Chapter 13 Age changes in the lumbar spine
Textbook descriptions imply that the structure of the lumbar spine conforms to some sort of standard or even ideal form, and that a standard description is applicable to all individuals. However, such descriptions only reflect the average, healthy, young adult spine. Yet, even then the lumbar spine is subject to variations, e.g. in the shape and orientation of the zygapophysial joints (see Ch. 3), the shape of the lumbar lordosis (see Ch. 5) and the possible ranges of movement (see Ch. 8). What is considered the ‘normal’ lumbar spine is only a composite of the mean values, or most common form, of these and other possible variables.
Biochemical changes
One of the most fundamental changes in the lumbar spine occurs in the nuclei pulposi. Changes in biochemistry are most dramatic from infancy to about the age of ten years.1,2 These seem to be triggered by the regression in infancy of the meagre blood supply to the disc, and they set the trend that occurs through later life as the disc adapts to anaerobic metabolism.1,2
With ageing, the rate of synthesis of proteoglycans decreases3 and the concentration of proteoglycans in the nucleus pulposus also decreases.4–7 In early adult life, proteoglycans amount to about 65% of the dry weight of the nucleus (see Ch. 2) but by the age of 60 they constitute only about 30%.8 Those proteoglycans that persist are smaller in size9,10 and have a smaller molecular weight.11,12 The proportion of aggregated proteoglycans decreases3 and the number of large proteoglycan aggregates decreases such that, by adolescence, the nucleus pulposus consists largely of clusters of short aggrecan molecules and non-aggregated proteoglycans.5 Associated with these latter changes is a decline in the concentration of functional link proteins.5
Apart from these changes in composition, the nature of the proteoglycans also changes. While the keratan sulphate content of the disc remains fairly constant, the concentration of chondroitin sulphate falls, and this results in a rise in the keratan sulphate/chondroitin sulphate (KS/CS) ratio.6,12–15
The other major change in the nucleus pulposus is an increase in its collagen content,5,16 and an increase in collagen–proteoglycan binding.9 The collagen content of the anulus fibrosus also increases17 but the concentration of elastic fibres in the anulus drops, from 13% at the age of 26 to about 8% at the age of 62.18
The collagen of the intervertebral disc not only increases in quantity but also changes in nature. The fibril diameter of collagen in the nucleus pulposus increases,5,19–22 such that the type II collagen of the nucleus starts to resemble the type I collagen of the anulus fibrosus. Reciprocally, the average fibril diameter in the anulus fibrosus decreases.20 Consequently, there is less distinction between the collagen of the nucleus pulposus and the anulus fibrosus.
The changes in collagen are related not only to age but also to location.23 While the collagen content of the anulus in general increases with age, there is a significant increase in the amount of type I collagen in the outermost laminae of the posterior quadrant of the anulus, and a reciprocal decrease in type II collagen. This suggests that some of the changes in collagen are not generalised age changes but are active metabolic responses to changes in the internal stresses of the anulus.23
The concentration of non-collagenous proteins in the nucleus pulposus increases,24–28 and ageing is characterised by the appearance of certain distinctive non-collagenous proteins.28 However, because the functions of non-collagenous proteins are not known (see Ch. 2), the significance of changes in these proteins remains obscure. In contrast, the changes in collagen, proteoglycans and elastic fibres have major biomechanical effects on the disc.
Because chondroitin sulphate is the major source of ionic radicals that bind water to proteoglycans (Ch. 2), it is tempting to expect that the change in the KS/CS ratio would result in a decrease in the water-binding capacity and the water content of the nucleus pulposus. Indeed, the water content of the nucleus does decrease with age.16 At birth, the water content of the nucleus pulposus is about 88%, and this drops to about 65–72% by the age of 75 years.6,29 However, most of this dehydration occurs during childhood and adolescence, and the water content of the nucleus pulposus decreases by only about 6% from early adult life to old age.30
Sophisticated biochemical studies indicate that it is not simply the loss of proteoglycans or the change in the KS/CS ratio that decreases water-binding in the nucleus. Rather, the increased collagen and increased collagen–proteoglycan binding leave fewer polar groups of the proteoglycans available to bind water,16 and the decrease in water-binding capacity of the nucleus is a function of the complex way in which the ionic interactions between proteoglycans and proteins are altered.10,11
Structural changes in the intervertebral discs
As the disc ages, the number of viable cells in the nucleus decreases, and the proportion of cells that exhibit necrosis changes from 2% in infancy to 50% in young adults and 80% in elderly individuals.5 Lipofuscin granules accumulate with advanced age.31
Macroscopically, as the intervertebral disc becomes more fibrous, the distinction between nucleus pulposus and anulus fibrosus becomes less apparent. The two regions coalesce and the nucleus pulposus appears to be encroached by the anulus fibrosus.32 After middle life, the nucleus pulposus becomes progressively more solid, dry and granular.32
As the nucleus pulposus dries out and becomes more fibrous, it is less able to exert fluid pressure.33,34 Thus, the nucleus is less able to transmit weight directly and less able to exert radial pressure on the anulus fibrosus (cf. Ch. 2). A greater share of any vertical load is therefore borne by the anulus fibrosus. Consequently, the anulus fibrosus is subject to greater stresses and undergoes changes reflecting the increasing and different strains it suffers.
With age, the collagen lamellae of the anulus increase in thickness and become increasingly fibrillated35–38 and cracks and cavities may develop,5,39 which may enlarge to become clefts and overt fissures.32 The number of incomplete lamellae increases.37 Such changes are not necessarily due to externally applied injuries to the spine but can simply be due to repeated minor insults sustained by the overloaded anulus fibrosus during trunk movements in the course of activities of daily living. Although the tensile strength of the anulus decreases with degeneration of the disc, there is no simple relationship between age and tensile properties.40
Narrowing of the intervertebral discs has previously been considered one of the signs of pathological ageing of the lumbar spine32,41,42 but large-scale post-mortem studies have now refuted this notion. The dimensions of the lumbar intervertebral discs increase with age. Between the second and seventh decades, the anteroposterior diameter of the lumbar discs increases by about 10% in females and 2% in males,30 and there is about a 10% increase in the height of most discs.30 Furthermore, the upper and lower surfaces of the discs increase in convexity,30 a change which occurs at the expense of the shape of the vertebral bodies (see below).
Maintenance of disc height is the ‘normal’ feature of ageing, and any loss of trunk stature with age is the result of decreases in vertebral body height.43–46 Overt disc narrowing invites the consideration of some process other than ageing, and this is considered in Chapter 15.
Changes in the vertebral endplate
In the newborn, the vertebral endplate is part of the growth plate of the vertebral body. Towards the intervertebral disc, the articular region of the endplate is formed by fibrocartilage, while on the vertebral body side, columns of proliferating cells extend into the ossifying vertebral body (see Ch. 12). By the age of 10–15 years, the articular region of the endplate becomes relatively thicker, while the growth zone decreases in thickness, and proliferating cells become fewer.47 As vertebral growth slows during the 17th–20th years, the vertebral endplate is gradually sealed off from the vertebral body by the development of the subchondral bone plate, and after the age of 20 only the articular region of the original growth plate persists.47 Between the ages of 20 and 65, the endplate becomes thinner47 and cell death occurs in the superficial layers of the cartilage.38
In the subchondral bone of the endplate, vascular channels are gradually occluded,47 resulting in a decrease in the permeability of the endplate region for nutrients to the disc. This impaired nutrition may be one of the factors that cause the biochemical changes in the nucleus pulposus, but it seems to come too late in life to be the fundamental cause.
With age, the apparent strength of the vertebral endplate decreases,34,48 but because the strength of the endplate depends on the strength of the underlying vertebral body, this change is better considered together with the other changes that affect the vertebral body.
Changes in the vertebral body
With age, there is an overall decrease in bone density in the lumbar vertebral bodies43,44,49 and a decrease in bone strength.34,50 These changes in density and strength correlate with changes in the size and pattern of trabeculae in the vertebral body.
Vertical trabeculae are slowly absorbed, although those that persist are said to be thickened.51 On the other hand, horizontal trabeculae are absorbed and not replaced.49,51 Consequently, ageing of the vertebral bodies is characterised by the loss of horizontal trabeculae49,51 and this is most marked in the central portion of the vertebral body (that part overlying the nucleus pulposus).
The loss of horizontal trabeculae removes their bracing effect on the vertical trabeculae (see Ch. 1), and the load-bearing capacity of the central portion of the vertebral body decreases. Overall, with weakening of the trabecular system, a greater proportion of the compressive load on vertebral bodies is borne by cortical bone. Over the age of 40, the trabecular bone bears only 35% of the load.34,50 However, cortical bone fails at only 2% deformation, whereas trabecular bone tolerates 9.5% deformation before failing.34 Consequently, with greater reliance on cortical bone, the vertebral body becomes less resistant to deformation and injury.
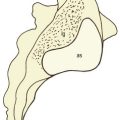
Lacking support from the underlying bone, the vertebral endplates deform by microfracture52 and gradually bow into the vertebral body, imparting a concave shape to the superior and inferior surfaces of the vertebral body.30,49 Moreover, the central portion of the vertebral endplate is rendered more liable to fracture in the face of excessive compressive loads applied to the disc, and with increasing age microfractures can be found in the endplates and vertical trabeculae of vertebral bodies.53–58
Fractures in the vertebral endplates may be large enough to allow nuclear material to extrude into the vertebral body, forming so-called Schmorl’s nodes. Schmorl’s nodes, however, are more a feature of the lower thoracic and thoracolumbar spines and have a low incidence below the level of L2.59,60 Per se, they are not symptomatic, nor are they related to age; their incidence is greatest in adolescence and they do not increase in frequency with age.59,60 Nevertheless, smaller protrusions of disc material into the vertebral bodies are not without significance and this is described in Chapter 15.
Changes in the zygapophysial joints
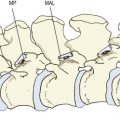
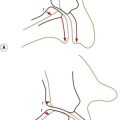
The subchondral bone of the lumbar zygapophysial joints increases in thickness during growth and reaches a maximum between the ages of 20 and 50 years.61,62 Thereafter, it gradually gets thinner.61,63 The articular cartilage, on the other hand, steadily increases in thickness with age but exhibits certain focal changes that start in the fourth decade and which can be related to the stresses applied to these joints.
In the anteromedial third of curved zygapophysial joints, the cartilage exhibits cell hypertrophy (particularly in the midzone layer), which progresses to vertical fibrillation of the cartilage associated with sclerosis of the subchondral bone plate.62 At any stage, these changes are more advanced in the concave, superior articular process than in the inferior articular process. It is the anteromedial, or backward-facing, portion of this facet that resists the forward shear stresses applied to the intervertebral joint during weight-bearing and flexion movements (see Chs 2 and 8), and it can be surmised that the fibrillation that develops with age in this region reflects the repeated stresses incurred in the course of normal activities of daily living.62–64 Severe or repeated pressures may result in erosion and focal thinning of the cartilage, while other regions may exhibit swelling that accounts for the general increase in thickness of the articular cartilage.63,64 Where cartilage is lost, fibro-fatty intra-articular inclusions may increase in size to fill the space vacated by the cartilage.62
The posterior section of the joint characteristically exhibits a different kind of splitting of cartilage – parallel to the joint surface. A split piece of cartilage may remain attached to the joint capsule and form a false intra-articular meniscoid.62
Cell hypertrophy is almost universal in the fourth decade, and minor fibrillation is common in the fourth and fifth decades. Older joints exhibit gross thickening and irregularity of the calcified zone of cartilage and increased collagen in the superficial layers. The cells are fewer and have smaller nuclei. The changes in cartilage are more severe in the polar regions of the joint than at its centre. In older joints, the distinction between changes in the anteromedial and posterior portions of the joint is lost.62
Changes in movements
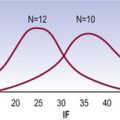
The biochemical and structural changes in the joints of the lumbar spine have an inevitable effect on the mechanical properties, and therefore movements, of the spine. Older lumbar spines show a greater amount of creep and hysteresis, and a greater set after creep deformation,65 but they show a decreased range of motion. A progressive decrease in range of motion with age has been demonstrated in cadavers59,66–68 and in living subjects using both clinical67 and radiographic59,66,69 methods, and is evident in the ranges of motion both of the entire lumbar spine67,68 and of individual intervertebral joints.59,66,69
Young children, strikingly, show the greatest lumbar mobility. At various segmental levels, they are between 50% and 300% more mobile than middle-aged subjects.67,69 Mobility decreases considerably by adolescence, and beyond the age of 30 there is a gradual but definite decrease in mobility.59,66,67,69
Because ‘release’ experiments show that removing the posterior ligaments and zygapophysial joints does not greatly increase the range of flexion in older cadavers,68 it appears that increased stiffness in the intervertebral discs is the principal cause of the reduction in mobility that develops with ageing. This can be readily ascribed to the dehydration and fibrosis of older intervertebral discs.
The greater hysteresis seen in older spines is probably due to the decreased water-binding capacity of their intervertebral discs.65 Less able to attract water, these discs take longer to resume their original configuration and structure after deformation.
Spondylosis and degenerative joint disease
The cardinal features of spondylosis are said to be the development of osteophytes (bony spurs) along the junction of vertebral bodies and their intervertebral discs.70–72 However, when viewed in the context of other changes that occur with ageing, it is evident that the features of spondylosis are not those of some aggressive disease that seemingly attacks the body, but are the natural consequences of the stresses applied to the spine throughout life. Whether they should be called ‘degenerative changes’ or ‘age changes’ may appear simply to be semantics but the development of osteophytes can be viewed as a reactive and adaptive change that seeks to compensate for biomechanical aberrations. The process is active and purposeful, and does not warrant the description as a degenerative process.
Perhaps the most crucial argument against viewing spondylosis and spinal osteoarthrosis as diseases is that they are so irregularly (if not infrequently) associated with symptoms and disability. The incidence of spondylosis and osteoarthrosis is just as great in patients with symptoms as in patients without symptoms.72–74
This raises the great paradox in the field of spinal pain, namely that while some patients with spondylosis or osteoarthrosis may present with pain, there are others with the same age changes who do not have pain, and many patients with pain do not have a trace of spondylosis or osteoarthrosis. Consequently, spondylosis or osteoarthrosis cannot legitimately be viewed as a pathological diagnosis. Some other, or additional, factor must be the cause of pain, and the resolution of this problem is addressed in Chapter 15.
1 Scott JE, Bosworth TR, Cribb AM, et al. The chemical morphology of age-related changes in human intervertebral disc glycosaminoglycans from cervical, thoracic and lumbar nucleus pulposus and annulus fibrosus. J Anat. 1994;184:73-82.
2 Taylor JR, Scott JE, Cribb AM, et al. Human intervertebral disc acid glycosaminoglycans. J Anat. 1992;180:137-141.
3 Johnstone B, Bayliss MT. The large proteoglycans of the human intervertebral disc. Spine. 1995;20:674-684.
4 Beard HK, Stevens RL. Biochemical changes in the intervertebral disc. In: Jayson MIV, editor. The Lumbar Spine and Backache. 2nd ed. London: Pitman; 1980:407-436. Ch. 14
5 Buckwalter JA. Spine update. Aging and degeneration of the human intervertebral disc. Spine. 1995;20:1307-1314.
6 Gower WE, Pedrini V. Age-related variation in protein polysaccharides from human nucleus pulposus, annulus fibrosus and costal cartilage. J Bone Joint Surg. 1969;51A:1154-1162.
7 Sylven B, Paulson S, Hirsch C, et al. Biophysical and physiological investigations on cartilage and other mesenchymal tissues. J Bone Joint Surg. 1951;33A:333-340.
8 Badgley CE. The articular facets in relation to low-back pain and sciatic radiation. J Bone Joint Surg. 1941;23:481-496.
9 Adams P, Muir H. Qualitative changes with age of human lumbar disks. Ann Rheum Dis. 1976;35:289-296.
10 Bushell GR, Ghosh P, Taylor TKF, et al. Proteoglycan chemistry of the intervertebral disks. Clin Orthop. 1977;129:115-123.
11 Comper WD, Preston BN. Model connective tissue systems. A study of the polyion-mobile ion and of excluded volume interactions of proteoglycans. Biochem J. 1974;143:1-9.
12 Urban J, Maroudas A. The chemistry of the intervertebral disc in relation to its physiological function. Clin Rheum Dis. 1980;6:51-76.
13 Adams P, Eyre DR, Muir H. Biochemical aspects of development and ageing of human lumbar intervertebral discs. Rheumatol Rehab. 1977;16:22-29.
14 Naylor A. Intervertebral disc prolapse and degeneration. The biochemical and biophysical approach. Spine. 1976;1:108-114.
15 Naylor A, Shental R. Biochemical aspects of intervertebral discs in ageing and disease. In: Jayson MIV, editor. The Lumbar Spine and Backache. New York: Grune & Stratton; 1976:317-326. Ch. 14
16 Hirsch C, Paulson S, Sylven B, et al. Biophysical and physiological investigation on cartilage and other mesenchymal tissues: characteristics of human nuclei pulposi during aging. Acta Orthop Scand. 1953;22:175-183.
17 Brinckmann P. Injury of the annulus fibrosus and disc protrusions: an in vitro investigation on human lumbar discs. Spine. 1986;11:149-153.
18 Johnson EF, Berryman H, Mitchell R, et al. Elastic fibres in the anulus fibrosus of the human lumbar intervertebral disc. A preliminary report. J Anat. 1985;143:57-63.
19 Bailey AJ, Herbert CM, Jayson MIV. Collagen of the intervertebral disc. In: Jayson MIV, editor. The Lumbar Spine and Backache. New York: Grune & Stratton; 1976:327-340. Ch. 12
20 Happey F. A biophysical study of the human intervertebral disc. In: Jayson MIV, editor. The Lumbar Spine and Backache. New York: Grune & Stratton; 1976:293-316. Ch. 13
21 Naylor A, Happey F, MacRae TP. The collagenous changes in the intervertebral disc with age and their effect on elasticity. BMJ. 1954;2:570-573.
22 Naylor A, Happey F, Turner RL, et al. Enzymic and immunological activity in the intervertebral disc. Orthop Clin North Am. 1975;6:51-58.
23 Brickley-Parsons D, Glimcher MJ. Is the chemistry of collagen in intervertebral discs an expression of Wolff’s law? A study of the human lumbar spine. Spine. 1984;9:148-163.
24 Blakely PR, Happey F, Naylor A, et al. Protein in the nucleus pulposus of the intervertebral disc. Nature. 1962;195:73.
25 Dickson IR, Happey F, Pearson CH, et al. Variations in the protein components of human intervertebral disk with age. Nature. 1967;215:52-53.
26 Ghosh P, Bushell GK, Taylor TFK, et al. Collagen, elastin, and non-collagenous protein of the intervertebral disk. Clin Orthop. 1977;129:123-132.
27 Naylor A. The biochemical changes in the human intervertebral disc in degeneration and nuclear prolapse. Orthop Clin North Am. 1971;2:343-358.
28 Taylor TKF, Little K. Intercellular matrix of the intervertebral disk in ageing and in prolapse. Nature. 1965;208:384-386.
29 Puschel J. Der Wassergehalt normaler und degenerierter Zwischenwirbelscheiben. Beitr path Anat. 1930;84:123-130.
30 Twomey L, Taylor J. Age changes in lumbar intervertebral discs. Acta Orthop Scand. 1985;56:496-499.
31 Yasuma T, Arai K, Suzuki F. Age-related phenomena in the lumbar intervertebral discs: lipofuscin and amyloid deposition. Spine. 1992;17:1194-1198.
32 Vernon-Roberts B, Pirie CJ. Degenerative changes in the intervertebral discs of the lumbar spine and their sequelae. Rheumatol Rehab. 1977;16:13-21.
33 Kulak RF, Belytschko TB, Schultz AB, et al. Non-linear behaviour of the human intervertebral disc under axial load. J Biomech. 1976;9:377-386.
34 White AA, Panjabi MM. Clinical Biomechanics of the Spine. Philadelphia: Lippincott; 1978.
35 Bernick S, Walker JM, Paule WJ. Age changes to the anulus fibrosus in human intervertebral discs. Spine. 1991;16:520-524.
36 Harris RI, MacNab I. Structural changes in the lumbar intervertebral discs. Their relationship to low back pain and sciatica. J Bone Joint Surg. 1954;36B:304-322.
37 Marchand F, Ahmed AM. Investigation of the laminate structure of lumbar disc anulus fibrosus. Spine. 1990;15:402-410.
38 Pritzker KPH. Aging and degeneration in the lumbar intervertebral discs. Orth Clin North Am. 1977;8:65-77.
39 Hirsch C, Schajowicz F. Studies on structural changes in the lumbar annulus fibrosus. Acta Orthop Scand. 1952;22:184-189.
40 Acaroglu ER, Iatridis JC, Setton LA, et al. Degeneration and aging affect the tensile behaviour of human lumbar anulus fibrosus. Spine. 1995;20:2690-2701.
41 Lawrence JS. Disc degeneration, its frequency and relationship to symptoms. Ann Rheum Dis. 1969;28:121-138.
42 Schmorl G, Junghanns H. The Human Spine in Health and Disease, 2nd American ed. New York: Grune & Stratton; 1971. 18
43 Ericksen MF. Ageing changes in the shape of the human lumbar vertebrae. Am J Phys Anthropol. 1974;41:477.
44 Ericksen MF. Some aspects of ageing in the lumbar spine. Am J Phys Anthropol. 1975;45:575-580.
45 Nachemson AL, Schultz AB, Berkson MH. Mechanical properties of human lumbar spinal segments. Spine. 1979;4:1-8.
46 Twomey LT, Taylor JR. The effects of ageing on the lumbar intervertebral discs. In: Grieve G, editor. Modern Manual Therapy. Edinburgh: Churchill Livingstone; 1986:129-137. Ch. 12
47 Bernick S, Cailliet R. Vertebral end-plate changes with aging of human vertebrae. Spine. 1982;7:97-102.
48 Perey O. Fracture of the vertebral end-plate in the lumbar spine. Acta Orthop Scand. 1957;Suppl. 25:1-101.
49 Twomey L, Taylor J, Furniss B. Age changes in the bone density and structure of the lumbar vertebral column. J Anat. 1983;136:15-25.
50 Rockoff SF, Sweet E, Bleustein J. The relative contribution of trabecular and cortical bone to the strength of human lumbar vertebrae. Calcif Tissue Res. 1969;3:163-175.
51 Atkinson PJ. Variations in trabecular structure of vertebrae with age. Calcif Tissue Res. 1967;1:24-32.
52 Hansson T, Roos B. Microcalluses of the trabeculae in lumbar vertebrae and their relation to the bone mineral content. Spine. 1981;6:375-380.
53 Brown T, Hansen RJ, Yorra AJ. Some mechanical tests on the lumbosacral spine with particular reference to the intervertebral discs. J Bone Joint Surg. 1957;39A:1135-1164.
54 Coventry MB, Ghormley RK, Kernohan JW. The intervertebral disc: its microscopic anatomy and pathology. Part I. Anatomy, development and physiology. J Bone Joint Surg. 1945;27:105-112.
55 Keyes DC, Compere EL. The normal and pathological physiology of nucleus pulposus of the intervertebral disc. J Bone Joint Surg. 1932;14:897-938.
56 Saunders JB, de CM, Inman VT. Pathology of the intervertebral disk. Arch Surg. 1940;40:380-416.
57 Vernon-Roberts B, Pirie CJ. Healing trabecular microfractures in the bodies of lumbar vertebrae. Ann Rheum Dis. 1973;32:406-412.
58 Whitehouse WJ, Dyson ED, Jackson CK. The scanning electron microscope in studies of trabecular bone from a human vertebral body. J Anat. 1971;108:481-496.
59 Hilton RC. Systematic studies of spinal mobility and Schmorl’s nodes. In: Jayson MIV, editor. The Lumbar Spine and Backache. 2nd ed. London: Pitman; 1980:115-134. Ch. 5
60 Hilton RC, Ball J, Benn RT. Vertebral end-plate lesions (Schmorl’s nodes) in the dorsolumbar spine. Ann Rheum Dis. 1976;35:127-132.
61 Taylor JR, Twomey LT. Age changes in the subchondral bone of human lumbar apophyseal joints. J Anat. 1985;143:233.
62 Taylor JR, Twomey LT. Age changes in lumbar zygapophyseal joints. Spine. 1986;11:739-745.
63 Twomey LT, Taylor JR. Age changes in the lumbar articular triad. Aust J Physiother. 1985;31:106-112.
64 Taylor JR, Twomey LT. Vertebral column development and its relation to adult pathology. Aust J Physiother. 1985;31:83-88.
65 Twomey L, Taylor J. Flexion creep deformation and hysteresis in the lumbar vertebral column. Spine. 1982;7:116-122.
66 Hilton RC, Ball J, Benn RT. In-vitro mobility of the lumbar spine. Ann Rheum Dis. 1979;38:378-383.
67 Taylor J, Twomey L. Sagittal and horizontal plane movement of the human lumbar vertebral column in cadavers and in living. Rheumatol Rehab. 1980;19:223-232.
68 Twomey LT, Taylor JR. Sagittal movements of the human lumbar vertebral column: a quantitative study of the role of the posterior vertebral elements. Arch Phys Med Rehab. 1983;64:322-325.
69 Tanz SS. Motion of the lumbar spine: a roentgenologic study. Am J Roentgenol. 1953;69:399-412.
70 Epstein BS. The Spine. A Radiological Text and Atlas, 4th ed. Philadelphia: Lea & Febiger; 1976.
71 Resnick D. Common disorders of the aging lumbar spine: radiographic-pathologic correlation. In: Genanat HK, editor. Spine Update 1984. San Francisco: Radiology Research and Education Foundation; 1983:35-42. Ch. 5
72 Torgerson WR, Dotter WE. Comparative roentgenographic study of the asymptomatic and symptomatic lumbar spine. J Bone Joint Surg. 1976;58A:850-853.
73 Lawrence JS, Bremner JM, Bier F. Osteoarthrosis: prevalence in the population and relationship between symptoms and X-ray changes. Ann Rheum Dis. 1966;25:1-24.
74 Magora A, Schwartz A. Relation between the low back pain syndrome and X-ray findings. 1. Degenerative osteoarthritis. Scand J Rehab Med. 1976;8:115-125.