption of chicken ≥ 3.5 times/week versus <1.5 times/week was inversely associated with late AMD. Moreover that the data suggested that different meats may differently affect AMD risk and that hence this may be a target for lifestyle modification.
Fats (animal fats versus fish fats)
A cross-sectional study that assessed whether dietary intake of fat or fish is associated with AMD showed that the amount and type of dietary fat intake may be associated with AMD.37, 68 That this could be the situation was apparent from a recent study that evaluated the associations between past dietary fat intake and the prevalence of AMD and found that a diet low in trans–unsaturated fat and rich in omega–3 fatty acids and olive oil may reduce the risk of AMD.69
A prospective cohort study was undertaken to advise patients with a high risk for advanced forms of AMD about preventive measures through an evaluation program of the relationships between dietary fat intake and the progression of early or intermediate AMD to the advanced stages of the disease associated with visual loss. This study showed that:70
An AREDS study that examined the association of dietary omega-3 long-chain polyunsaturated fatty acid and fish intake with incident neovascular AMD and central geographic atrophy (CGA) reported that the data strongly suggested that dietary omega-3 long-chain polyunsaturated fatty acid intake was associated with a decreased risk of progression from bilateral drusen to CGA.71
A systematic review and meta-analysis from the same year confirmed that consumption of fish and foods rich in omega-3 fatty acids may be associated with a lower risk of AMD. However, it also reported that there was insufficient evidence from the current literature, with few prospective studies and no randomised clinical trials, to support their routine consumption for AMD prevention.72
In an elderly Australian cohort from 2009, 3654 participants that were examined at baseline and 2454 participants who were examined 5 and/or 10 years later were assessed for AMD.73 The study provided significant evidence for protection against early AMD from regular consumption of fish. Greater consumption of omega-3 polyunsaturated fatty acids and low intakes of foods rich in linoleic acid afforded a similar protection. It was also noted that regular consumption of nuts may also reduce AMD risk.
Salt consumption
A large Australian population-based cross-sectional study74 of 2873 patients (mean age 65 years), from the Blue Mountains area, reported that low intakes of salt in the diet reduced the risk of cataracts. Of these participants, 620 already had cortical cataracts, 350 had nuclear cataracts and 160 had posterior subcapsular cataracts. After controlling for additional risk factors for cataract formation, people with the highest quintile of sodium intake had twice the risk of developing posterior subcapsular cataracts than those in the lowest quintile of sodium intake.
Supplements
Vitamins and minerals
AMD
Antioxidants (Beta-carotene, vitamin C, vitamin E, Zinc)
The Beaver Dam Eye Study pointed to the importance of micronutrients in the prevention of progression of eye diseases. Significant, but modest, inverse associations were observed between intakes of pro–vitamin A carotenoids and dietary vitamin E and the incidence of large drusen and between zinc and the incidence of pigmentary abnormalities. No significant inverse associations though were found between antioxidants or zinc intake and the incidence of overall early AMD.75
Randomised placebo-controlled trials (by the AREDS study group) investigating high levels of antioxidant consumption of vitamins and minerals such as C and E, beta-carotene, and zinc have reported that people without contraindications, such as smoking, should consider taking a supplement of antioxidants plus zinc that could be of benefit in preventing progression of AMD.76
Systematic reviews provide additional recommendations that include that people with AMD, or early signs of the disease, could experience some benefit from taking supplements as used in the AREDS trial.77 The author also alluded to the potential harms of high-dose antioxidant supplementation which included an increased risk of lung cancer in smokers with beta-carotene, heart failure in people with vascular disease or diabetes using high dose vitamin E, and hospitalisation for genitourinary conditions for those people using high dose zinc.77, 78
Recent randomised trials with single vitamins, namely beta–carotene, has demonstrated no beneficial or harmful effects on the incidence of AMD.79 These randomised data relative to 12 years of treatment among a large population of apparently healthy men indicated that long-term supplementation with beta-carotene neither decreased nor increased the risk of AMD.
The evidence is somewhat contentious though given that systematic reviews have reported that there is insufficient evidence to support the role of dietary antioxidants, including the use of dietary antioxidant supplements, for the primary prevention of early AMD or to delay its progression.78, 80
However, while there is no definitive treatment presently available for AMD, the most recent evidence recommends that vitamin supplements with high doses of antioxidants used for primary prevention may reduce the incidence of AMD.81 This together with previous research by the National Eye Institute (AREDS) and others suggest benefit in the secondary prevention of dry AMD for some patients and some improvement in visual acuity.
B Group Vitamins
Observational epidemiologic and clinical studies indicate a direct association between homocysteine concentration in the blood and the risk of AMD.78, 82, 83, 84
A recent US study conducted a randomised double-blind placebo-controlled trial including 5442 female health care professionals 40 years or older with pre-existing cardiovascular disease or 3 or more cardiovascular disease risk factors. They examined the effect of combined folic acid (2.5mg/day), pyridoxine hydrochloride (vitamin B6 50mg/day), and cyanocobalamin (vitamin B12, 1mg/day) therapy to lower homocysteine levels versus placebo. A total of 5205 of these women did not have a diagnosis of AMD at baseline.81 At the end of the study they reported that after an average of 7.3 years of treatment and follow-up, there were 55 cases of AMD in the combination treatment group and 82 in the placebo group. For visually significant AMD, there were 26 cases in the combination treatment group and 44 in the placebo group These randomised trial data from a large cohort of women at high risk of cardiovascular disease indicates that daily supplementation with a combination of folic acid, pyridoxine, and cyanocobalamin may significantly reduce the risk of AMD.
Vitamin D
A cross-sectional association study of serum vitamin D and early and advanced AMD, assessed from non-mydriatic fundus photographs, were evaluated in the third National Health and Nutrition Examination Survey.85 This was a multistage nationally representative probability sample of non-institutionalised individuals (n = 7752; 11% with AMD). This study showed that consistent use versus non-use of vitamin D from supplements was inversely associated with early AMD only in individuals who did not consume milk daily. Moreover, even though additional studies are required to confirm these results, it was concluded that this study provided evidence that vitamin D may protect against AMD.
Cataract
Antioxidants (Vitamins C, E)
Population studies investigating the use of antioxidant vitamins and cataract incidents reported that the results provided significant evidence that there was a lower risk for cataract among users of multivitamin supplements and a stronger relationship for long-term use.86, 87 A nutritional assessment study of long-term intake of vitamins and carotenoids reported that the data support a role for vitamin C in diminishing the risk of cortical cataracts in women aged < 60 years and for carotenoids in diminishing the risk of posterior subcapsular cataracts in women who had never smoked.88
Randomised clinical trials with antioxidants have also been conducted for the prevention of cataract. The AREDS group has also reported that the use of a high-dose formulation of vitamin C, vitamin E, and beta-carotene in a relatively well-nourished older adult cohort had no apparent effect on the 7-year risk of development or progression of age-related lens opacities or visual acuity loss.89
Furthermore, an additional large randomised trial of apparently healthy female health professionals with 9.7 years of treatment and follow-up indicated that 600IU natural-source vitamin E taken every other day provided no benefit for age-related cataract or subtypes.90
Other nutritional supplements
Omega-3 Essential Fatty Acids (EFAs)
EFAs that humans require for healthy metabolic functions include alpha-linolenic acid, (short-chain omega-3 fatty acid) docosahexaenoic acid (DHA), and eicosapentaenoic acid (EPA) (both long-chain omega-3 fatty acids). Alpha-linolenic acid is the dietary precursor to both DHA and EPA and can be converted to a long-chain omega-3 fatty acid.91, 92, 93 Importantly and related to eye physiological structure and function, DHA is present in high concentrations in the retinal outer segments, and its deficiency has been reported to be associated with the initiation of the onset of AMD.94 Moreover, long-chain omega-3 fatty acids may also protect against unbalanced oxygenic,95 inflammatory and age-related retinal damage,93 which have been reported to be key pathogenic processes in AMD development.96
To date there have been few prospective studies37, 97–101 and no randomised clinical trials investigating the efficacy of EFAs in preventing AMD. However a recent systematic review and meta-analysis does suggest that consumption of fish and foods rich in omega-3 fatty acids may be associated with a lower risk of AMD.102
Recently it was reported that dietary lipid intake in the form of EFAs is a lifestyle and/or nutritional modifiable factor that could significantly influence the likelihood of developing sight-threatening forms of AMD.103
Phytochemicals
Lutein and zeaxanthin xanthophyll
Lutein and zeaxanthin give the macula lutea its characteristic yellow appearance.
There is cumulative evidence on the consumption of lutein carotenoid and zeaxanthin xanthophyll (in whole food or supplemental form), the resulting concentrations in the serum, and tissue distribution throughout the body, particularly in the retina, play a role in the development of AMD.104
The observational study, Carotenoids in Age-Related Eye Disease (CAREDS), concluded that diets rich in lutein plus zeaxanthin may protect against intermediate AMD in healthy women.105, 106 In addition, the population-based Pathologies Oculaires Liees-a l’Age (POLA) study also was strongly suggestive of a protective role of the xanthophylls, in particular zeaxanthin, for the protection against AMD as well as cataract.107
Other epidemiological studies have shown that elevated serum levels and/or intake of several antioxidants, such as carotenoids, vitamin E and ascorbic acid, are associated with a diminished risk for cataracts.108, 109 A case control study though failed to show that the serum status of carotenoids and tocopherols in patients with age-related cataracts was significantly different between cases and controls.110
Studies have found that lutein and xeanthins can be increased in the serum following an oral dose.111 A more recent study that investigated the effect of the carotenoid, lutein, supplemented at doses of 2.5, 5.0, and 10mg/day for 6 months on distribution of these carotenoids and their metabolites in the serum of elderly human participants, with and without AMD reported similar findings.112 The increase in the serum levels of lutein and/or zeaxanthin correlated with increases in the serum levels of their metabolites that have previously been identified in the ocular tissues. Hence it was concluded that elderly human participants with and without AMD can safely take supplements of lutein up to 10mg/day for 6 months with no apparent toxicity or side-effects.
A small study that investigated 17 patients clinically diagnosed with age-related cataracts were randomised in a double-blind study involving dietary supplementation with lutein (15mg; n = 5), alpha-tocopherol (100mg; n = 6), or placebo (n = 6), 3 times a week for up to 2 years demonstrated that visual function in patients who received the lutein supplements improved, suggesting that a higher intake of lutein, through lutein-rich fruit and vegetables or supplements, may have significantly beneficial effects on the visual performance of people with age-related cataracts.113
A further small study that investigated the long-term supplementation of lutein (mean 13 months range 4–20 months for participants diagnosed with AMD; mean 26 months range 16–36 months for participants diagnosed with cataract) found that serum lutein levels were maintained and that it was associated with improved visual function of the participants.114
Notwithstanding this evidence the US Food and Drug Administration (FDA) reviewed intervention and observational studies that evaluated the role of lutein and zeaxanthin in reducing the risk of AMD and cataracts and concluded that no credible evidence exists for a health claim about the intake of lutein or zeaxanthin and the risk of AMD or cataracts.115
A recent report post the FDA’s review, the 6–12 month Lutein Xanthophyll Eye Accumulation study involving 102 healthy caucasian males who received daily supplementation of either lutein (11mg), zeaxanthin (13mg), or a combination (10mg lutein, 12mg zeaxanthin), or placebo, a significant improvement was reported in macular pigment optical density responses were observed compared to placebo. This clinical trial did not report any adverse event due to the supplemental phytonutrient doses and it reported increased bioavailability, showing a 27–fold increase in plasma xanthophyll concentrations.116 In previous clinical trials, lutein and zeaxanthin were also reported to be safe at higher doses and to improve macular pigment optical density, visual acuity, and glare sensitivity.
Furthermore, it should be noted that currently evaluation of nutritional factors, specifically lutein and/or zeaxanthin and omega-3 fatty acids, is being tested in a multi-centre controlled, randomised trial — the Age-Related Eye Disease Study 2 (AREDS2).106 This is a randomised controlled clinical trial which started enrolling participants in the northern hemisphere in the autumn of 2006. The primary outcome of this trial is to determine whether oral supplementation with macular xanthophylls (lutein at 10mg/day and zeaxanthin at 2mg/day) or omega-3 long-chain polyunsaturated fatty acids (LCPUFAs; DHA eicosapentaenoic acid at a total of 1g/day) will decrease the risk of progression to advanced AMD, as compared with placebo. This study should further clarify the role, especially of these carotenoids in preventing AMD and or cataract.
A recent review has recommended108 that for persons with intermediate risk of AMD (such as bilateral large drusen) or advanced AMD (such as unilateral neovascular AMD or geographic atrophy involving the centre of fovea), the AREDS-type supplements are recommended because they are proven to reduce the risk of developing advanced AMD by 25% according to the following formulation.108
There are a number of additional emerging phytochemicals that have been reported to be promising in ocular physiology health.117
Anthocyanins
Blackcurrant anthocyanins have been found to be widely distributed in their intact forms in the plasma, ocular tissues, and whole eye after oral and intraperitoneal administration in animal models. Four anthocyanins were identified after intravenous administration in the aqueous humor, cornea, choroid, and retina, as well as small amounts in the vitreous and lens areas demonstrating the potential ocular protective benefits from oral intakes of anthocyanins.118
Anthocyanins are water-soluble flavonoid pigments that have been reported to have potent antioxidants and are known to reduce inflammatory processes and regulate redox signalling.119
Participants in a parallel-designed, placebo-controlled, clinical trial who were given 300mg/day of capsules containing purified anthocyanins isolated from bilberry (Vaccinium myrtillus) and blackcurrant (Ribes nigrum) for 3 weeks displayed 15–45% reductions of NFkB inflammatory markers.120
Resveratrol
Resveratrol, a polyphenolic compound isolated from red wine, has been shown to have protective benefits against inflammation and also involved in the regulation of redox signalling both in vitro and in vivo.121, 122
Studies have demonstrated that treatment with 50 and 100 μmol/L resveratrol significantly reduced proliferation of retinal pigment epithelium cells by 10% and 25%, respectively.123 Further, in an animal model, rats supplemented with 40mg/kg body weight of resveratrol for 4 days were reported to have a reduced frequency of cataract development.124
Herbal medicines
Ginkgo biloba
Small early studies, such as phase I placebo-controlled cross-over, have demonstrated benefit in ocular functions. A trial with 11 patients investigated the effects of 40mg of Ginkgo biloba extract administered 3 times per day for 2 days on ocular blood flow. Ginkgo biloba extract increased blood flow to the ophthalmic artery, while no change was observed in the placebo group.125 In a further prospective, randomised, placebo-controlled, cross-over trial that investigated the effects of 40mg of Ginkgo biloba extract administered for 4 weeks demonstrated improvements in pre-existing visual field damage in patients, but no improvements in intraocular pressure.126
Epigallocatechin gallate (EGCG)
EGCG is the principal flavonoid present in green tea. EGCG has been reported to confer neuroprotection of retinas injured by ischemia/reperfusion.127
Moreover, a recent investigation was conducted to deduce as to whether inclusion of EGCG in the drinking water of albino rats attenuated the effect of a light insult to the retina.128 This study showed that orally administered EGCG blunted the detrimental effect of light to the retina of albino rats where the photoreceptors were primarily affected.
A recent human study with 18 ocular hypertension patients and 18 patients with open-angle glaucoma were randomly assigned to an oral placebo or EGCG over a 3-month period in a randomised, placebo-controlled, double-blind, cross-over design clinical trial.129 The study concluded that although it could not provide evidence for long-term benefit of EGCG supplementation in open angle glaucoma, and the observed effect was small, the results suggest that EGCG might favourably influence inner retinal function in eyes with early to moderately advanced glaucomatous damage.
Physical therapies
Acupuncture
Experimental studies with laboratory animals have reported the usefulness of electro-acupuncture in preventing selenite-induced cataract formation.130 The study showed that electro-acupuncture effectively decreased selenite-induced cataract formation rate in pup rats when needles were applied at specific acu-points.
A recent study assessed the effectiveness of acupuncture in reducing anxiety in patients having cataract surgery under topical anaesthesia.131 The study design was a prospective randomised double-blind controlled trial. Anxiety levels before and after cataract surgery in 3 groups (A = no acupuncture, B = true acupuncture starting 20 minutes before surgery, C = sham acupuncture starting 20 minutes before surgery) were compared using the visual analogue scale. Twenty-five patients scheduled for inpatient phacoemulsification surgery were enrolled in each group. All surgeries were performed using topical anaesthesia. The study concluded that acupuncture was effective in reducing anxiety related to cataract surgery under topical anaesthesia.
Conclusion
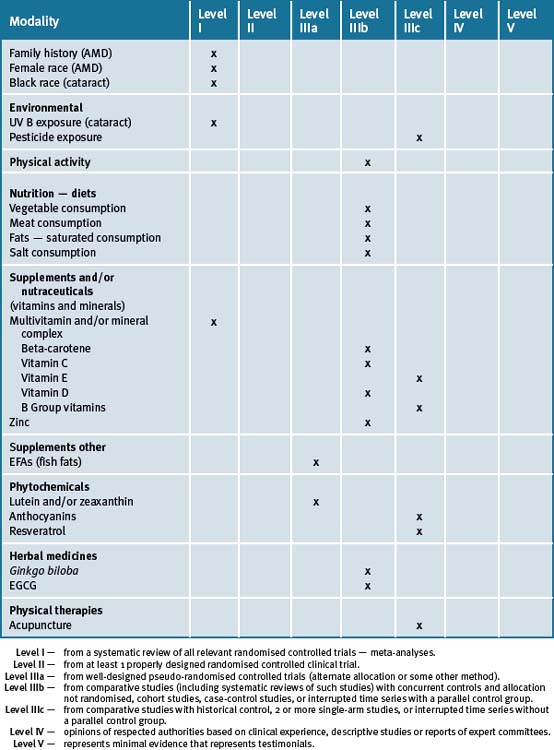
The use of a good multivitamin and/or mineral supplement has numerous advantages in reducing the risk of developing eye diseases by assisting to maintain metabolic health. Within this framework of scientific evidence there is an emerging literature that suggests that phytochemicals such as lutein and zeaxanthins, and to a lesser extent anthocyanins, resveratrol and some herbal medicines such as Ginko biloba and tea catechins, may provide protection and significantly delay the progression of eye diseases — extended clinical trials are however necessary. Table 3.2 summarises the best evidence for lifestyle, CM and therapies for AMD and cataracts.
Clinical tips handout for patients — AMD and cataracts
1 Lifestyle advice
Sunshine
2 Physical activity/exercise
3 Mind–body medicine
5 Dietary changes
7 Supplements
Fish oils
Vitamins B group
Vitamins B6 and B12
Vitamin E (natural form)
Zinc
Selenium (sodium selenite, organic selenium found in yeast)
1 World Health Organization Report. http://www.who.int/blindness/causes/priority/en/print.html (Accessed July 2009).
2 Magnitude and causes of visual impairment, World Health Organization, Fact Sheet No 282.
3 Resnikoff S., Pascolini D., Etya’ale D., et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844-851.
4 Klein R., Peto T., Bird A., Vannewkirk M.R. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004;137:486-495.
5 Friedman D.S., O’Colmain B.J., Munoz B., et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564-572.
6 Wolfs R.C., Borger P.H., Ramrattan R.S., et al. Changing views on openangle glaucoma: definitions and prevalences—The Rotterdam Study. Invest Ophthalmol Vis Sci. 2000;41:3309-3321.
7 Mitchell P., Wang J.J., Foran S., et al. Five-year incidence of age-related maculopathy lesions: the Blue Mountains Eye Study. Ophthalmology. 2002;109:1092-1097.
8 Taylor H., Guymer R., Keeffe J. The Impact of Age-Related Macular Degeneration. Access Economics Pty Limited. Melbourne: University of Melbourne; 2006. 1–72
9 Taylor H.R., Keeffe J.E., Vu H.T., et al. Vision loss in Australia. Med J Aust. 2005;182:565-568.
10 Friedman D.S., O’Colmain B.J., Munoz B., et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564-572.
11 Jonasson F., Arnarsson A., Peto T., et al. 5-year incidence of age-related maculopathy in the Reykjavik Eye Study. Ophthalmology. 2005;112:132-138.
12 Andersen N. Age-related macular degeneration among the Inuit in Greenland. Int J Circumpolar Health. 2004;63(Suppl 2):320-323.
13 Vingerling J.R., Dielemans I., Hofman A., et al. The prevalence of age-related maculopathy in the Rotterdam Study. Ophthalmology. 1995;102:205-210.
14 Krishnaiah S., Das T., Nirmalan P.K., et al. Risk factors for age-related macular degeneration: findings from the Andhra Pradesh eye disease study in South India. Invest Ophthalmol Vis Sci. 2005;46:4442-4449.
15 Munoz B., Klein R., Rodriguez J., et al. Prevalence of age-related macular degeneration in a population-based sample of Hispanic people in Arizona: Proyecto VER. Arch Ophthalmol. 2005;123:1575-1580.
16 Leske M.C., Wu S.Y., Hennis A., et al. Nine-year incidence of age-related macular degeneration in the Barbados Eye Studies. Ophthalmology. 2006;113:29-35.
17 Wong T.Y., Loon S.C., Saw S.M. The epidemiology of age-related eye diseases in Asia. Br J Ophthalmol. 2006;90:506-511.
18 Asbell P.A., Dualan I., Mindel J., et al. Age-related cataract. Lancet. 2005;365:599-609.
19 Kahn H.A., Leibowitz H.M., Ganley J.P., et al. The Framingham Eye Study: outline and major prevalence findings. Am J Epidemiol. 1977;106:17-32.
20 Klein B.E., Klein R., Linton K.L. Prevalence of age-related lens opacities in a population. Ophthalmology. 1992;99:546-552.
21 Sommer A., Tielsch J.M., Katz I., et al. Racial differences in the cause specific prevalence of blindness in east Baltimore. NEJM. 1991;325:1412-1417.
22 Asbell P.A., Dualan I., Mindel J., et al. Age-related cataract. Lancet. 2005;365(9459):599-609.
23 Hankinson S.E. Epidemiology of age-related cataract. In: Albert D.M., Jakobiec F.A., editors. Principles and Practice of Ophthalmology. 2nd edn. Philadelphia: WB Saunders; 2000:511-519.
24 Chou R., Dana T., Bougatsos C. Screening older adults for impaired visual acuity: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151(1):44-58.
25 Kaufman S.R. Developments in age-related macular degeneration: Diagnosis and treatment. Geriatrics. 2009;64(3):16-19.
26 Seddon J., Ajani U., Mitchell B. Familial aggregation of age-related maculopathy. Am J Ophthalmol. 1997;123:199-206.
27 Meyers S.M., Greene T., Gutman F.A. A twin study of age-related macular degeneration. Am J Ophthalmol. 1995;120:757-766.
28 Hammond C.J., Webster A.R., Snieder H., et al. Genetic influence on early agerelated maculopathy: a twin study. Ophthalmology. 2002;109:730-736.
29 Friedman D.S., O’Colmain B.J., Munoz B., et al. The Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564-572.
30 Friedman D.S., Katz J., Bressler N.M., et al. Racial differences in the prevalence of age-related macular degeneration. The Baltimore Eye Survey Ophthalmology. 1999;106(6):1049-1055.
31 Schachat A.P., Hyman L., Leske C., et al. Barbados Eye Study Group. Features of age-related macular degeneration in a black population. Arch Ophthalmol. 1995;113(6):728-735.
32 Bressler S.B., Muñoz B., Solomon S.D., et al. Salisbury Eye Evaluation (SEE) Study Team. Racial differences in the prevalence of age-related macular degeneration: the Salisbury Eye Evaluation (SEE) Project. Arch Ophthalmol. 2008;126(2):241-245.
33 West S.K., Munoz B., Schein O.D., et al. Racial differences in lens opacities: the Salisbury Eye Evaluation (SEE) project. Am J Epidemiol. 1998;148(11):1033-1039.
34 Friedman D.S., West S.K., Munoz B., et al. Racial variations in causes of vision loss in nursing homes: The Salisbury Eye Evaluation in Nursing Home Groups (SEEING) Study. Arch Ophthalmol. 2004;122(7):1019-1024.
35 Cook H.L., Patel P.J., Tufail A. Age-related macular degeneration: diagnosis and management. Br Med Bull. 2008;85:127-149.
36 Abraham A.G., Condon N.G., West Gower E. The new epidemiology of cataract. Ophthalmol Clin North Am. 2006;19(4):415-425.
37 Seddon J.M., George S., Rosner B. Cigarette smoking, fish consumption, omega-3 fatty acid intake, and associations with age-related macular degeneration: the US Twin Study of Age-Related Macular Degeneration. Arch Ophthalmol. 2006;124(7):995-1001.
38 Chong E.W., Kreis A.J., Wong T.Y., et al. Alcohol consumption and the risk of age-related macular degeneration: a systematic review and meta-analysis. Am J Ophthalmol. 2008;145(4):707-715.
39 Hiratsuka Y., Li G. Alcohol and eye diseases: a review of epidemiologic studies. J Stud Alcohol. 2001;62(3):397-402.
40 Wang S., Wang J.J., Wong T.Y. Alcohol and eye diseases. Surv Ophthalmol. 2008;53(5):512-525.
41 West S. Ocular ultraviolet B exposure and lens opacities: a review. J Epidemiol. 1999;9(6 Suppl):S97-S101.
42 Meyer-Rochow V.B. Risks, especially for the eye, emanating from the rise of solar UV-radiation in the Arctic and Antarctic regions. Int J Circumpolar Health. 2000;59(1):38-51.
43 McCarty C.A., Taylor H.R. A review of the epidemiologic evidence linking ultraviolet radiation and cataracts. Dev Ophthalmol. 2002;35:21-31.
44 Buch H., Vinding T., Nielsen N.V. Prevalence and causes of visual impairment according to World Health Organization and United States criteria in an aged, urban Scandinavian population: the Copenhagen City Eye Study. Ophthalmology. 2001;108(12):2347-2357.
45 Ryskulova A., Turczyn K., Makuc D.M., et al. Self-reported age-related eye diseases and visual impairment in the United States: results of the 2002 national health interview survey. Am J Public Health. 2008;98(3):454-461.
46 Black R.L., Ogleby R.B., Von Sallmann L., et al. Posterior subcapsular cataracts induced by corticosteroids in patients with rheumatoid arthritis. JAMA. 1960;174:166-171.
47 Dickerson J.E.Jr., Dotzel E., Clark A.F. Steroid-induced cataract: new perspective from in vitro and lens culture studies. Exp Eye Res. 1997;65(4):507-516.
48 Jobling A.I., Augusteyn R.C. What causes steroid cataracts? A review of steroid-induced posterior subcapsular cataracts. Clin Exp Optom. 2002;85(2):61-75.
49 James E.R. The etiology of steroid cataract. J Ocul Pharmacol Ther. 2007;23(5):403-420.
50 Seddon J.M., Cote J., Davis N., et al. Progression of age-related macular degeneration: association with body mass index, waist circumference, and waist-hip ratio. Arch Ophth. 2003;121(6):785-792.
51 Peeters A., Magliano D.J., Stevens J., et al. Changes in abdominal obesity and age-related macular degeneration: the Atherosclerosis Risk in Communities Study. Arch Ophthalmol. 2008;126:1554-1560.
52 Harris M.I. Diabetes in America: epidemiology and scope of the problem. Diabetes Care. 1998;21:C11-C14.
53 Kaushik S., Wang J.J., Flood V., et al. Dietary glycaemic index and the risk of age-related macular degeneration. AJCN. 2008;88(4):1104-1110.
54 Chiu C.J., Milton R.C., Klein R., et al. Dietary carbohydrate and the progression of age-related macular degeneration: a prospective study from the Age-Related Eye Disease Study. AJCN. 2007;86:1210-1218.
55 Chiu C.J., Milton R.C., Klein R., et al. Dietary compound score and risk of age-related macular degeneration in the age-related eye disease study. Ophthalmology. 2009;116(5):939-946.
56 Tomany S.C., Cruickshanks K.J., Klein R., et al. Sunlight and the 10-year incidence of age-related maculopathy: the Beaver Dam Eye Study. Arch Ophthalmol. 2004;122(5):750-757.
57 Cruickshanks K.J., Klein R., Klein B.E., et al. Sunlight and the 5 year incidence of early age-related maculopathy: the Beaver dam eye study. Archives of Ophthalmology. 2001;119(2):246-250.
58 Darzins P., Mitchell P., Heller R.F. Sun exposure and age-related macular degeneration. An Australian case-control study. Ophthalmology. 1997;104(5):770-776.
59 Dementi B. Ocular effects of organophosphates: a historical perspective of Saku disease. J Appl Toxicol. 1994;14:119-129.
60 Misra U.K., Nag D., Misra N.K., et al. Some observations on the macula of pesticide workers. Hum Toxicol. 1985;4:135-145.
61 Knudtson M.D., Klein R., Klein B.E. Physical activity and the 15-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Br J Ophthalmol. 2006;90(12):1461-1463.
62 Williams P.T. Prospective study of incident age-related macular degeneration in relation to vigorous physical activity during a 7-year follow-up. Invest Ophthalmol Vis Sci. 2009;50(1):101-106.
63 Williams P.T. Prospective epidemiological cohort study of reduced risk for incident cataract with vigorous physical activity and cardiorespiratory fitness during a 7-year follow-up. Invest Ophthalmol Vis Sci. 2009;50(1):95-100.
64 Seddon J.M., Ajani U.A., Sperduto R.D., et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA. 1994;272(18):1413-1420.
65 van Leeuwen R., Boekhoorn S., Vingerling J.R., et al. Dietary intake of antioxidants and risk of age-related macular degeneration. JAMA. 2005;294(24):3101-3107.
66 Christen W.G., Liu S., Glynn R.J., et al. Dietary carotenoids, vitamins C and e, and risk of cataract in women: a prospective study. Arch Ophthalmol. 2008;126(1):102-109.
67 Chong E.W., Simpson J.A., Robman L.D., et al. Red meat and chicken consumption and its association with age-related macular degeneration. Am J Epidemiol. 2009;169(7):867-876.
68 Smith W., Mitchell P., Leeder S.R. Dietary fat and fish intake and age-related maculopathy. Arch Ophthalmol. 2000;118(3):401-404.
69 Chong E.W., Robman L.D., Simpson J.A., et al. Fat consumption and its association with age-related macular degeneration. Arch Ophthalmol. 2009;127(5):674-680.
70 Seddon J.M., Cote J., Rosner B. Progression of age-related macular degeneration: association with dietary fat, transunsaturated fat, nuts, and fish intake. Arch Ophthalmol. 2003;121(12):1728-1737.
71 San Giovanni J.P., Chew E.Y., Agrón E., et al. The relationship of dietary omega-3 long-chain polyunsaturated fatty acid intake with incident age-related macular degeneration: AREDS report no. 23. Arch Ophthalmol. 2008;126(9):1274-1279.
72 Chong E.W., Kreis A.J., Wong T.Y., et al. Dietary omega-3 fatty acid and fish intake in the primary prevention of age-related macular degeneration: a systematic review and meta-analysis. Arch Ophthalmol. 2008;126(6):826-833.
73 Tan J.S., Wang J.J., Flood V., et al. Dietary fatty acids and the 10-year incidence of age-related macular degeneration: the Blue Mountains Eye Study. Arch Ophthalmol. 2009;127(5):656-665.
74 Cumming R.G., Mitchell P., Smith W. Dietary sodium intake and cataract: the Blue Mountains Eye Study. Am J Epidemiol. 2000;151(6):624-626.
75 Vanden Langenberg G.M., Mares-Perlman J.A., Klein R., et al. Associations between antioxidant and zinc intake and the 5-year incidence of early age-related maculopathy in the Beaver Dam Eye Study. Am J Epidemiol. 1998;148:204-214.
76 Age-Related Eye Disease Study Research Group. A randomised, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta-carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119(10):1417-1436.
77 Evans J. Antioxidant supplements to prevent or slow down the progression of AMD: a systematic review and meta-analysis. Eye. 2008;22(6):751-760.
78 Evans J.R., Henshaw K. Antioxidant vitamin and mineral supplements for preventing age-related macular degeneration. Cochrane Database Syst Rev. (1):2008 Jan 23. CD000253
79 Christen W.G., Manson J.E., Glynn R.J., et al. Beta-carotene supplementation and age-related maculopathy in a randomised trial of US physicians. Arch Ophthalmol. 2007;125(3):333-339.
80 Chong E.W., Wong T.Y., Kreis A.J., et al. Dietary antioxidants and primary prevention of age-related macular degeneration: systematic review and meta-analysis. BMJ. 2007;335(7623):755.
81 Christen W.G., Glynn R.J., Chew E.Y., et al. Folic acid, pyridoxine, and cyanocobalamin combination treatment and age-related macular degeneration in women: the Women’s Antioxidant and Folic Acid Cardiovascular Study. Arch Intern Med. 2009;169(4):335-341.
82 Nowak M., Szapska B., Swietochowska E., et al. (Blood concentration of homocysteine, vitamin B (12), and folic acid in patients with exudative age-related macular degeneration). Klin Oczna. 2004;106(3 Suppl):429-430.
83 Kamburoglu G., Gumus K., Kadayifcilar S., et al. Plasma homocysteine, vitamin B12 and folate levels in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2006;244(5):565-569.
84 Rochtchina E., Wang J.J., Flood V.M., et al. Elevated serum homocysteine, low serum vitamin B12, folate, and age-related macular degeneration: the Blue Mountains Eye Study. Am J Ophthalmol. 2007;143(2):344-346.
85 Parekh N., Chappell R.J., Millen A.E., et al. Association between vitamin D and age-related macular degeneration in the Third National Health and Nutrition Examination Survey, 1988 through 1994. Arch Ophthalmol. 2007;125(5):661-669.
86 Mares-Perlman J.A., Lyle B.J., Klein R., et al. Vitamin supplement use and incident cataracts in a population-based study. Arch Ophthalmol. 2000;118(11):1556-1563.
87 Lyle B.J., Mares-Perlman J.A., Klein B.E., et al. Antioxidant intake and risk of incident age-related nuclear cataracts in the Beaver Dam Eye Study. Am J Epidemiol. 1999;149(9):801-809.
88 Taylor A., Jacques P.F., Chylack L.T.Jr., et al. Long-term intake of vitamins and carotenoids and odds of early age-related cortical and posterior subcapsular lens opacities. AJCN. 2002;75(3):540-549.
89 Age-Related Eye Disease Study Research Group. A randomised, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta-carotene for age-related cataract and vision loss: AREDS report no. 9. Arch Ophthalmol. 2001;119(10):1439-1452.
90 Christen W.G., Glynn R.J., Chew E.Y., et al. Vitamin E and age-related cataract in a randomised trial of women. Ophthalmology. 2008;115(5):822-829. e1
91 Vitetta L., Sali A. Omega–3 Fatty Acids PUFA – A Review PART I. Journal of Complementary Medicine. 2006;5(6):52-59.
92 SanGiovanni J.P., Chew E.Y. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res.. 2005;24(1):87-138.
93 Nettleton J.A. Omega-3 fatty acids: comparison of plant and seafood sources in human nutrition. J Am Diet Assoc.. 1991;91(3):331-337.
94 Bazan N.G. The metabolism of omega-3 polyunsaturated fatty acids in the eye: the possible role of docosahexaenoic acid and docosanoids in retinal physiology and ocular pathology. Prog Clin Biol Res. 1989;312:95-112.
95 Linnane A.W., Kios M., Vitetta L. Healthy Ageing: Regulation of the Metabolome by Cellular Redox Modulation and Prooxidant Signaling Systems. The Essential Roles of Superoxide Anion and Nitric Oxide. Biogerontology. 2007;8(5):445-467.
96 Kirschfeld K. Carotenoid pigments: their possible role in protecting against photooxidation in eyes and photoreceptor cells. Proc R Soc Lond B Biol Sci. 1982;216(1202):71-85.
97 Mares-Perlman J.A., Brady W.E., Klein R., et al. Dietary fat and age-related maculopathy. Arch Ophthalmol. 1995;113(6):743-748.
98 Heuberger R.A., Mares-Perlman J.A., Klein R., et al. Relationship of dietary fat to age-related maculopathy in the Third National Health and Nutrition Examination Survey. Arch Ophthalmol. 2001;119(12):1833-1838.
99 Delcourt C., Carriere I., Cristol J.P., et al. Dietary fat and the risk of age-related maculopathy: the POLANUT Study. Eur J Clin Nutr. 2007;61(11):1341-1344.
100 Seddon J.M., Rosner B., Sperduto R.D., et al. Dietary fat and risk for advanced age-related macular degeneration. Arch Ophthalmol. 2001;119(8):1191-1199.
101 SanGiovanni J.P., Chew E.Y., Clemons T.E., et al. Age-Related Eye Disease Study Research Group. The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: AREDS report No. 20. Arch Ophthalmol. 2007;125(5):671-679.
102 Chong E.W., Kreis A.J., Wong T.Y., et al. Dietary omega-3 fatty acid and fish intake in the primary prevention of age-related macular degeneration: a systematic review and meta-analysis. Arch Ophthalmol. 2008;126(6):826-833.
103 SanGiovanni J.P., Chew E.Y., Agrón E., et al. The relationship of dietary omega-3 long-chain polyunsaturated fatty acid intake with incident age-related macular degeneration: AREDS report no. 23. Arch Ophthalmol. 2008;126(9):1274-1279.
104 Carpentier S., Knaus M., Suh M. Associations between lutein, zeaxanthin, and age-related macular degeneration: an overview. Crit Rev Food Sci Nutr. 2009;49(4):313-326.
105 Moeller S.M., Parekh N., Tinker L., et al. CAREDS Research Study Group. Associations between intermediate age-related macular degeneration and lutein and zeaxanthin in the Carotenoids in Age-related Eye Disease Study (CAREDS): ancillary study of the Women’s Health Initiative. Arch Ophthalmol. 2006;124:1151-1162.
106 Moeller S.M., Voland R., Tinker B., et al. CAREDS Study Group. Women’s Health Initiative. Associations between age-related nuclear cataract and lutein and zeaxanthin in the diet and serum in the Carotenoids in the Age-Related Eye Disease Study (CAREDS), an ancillary study of the Women’s Health Initiative. Arch Ophthalmol. 2008;126(3):354-364.
107 Delcourt C., Carriere I., Delage M., et al. POL A Study Group. Plasma lutein and zeaxanthin and other carotenoids as modifiable risk factors for age-related maculopathy and cataract: the POLA Study. Invest Ophthalmol Vis Sci. 2006;47:2329-2335.
108 Coleman H., Chew E. Nutritional supplementation in age-related macular degeneration. Curr Opin Ophthalmol. 2007;18(3):220-223.
109 Bartlett H., Eperjesi F. Age-related macular degeneration and nutritional supplementation: a review of randomised controlled trials. Ophthalmic Physiol Opt. 2003;23(5):383-399.
110 Olmedilla B., Granado F., Blanco I., et al. Serum status of carotenoids and tocopherols in patients with age-related cataracts: a case-control study. J Nutr Health Ageing. 2002;6(1):66-68.
111 Bone R.A., Landrum J.T., Guerra L.H., et al. Lutein and zeaxanthin dietary supplements raise macular pigment density and serum concentrations of these carotenoids in humans. J Nutr. 2003;133:992-998.
112 Khachik F., de Moura F.F., Chew E.Y., et al. The effect of lutein and zeaxanthin supplementation on metabolites of these carotenoids in the serum of persons aged 60 or older. Invest Ophthalmol Vis Sci. 2006;47(12):5234-5242.
113 Olmedilla B., Granado F., Blanco I., et al. Lutein, but not alpha-tocopherol, supplementation improves visual function in patients with age-related cataracts: a 2-y double-blind, placebo-controlled pilot study. Nutrition. 2003;19(1):21-24.
114 Olmedilla B., Granado F., Blanco I., et al. Lutein in patients with cataracts and age-related macular degeneration: a long term supplementation study. J Sci Food Agric. 2001;81:904-909.
115 Trumbo P.R., Ellwood K.C. Lutein and zeaxanthin intakes and risk of age-related macular degeneration and cataracts: an evaluation using the Food and Drug Administration’s evidence-based review system for health claims. AJCN. 2006;84:971-974.
116 Schalch W., Cohn W., Barker F.M., et al. Xanthophyll accumulation in the human retina during supplementation with lutein or zeaxanthin — the LUXEA (Lutein Xanthophyll Eye Accumulation) study. Arch Biochem Biophys. 2007;458:128-135.
117 Rhone M., Basu A. Phytochemicals and age-related eye diseases. Nutr Rev. 2008;66(8):465-472.
118 Matsumoto H., Nakamura Y., Iida H., et al. Comparative assessment of distribution of blackcurrant anthocyanins in rabbit and rat ocular tissues. Exp Eye Res. 2006;83:348-356.
119 Rahman I., Biswas S.K., Kirkham P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol. 2006;72:1439-1452.
120 Karlsen A., Retterstol L., Laake P., et al. Anthocyanins inhibit nuclear factor-kappaB activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J Nutr. 2007;137:1951-1954.
121 Miura D., Miura Y., Yagasaki K. Resveratrol inhibits hepatoma cell invasion by suppressing gene expression of hepatocyte growth factor via its reactive oxygen species-scavenging property. Clin Exp Metastasis. 2004;21:445-451.
122 Sun W., Wang W., Kim J., et al. Anti-cancer effect of resveratrol is associated with induction of apoptosis via a mitochondrial pathway alignment. Adv Exp Med Biol. 2008;614:179-186.
123 King R.E., Kent K.D., Bomser J.A. Resveratrol reduces oxidation and proliferation of human retinal pigment epithelial cells via extracellular signal-regulated kinase inhibition. Chem Biol Interact. 2005;151(2):143-149.
124 Doganay S., Borazan M., Iraz M., et al. The effect of resveratrol in experimental cataract model formed by sodium selenite. Curr Eye Res. 2006;31:147-153.
125 Chung H.S., Harris A., Kristinsson J.K., et al. Ginkgo biloba extract increases ocular blood flow velocity. J Ocul Pharmacol Ther. 1999;15:233-240.
126 Quaranta L., Bettelli S., Uva M.G., et al. Effect of Ginkgo biloba extract on preexisting visual field damage in normal tension glaucoma. Ophthalmology. 2003;110:359-364.
127 Zhang B., Safa R., Rusciano D., et al. Epigallocatechin gallate, an active ingredient from green tea, attenuates damaging influences to the retina caused by ischemia/reperfusion. Brain Res. 2007;1159:40-53.
128 Costa B.L., Fawcett R., Li G.Y., et al. Orally administered epigallocatechin gallate attenuates light-induced photoreceptor damage. Brain Res Bull. 2008;76(4):412-423.
129 Falsini B., Marangoni D., Salgarello T., et al. Effect of epigallocatechin-gallate on inner retinal function in ocular hypertension and glaucoma: A short-term study by pattern electroretinogram. Graefes Arch Clin Exp Ophthalmol. 2009;247(9):1223-1233.
130 Cariello A.J., Casanova F.H., Lima Filho A.A., et al. Effect of electroacupuncture to prevent selenite-induced cataract in Wistar rats. Arq Bras Oftalmol. 2006;69(3):299-303.
131 Gioia L., Cabrini L., Gemma M., et al. Sedative effect of acupuncture during cataract surgery: prospective randomised double-blind study. J Cataract Refract Surg. 2006;32(11):1951-1954.