Chapter 278 African Trypanosomiasis (Sleeping Sickness; Trypanosoma brucei Complex)
Clinical Manifestations
Hemolymphatic Stage (Stage 1)
The most common presenting features of acute HAT occur at the time of invasion of the bloodstream by the parasites, 2-3 wk after infection. Patients usually present with irregular episodes of fever, each lasting up to 7 days, accompanied by headache, sweating, and generalized lymphadenopathy. Attacks may be separated by symptom-free intervals of days or even weeks. Painless, nonmatted lymphadenopathy, most commonly of the posterior cervical and supraclavicular nodes, is 1 of the most constant signs, particularly in the Gambian form. A common feature of trypanosomiasis in Caucasians is the presence of blotchy, irregular, nonpruritic, erythematous macules, which may appear any time after the 1st febrile episode, usually within 6-8 wk. The majority of macules have a normal central area, giving the rash a circinate outline. This rash is seen mainly on the trunk and is evanescent, fading in 1 place only to appear at another site. Examination of the blood during this stage may show anemia, leukopenia with relative monocytosis, and elevated levels of IgM. Cardiac manifestations of HAT have also been reported but are generally limited to nonspecific ST-T wave electrocardiographic abnormalities. Histopathologic characterization shows a lymphomonohistiocytic infiltrate in the interstitium and there is no penetration of the myocardial cells, unlike that for American trypanosomiasis (Chapter 279). Progression of cardiac pathology to congestive heart failure has not been reported, and the perimyocarditis is usually self-limited and/or readily resolves with treatment.
Diagnosis
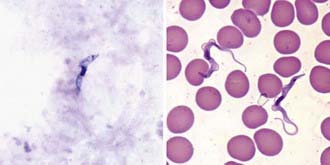
Definitive diagnosis can be established during the early stages by examination of a fresh, thick blood smear, which permits visualization of the motile active forms (Fig. 278-1). HAT can also be detected from blood using a variety of sensitive techniques: quantitative buffy coat smears and mini anion exchange resins are common examples. The card agglutination trypanosomiasis test (CATT) is of value for epidemiologic purposes and in screening for T. brucei gambiense. Dried, Giemsa-stained smears should be examined for the detailed morphologic features of the organisms. If a thick blood or buffy coat smear is negative, concentration techniques may help.
Blum JA, Zellweger MJ, Burri C, et al. Cardiac involvement in African and American trypanosomiasis. Lancet Infect Dis. 2008;8:631-641.
Brun R, Blum J, Chappuis F, et al. Human African trypanosomiasis. Lancet. 2010;375:148-156.
Checchi F, Barrett MP. African sleeping sickness. BMJ. 2008;336:679-680.
Checchi F, Filipe JA, Barrett MP, et al. The natural progression of gambiense sleeping sickness: what is the evidence? PLoS Negl Trop Dis. 2008;2:e303.
Fèvre EM, Wissmann BV, Welburn SC, et al. The burden of human African trypanosomiasis. PLoS Negl Trop Dis. 2008;2:e333.
Inojosa WO, Augusto I, Bisoffi Z, et al. Diagnosing human African trypanosomiasis in Angola using a card agglutination test: observational study of active and passive case finding strategies. Br Med J. 2006;332:1479-1481.
Maclean L, Odiit M, Macleod A, et al. Spatially and genetically distinct African Trypanosome virulence variants defined by host interferon-gamma response. J Infect Dis. 2007;196:1620-1628.
Pais R, Lohs C, Wu Y, et al. The obligate mutualist Wigglesworthia glossinidia influences reproduction, digestion, and immunity processes of its host, the tsetse fly. Appl Environ Microbiol. 2008;74:5965-5974.
Thumbi SM, McOdimba FA, Mosi RO, et al. Comparative evaluation of three PCR base diagnostic assays for the detection of pathogenic trypanosomes in cattle blood. Parasit Vectors. 2008;1:46.
Welburn S, Picozzi K, Coleman PG, et al. Patterns in age-seroprevalence consistent with acquired immunity against Trypanosoma brucei in Serengeti lions. PLoS Negl Trop Dis. 2008;2:e347.