Chapter 14 Aesthetic Contouring of the Craniofacial Skeleton
Introduction
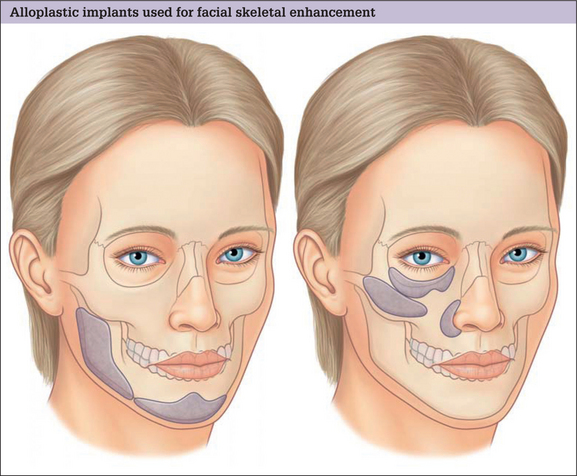
Augmentation is the predominant means of aesthetic contouring of the facial skeleton in non-Asian populations. Conceptually, autogenous bone would be the best material for augmentation, because it has the potential to be revascularized and, then, incorporated into the facial skeleton. In time, it could be biologically indistinguishable from the adjacent native skeleton. Practically, the use of autogenous bone is limited. The morbidity, time and hospital costs associated with autogenous bone graft harvest can be significant. Furthermore, the inevitable resorption and the poor handling characteristics of autogenous bone grafts also limit the quality and predictability of the aesthetic result. For these reasons, almost all facial skeletal augmentation is done with alloplastic implants. A diagrammatic survey of the facial skeletal areas amenable to augmentation with alloplastic implants is presented in Fig. 14.1.
Indications
Preoperative Evaluation
Radiology
Most aesthetic procedures are done without preoperative radiology assessment. In general, the size and position of the implant are largely aesthetic judgments. Cephalometric X-rays are most often used for planning chin and mandible augmentation surgery. These studies define skeletal dimensions and asymmetries as well as the thickness of the chin pad. Computerized tomographic (CT) scans provide the ability to view the skeleton in different planes and, through computer manipulation, in three dimensions. CT imaging provides digitized information that can be transferred to design software. This can be used to create life-sized models and custom implants, which are particularly helpful when augmenting facial skeletons with significant asymmetries.
Surgical Planning
Facial anthropometries and neoclassical canons
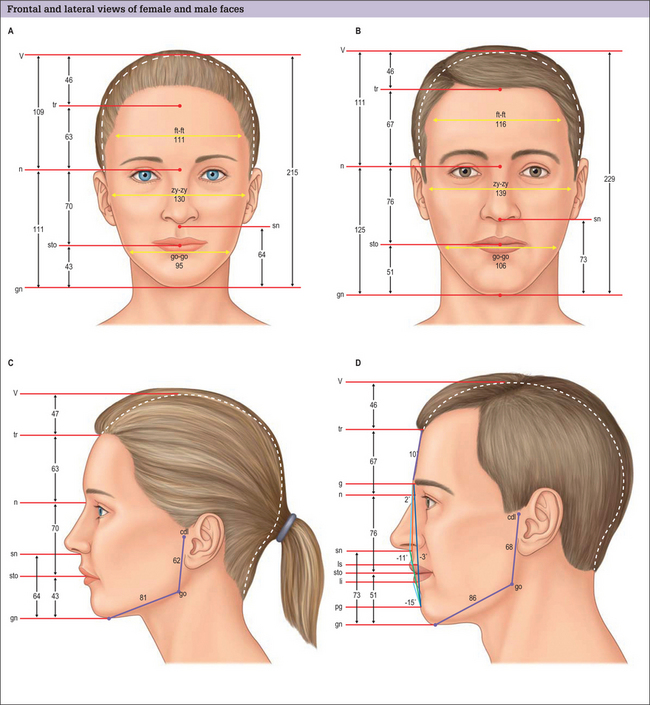
For purposes of painting and sculpture, Renaissance scholars and artists formulated ideal proportions and relations of the head and face. These were largely based on classical Greek canons. Although usually referenced in texts discussing facial skeletal augmentation, neoclassical canons have a limited role in surgical evaluation and planning, because they are based on idealizations. When the dimensions of normal males and females were evaluated and compared to these artistic ideals, it was found that some theoretic proportions are never found, and others are one of many variations found in healthy normals, or those determined more attractive than normals.1,2 The neoclassical canons do not allow for the facial dimensions that are known to differ with sex and age. Most of these canons of proportion (e.g. the width of the upper face is equal to five eye widths), are interesting but hold for few individuals and cannot be obtained surgically or, if obtainable, only with extremely sophisticated craniofacial procedures. For these reasons, we have found it more useful to use the anthropometric measurements of normal individuals to guide our Gestalt in the selection of implants for facial skeletal augmentation (Fig. 14.4).3 Normal dimensioned faces are intrinsically balanced. That is, the relations between the various areas of the face relate to one another in a way that is not distracting to the observer. By comparing a patient’s dimensions to the average, the surgeon has some objective basis as to what anatomic area may be amenable to augmentation, and by how much.
Alloplastic Materials
Implant materials used for facial skeletal augmentation are biocompatible – that is, there is an acceptable reaction between the material and the host. In general, the host has little or no enzymatic ability to degrade the implant with the result that the implant tends to maintain its volume and shape. Likewise, the implant has a small and predictable effect on the host tissues that surround it. This type of relationship is an advantage over the use of autogenous bone or cartilage which, when revascularized, will be remodeled to varying degrees, thereby changing volume and shape.
The presently used alloplastic implants used for facial reconstruction have not been shown to have any toxic effects on the host.4 The host responds to these materials by forming a fibrous capsule around the implant, which is the body’s way of isolating the implant from the host. The most important implant characteristic that determines the nature of the encapsulation is the implant’s surface characteristics. Smooth implants result in the formation of smooth-walled capsules. Porous implants allow varying degrees of soft tissue ingrowth that results in a less dense and defined capsule. It is a clinical impression that porous implants, as a result of fibrous incorporation rather than encapsulation, have a lower tendency to erode underlying bone or migrate due to soft tissue mechanical forces and, perhaps, are less susceptible to infection when challenged with an inoculum of bacteria. The most commonly used, commercially available materials today for facial skeletal augmentation are solid silicone, and porous polyethylene -Medpor (Porex, Fairborn, GA). Eppley5 has summarized the attributes of these materials.
Requisites of Implant Shape, Positioning, and Immobilization
Immobilization
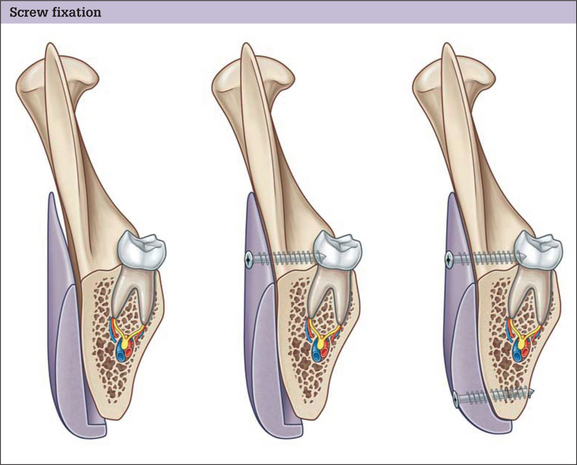
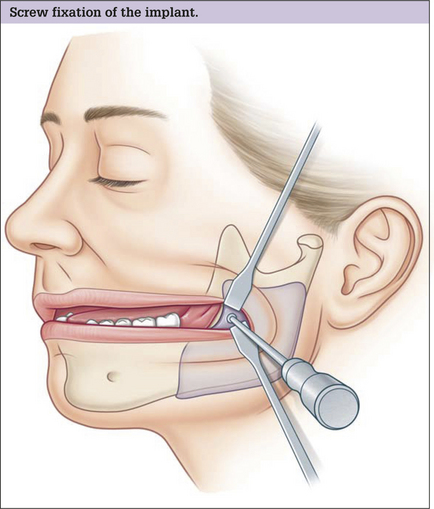
Facial implants should be immobilized. Many surgeons stabilize the position of the implant by suturing it to surrounding soft tissues or by using temporary transcutaneous pull-out sutures. Screw fixation of the implant to the skeleton is preferred. Screw fixation prevents any movement of the implant and also assures application of the implant to the surface of the bone. Because each facial skeleton has a unique and varying surface topography, a non-conforming implant will leave gaps between the implants and the skeleton. These gaps between the implant and the skeleton are problematic for two reasons. The space between the implant and the skeleton is equivalent to an additional augmentation. This can lead to over-augmentation and asymmetries. Gaps are also potential spaces for hematoma and seroma formation (Fig. 14.5). Finally, screw fixation allows for final contouring with the implant in position. This final contouring is particularly important where the implant interfaces with the skeleton. Any step-off between the implant and the skeleton will be palpable and possibly visible in patients with thin soft tissue cover.
Midface Implants
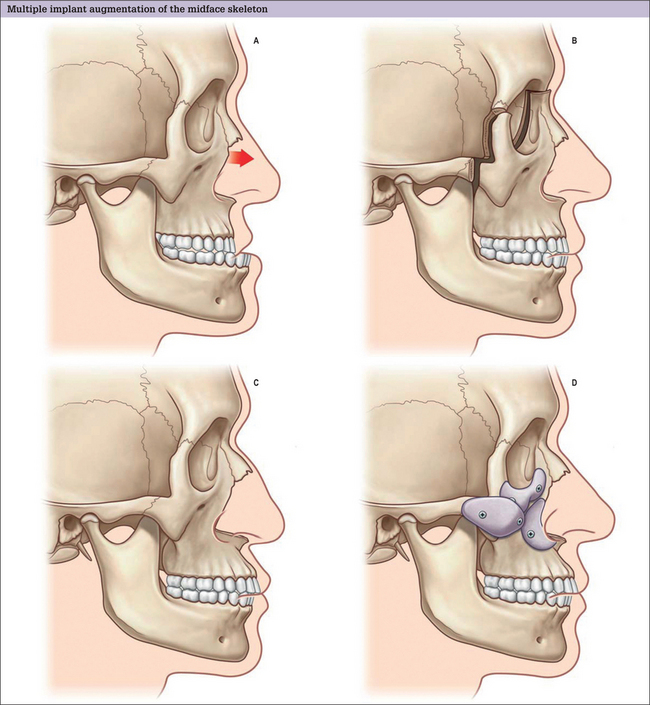
Virtually all aesthetic augmentation is performed on the middle and lower thirds of the facial skeleton. The midface is best conceptualized as having three zones, alone, or in combination, appropriate for augmentation. Implants are designed to augment the infraorbital rim, the malar area, and the pyriform aperture.
Infraorbital rim
Deficient infraorbital rim projection predisposes to lower-lid descent with aging.
Deficient infraorbital rim projection predisposes to lower-lid descent after blepharoplasty.
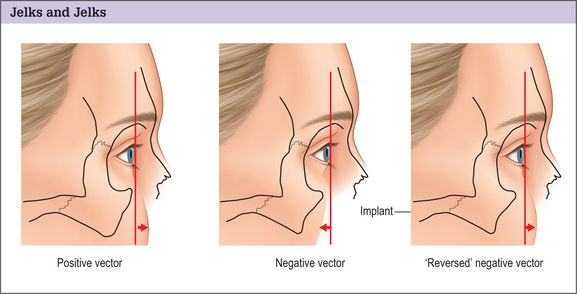
Jelks and Jelks6 categorized globe-orbital rim relationships and the tendency for the development of lower-lid malposition after blepharoplasty. On sagittal view, they placed a line or “vector” between the most anterior projection of the globe and the malar eminence and lid margin. A “positive” vector relationship exists when the most anterior projection of the globe is posterior to the lid margin and the malar eminence. A “negative” vector relationship exists when the most anterior projection of the globe lies anterior to the lower lid and the malar eminence. See Fig. 14.6. They warned that, patients whose orbital morphology has a negative vector relationship are prone to lid malposition after lower blepharoplasty.
Augmentation of the infraorbital rim in patients with a retruded infraorbital rim can bring it into a better relationship with the globe, thereby “reversing the negative vector”.7 See Fig. 14.6.
Infraorbital rim augmentation is part of the strategy for normalizing the appearance in patients who are “morphologically prone”. It can be adapted for morphologically prone patients who are first seeking improvement in their periorbital appearance or for those whose lid malposition and round-eye appearance that has been exaggerated by previous lower blepharoplasty.8
Surgical technique
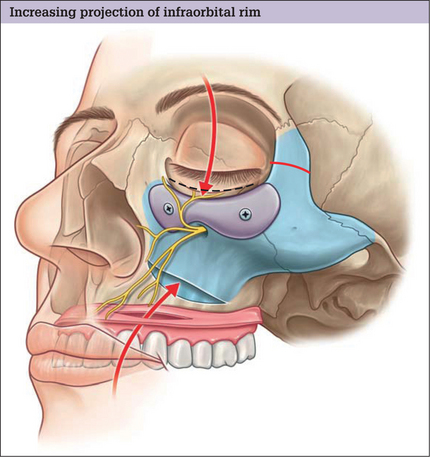
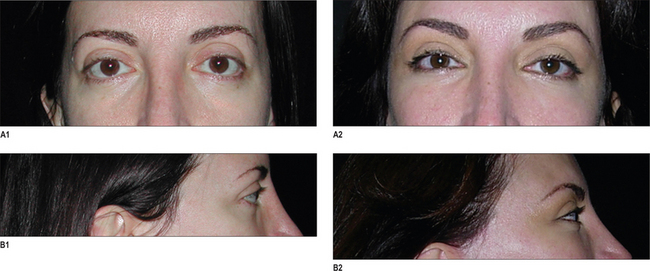
The infraorbital rim and adjacent anatomy must be exposed sufficiently to assure ideal implant placement, smooth implant facial skeleton transition, and screw fixation. Direct, subciliary skin or skin muscle-flap incisions can provide this exposure. A transconjunctival incision alone is inadequate for implant placement or screw stabilization. Use of a transconjunctival retroseptal incision requires lengthening with a lateral canthotomy or combination with intraoral and coronal incision. It is important to identify the infraorbital nerve that exits from the infraorbital foramen about 1 cm below the margin of the orbit. This may be 3–6 mm in patients with significant maxillary hypoplasia – usual candidates for this surgery. The orbicularis oculi and the origins of the lip elevators are separated from the underlying skeleton in a subperiosteal plane to expose the infraorbital rim. The implant is carved to fit the specific needs of the patient. Segmenting the implant may facilitate placement of the implant through limited skeletal access. The implant is fixed to the skeleton with titanium screw. This technique is summarized in Fig. 14.7. A clinical example is shown in Fig. 14.8.
Pyriform aperture
The average North American Caucasian face is convex (Figs 14.4). A relative deficiency in lower midface projection may be congenital or may be acquired, particularly after cleft surgery and trauma. Patients with satisfactory occlusion and lower midface concavity can have their aesthetic desires satisfied with skeletal augmentation. Implantation of alloplastic material in the paranasal area can simulate the visual effect of LeFort I advancement and other skeletal manipulations.9
Surgical technique
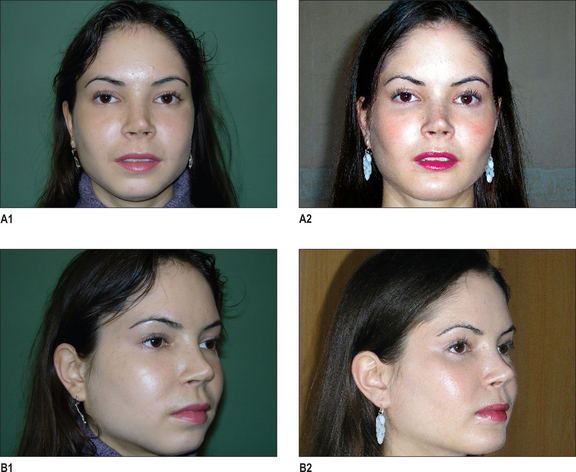
Paranasal augmentation is done through an upper gingivobuccal sulcus incision made just lateral to the piriform aperture to avoid placing incisions directly over the implant. An adequate cuff of mucosa is left to allow layered closure. Subperiosteal dissection exposes the area to be augmented. The infraorbital nerve is identified and preserved. The patient’s anatomy will determine whether the entire crescent or just the horizontal or vertical limb of the crescent will be utilized. The root of the canine usually lies in the field to be augmented and should be avoided if the implant is immobilized with screws. A patient who underwent aesthetic rhinoplasty and augmentation of the pyriform aperture area is shown in Fig. 14.9.
Malar
Since full cheeks are associated with youth, malar augmentation is often performed to provide a youthful appearance. This may provide an aesthetic benefit if there is a relative malar hypoplasia or if the implants are of modest size and projection. This skeletal augmentation is not equivalent to a soft tissue augmentation or resuspension. Similarly, malar implants are often advocated as a means to obliterate lower-eyelid wrinkles or secondary bags. Malar augmentation impacts poorly on these surface irregularities. More often, they detract from periorbital aesthetics by contributing to lower-lid malposition, particularly when placed through an eyelid approach.10
Surgical technique
Although implants with greater projection are available, I rarely provide more than 3 mm of augmentation at the point of maximum projection. Larger implants will become obvious with time as the overlying soft tissues atrophy and sag. The capsule formation that accompanies smooth-surfaced implants further exaggerates the tendency towards implant visibility.
In my experience, implants with large surface areas do not mimic natural skeletal topography and cause unnatural implant-dictated contours. The intraoperative position of both smooth and porous implants can be guaranteed with screw fixation of the implant to the skeleton. See Figs 14.10 and 14.11.
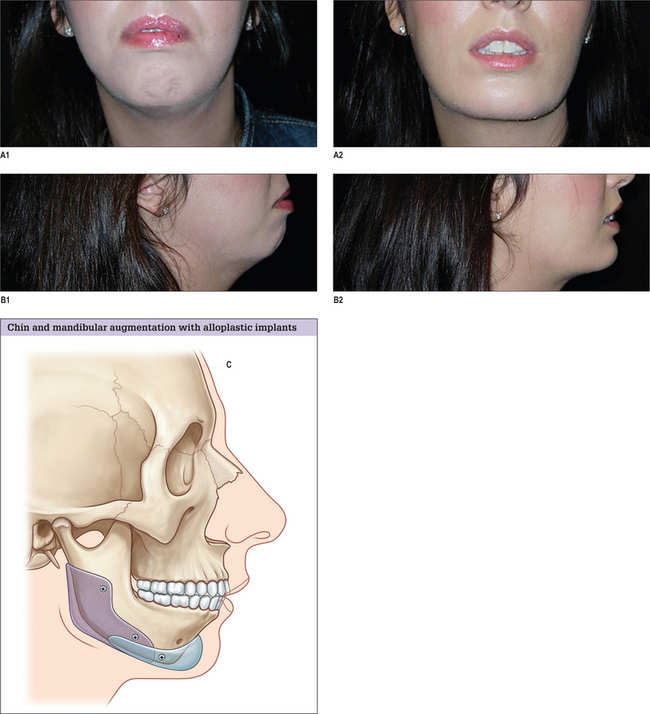
Mandible Implants
Each of the anatomic areas of the mandible – chin, body, angle and ramus – may be deficient and are amenable to augmentation with alloplastic materials. For further information on the chin please see Chapter 13, Genioplasty.
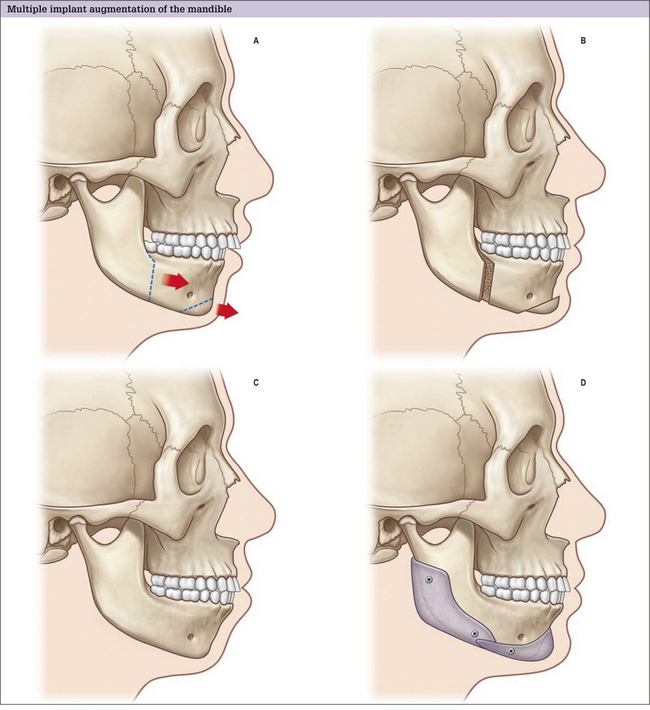
Ramus, angle, and body
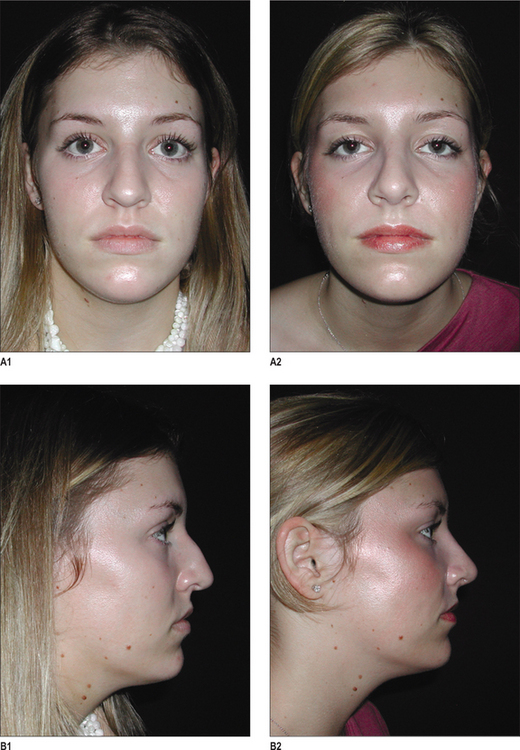
Alloplastic augmentation of the mandibular ramus and body can have a dramatic impact on the appearance of the lower third of the face. Two patient populations have had their aesthetic concerns satisfied with mandibular augmentation procedures. One group has mandibular dimensions that relate to the upper and middle thirds of the face within the normal range. These patients perceive a wider lower face as an enhancement to their appearance. Patients in this treatment group often present with a desire to emulate the appearance of models and actors who have a defined, angular lower face. This patient group benefits from implants designed to augment the ramus and posterior body of the mandible and, in so doing, increase the bigonial distance (Fig. 14.4 and 14.5).
The other major group of patients who benefit from augmentation of the mandibular ramus and body are those patients with skeletal mandibular deficiency who have their malocclusion treated with orthodontics. The skeletal anatomy associated with mandibular deficiency, which can be camouflaged with implants designed to augment the ramus and body of the mandible include the obtuse mandibular angle with steep mandibular plane, as well as the decreased vertical and transverse ramus dimensions. The addition of an extended chin implant will camouflage the poorly projecting chin.14
Surgical technique
To assure the desired placement of the implant and its application to the surface of the mandible, the implant is fixed to the mandible with titanium screws. With vigorous retaction, this is performed intraorally (Fig. 14.12). This allows strategic unicortical screw fixation of the implant to the mandible. Usually two or three screws are used to obliterate any gaps between the mandible and the implant. Screws are placed to avoid the anticipated path of the inferior alveolar nerve prior to its exit from the mental foramen.
Implants used to camouflage soft tissue depressions
The implants discussed in this chapter are designed to increase the surface projection of the facial skeleton. Some surgeons have used implants placed on the facial skeleton to disguise overlying soft tissue volume inadequacy, usually due to involutional changes brought on by age. Submalar implants are used to expand midfacial depression and, in so doing, may reduce the prominence of the nasolabial fold.15 Mittleman designed an extended chin implant shaped to camouflage the prejowl sulcus.16 The sulcus develops due to some bone resorption along the border of the mandible and soft tissue atrophy directly over it. This depression exaggerates the jowl. Flowers designed an implant to fill out the “tear trough”, the area that develops in some individuals medial to the infraorbital foramen between the thick cheek skin and thin eyelid skin.17
Complications
There are no truly scientific data to document the surgical complication rate related to facial skeletal augmentation. Prospective studies that control for surgical technique, implant site, patient selection, and follow-up time do not exist. We reviewed almost 200 reports in the literature that provided sufficient data to compare complication rates related both to implant material and implant site.4 The accumulation of these clinical data can provide some useful information about factors that can contribute to morbidity with implants. For example, the quality of the soft tissue coverage is clearly related to morbidity. Complication rates are lowest in the chin and malar region, where the soft tissue cover is relatively thick, and highest in the nose and ear, where the soft tissue cover is thin and often under tension from the underlying device. The surface characteristic of an implant may also be important. Porous implants allow a certain ingrowth of tissue which may allow the presence of host defenses within the implant to decrease the risk of infection. Given that several biomaterials are well tolerated in the human body, the actual chemical structure of an implant is important only to the degree that it influences the consistency and surface characteristics of the device.
In 2003, I reported my personal experience with porous implants used for facial skeletal reconstruction.8 This report was based on experiences with 162 patients who were operated on over an 11-year period (1990–2001). In this series no implants migrated or were extruded, formed clinically apparent capsules, or caused symptoms attributable to bio-incompatibility. The overall reoperation was 10% (n = 16), which included operations to remove implants because of acute infection (2% – n = 3) or late infection (1% – n = 1), or to remove implants causing displeasing contours (2% – n = 3). The rate of complications has not changed in facial implant procedures in the subsequent 100 patients.
The presence of a foreign body decreases the minimal infecting dose of Staphylococcus aureus in an animal model due to impaired bacterial clearance.19 If microorganisms are not eliminated rapidly from an implant surface they will adhere to the implant initially by non-specific physical forces and then by the formation of biofilms characterized by clustering together in an extracellular matrix attached to the implant.20 Biofilms protect bacteria from host defenses and antibiotics. Only aggressive debridement and long-term suppressive therapy have been effective in treating orthopedic implant-related infections. This approach is usually not appropriate in facial implant patients since both debridement and chronic infection may be deforming in this appearance conscious population. Since antibiotic treatment alone is usually not successful, facial implant-related infections are treated by implant removal and appropriate wound care. Implants may be replaced in 6 to 12 months.
1. Farkas L., Hreczko T.A., Kolar J.C., Munro I.R. Vertical and horizontal proportions of the face in young adult North American Caucasians: revision of neoclassical canons. Plast Reconstr Surg. 1985;75:328.
2. Farkas L.G., Kolar J.C. Anthropometries and art in the aesthetics of women’s faces. Clin Plast Surg. 1987;14:599.
3. Farkas L.G., Hreczko T.A., Katie M.J. Craniofacial norms in North American Caucasians from birth (one year) to adulthood. Appendix A. In Farkas L.G., editor: Anthropometry of the Head and Face, 2nd edn., New York: Raven Press, 1994.
4. Rubin J.P., Yaremchuk M.J. Complications and toxicities of implantable biomaterials used in facial reconstructive and aesthetic surgery: a comprehensive review of the literature. Plast Reconstr Surg. 1997;100:1336.
5. Eppley B.L. Alloplastic implantation. Plast Reconstr Surg. 1999;104:1761.
6. Jelks G.W., Jelks E.B. The influence of orbital and eyelid anatomy on the palpebral aperture. Clin Plast Surg. 1991;18:193.
7. Yaremchuk M.J. Infraorbital rim augmentation. Plast Reconst Surg. 2001;107:1585.
8. Yaremchuk M.J. Restoring palpebral fissure shape after previous lower blepharoplasty. Plast Reconst Surg. 2003;111:441.
9. Yaremchuk M.J., Israeli D. Paranasal implants for correction of midface concavity. Plast Reconst Surg. 1998;102:1676.
10. Yaremchuk M.J. Malar implants. In Atlas of Facial Implants. Philadelphia: Elsevier-Saunders; 2007. Ch. 7
11. McCarthy J.G., Ruff J.G. The chin. Clin Plast Surg. 1988;15:125.
12. Yaremchuk M.J. Improving aesthetic outcomes after alloplastic chin augmentation. Plast Reconst Surg. 2003;112:1422-1432.
13. Zide B.M. The mentalis muscle: An essential component of chin and lower lip position. Plast Reconst Surg. 1989;83:413.
14. Yaremchuk M.J. Mandibular augmentation. Plast Reconstr Surg. 2000;106:697.
15. Binder W. Submalar augmentation: an alternative to facelift surgery. Arch Otolaryngol. 1989;115:797.
16. Mittleman H. The anatomy of the aging mandible and its importance to facelift surgery. Facial Plast Surg Clin North Am. 1994;2:301.
17. Flowers R.S. Tear trough implants for correction of tear trough deformity. Clin Plast Surg. 1993;20:403.
18. Yaremchuk M.J. Facial skeletal reconstruction using porous polyethylene implants. Plast Reconstr Surg. 2003;111:1818.
19. Zimmerli W., Waldvogel F.A., Vaudaux P., Nydegger U.E. Pathogenesis of foreign body infection: description and characteristics of an animal model. J Infect Dis. 1982;146:487-497.
20. Costerton J.W., Stewart P.S., Greenberg E.P. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318-1322.