CHAPTER 2 Adverse Sequelae and Complications of Venous Hypertension
Pathogenesis
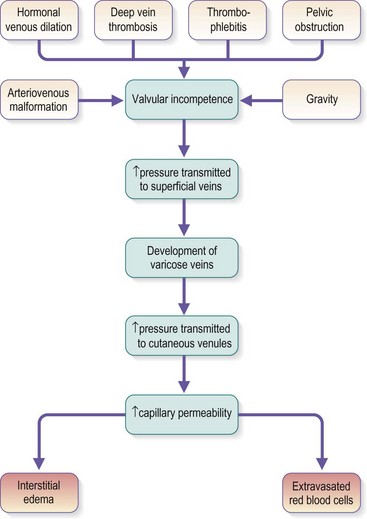
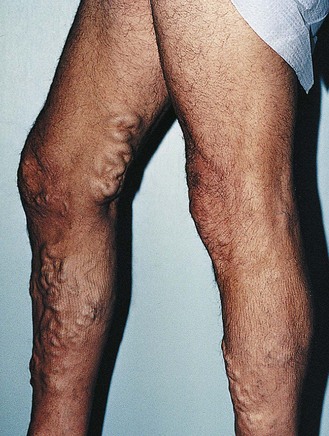
Chronic venous insufficiency, which must be semantically distinguished from venous disease or disorder, may be defined as relative impedance of venous flow back to the heart and responsible for clinical consequences. When this occurs in the lower extremities, the normal reabsorption of perivascular fluids by osmotic and pressure gradients is impaired, resulting in accumulation of perivascular and lymphatic fluid. This leads to edema and impaired oxygenation of surrounding tissue (Figs 2.1 and 2.2). This disruption of the normal vascular and lymphatic flow of the lower extremities may result in pain, cramping (especially at night), restlessness, pigmentary changes, dermatitis, and ulceration (Fig. 2.3).1–3 The association of abnormal venous flow with various signs and symptoms has been noted for centuries: first by Hippocrates in the fourth century BC and by Wiseman in England in 1676.4 It has been estimated that chronic venous insufficiency will develop in almost 50% of patients with major varicose veins.5,6
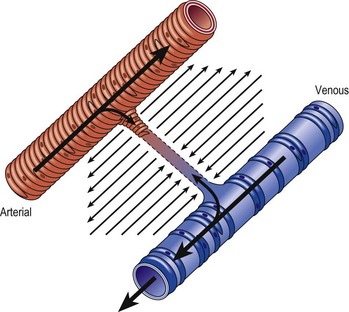
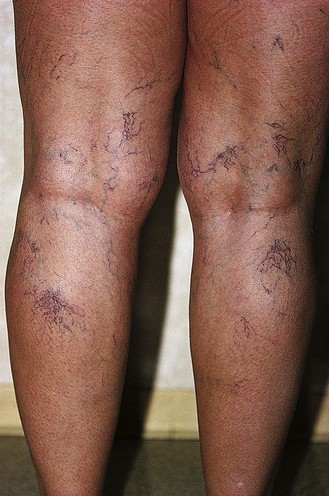
Several alterations of normal venous flow cause venous hypertension. Such hypertension in the lower extremities is usually caused by a loss or disruption of the normal one-way valvular system. This may occur because of deep vein thrombosis (DVT), thrombophlebitis, or a dilation of veins from other causes.7,8 When perforating vein valvular function becomes incompetent, there may be shunting of blood flow from the deep to the superficial venous system through the incompetent perforating veins, with resultant adverse sequelae.9–11 The superficial veins respond by dilating to accommodate the increased blood flow, which produces superficial valvular incompetence leading to the development of varicosities.12 In addition, with muscular movement in the lower limbs, the high venous pressure normally occurring within the calf is transmitted straight to the superficial veins and subcutaneous tissues.13,14 Venous pressure in the cuticular venules may greatly exceed the normal 100 mmHg in the erect position.15 This causes venular dilation over the whole area, resulting in capillary dilation, increased permeability,16–19 and increase in the subcutaneous capillary bed.17,20 This is manifested as telangiectasia and venectasia (Fig. 2.4). Venous hypertension has also been demonstrated to destroy the venous valves that are present in the subcuticular vascular system.21 This destruction promotes persistent and progressive changes in the venous drainage system of the skin and subcutaneous tissues. The greater the degree of venous hypertension, the greater the risk of venous ulcer development.22,23 Fortunately, both sclerotherapy and surgical treatment are capable of normalizing abnormal venous hypertension.
Venous hypertension and subsequent insufficiency may also derive from venous obstruction, either at lower limb level or at iliocaval level, usually associated with reflux.24
Molecular Mechanisms
The molecular mechanisms involved in leukocyte adhesion and activation in chronic venous disease (CVD) are beginning to be elucidated. Circulating leukocytes and vascular endothelial cells express membrane adhesion molecules. For example, binding of L-selectin on the leukocyte surface to E-selectin on endothelial cells may be involved in leukocyte ‘rolling’ along the endothelial surface (Fig. 2.5A). Then, when leukocytes are activated, they shed L-selectin into the plasma and express molecules of the integrin family, including CD11b, which binds firmly to intercellular adhesion molecule-1 (ICAM-1). Integrin binding can promote firm adhesion of leukocytes, the starting point for their degranulation8 and migration through the endothelium.
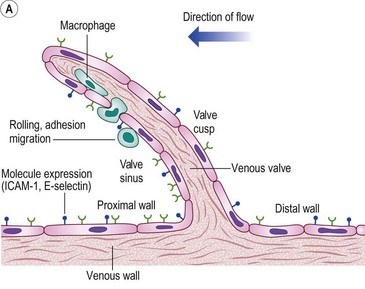
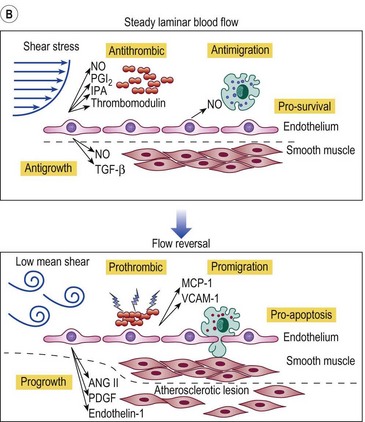
Figure 2.5 A: Leukocyte-endothelial interactions on a venous valve. ICAM-1, intercellular adhesion molecule-1. B: Summary of contrasting effects of steady, laminar shear stress (upper panel) and turbulent or reversing shear stress (lower panel) on vessel walls. NO, nitric oxide; PGI2, prostacyclin; t-PA, tissue plasminogen activator; TGF-β, transforming growth factor beta; MCP-1, monocyte chemotactic protein-1; VCAM-1, vascular cell adhesion molecule-1; ANG II, angiotensin II; PDGF, platelet-derived growth factor.
(Modified from Traub O, Berk BC: Arterioscler Thromb Vasc Biol 18:677, 1998, with permission.)
(Modified from Takase S, Bergan JJ, Schmid-Schönbein GW: Ann Vasc Surg 14:427, 2000 and from Coleridge Smith PD, Bergan JJ: Inflammation in venous disease: In Schmid-Schönbein GW, Granger N, editors, Molecular basis for microcirculatory disorders, Paris, 2003, Springer-Verlag, pp 489–500, with permission.)
Several studies have looked at markers of leukocyte and endothelial adhesion and activation in CVD. After venous hypertension was induced by standing for 30 minutes, levels of L-selectin and integrin CD11b on circulating neutrophils and monocytes in CVD patients were found to decrease, reflecting the trapping of these cells in the microcirculation. At the same time, plasma levels of soluble L-selectin increased, reflecting the shedding of these molecules from leukocyte surfaces during leukocyte–endothelial adhesion.24 Similarly, basal plasma levels of the adhesion molecules ICAM-1, endothelial leukocyte adhesion molecule-1 (ELAM-1), and vascular cell adhesion molecule-1 (VCAM-1) were higher in CVD patients than controls and increased significantly in response to venous hypertension provoked by standing.25 Baseline levels of plasma von Willebrand factor, a marker for endothelial cell damage, were also higher in patients with lipodermatosclerosis than in those without skin changes.26 Lactoferrin and neutrophil elastase are enzymes released from neutrophil granules. Plasma levels of these molecules are therefore markers of neutrophil activation, and both were found to be higher in patients with CVD than in age- and sex-matched controls.27
In most of these studies, prolonged standing induced venous hypertension. However, in patients with chronic venous insufficiency, increases in plasma levels of ICAM-1 and VCAM-1 can be induced by walking,28 presumably in response to hydrodynamic pressure increases generated by the musculovenous pump being transmitted to subcutaneous and cutaneous small vessels through incompetent perforating veins.
In addition to local factors operating in relation to venous hypertension, CVD patients have a tendency for systemically elevated leukocyte adhesion. Venous hypertension in the upper limb (where the local vessels and tissues are presumably normal) produced by means of a pressure cuff causes greater leukocyte accumulation in patients with venous leg ulcers than in normal subjects.29 Evidence has been found for an activation factor in the plasma of CVD patients.15 Plasma obtained from CVD patients induced greater degrees of activation as shown by oxygen-free radical production and pseudopod formation in healthy, naive granulocytes than did plasma taken from normal subjects. Also, there was a trend towards more activation in more severe cases of chronic venous insufficiency. The nature of the plasma factor responsible for leukocyte adhesion and activation is presently unknown.
Inflammation and Skin Changes
There has also been progress in linking the chronic inflammatory state seen in CVD patients with the specific skin changes typical of the condition. In lipodermatosclerosis, the skin capillaries become elongated and tortuous, giving the appearance in histologic sections of elevated capillary density.30 In advanced skin disease especially in the ulcerative stages, the capillaries may take on a glomerular appearance,31 and it seems clear that substantial proliferation of the capillary endothelium occurs. Several factors could contribute to endothelial proliferation, but vascular endothelial growth factor (VEGF) is an obvious candidate. VEGF is known to be involved in inflammatory and healing processes in the skin and has been shown to increase microvascular permeability both acutely and chronically.32 Plasma levels of VEGF have been shown to increase during the venous hypertension induced by 30 minutes of standing both in normal subjects and in CVD patients. Both supine and standing VEGF levels are higher in patients than in normal controls.33 Furthermore, plasma VEGF levels are higher in CVD patients with skin changes than in CVD patients with normal skin.34
Another feature of the skin changes associated with CVD is dermal tissue fibrosis. Transforming growth factor-β1 (TGF-β1) is a known fibrogenic cytokine. Detailed analysis of punch biopsy specimens has shown that skin from the lower calf of CVD patients had significantly elevated active TGF-β1 levels compared with normal skin. In addition, this was true relative to skin taken from the lower thigh region of the same patients.35 Immunohistochemistry and immunogold labeling showed the TGF-β1 to be located in leukocytes, fibroblasts, and on collagen fibrils. Pappas el al36 proposed that activated leukocytes migrate out of the vasculature and release TGF-β1, which stimulates increased collagen production by dermal fibroblasts. Over an extended period, such a process could contribute to the typical dermal fibrosis seen in CVD.
Altered collagen synthesis has also been reported for dermal fibroblasts taken from apparently healthy areas of skin in patients with varicose veins.37 It has been possible to correlate altered levels and distributions of growth factors, including basic fibroblast growth factor (bFGF), transforming growth factor-3 (TGF-3), and the receptor for epidermal growth factor (EGF), with different types of skin change, including venous eczema, pigmentation, lipodermatosclerosis, and ulceration.38
Advanced cutaneous changes of lipodermatosclerosis appear as an erythematous, telangiectatic, edematous plaque with mottled hyperpigmentation. Histologic examination shows advanced stasis changes, including zones of ischemic necrosis in the central part of fat lobules. Septal fibrosis, fat microcysts, membranous fat necrosis, and sclerosis occur later. This advanced clinical–histologic change is termed sclerosing panniculitis.39 With superficial venous insufficiency alone, changes in the histologic appearance of capillaries are moderate. The combination of deep venous insufficiency, with or without superficial venous insufficiency, produces more profound changes.40,41
Labropoulos et al42 studied 255 limbs in 217 patients. These had superficial venous insufficiency alone, with normal perforating veins and deep veins. The color-flow duplex imaging techniques were used. The researchers concluded that aching, ankle edema and skin changes in limbs with reflux confined to the superficial venous system were associated predominantly with reflux in veins below the knee. An important finding was that ulceration occurred only when the entire great saphenous vein (GSV) was involved or when reflux was extensive in both the great and small saphenous systems.
Classification of Venous Disease
An international ad hoc committee of the American Venous Forum developed the CEAP classification for CVD in 1994. The goal was to stratify clinical levels of venous insufficiency. The four categories selected for classification were: clinical state (C), etiology (E), anatomy (A), and pathophysiology (P). The CEAP classification has been endorsed worldwide despite its acknowledged deficiencies. It has been adopted as a standard in many clinics in Europe, Asia and South America and is considered the only modern method for reporting data in the United States.43–45
The CEAP classes are shown in Box 2.1. Each clinical class is further characterized by a subscript for the presence of symptoms (S, symptomatic) or their absence (A, asymptomatic). Symptoms include aching, pain, tightness, skin irritation, heaviness, and muscle cramps, as well as other complaints attributable to venous dysfunction. A basic CEAP is suggested. For the practicing physician, CEAP is an instrument for correct diagnosis, to guide the treatment and assess the prognosis. Venous disease is complex, but can be described. In modern phlebologic practice, the vast majority of patients will undergo a duplex scan of the venous system of the leg, which will provide data on E, A and P. In basic CEAP where a duplex scan is performed, E, A and P should be utilized. For the anatomical classification A in basic CEAP, the simple s = superficial, p = perforator and d = deep descriptors should be used. Multiple descriptors should be permitted for all four components in basic CEAP; for example a patient could be classified as C234s Ep Asd Pr. Use of all components of CEAP is encouraged. Definitions that apply to the CEAP classification are shown in Box 2.2.
Box 2.2 CEAP definitions
But the CEAP is a description of the disease, not an assessment of its severity. It serves for classification, not evaluation. For this reason, several scores have been added to the CEAP, such as the VCSS (venous clinical severity score).46 However, a physician reported outcome such as the VCSS is influenced by the ‘experimenter-expectancy effect’ and so the need for the application of patient reported outcomes (PROs) to clinical trials is now recognized.47 Revicki summarizes: ‘… the patient’s perspective and patient-reported HRQL (health related quality of life) is the ultimate outcome for health care interventions.’48 Basically, a PRO will increase the validity of randomized controlled trials (RCTs) especially when they cannot be double or even single blinded. The influence of the experimenter’s opinion about the efficacy of a treatment significantly changes the RCT results in many different fields of medicine.49 An additional precaution for outcome evaluation could be the use of an external evaluation, but this would not take into account the patient’s point of view and self-appraisal.
The patient’s opinion is a point that, albeit obvious, has been too long neglected. Several Quality of Life questionnaires, generic (e.g. SF1250) and specific (e.g. CIVIQ,51 AVVQ) already exist and have been successfully used.52 However, they have not take into account the only sure thing we could ever state: if the patient is not happy with the result of the treatment, it means it (you) failed. Therefore, the need to assess the patient’s own appraisal in detail, with satisfactory sensitivity and specificity, appears of utmost importance. Guex et al addressed this point and observed that CVD patients had one main concern belonging to one of the following five groups: discomfort/pain, appearance/attractiveness, risk/threat to health, restriction of movements/activities, emotional distress.53 They have therefore constructed a novel PRO (the SQOR-V), specially dedicated to CVD and based on these five patient concerns.54 Its values range from 20 to 100, 20–30 being normal, 30–50 moderate consequences of CVD and >50 being severe CVD. This questionnaire is free to use and is available in several languages, including French, English and Spanish.
Pittaluga et al have established a classification of venous refluxes based on the extent of saphenous vein reflux.55 They noted a positive correlation between age and progression of superficial venous insufficiency.
Incidence
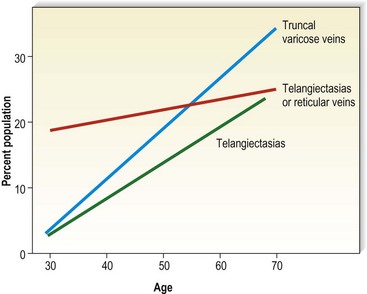
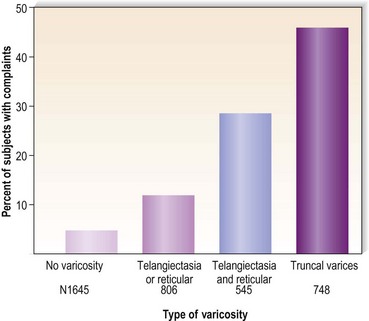
Varicose veins have been noted to increase in incidence with age and have been estimated to occur in 7% to 60% of the adult population in the United States.5,15,56–60 Varicose veins occur in 8% of women aged of 20 to 29 years, increasing to 41% in the fifth decade and 72% in the seventh decade of life.57,61,62 A similar rate of increase in the incidence of varicose veins occurs in men: 1% in the third decade, increasing to 24% in the fourth decade and 43% in the seventh decade.57,61 The Basle study III found that the greatest correlation between age and incidence of varicose veins occurred in those with varicose veins only, rather than in those with the presence of telangiectasias (hyphenwebs) or reticular veins (Fig. 2.6).5 The Framingham study,63 curiously, showed no difference in the incidence of varicose veins with age.
Varicosities in childhood are rare, occurring almost exclusively in association with congenital vascular malformations (see Chapter 3). When varicosities occur, they usually appear as a subtle physical finding, such as a slight bulge over the popliteal fossa (Fig. 2.7). One estimation on the incidence of telangiectasias in children was performed by Oster and Nielsen64 in Denmark. Of 2171 Danish schoolchildren examined, 46.2% of the girls and 35.1% of the boys had telangiectasias on the nape of the neck; 1% of these children had pronounced telangiectasias elsewhere; that is, on the shoulders, thorax, cheeks and ears. No mention was made of the occurrence of telangiectasias on the legs. An examination of 403 children in the former East Germany, aged 8–18 years, disclosed a 50% incidence of ‘very discrete’ venous abnormalities of leg veins. Of the children examined, 15% had clear symptoms (without visible varices) that could be assigned to a prospective varicose disease. Only between the ages of 17 and 18 years could reticular varicose veins be identified. In the 403 children studied by venous Doppler, 2.3% had incompetent communicating veins and 3.2% had an incompetent saphenofemoral junction.65 An epidemiologic study of 419 Czechoslovakian children aged 8 to 13 years, with clinical examination plus digital photoplethysmography, showed clinically apparent varices in 8.7% and an impairment of venous function in 14.3% of the examined extremities.66
The most recent and complete studies of 518 children aged 10 to 20 years, using photoplethysmography in addition to venous Doppler, are the Bochum I, II, and III studies. This continuing investigation initially demonstrated a 10.2% incidence of reticular varicose veins without other venous abnormalities in children who were 10 to 12 years of age.67 When these children were examined 4 years later, the incidence of reticular veins was 30.3%; 2% of the children had developed varicose veins and 4% had developed incompetent communicating veins. When they were examined another 4 years later, the frequency of saphenous refluxes and large varices was still increasing (19.8% saphenous refluxes, 3.3% truncal varices, 5% tributary varices, and 5.2% incompetent perforators). The incidence of reticular veins increased to 35.5%, and the incidence of telangiectasia increased to 12.9%.68 Therefore, venous disease can be demonstrated in a small number of children, its progression can be documented, and saphenous reflux is a risk factor for the development of truncal varices.
Symptoms
Varicose veins may be symptomatic in addition to being large and unsightly (Box 2.3). A study of 4280 town and country inhabitants of Tübingen, Germany, found symptoms in 98% of patients with clinically relevant venous alterations.69 One American health survey found that nearly 50% of those with varicose veins were bothered by their symptoms once in a while, and 18% noted frequent to continuous symptoms.70 Galen71 described the symptoms of varicose veins as ‘a heavy and depressing pain’. Pain is related most likely to pressure on the dense network of somatic nerve fibers present in the subcutaneous tissues adjacent to the affected nerve. Alternatively (or in addition), pain may occur from the dilated vein compressing adjacent nerves or from lactic acid accumulation that results from retrograde, circular, or slower venous blood flow clearance. Symptoms may precede the clinical appearance of the varicosity and are proportional to the presence of intermittent edema. At this point, discomfort usually occurs during warm temperatures when the patient is standing for prolonged periods.72 These patients may respond to systemic hydroxyrutosides, which decrease inflammation of the vein wall.73–75 Paroven – a mixture that mainly consists of mono-, di-, tri-, and tetra-O-β-hydroxyethyl rutosides containing at least 45% troxerutin (Zyma, United Kingdom) – 250 mg three or four times a day has been reported to help.76 Reduction in venous hypertension or excision of the involved segment usually causes prompt relief of pain.4
Almost all patients with postthrombotic obstruction complain of ‘bursting’ calf pain that is exacerbated by exercise.77 Venous claudication is an appropriate term to use in this situation to emphasize the relationship of exercise to pain. Pain during exercise may be due to nociceptor stimulation of the distended vein wall.78 Alternatively, exercise pain may be due to an accumulation of tissue metabolites or an increase in interstitial pressure. Patients with acute DVT have an increased intramuscular pressure that is proportional to the degree of thrombosis.79,80
Kistner81 has found that perforator incompetence leads to indurated skin with ulceration, whereas incompetence of the tibial, popliteal, or femoral system produces aching and swelling in the leg. There are patients who have a significant amount of valvular incompetence without symptoms. Clinical symptoms vary based on the effectiveness of the calf muscle pump to compensate for venous hypertension. Young, thin, and more athletic patients have fewer symptoms than older and obese patients.
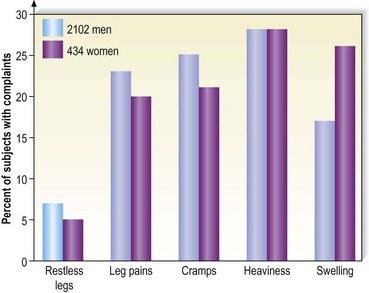
Varicose vein pain is described usually as a dull aching of the legs, particularly after prolonged standing or during certain times of the menstrual cycle (especially during menses).82 A small number of women also experience painful varicose veins after sexual intercourse.83 It is proposed that the increase in venous pressure with distension of the varicose vein contributes to heaviness and tightness of the lower legs.4,84 The Basel study III found a similar range of complaints in both men and women (Fig. 2.8) and an increased incidence of complaints among women and older patients.5 Many patients in the Basel study III had complaints without evidence of peripheral vascular disease. Strandness and Thiele4 suggest that symptoms specifically caused by varicose veins improve with aging as the level of activity declines.
Symptoms in varicose veins are often disproportionate to the amount of actual pathologic change. Patients with small, early-stage varices may complain more than those with large, longstanding varicosities.85–89 A survey of 350 patients who presented for sclerotherapy treatment of veins that were less than 1 mm in diameter noted that 53% of these patients complained of swelling, burning, throbbing and cramping of the legs, in addition to a ‘tired feeling’.90 A retrospective analysis of 401 patients who sought treatment solely for telangiectasia showed 69% with various symptoms, including pain, cramping, burning, throbbing and heaviness.91 These symptoms are so insidious that most patients fail to realize how good their legs can feel until after treatment of these blood vessels by either compression sclerotherapy or by wearing lightweight (20 mmHg) graduated compression stockings.59 Patients with nonsaphenous reflux had twice the incidence of painful legs (43%) than did patients with saphenous reflux (22%).92
The increased incidence of symptomatic varicose veins in women may have a hormonal etiology. It has been estimated that 27.7% of women with varicose veins have premenstrual pain in their varices.93 Varicose veins during pregnancy appear to be more symptomatic than those unassociated with pregnancy. In a study of 150 pregnant women with varicose veins, 125 noted pain and 26 were unable to stand for more than 1 to 2 hours because of the pain.94
The differential diagnosis of leg pain is extensive and not necessarily attributable to a patient’s varicosities (Box 2.4). Symptoms derived from varicose veins can usually be distinguished from arterial symptoms. Pain associated with arterial diseases often disappears at rest and is exacerbated with walking. Pain associated with varicosities is dull, vague and localized on the medial side of the legs. It is usually relieved with walking. In addition to the dull aching, varicose veins associated with venous hypertension may produce cramping or painful spasms of the legs and an increase in leg fatigue and restless legs, especially at night. Unfortunately, a patient’s perception and reporting of pain are subjective in nature and difficult to document. Browse et al95 advocate the use of compression hosiery as a diagnostic test to determine if the pain is of venous origin. The authors have found that if a patient’s symptoms are alleviated with 20 mmHg graduated compression stockings, sclerotherapy treatment also alleviates the symptoms.
In a study that compared groups matched by age as well as gender, it was found that subjects with venous disease were markedly symptomatic compared with control individuals. Symptoms specific to venous disease were found to correlate with the presence of both small vein and large vein disease. Vein size did not predict the presence or severity of symptoms.96 A study of 1366 people in Edinburgh, Scotland, found that women were more likely than men to report a wide range of lower limb symptoms.97 In men, only itching was significantly related to the presence of varicose veins. In women, there was a significant relation between varicose veins and heaviness or tension, aching and itching.
Using the Aberdeen varicose vein questionnaire, a prospective cohort of 137 patients was studied and the results compared with the well-accepted short form (SF-536). The two questionnaires correlated highly. Both showed the patients having worse health preoperatively than postoperatively. After surgery, all domains of health were improved and reached significance, chiefly in mental health. The conclusion of the authors of this study was that persons with varicose veins have a reduced quality of life (QOL) compared with the general population and that this discrepancy is significantly improved at 6 weeks after surgery.98
Two recent QOL studies were conducted on people with varicose veins. An analysis of 5688 consecutive outpatients in Belgium, Canada, France, and Italy found that the QOL in patients with varicose veins is associated with concomitant venous disease, rather than the presence of varicose veins per se.99 Sixty-five percent of patients with varicose veins also have concomitant venous diseases such as edema, skin changes or ulcers. A study of 2404 employees of the University of California, San Diego Medical Center, also found an adverse effect on QOL in patients with CVD.100 The effect of venous disease is more on the functional scale (what a person can do) and does not seem to affect the well-being aspects (how a person feels). Thus, venous disease is more than simply a cosmetic problem to most patients.
Signs
In addition to the direct symptoms of varicose and telangiectatic veins, varicose veins may be a cutaneous marker for venous insufficiency, where they occur in more than 50% of patients (Box 2.5).101–104 Certainly, deep venous insufficiency as a result of valvular incompetence is the major etiologic factor for the cutaneous manifestations of chronic venous insufficiency. In fact, when descending venography was used to examine patients with chronic venous insufficiency, reflux occurred in the superficial system alone in only 2% of the 644 examined limbs. Eighteen percent of the limbs had combined deep and superficial reflux.105 Studies using ascending venography have shown that more than 20% of patients with chronic venous insufficiency have isolated perforator incompetence as the only demonstrable abnormality.106 However, a significant group of patients has superficial venous insufficiency alone (13–38%) or in combination with deep venous insufficiency (28–78%).104,107–112 In addition, Walsh et al113 found that treating the incompetent superficial venous system with ligation and stripping the GSV to the knee with stab avulsion of distal varices restored competency to the femoral vein. Therefore, identification of patients with superficial venous insufficiency is important because they may respond to sclerotherapy or surgical treatment of the superficial venous system alone.
It has been estimated that between 17% and 50% of the population with varicose veins have cutaneous findings.2,114 Approximately 70% of limbs with chronic venous insufficiency have clinical findings.108 There is a strong association between the severity of clinical signs (described later in this chapter) and superficial venous incompetence.108 Almost all patients with cutaneous abnormalities have incompetence of perforator veins and all patients with either active or healed venous ulcerations have evidence of perforator incompetence.114 The cutaneous manifestation appears as edema, hyperpigmentation, dermatitis or ulceration. More than a million Americans suffer from skin ulcers caused by abnormal circulation that results from varicose veins, and nearly 100,000 Americans are totally disabled by this condition.57 Thus, varicose and telangiectatic leg veins are not merely of cosmetic concern but represent a widespread and potentially serious medical problem.
Edema
Ankle edema is usually the first manifestation of chronic venous insufficiency.115 Ankle edema tends to be worse in warm weather2 and towards the end of the day.59 It is especially common in persons who stand a great deal.59 True ‘pitting’ edema is rare,85 perhaps resulting from increased dermal fibrosis present in lipodermatosclerosis. The edema usually found is restricted to a limited area drained by capillaries that empty directly into the varicose or incompetent perforating veins.116 This area has been termed the gaiter area and refers to the ankle and lower calf. (In the 1800s it was commonly covered by a cloth or leather material (gaiter) to protect the ankle and instep from the environmental elements. Such protection is still used today by cross-country skiers.) Ankle edema caused by venous hypertension and varicose veins must be differentiated from that caused by other conditions (Box 2.6). However, as previously described, lymphatic edema may also be present in patients with chronic venous leg ulcers.117
The incidence of leg edema may not be related to the extent of varicose vein disease. A statistical study of 9100 civil servants in the German cities of Düsseldorf and Essen disclosed a statistically significant increase only in leg swelling in those with leg veins less than 1 mm in diameter compared to in those without such veins.118 There was no difference in muscle cramps, restless legs, and itching. Pain was not evaluated.
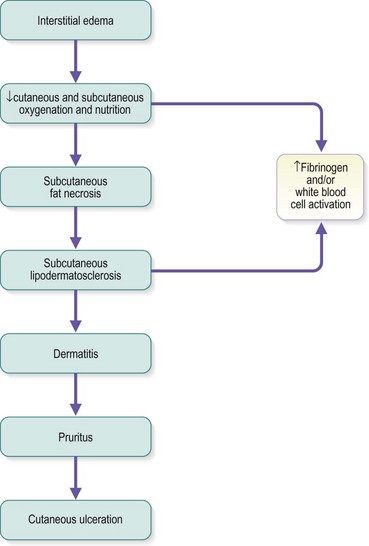
The protein-rich edema fluid stimulates fibroblastic activity, which entangles blood vessels and lymphatics into a fibrous mass.119 Histologically, a microedema around capillaries is seen.120 The edema, which contains fibrin, proteins and neutral polysaccharides, is probably the main reason for the lack of nutrition of the skin.121,122 The resulting lymphedema and the hypertrophy of the skin and subcutaneous tissues disrupt the flow of cutaneous nutrition. In women with venous stasis and fat, hairless, erythrocyanoid-type ankles, the resulting decrease in nutrition for the sizable fatty subcutaneous tissue and the decrease in local tissue oxygenation may cause sudden and massive fat necrosis of the subcutaneous tissue.2,15 The affected area may then appear erythematous, indurated, and tender to the touch.
Guex et al have correlated ankle circumference, symptoms and quality of life scores in 1036 patients with venous symptoms.123 They demonstrated the relevance of moderate ankle swelling to be secondary to chronic venous insufficiency.
Treatment of ankle edema caused by increased venous pressure, with or without lipodermatosclerosis, is directed primarily towards the prevention of trauma and alleviation of superficial venous hypertension.19 Temporizing treatments include leg elevation, systemic diuretics and localized compression bandaging.19 Graduated compression bandaging can normalize lymphatic flow over time. Thus this ‘conservative’ form of treatment is actually therapeutic as well (see Chapter 6). Recently, fibrinolytic enhancement has reportedly been successful in improving symptoms, induration and cutaneous thickening in patients with lipodermatosclerosis.124 The authors of that report used stanozolol (5 mg by mouth, twice daily) in 14 patients with longstanding lipodermatosclerosis resulting from venous disease. Of these 14 patients, 11 noted improvement within 3 months. Long-term follow-up was not given, nor has this treatment been accepted elsewhere.
Pigmentation
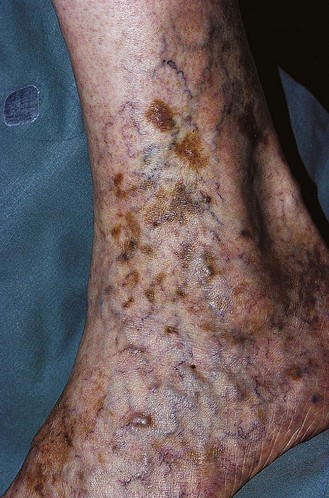
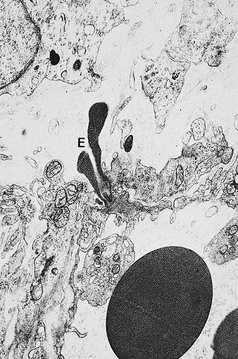
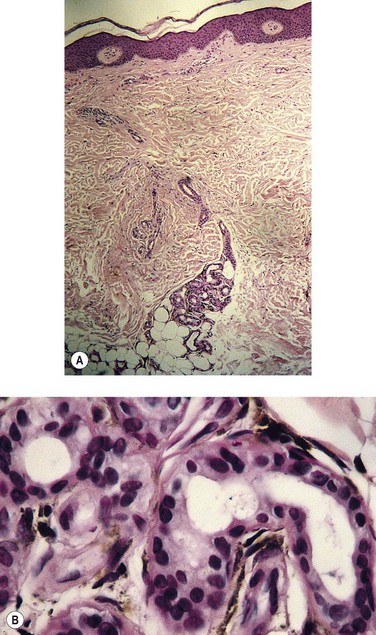
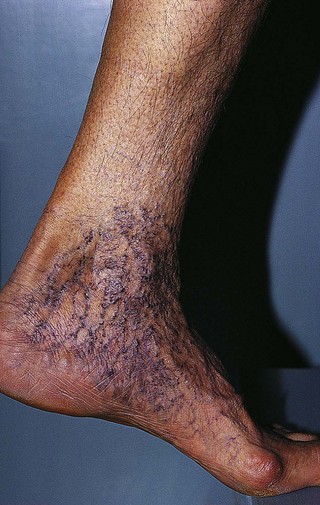
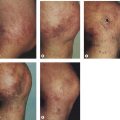
Pigmentation (Fig. 2.9) is a sign of venous stasis disease. The elongated, distended vascular system underlying areas of stasis is more susceptible to trauma than are normal vessels. Even minor blunt injuries may cause rupture of the vascular wall with extravasation of erythrocytes into the cutis.12,18,119 Histologically, cutaneous hyperpigmentation represents increased melanin early on and extravasated erythrocytes and hemosiderin-laden macrophages interspersed between dilated and tortuous capillaries in later stages and in inflammatory stages.12,125,126 Erythrocytes appear either intact or to have become fragmented during their passage (Fig. 2.10). Extravasation appears to be due to increased intravascular pressure and not chemotaxis, as occurs with white blood cells.125
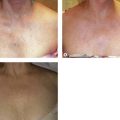
Extravasated erythrocytes may be found in the deep dermis around adnexal structures (Fig 2.11). When present clinically, they are a reliable guide to the existence of microangiopathy. A more acute ‘eruptive’ cutaneous pigmentation has also been noted as a ‘blow-out’ of erythrocytes into the dermis as a result of tremendous back pressure within the cutaneous microvasculature.127 This phenomenon has been ascribed as the cause of lichen aureus (Fig. 2.12).128 The only treatment for this condition is correction of the underlying venous hypertension (sclerotherapy and/or surgery), including graduated compression stockings and leg elevation. Fortunately, when venous hypertension is treated, dark pigmentation gradually fades. After correction of the etiology for the venous hypertension, a Q-switched laser specific for hemosiderin can then be used to remove or minimize the pigmentation (see Chapter 8). In addition, a noncoherent intense pulsed light source has also been demonstrated to lighten pigmentation in one case report.129
Venous (stasis) dermatitis
The next dermatologic manifestation to occur in the chain of events following venous hypertension–stasis is ‘stasis’ dermatitis. This dermatitis has been given many names, including stasis eczema, varicose eczema, stasis syndrome, hypostatic eczema, congestive eczema and dermatitis hypostatica. Because there may not be a true ‘stasis’ in the lower leg with venous insufficiency, but rather venous hypertension, this condition is best referred to as ‘venous’ dermatitis. Venous dermatitis occurs more frequently in women, obese people, middle-aged men and women and those with a history of DVT and thrombophlebitis.3 Edema, which causes the change from the normal cutaneous venous circulation to a high pressure system with increased vascular permeability, is the precursor of this dermatitis.128–132
A random sample of 476 people over the age of 65 years in Sheffield, England, found an incidence of venous dermatitis of 21% in men and 25% in women.133 In Denmark, the incidence in a random sample of people in homes for the aged (55–106 years old; mean age, 80 years) was 6.9%.134 The authors speculated that the difference was due to better nutrition in the Danish population. A similar incidence of venous dermatitis (5.9%) was found in a randomized examination of noninstitutionalized volunteers aged 50 to 91 years (average age, 74 years) in Boston, Massachusetts.135 Interestingly, a survey of dermatologic patients in the Filipino population aged 60 years and over disclosed a 12% incidence of venous dermatitis, with 49% of all patients having varicosities.62 This is contrary to the popular belief (see Chapter 3) that there is a lower incidence of venous disease in non-Western races.
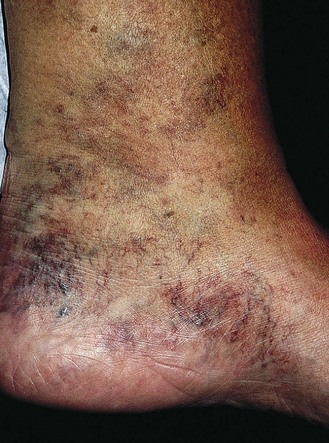
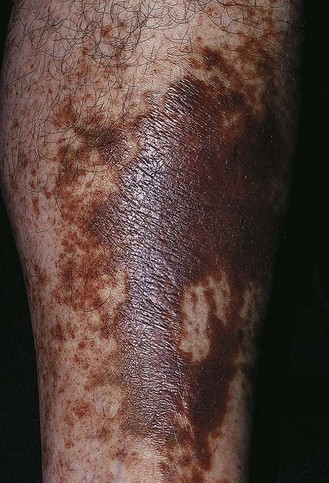
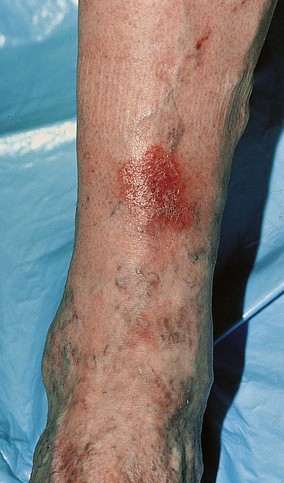
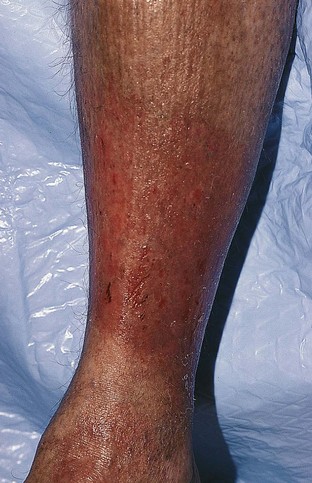
The dermatitis usually begins in the medial paramalleolar region. This region is particularly vulnerable because the vascular supply, skin nutrition and subcutaneous tissue are less abundant here than in other areas of the lower extremity.136 Venous dermatitis appears clinically as a sharply marginated, erythematous, crusted plaque (Fig. 2.13). With time and cutaneous trauma resulting from pruritus, overlying lichenification may occur,3 as well as exudation, depending on the extent of the inflammation and associated edema. However, an indolent venous flow may make the skin assume a much paler color with less moisture.137 The color of the lesion darkens as a result of an increase in melanocytic postinflammatory hyperpigmentation and dermal hemosiderin (Fig. 2.14).2,138 The dermatitis may also be complicated by a generalized systemic hypersensitivity or ‘ID’ reaction.2
Venous dermatitis may manifest as a solitary lesion in 7% of patients and may mimic neoplasms including squamous cell carcinoma and basal cell carcinoma.139 Early recognition of the solitary venous dermatitis lesion should lead to appropriate treatment of venous hypertension, which may prevent further morbidity.
Recently, an association of venous dermatitis with amlodipine (a long-acting calcium channel blocker used in the treatment of hypertension, chronic stable angina and vasospastic angina) has been reported.140 It is thought that amlodipine use may predispose those with venous insufficiency to leg edema and venous hypertension. Therefore, it is prudent to question patients with new onset stasis dermatitis about medication use.
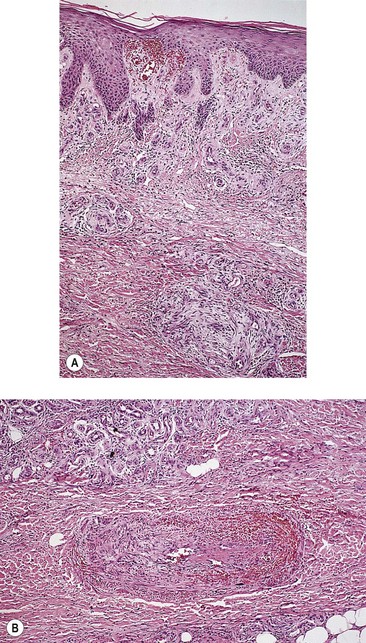
Histologic examination (Fig. 2.15) of the dermis demonstrates a diffuse homogenization of collagen, fragmentation or absence of elastic fibers, thickening and partial occlusion of arterioles, and atrophic changes of appendages.12,141 There may also be an associated acanthosis and hyperkeratosis of the epidermis, which rarely results in pseudoepitheliomatous hyperplasia.3 The lymphatic vessels are usually thickened and fibrotic, and there may be an associated dermal inflammatory infiltrate.3 Because these changes are those of a nonspecific dermatitis, the histologic diagnosis of venous dermatitis can be certain only with clinical correlation.
Other conditions can give the appearance of venous dermatitis and should be ruled out; three cases of myelogenous leukemia cutis have been reported which appeared to be venous dermatitis.142–144 Skin biopsy made the correct diagnosis in these cases.
Atrophie blanche
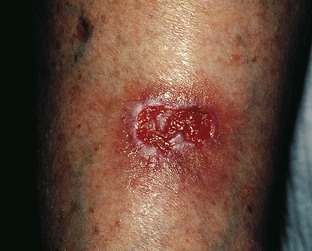
Atrophie blanche is the descriptive name given to the appearance of porcelain-white scars seen on the lower extremities as a result of infarctive lesions of the skin (Fig. 2.16). This condition was attributed originally to syphilis or tuberculosis in 1929.145 The white plaques are bordered by hyperpigmentation and telangiectasias. Histologically, meandering capillaries are detected at the border of lesions, with their apex oriented towards the avascular center.146
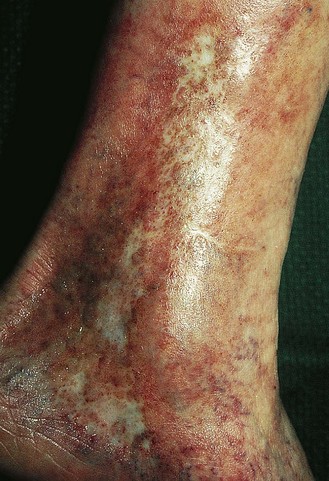
Figure 2.16 Middle-aged woman with venous insufficiency and atrophie blanche of the medial malleolar area.
(Courtesy Kim Butterwick, MD)
Patients with atrophie blanche have been compared with patients having severe venous insufficiency but without atrophie blanche. In turn, these have been compared with 10 healthy controls. Laser Doppler perfusion imaging was used, as were transcutaneous oxygen tension measurements. The overall results showed that resting blood perfusion was greater in atrophie blanche areas than in healthy controls, and the venoarterial response was significantly increased in these atrophie blanche areas. In contrast, there was a decrease in transcutaneous oxygen pressure values in areas of atrophie blanche lesions and in the skin of patients with chronic venous insufficiency without atrophie blanche. The authors of this study concluded that basic resting flow in atrophie blanche is higher compared to that in normal skin and in patients with chronic venous incompetence, but there is also marked decrease in flow in response to venous occlusion in these affected areas.147 In addition to treating venous hypertension, treatment with antifibrinolytics (i.e. aspirin, dipyridamole); anti-inflammatory agents (i.e. as dapsone) and pentoxifylline have demonstrated to be helpful especially in patients with idiopathic forms.148
Ulceration
Cutaneous ulceration represents the end-stage manifestation of venous stasis disease. This relationship has been noted for millennia. Hippocrates was the first to record the association.149 More than 300 years ago, Wiseman150 noted that valvular incompetence caused by venous thrombosis could result in a circulatory defect leading to ulceration of the skin.
The prevalence of varicose or post-thrombophlebotic ulcers has been estimated to be as high as 1% of the United States population and 2% of the Swedish population (Fig. 2.17).3,151–153 Although venous ulcers may have an onset in early adulthood, they increase in frequency with age and peak at approximately 70 years.152–155 The ratio of females to males is approximately 3 : 1 after the age of 40, with an equal incidence before 40 years of age.154
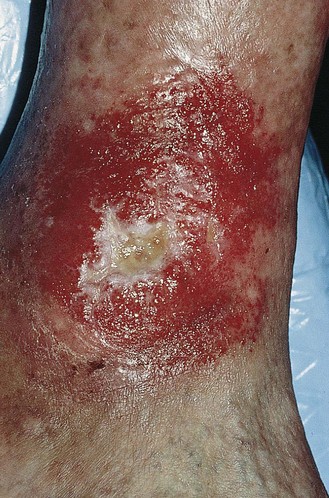
Figure 2.17 Chronic venous insufficiency with cutaneous ulceration in a 68-year-old man before treatment.
Seventy-two different causes of leg ulcers have been recognized and grouped into three categories.155 Between 75% and 90% are of venous etiology,3,156,157 40% to 60% of which are associated with varicose veins,157–159 and 35% to 90% are associated with a history of DVT.3,157–161 Nonvenous causes of leg ulceration include arterial disease (8%) and ulcers caused by trauma or those that have bacteriologic, mycotic, hematologic, neoplastic, neurologic or systemic origins (2%).156
The cost of treating leg ulcers in the United States was estimated in 1991 to be between $775 million and $1 billion, based on the annual cost of ulcer care in Sweden.162 In addition to this cost, there is an estimated loss of 2 million working days annually in the United States because of leg ulcers.163 Therefore, optimizing treatment, or better yet, prevention, is important. Curiously, despite its importance, very little government funding is allocated towards ulcer treatment. Patients diagnosed with leg ulcers in the United States spend, on average, 12.1 days in hospital.164 Because leg ulcers frequently take much longer to heal with bed rest and ancillary care, this form of therapy is impractical and not properly reimbursable.
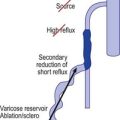
Color duplex investigation of limbs with reflux and ulceration has shown that distal venous reflux has an important influence over skin changes and ulceration, and reflux in the superficial veins appears to be more harmful than that confined to the deep veins even when such deep venous reflux extends through the length of the limb.165 Reflux in the local area near the ulcer also influences ulceration. Local reflux may be influenced by perforating vein incompetence and outward flow. Isolated perforating vein outward flow without accompanying superficial reflux or deep reflux is seen in 4% to 6% of limbs with ulceration.166 There is much controversy about the role of surgery in treating such reflux. However, most of the available data suggest that ablation of superficial venous reflux and ablation of outward flow through perforating veins is an appropriate method for the management of patients with primary venous leg ulceration.167
Between 20% and 25% of ulcerations have superficial venous insufficiency, either alone with perforating vein incompetence or as a significant component combined with deep venous insufficiency.94,98,99,168,169 In one practice of more than 20,000 lifetime patients, it was estimated that nearly 15% of patients with major varicose veins developed ulcerations.6 Although ulcerations are more common in patients with DVT, 13% of all venous ulcerations are observed in limbs with superficial venous insufficiency alone.
Cutaneous ulceration usually occurs 10 to 35 years (mean, 24 years) after the onset of varicose veins170 and is commonly assumed to be specifically associated with incompetent calf perforating veins (see Chapter 9).15,171–173 Dodd and Cockett15 surgically explored 135 limbs with ankle ulceration and found that the most severe lesions were always associated with an incompetent perforating vein. Lawrence et al172 studied patients with varicosities, both with and without associated ulceration, using Doppler ultrasound. They found sustained retrograde flow in incompetent veins in eight of nine ulcer patients but found it in only one of seven patients with varicose veins without ulceration. However, a recent study using duplex sonography showed no direct correlation between incompetent perforators and venous ulceration. Plethysmographic examination indicated that venous hypertension in superficial veins was the more important factor.174 One study of 213 consecutive patients with venous ulceration demonstrated that 90% of patients had sustained ulcer healing (with a mean follow-up period of 3.4 years) when treated with saphenous ligation alone, without perforating vein treatment, even when incompetence of the perforating veins had been demonstrated.175 Thus, any operative procedure on varicose ulcers must correct the underlying abnormal communicating superficial or perforating veins. Interestingly, no evidence or history of DVT was reported in up to 24% of patients with chronic venous leg ulcers;159,176 therefore, the etiology may be multifactorial, with the majority of patients having a similar initiating event: superficial venous hypertension. This may arise from incompetent perforating veins alone, associated with an abnormal deep venous system, or with an incompetent superficial venous system.177,178
Stasis ulcerations, unlike most other causes of cutaneous ulcerations, appear in the gaiter area.159,179 The ulcers appear cyanotic, edematous, and friable. The base is usually covered with thick granulation tissue that rarely penetrates the deep fascia. The skin edges are painless, thickened and bleed easily. Adjacent skin is edematous and inflamed, with associated dilated venules, eczematous changes and pigmentation.161,180 Calcification of the subcutaneous tissue, often not even adjacent to the ulceration, occurs in a significant number of patients,130,181,182 up to 25% in one study (Fig. 2.18).183 The calcium, acting as a foreign body, may perpetuate the ulceration or actually may be an essential cause of the lesion. Calcification is probably caused by venous insufficiency and represents the last stage of the inflammatory response. It almost always precedes the ulceration.130
In contrast to venous stasis ulceration, ischemic ulcers occur most commonly on the anterior and/or lateral leg and ankle. However, lateral ankle ulcerations may arise from incompetent small saphenous veins.184 The base of an ischemic ulcer is often obscured by a pale yellow, purulent exudate. Often the borders are poorly epithelialized, with a ‘punched-out’ appearance, and are necrotic with islands of gangrenous skin. Deep fascia and tendon may be exposed at the base, with little or no spontaneous granulation tissue.
Leg ulcers can also have multiple causes. Two case reports of mixed skin ulcers misdiagnosed as pyoderma gangrenosum and rheumatoid ulcer successfully treated with ultrasound-guided injection of polidocanol microfoam has recently been reported.185
Malignant degeneration
A potentially fatal, but fortunately rare, secondary change in venous ulcers is malignant degeneration (Fig. 2.19). There have been more than 100 case reports of malignant degeneration of a stasis ulcer in the world literature.173,186–194
The most common cancers reported are carcinomas (squamous and basal cell) and sarcomas (fibrosarcoma, osteosarcoma and angiosarcoma). Even malignant melanoma has been reported to occur in chronic venous ulceration.195 The incidence of malignant degeneration of venous stasis ulcerations is 0.4% to 1%.193,196–198 The average duration of the ulcer before tumor growth is 21 years, with a reported span of 10 to 40 years.198 The onset of malignant change usually appears as a rapid growth of exuberant cauliflower-like masses, an increase in pain, or, in a smaller number of cases, a rapid extension of the ulcer crater.198 An increase in induration of the ulcer borders and surrounding tissue and a failure of the ulcer to respond to prolonged conservative treatment are also suspect.199 Transition into a malignant growth is thought to be stimulated by many factors, including chronic dermatitis, irritation and infection.199,200 Implanted epithelial cells may produce a chronic foreign-body reaction with subsequent neoplasia.201 Chronic scarring from the sequelae of ulceration mentioned above may obliterate lymphatic channels, leading to decreased immune surveillance of the scar by immunologically competent cells. This relatively localized immune deficiency provides less protection against cellular mutation, which allows cellular progression into neoplasia.202 Therefore it seems prudent to perform a biopsy of the base and border areas of ulcers with these characteristics or of ulcers that persist for more than 4 months. This is particularly important when surgically correcting the ulceration with a skin graft. One patient developed a squamous cell carcinoma following split skin grafting and, in spite of amputation and radiotherapy, died from multiple metastasis.173
Although the appearance of malignant degeneration in a nonhealing leg ulcer is often characteristic, basal cell carcinoma in the ulcer may either appear as exuberant and translucent ‘granulation tissue’ or may have no clinical features to suggest malignancy.188,191,203
A study of squamous cell carcinomas complicating chronic venous leg ulcer has revealed some interesting facts. The mean age at cancer diagnosis was 78.5 years; the median survival was 1 year. Of these tumors, 11 were well differentiated, 10 moderately differentiated, and 4 were poorly differentiated. All patients with poorly differentiated tumors died within 1 year. Metastases were certain in 8 cases.204 The disease was lethal in 10 cases, which included all of the poorly differentiated tumors. This suggests that when squamous cell carcinoma in chronic leg ulcers is found, a thorough investigation must include the degree of differentiation and a definition of extent of spread. Aggressive treatment is indicated because poorly differentiated tumors and some moderately differentiated tumors are fatal.
Secondary complications of venous hypertension–stasis
Hemorrhage
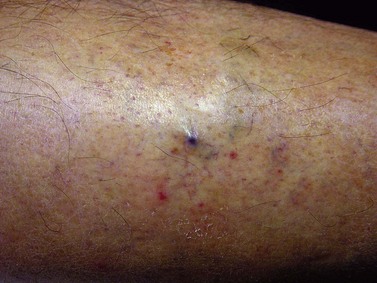
Hemorrhage from varicose veins may not be a rare event. Tretbar205 reported treating 12 patients in 3 years for 18 episodes of hemorrhagic varicose veins. All but two of his patients had had varicose veins for more than 20 years. The bleeding area typically consisted of a mat of ‘blue blebs’, each of 1 to 2 mm in diameter, on the medial ankle. Doppler examination usually disclosed an underlying incompetent communicating vein. None of Tretbar’s patients developed serious sequelae from the bleeding episodes and all were treated successfully with compression sclerotherapy of the affected veins. However, bleeding can be profuse and, if unnoticed or improperly treated, can be fatal.15,206–208 A report on the mortality of varicose veins from Australia between 1997 and 2000 disclosed 51 deaths where varicose veins were indicated to be the primary cause of death.209 In one-third of these cases, hemorrhage was the cause.
Hemorrhage is usually spontaneous but may also occur when the skin overlying a varicose vein becomes traumatized or eroded. Most cases described in the literature occur in patients with ulcers overlying varices, but profuse bleeding may also occur from varicosities 1 to 2 mm in diameter (Fig. 2.20). Twenty-three fatal cases of hemorrhage were reported in England and Wales in 1971.206 The patients most at risk are solitary elderly patients with longstanding varicose veins. These patients usually live alone and are unable to apply pressure to the bleeding varix or to get help because of physical disabilities. Rarely, patients may have no history of longstanding varices or overlying ulcers. Hemorrhage in this setting is usually attributable to the rapid development of venous hypertension that occurs from DVT.210
Direct pressure over the area of hemorrhage stops the bleeding, and maintaining that pressure for 5 to 7 days allows complete healing of the epidermis over the area of the hemorrhage.211 It has been found that a suture of the area of hemorrhage, usually done in a hospital emergency department, causes venous ulceration. Direct suture, therefore, should be avoided.
Injection of potentially hemorrhagic veins is mandatory (Fig. 2.21). In these cases, usual aesthetic concerns do not apply and it is logical to inject the fragile venules at the very beginning of the treatment, prior to the necessary reduction of venous hypertension. Sclerosing injections of bleeding veins provide an elegant solution to the problem; provided the sclerosing agent induces a spasm (polidocanol, sodium tetradecyl sulfate), it stops the hemorrhage and usually prevents a recurrence. The leg should be elevated during injection, and higher than usual concentrations of sclerosing solution are often required. Foamed sclerosant may also help stop the bleeding more quickly.
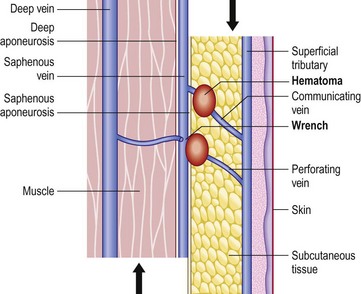
The ‘shear off’ phenomenon can explain ‘spontaneous’ acute pains of the calf, known in the French literature as ‘whip pains of the calf’. During a quick movement of the lower limb, inertia of muscle and fat tissues creates a relative displacement of the different anatomical layers (Fig. 2.22) responsible for wrench of communicating or perforating veins, resulting in pain, hematoma and ecchymosis. This atraumatic lesion is more common on varicose veins because of the venous wall remodeling and dysplasia, and because of venous hypertension. Duplex ultrasound shows edema and a small hematoma, and usually no sign of superficial thrombophlebitis. Local compression and massage with nonsteroidal anti-inflammatory cream is the sole treatment.
Superficial thrombophlebitis
Superficial thrombophlebitis (ST) is a painful condition that fortunately seldom results in serious embolic complications. In the absence of malignancy, thrombophlebitis of the leg is almost invariably associated with varicose veins.4,212–214 Patients with varicose vein ST are younger and have a decreased incidence of coexistent DVT (9.75% versus 43.75%).213 The condition results from the development of a clot in a varicose vein caused by one or more of the following factors: trauma to the varicosity, stasis of blood flow or occlusion of blood flow. Fifty percent of cases may occur spontaneously.212,215 An evaluation of 51 consecutive patients with venous thrombosis and varicose veins found 8% with an underlying malignancy, 7% with an antiphospholipid syndrome and a total of 26% with other systemic illnesses.216 Therefore it is recommended that a search for an underlying cause be made.
The great saphenous system is the usual site of ascending ST. Clinically, one notes a painful, tender, hot erythematous swelling along the course of the vein, with a variable amount of perivascular edema. The pain associated with ST is often severe, probably resulting from inflammation of the dense network of somatic nerve fibers in the associated subcutaneous tissue.4
Incidence of ST, irrespective of the presence of varicose veins, increases with advancing age and inactivity, and with bed rest as the result of surgery, childbirth or cardiac disease.217,218 In patients in whom the incidence of varicose veins is not stated, ST has been estimated to occur in 0.7% of women in their fourth decade of life, increasing to 2.6% of women in the seventh decade.57 In men, the incidence of ST has been estimated to be 0.4% in the fourth decade, increasing to 1.7% in the seventh decade.57 The actual number of patients with ST in the United States was estimated in 1973 to be 123,000 yearly. The incidence of ST is substantially higher when related to the presence of varicose veins.4 A review of the lifetime work of one physician with more than 20,000 patients notes an incidence of ST in as many as 20% of patients with prominent major varicosities.6 Older papers have estimated a 50% lifetime incidence of thrombophlebitis in patients with varicose veins.219 Fegan83 estimates that ST occurs in approximately 4% of those with varicose veins.
Although the condition is usually treated as a benign complication of varicose veins, the development of DVT, venous hypertension and pulmonary emboli may occur in a significant percentage of patients.212,215,220–224 A review of 340 cases of ST in a university hospital disclosed a 10% incidence of pulmonary emboli with five deaths,215 and this risk has been confirmed by others.225 The development of pulmonary emboli may also be related in some cases to a coexistent DVT.4,226,227 One study of 44 consecutive patients with ST found coexistent DVT in 23%. All of these cases were occult clinically, with the site of the ST not predictive of DVT.214 Thus, noninvasive deep venous studies are recommended for all patients with ST.
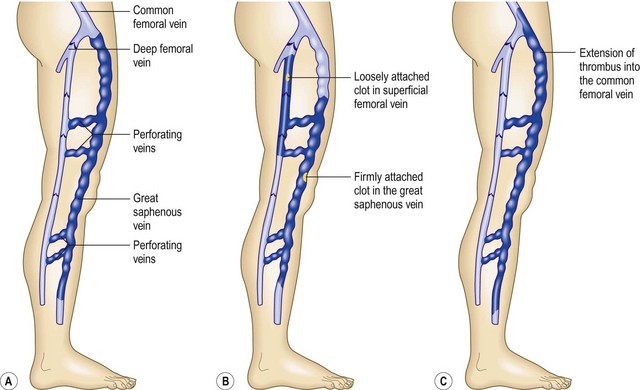
Pulmonary emboli and DVT, by definition, were supposed not to complicate ST unless the thrombus progressed into the deep venous system, but it has been observed that DVT can occur in other venous networks. This may happen because of either progression into a perforating vein or ascending involvement of the common femoral vein at the saphenofemoral junction (Fig. 2.23) or simply due to the presence of a hypercoagulable state.228 When either of these events occurs, superficial or deep venous hypertension develops as a result of valvular destruction.218 Propagation of the thrombotic process into the deep system has been reported to occur in 6%229 to 32%220 of all cases of ST. In an 11-year retrospective series, 17% of 133 patients were noted to have extension of the clot into the deep system.230 Surgical exploration of the saphenofemoral junction, followed by ligation, thrombectomy and limited vein stripping, has been advocated, particularly if clinical signs of thrombophlebitis reach the midthigh. We recommend full anticoagulation. Surgical removal of the thrombosed vein segments and associated varicosities shortens the convalescence and mitigates recurrences.229,231,232 Unfortunately, this latter form of treatment usually results in extensive scarring. Finally, because DVT may manifest partly in the appearance of ST, patients should be examined carefully.
Surgical treatment of ST has been dominant when the ST has affected the GSV and ascended towards the saphenofemoral junction. It is thought that the incidence of DVT is three times that of normal individuals, and, in the past, with ascending thrombophlebitis of the GSV, an operation under local anesthesia to ligate and divide the vein was recommended.233 However, gradually, anticoagulant treatment has dominated clinical practice. The advantage, of course, is that the ST is treated simultaneously by the anticoagulation, compression and rest. Nonsteroidal anti-inflammatory medications have been advocated but may have potentially severe side effects. Since ST is associated with a higher risk of DVT, and since ST of the GSV when ascending to the junction leads to progression of the clot into the femoral vein, the use of low molecular weight heparins must be considered – prophylactically for 2 weeks when the clot does not threaten the deep system, but therapeutic, like for a DVT, when it does.234 External elastic compression is recommended by most authorities on this subject.228
Deep venous thrombosis
Varicose veins, by virtue of their low blood flow, are considered a high risk factor for DVT.4 Without other predisposing factors, patients with varicose veins have an incidence of DVT ninefold that of the normal population.235 Platelet aggregation is thought to occur behind valve cusps, especially when the valves are incompetent in varicose veins.236,237 Thrombosis on the valve cusps then triggers the coagulation cascade and results in clot propagation.
Stasis of blood flow may cause activation of factors XII, XI, and IX, which initiates thrombin activity to propagate thrombus formation through fibrin formation and platelet aggregation.238 Stasis may also result in a significant amount of endothelial sloughing, with exposure of subendothelial collagen and subsequent activation of platelets.239 An additional reason for the propensity for DVT in patients with chronic venous insufficiency (which is commonly associated with the presence of varicose veins) may be related to a faulty fibrinolytic system, correlated with pericapillary deposition of fibrin.240 This hypothesis has been questioned because up to 70% of patients with idiopathic DVT have a decreased tissue plasminogen activator that probably reflects endothelial dysfunction and a decreased clearance of clotting factors.240–243
An increased incidence of DVT is also found in the postoperative period.244–248 This may be related to the increased incidence of thrombophlebitis in varicose veins in the postoperative period, with an incidence estimated at 6% versus the normal 0.5% to 0.7% incidence.249 This is of particular significance in patients less than 60 years of age. With fibrinogen scanning, DVT occurs in 56% of patients over 60 with varicose veins versus 41% in patients over 60 without varicose veins. This can be compared with 56% in patients younger than 60 years with varicose veins versus 19% in patients less than 60 without varicose veins.250 Therefore, all patients with varicose veins who are about to undergo surgery, or who are bedridden or pregnant, should receive thrombosis prophylaxis, such as wearing a graduated support stocking, to prevent this potentially fatal, albeit rare, complication of varicose veins.
In summary, varicose veins are associated with a number of serious medical problems and are not just of cosmetic concern. Basle study III5 found that the incidence of the major complications of varicose veins – chronic venous insufficiency, phlebitis and pulmonary embolism – increases with the severity of the varicosity (Fig. 2.24). Even patients with minor telangiectasias and reticular veins in combination demonstrated a significant increase of these serious medical complications when compared with patients without these types of veins.
Classification
A classification of varicose veins should be based on anatomic or subsequent therapeutic considerations. The first anatomic classification was proposed by Heyerdale and Stalker251 in 1941 (Table 2.1). This classification is useful in determining when surgical ligation of the GSV is advantageous before performing sclerotherapy. The list of advantages presented by Heyerdale and Stalker still holds true today (Box 2.7). The Basle study5 classified varicose veins into three groups:
Table 2.1 Classification of varicosities of the lower extremities
| Group | Varicosities | Saphenous System |
|---|---|---|
| 1 | Spider bursts; telangiectatic veins | Competent |
| 2 | Mild or moderate varicosities | Competent |
| 3 | Mild, moderate, or marked varicosities | Incompetent |
Box 2.1 CEAP classes of clinical state
From Heyerdale WW, Stalker LK: Ann Surg 114:1042, 1941.
Duffy252 proposed a more complete classification of ‘unwanted leg veins’. Since one purpose of a classification is to provide a mechanism for evaluating pathophysiology and treatment, a modification of the Duffy classification appears useful. It provides comprehensive clinical and therapeutic criteria in an effort to optimize treatment (Box 2.8 and Figs 2.25 to 2.30).
Box 2.8
Vessel classification
Modified from Duffy DM: Small vessel sclerotherapy: an overview. In Callen JP et al, editors: Advances in dermatology, vol 3, Chicago, 1988, year Book.
The development of varicose vein disease is generally progressive. Six different patterns of GSV varicosity have been described.114 These relate to the duration of varicose vein disease (Fig. 2.31). Certain patients may experience spontaneous stabilization of the disease in the early stages. Treatment of early-stage disease may prevent the progression and cause regression of the disease process. A complete understanding of the anatomy and pathophysiology of the venous system with regard to varicose veins allows the development of a rational treatment plan.
Figure 2.31 Patterns of great saphenous vein incompetence in 296 limbs with primary varicose veins.
(Modified from Almgren B, Eriksson I: Acta Chir Scand 156:69, 1990. © British Journal of Surgery Society Ltd. Reproduced with permission. Permission is granted by John Wiley & Sons Ltd on behalf of the BJSS Ltd.)
1 Baron HC. Varicose veins. Consultant. May 1983:108.
2 Hobbs JT. The problem of the post-thrombotic syndrome. Postgrad Med J. 1973;Aug suppl:48.
3 Beninson J, Livingood CS. Stasis dermatitis. In: Demis DJ, editor. Clinical dermatology, vol 2. Philadelphia: Harper & Row; 1985.
4 Strandness DEJr, Thiele BL. Selected topics in venous disorders. Mt Kisco, New York: Futura; 1981.
5 Widmer LK. Peripheral venous disorders: prevalence and socio-medical importance: observations in 4529 apparently healthy persons, Basle study III. Bern, Switzerland: Hans Huber; 1978.
6 Gallagher PG. Major contributing role of sclerotherapy in the treatment of varicose veins. J Vasc Surg. 1986;20:139.
7 Homans J. The operative treatment of varicose veins and ulcers, based upon a classification of these lesions. Surg Gynecol Obstet. 1916;22:143.
8 Haeger KHM, Bergman L. Skin temperature of normal and varicose veins and some reflections on the etiology of varicose veins. Angiology. 1963;14:473.
9 Linton R. The post-thrombotic ulceration of the lower extremities: its etiology and surgical treatment. Ann Surg. 1953;138:415.
10 Burnand KG, O’Donnell TFJr, Thomas ML, Browse NL. The relative importance of incompetent communicating veins in the production of varicose veins and venous ulcers. Surgery. 1977;82:9.
11 Warren R, White EA, Belcher CB. Venous pressure in the saphenous system in normal, varicose, and post-phlebitic extremities. Surgery. 1949;26:435.
12 Farber EM, Batts EE. Pathologic physiology of stasis dermatitis. Arch Dermatol. 1954;70:653.
13 Smith HG. Complicating factors in the surgical management of varicose veins. Surgery. 1945;17:590.
14 Cockett FB, Jones BE. The ankle blow-out syndrome: a new approach to the varicose ulcer problem. Lancet. 1953;261:17.
15 Dodd H, Cockett FB. The pathology and surgery of the veins of the lower limbs, 2nd ed. London: Churchill Livingstone; 1976.
16 Landis EM. Factors controlling the movement of fluid through the human capillary wall. Yale J Biol Med. 1933;5:201.
17 Burnand KG, Clemenson G, Whimster I, et al. The effect of sustained venous hypertension of the skin capillaries of the canine hind limb. Br J Surg. 1982;69:41.
18 Ryan TJ, Wilkinson DS. Diseases of the veins: venous leg ulcers. In Rook A, Wilkinson DS, Ebling FTG, editors: Textbook of dermatology, 3rd ed, London: Blackwell Scientific, 1979.
19 Ryan TJ. Diseases of the skin: management of varicose ulcers and eczema. Br Med J. 1974;1:192.
20 Whimster I. The pathology and surgery of the veins of the lower limbs. cited in Dodd H, Cockett FP. Churchill Livingstone, Edinburgh, 1956..
21 Van Bemmelen SP, Hoynck van Papendrecht AA, Hodde KC, Klopper PJ. A study of valve incompetence that developed in an experimental model of venous hypertension. Arch Surg. 1986;121:1048.
22 Nicolaides AN, Hussein MK, Szendro G. The relation of venous ulceration with ambulatory venous pressure measurements. J Vasc Surg. 1993;17:414.
23 Christopoulos DG, Nicolaides AN, Szendro G, et al. Air-plethysmography and the effect of elastic compression on hemodynamics of the leg. J Vasc Surg. 1987;5:148.
24 Saharay M, Shields DA, Porter JB, et al. Leukocyte activity in the microcirculation of the leg in patients with chronic venous disease. J Vasc Surg. 1997;25:265.
25 Saharay M, Shields DA, Georgiannos SN, et al. Endothelial activation in patients with chronic venous disease. Eur J Vasc Endovasc Surg. 1998;15:342.
26 Shields DA, Andaz SK, Abeysinghe RD, et al. Plasma lactoferrin as a marker of white cell degranulation in venous disease. Phlebology. 1994;9:55.
27 Shields DA, Andaz SK, Sarin S, et al. Plasma elastase in venous disease. Br J Surg. 1994;81:1496.
28 Ciuffetti G, Lombardini R, Pasqualini L, et al. Circulating leucocyte adhesion molecules in chronic venous insufficiency. Vasa. 1999;28:156.
29 Vanscheidt WQ, Kresse O, Hach-Wunderle V, et al. Leg ulcer patients: no decreased fibrinolytic response but white cell trapping after venous occlusion of the upper limb. Phlebology. 1992;7:92.
30 Takase S, Schmid-Schönbein G, Bergan JJ. Leukocyte activation in patients with venous insufficiency. J Vasc Surg. 1999;30:148.
31 Burnand KG, Whimster I, Clemenson G, et al. The relationship between the number of capillaries in the skin of the venous ulcer-bearing area of the lower leg and the fall in vein pressure during exercise. Br J Surg. 1981;68:297.
32 Junger M, Hahn U, Bort S, et al. Significance of cutaneous microangiopathy for the pathogenesis of dermatitis in venous congestion due to chronic venous insufficiency. Wien Med Wochenschr. 1994;144:206.
33 Bates DO, Curry FE. Vascular endothelial growth factor increases hydraulic conductivity of isolated perfused microvessels. Am J Physiol. 1996;271:H2520.
34 Shoab SS, Scurr JH, Coleridge Smith PD. Increased plasma endothelial growth factor among patients with chronic venous disease. J Vasc Surg. 1998;28:535.
35 Shoab SS, Scurr JH, Coleridge Smith PD. Plasma VEGF as a marker of therapy in patients with chronic venous disease treated with oral micronised flavonoid fraction – a pilot study. Eur J Endovasc Surg. 1999;18:334.
36 Pappas PJ, You R, Rameshwar P, et al. Dermal tissue fibrosis in patients with chronic venous insufficiency is associated with increased transforming growth factor β1 gene expression and protein production. J Vasc Surg. 1999;30:1129.
37 Sansilvestri-Morel P, Rupin A, Jaisson S, et al. Synthesis of collagen is dysregulated in cultured fibroblasts derived from skin of subjects with varicose veins as it is in venous smooth muscle cells. Circulation. 2002;106:479.
38 Peschen M, Grenz H, Grothe C, et al. Patterns of epidermal growth factor receptor, basic fibroblast growth factor and transforming growth factor-3 expression in skin with chronic venous insufficiency. Eur J Dermatol. 1998;8:334.
39 Jorizzo JL, White WL, Zanolli MD, et al. Sclerosing panniculitis: a clinicopathologic assessment. Arch Dermatol. 1991;127:554.
40 Fagrell B. Local microcirculation in chronic venous incompetence and leg ulcers. Vasc Surg. 1979;13:217.
41 Speiser DE, Bollinger A. Microangiopathy in mild chronic venous incompetence (CVI): morphological alterations and increased transcapillary diffusion detected by fluorescence video-microscopy. Int J Microcirc Clin Exp. 1991;10:55.
42 Labropoulos N, Leon M, Nicolaides AN, et al. Superficial venous insufficiency: correlation of anatomic extent of reflux with clinical symptoms and signs. J Vasc Surg. 1994;20:953.
43 Eklof B, Rutherford RB, Bergan JJ, et al. American Venous Forum International Ad Hoc Committee for Revision of the CEAP Classification: Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004;40:1248.
44 American Venous Forum. Classification and grading of chronic venous disease in the lower limbs. A consensus statement. Ad Hoc Committee, American Venous Forum. J Cardiovasc Surg (Torino). 1997;38:437.
45 Rutherford RB, Padberg FTJr, Comerota AJ, et al. Venous severity scoring: an adjunct to venous outcome assessment. J Vasc Surg. 2000;31:1307.
46 Rutherford RR, Moneta GL, Padberg FT, Meissner MH. Outcome assessment in chronic venous disease. In: Gloviczki P, editor. Handbook of venous disorders. 3rd ed. London: Hodder Arnold; 2009:684-693.
47 Vasquez MA, Munschauer CE. Venous clinical severity score and quality-of-life assessment tools: application to vein practice. Phlebology. 2008;23:259.
48 Revicki DA. Seven interesting things I’ve learned about health outcomes research. President’s award address. http://www.isoqol.org, 2007. available at
49 Wigal JK, Stout C, Kotse H, et al. Experimenter expectancy in resistance to respiratory air flow. Psychosom Med. 1997;59:318.
50 Ware JEJr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220.
51 Launois R, Reboul-Marty J, Henry B. Construction and validation of a quality of life questionnaire in chronic lower limb venous insufficiency (CIVIQ). Qual Life Res. 1996;5:539.
52 Guex JJ, Myon E, Didier L, et al. Chronic venous disease: health status of a population and care impact on this health status through quality of life questionnaires. Int Angiol. 2005;24:258.
53 Guex JJ, Zimmet SE, Boussetta S, et al. Construction and validation of a patient reported outcome dedicated to chronic venous disorders: SQOR-V (Specific Quality of Life & Outcome Response – Venous). J Mal Vasc. 2007;32:135.
54 Guex JJ, Zimmet SE, Boussetta S, et al. Patient-reported SQOR-V quality of life questionnaire in venous disorders. In: Preedy V, Watson RR, editors. Handbook of disease burdens and quality of life measures. Springer Verlag, 2009.
55 Pittaluga P, Chastanet S, Rea B, Barbe R. Classification of saphenous refluxes: implications for treatment. Phlebology. 2008;23:2.
56 Strandness DEJr. Varicose veins. In: Dermis DJ, editor. Clinical dermatology. Philadelphia: Harper & Row, 1985.
57 Coon WW, Willis PWIII, Keller JB. Venous thromboembolism and other venous disease in the Tecumseh community health study. Circulation. 1973;48:839.
58 Engel A, Johnson ML, Haynes SG. Health effects of sunlight exposure in the United States: results from the first national health and nutrition examination survey, 1971–1974. Arch Dermatol. 1988;124:72.
59 Dinn A, Henry M. Value of lightweight elastic tights in standing occupations. Phlebology. 1989;4:45.
60 Langer RD, Criqui MH, Denenberh J, Fronel A. The prevalence of venous disease by gender and ethnicity in a balanced sample of four ethnic groups in southern California. Phlebology. 2000;15:99.
61 Lake M, Pratt GH, Wright IS. Arteriosclerosis and varicose veins: occupational activities and other factors. JAMA. 1942;119:696.
62 Tianco EAV, Buendia-Teodosio G, Alberto NL. Survey of skin lesions in the Filipino elderly. Int J Dermatol. 1992;31:196.
63 Brand FN, Dannenberg AL, Abbott RD, Kannel WB. The epidemiology of varicose veins: the Framingham study. Am J Prevent Med. 1988;4:96.
64 Oster J, Nielsen A. Nuchal naevi and intrascapular telangiectasies. Acta Paediatr Scand. 1970;59:416.
65 Heede G. Prevaricose epidemiological symptoms in 8 to 18 aged pupils. In: Davy A, Stemmer R, editors. Phlébologie ’89. Montrouge, France: John Libbey Eurotext, 1989.
66 Strejcek J. The first experience with using digital photoplethysmography in epidemiological study of children (Bohemian Study I). Prakt Flebol. 1992;2:5.
67 Schultz-Ehrenburg U, Weindorf N, von Usler D, et al. Prospective epidemiological investigations on early and preclinical stages of varicosis. In: Davy A, Stemmer R, editors. Phlébologie ’89. Montrouge, France: John Libbey Eurotext, 1989.
68 Schultz-Ehrenburg U, Weindorf N, Matthes U, et al. New epidemiological findings with regard to initial stages of varicose veins (Bochum Study I-III). In: Raymond-Martimbeau P, Prescott R, Zummo M, editors. Phlébologie ’92. Paris: John Libbey Eurotext, 1992.
69 Fischer H. Socio-epidemiological study on distribution of venous disorders among a residential population. Int Angiol. 1984;3:89.
70 Wilder CS. Prevalence of selected chronic circulatory conditions. Vital Health Stat. 1974;94:1.
71 Galen. Galen, on the affected parts. Translated by Rudolph E Siegel. Basel, Switzerland: Karger; 1976.
72 Delater G, Hugel R. Apercu de pathologie veineuse le systeme veineux peripherique superficiel. Rev Gen Clin Ther. 1927;41:166.
73 Neumann HAM, van den Broek MJTB. Evaluation of O-(β-hydroxy-ethyl)-rutosides in chronic venous insufficiency by means of non-invasive techniques. Phlebology. 1990;5(Suppl 1):13.
74 De Jongste AB, Jonker JJC, Huisman MV, et al. A double-blind trial on the short-term efficacy of HR in patients with the post-thrombotic syndrome. Phlebology. 1990;5(Suppl 1):21.
75 Nocker W, Diebschlag W, Lehmacher W. Clinical trials of the dose-related effects of O-(β-hydroxyethyl)-rutosides in patients with chronic venous insufficiency. Phlebology. 1990;5(Suppl 1):23.
76 Conrad P. Painful legs: the GP’s dilemma. Aust Fam Physician. 1980;9:691.
77 Negus D. Calf pain in the post-thrombotic syndrome. Br Med J. 1968;2:156.
78 Cocket FB, Lea Thomas M. The iliac compression syndrome. Br J Surg. 1965;52:816.
79 Qvarfordt P, Eklof B, Ohlin P. Intramuscular pressure in the lower extremity in deep venous thrombosis and phlegmasia cerulea dolens. Ann Surg. 1983;197:450.
80 Qvarfordt P, Eklof B, Ohlin P, et al. Intramuscular pressure, blood flow and skeletal muscle metabolism in patients with venous claudication. Surgery. 1984;95:191.
81 Kistner RL. Primary venous valve incompetence of the leg. Am J Surg. 1980;140:218.
82 McPheeters HO. The value of oestrogen therapy in the treatment of varicose veins complicating pregnancy. Lancet. 1949;69:2.
83 Fegan G. Varicose veins. London: William Heinemann; 1967.
84 Summer DS. Hemodynamics and pathophysiology of venous disease. In: Rutherford RB, editor. Vascular surgery. Philadelphia: Saunders, 1984.
85 Lofgren KA. Varicose veins: their symptoms, complications, and management. Postgrad Med. 1979;65:131.
86 Colgan MP, Dormandy JA, Jones PW, et al. Oxypentifylline treatment of venous ulcers of the leg. Br Med J. 1990;300:972.
87 Allen JC. The micro-circulation of the skin of the normal leg in varicose veins and in the post-thrombotic syndrome. S Afr J Surg. 1972;10:29.
88 Sumner DS. Venous dynamics – varicosities. Clin Obstet Gynecol. 1981;24:743.
89 Burnand KG. Management of varicose veins of the legs. Nursing Mirror. 1977;144:45.
90 Weiss R, Weiss M. Resolution of pain associated with varicose and telangiectatic leg veins after compression sclerotherapy. J Dermatol Surg Oncol. 1990;16:333.
91 Murray RY. A retrospective analysis of varying symptoms in patients presenting for sclerotherapy of telangiectasia. J Dermatol Surg Oncol. 1994;20:70. (abstract)
92 Parish TD. Varicosis, aesthetics, and pain. Am J Cosmetic Surg. 1998;15:143.
93 Fegan WG, Lambe R, Henry M. Steroid hormones and varicose veins. Lancet. 1967;290:1070.
94 MacCausland AM. Varicose veins in pregnancy. Calif West Med. 1939;50:258.
95 Browse NL, Burnand KG, Thomas ML. Diseases of the veins: pathology, diagnosis, and treatment. London: Edward Arnold; 1988.
96 Isaacs MN. Symptomatology of vein disease. Dermatol Surg. 1995;21:321.
97 Bradbury A, Evans C, Allan P, et al. What are the symptoms of varicose veins? Edinburgh vein study cross sectional population survey. Br J Med. 1999;318:353.
98 Smith JJ, Garratt AM, Guest M, et al. Evaluating and improving health-related quality of life in patients with varicose veins. J Vasc Surg. 1999;30:710.
99 Kurz X, Lamping DL, Kahn SR, et al. Do varicose veins affect the quality of life? Results of an international population-based study. J Vasc Surg. 2001;34:641.
100 Kaplan RM, Criqui MH, Denenberg JO, et al. Quality of life in patients with chronic venous disease: San Diego population study. J Vasc Surg. 2003;37:1047.
101 Veal JR, Hossey H. The pathologic physiology of the circulation in the post-thrombotic syndrome. Am Heart J. 1942;23:390.
102 Gay J. On varicose disease of the lower extremities. London: J Churchill; 1868.
103 Pleuss J. Contribution a l’etude etiopathogenique des ulceres de jambe. Bull Soc Fr Phlebol. 1952;4:137.
104 McEnroe CS, O’Donnell TFJr, Mackey WC. Correlation of clinical findings with venous hemodynamics in 386 patients with chronic venous insufficiency. Am J Surg. 1988;156:148.
105 Morano JU, Raju S. Chronic venous insufficiency: assessment with descending venography. Radiology. 1990;174:441.
106 Train JS, Schanzer H, Peirce ECII, et al. Radiological evaluation of the chronic venous stasis syndrome. JAMA. 1987;258:941.
107 Darke SG, Andress MR. The value of venography in the management of chronic venous disorders of the lower limb. In: Greenhalgh RM, editor. Diagnostic techniques and assessment procedures in vascular surgery. London: Grune & Stratton, 1985.
108 Moore DJ, Himmel PD, Sumner DS. Distribution of venous valvular incompetence in patients with postphlebotic syndrome. J Vasc Surg. 1986;3:49.
109 Mastroroberto M, Chello M, Marchese AR. Distribution of valvular incompetence in patients with venous stasis ulceration. J Vasc Surg. 1992;14:307.
110 Hanrahan LM, Kechejian GJ, Cordts PR, et al. Patterns of venous insufficiency in patients with varicose veins. Arch Surg. 1991;126:687.
111 Schanzer H, Peirce EII. A rational approach to surgery of the chronic venous stasis syndrome. Ann Surg. 1982;195:25.
112 Raju S, Fredericks R. Evaluation of methods for detecting venous reflux. Arch Surg. 1990;125:1463.
113 Walsh JC, Bergan JJ, Beeman S, Comer T. Femoral venous reflux is abolished by greater saphenous vein stripping. J Dermatol Surg Oncol. 1994;20:65.
114 Almgren B, Eriksson I. Valvular incompetence in superficial, deep and perforator veins of the limbs with varicose veins. Acta Chir Scand. 1990;156:69.
115 Allen EV, Barker NW, Hines EAJr. Peripheral vascular diseases. Philadelphia: Saunders; 1946.
116 Hojensgard IC, Sturup H. On the function of the venous pump and the venous return from the lower limbs. Acta Derm Venerol Stockh. 1952;29(Suppl):169.
117 Bull RH, Gane JN, Evans JE, et al. Abnormal lymph drainage in patients with chronic venous leg ulcers. J Am Acad Dermatol. 1993;28:585.
118 Kroger K, Ose C, Rudofsky G, et al. Symptoms in individuals with small cutaneous veins. Vasc Med. 2002;7:13.
119 Ryan TJ. Microvascular injury: vasculitis, stasis, and ischemia. London: Saunders; 1976.
120 Fagrell B. Vital microscopy in the pathophysiology of deep venous insufficiency. In: Eklof B, et al, editors. Controversies in the management of venous disorders. London: Butterworth, 1989.
121 Burnand KG, Whimster I, Naidoo A, Browse NL. Pericapillary fibrin in the ulcer-bearing skin of the leg: the cause of lipodermatosclerosis and venous ulcerations. Br Med J (Clin Res Ed). 1982;285:1071.
122 Fagrell B. Microcirculatory disturbances: the final cause for venous leg ulcers? Vasa. 1982;11:101.
123 Guex JJ, Enrici E, Boussetta S, Avril L, Lis C, Taieb C. Correlations between ankle circumference, symptoms, and quality of life demonstrate the clinical relevance of minimal leg swelling reduction: results of a study in 1,036 Argentinean patients. Dermatol Surg. 2008;34:1666.
124 Browse NL, Jarrett PE, Morland M, Burnand K. The treatment of liposclerosis of the leg by fibrinolytic enhancement: a preliminary report. Br Med J. 1977;2:434.
125 Wenner A, Leu HJ, Spycher M, Brunner U. Ultrastructural changes of capillaries in chronic venous insufficiency. Exp Cell Biol. 1980;48:1.
126 Caggiati A, Rosi C, Franceschini M, Innocenzi D. The nature of skin pigmentations in chronic venous insufficiency: a preliminary report. Eur J Endovasc Surg. 2008;35:111.
127 Hobbs JT. The post-thrombotic syndrome. In: Hobbs JT, editor. The treatment of venous disorders. Philadelphia: JB Lippincott, 1977.
128 Shelley WB, Swaminathan R, Shelley ED. Lichen aureus: a hemosiderin tattoo associated with perforator vein incompetence. J Am Acad Dermatol. 1984;11:260.
129 Pimentel CL, Rodriguez-Salido MJ. Pigmentation due to stasis dermatitis treated successfully with a noncoherent intense pulsed light source. Dermatol Surg. 2008;34:950.
130 Burton JL, Rook A, Wilkinson DS. Gravitational eczema. In Rook A, Wilkinson DS, Ebling FJG, et al, editors: Textbook of dermatology, 4th ed, Melbourne: Blackwell Scientific, 1986.
131 McPheeters HO, Anderson JK. Injection treatment of varicose veins and hemorrhoids. Philadelphia: FA Davis; 1943.
132 Zimmerman LM, Rattner H. Infra-red photography of subcutaneous veins. Am J Surg. 1935;27:502.
133 Droller H. Dermatologic findings in a random sample of old persons. Geriatrics. 1955;10:421.
134 Weismann K, Krakauer R, Wanscher B. Prevalence of skin disease in old age. Acta Derm Venerol. 1980;60:352.
135 Beauregard S, Gilchrest BA. A survey of skin problems and skin care in the elderly. Arch Dermatol. 1987;123:1638.
136 Larsen WG, Maibach HI. Dermatitis and eczema. In Mosschella SL, Hurley HJ, editors: Dermatology, 2nd ed, Philadelphia: Saunders, 1985.
137 Biegeleisen HI. Varicose veins, related diseases, and sclerotherapy: a guide for practitioners. London: Eden; 1984.
138 Jeghers H, Edelstein L. Skin color in health and disease. In MacBryde CM, Blackslow RS, editors: Signs and symptoms, 6th ed, Philadelphia: JB Lippincott, 1983.
139 Weaver J, Billings SD. Initial presentation of stasis dermatitis mimicking solitary lesions: a previously unrecognized clinical scenerio. J Am Acad Dermatol. 2009;61:1028.
140 Gosnell AL, Nedorost ST. Stasis dermatitis as a complication of amlodipine therapy. J Drugs Dermatol. 2009;8:135.
141 Kulwin MH, Hines EAJr. Blood vessels of the skin in chronic venous insufficiency: clinical pathologic studies. Arch Dermatol Syph. 1950;62:293.
142 Braverman IM. Skin signs of systemic disease. Philadelphia: WB Saunders; 1998. p. 134
143 Butler DF, Berger TG, Rodman OG. Leukemia cutis mimicking stasis dermatitis. Cutis. 1985;35:47.
144 Chang HY, Wong KM, Bosenberg M, et al. Myelogenous leukemia cutis resembling stasis dermatitis. J Am Acad Dermatol. 2003;49:128.
145 Milian G. Les atrophies cutanees syphilitiques. Bull Soc Fr Dermatol Syph. 1929;36:865.
146 Bollinger A. Transcapillary and interstitial diffusion of Na-fluoroscein in chronic venous insufficiency with white atrophy. Int J Microcirc Clin Exp. 1982;1:5.
147 Maessen-Visch MB, Sommer A, De Paepe JA, Neumann HAM. Change in microcirculation in patients with atrophie blanche visualized by laser Doppler perfusion imaging and transcutaneous oxygen measurement. Phlebology. 1998;13:45.
148 Amato L, Chiarini C, Berti S, et al. Idiopathic atrophie blanche. Skinmed. 2006;5:151.
149 Anning ST. Leg ulcers: their causes and treatment. London: J & A Churchill; 1954.
150 Wiseman R. Severall chirurgical treatises. London: Royston & Took; 1676.
151 Dale WA, Foster J. Leg ulcers: comprehensive plan of diagnosis and management. Med Sci. 1964;15:56.
152 Callam MJ, Ruckley CV, Harper DR, Dale JJ. Chronic ulceration of the leg: extent of the problem and provision of care. Br Med J. 1985;290:1855.
153 Margolis DJ, Bilker W, Santanna J, Baumgarten M. Venous leg ulcer: incidence and prevalence in the elderly. J Am Acad Dermatol. 2002;46:381.
154 Baker SR, Stacey MC, Jopp-McKay AG, et al. Epidemiology of chronic venous ulcers. Br J Surg. 1991;78:864.
155 Nelzen O, Bergqvist D, Lindhagen A, Hallbook T. Chronic leg ulcers: an underestimated problem in primary health care among elderly patients. J Epidemiol Community Health. 1991;45:1847.
156 Haeger K, editor. Venous and lymphatic disorders of the leg. Lund, Sweden: Bokforlaget Universitet och Skola, 1966.
157 Sicard JA, Forestier J, Gaugier L. Treatment of varicose ulcers. Proc R Soc Med. 1929;21:1837.
158 Sigg K. Varizen, ulcus cruris, und thrombose. Berlin: Springer-Verlag; 1958.
159 Ruckley CV, Dale JJ, Callam MJ, Harper DR. Causes of chronic leg ulcer. Lancet. 1982;2:615.
160 Bauer G. Heparin as a therapeutic against thrombosis: results of a one-year treatment at Mariestal Hospital. Acta Chir Scand. 1942;86:217.
161 Dodd H, Cockett FB. The pathology and surgery of the veins of the lower limbs. Edinburgh: Churchill Livingstone; 1956.
162 Gjores JE. Symposium on venous ulcers: opening comments. Acta Chir Scand. 1988;544(Suppl):7.
163 Browse NL, Burnand KG. The postphlebetic syndrome: a new look. In: Bergan JJ, Yao JST, editors. Venous problems. Chicago: year Book, 1978.
164 Neldner KH. The management of venous leg ulcers. Curr Concepts Skin Disord. 1987;8:5.
165 Labropoulos N, Leon M, Nicolaides AN, et al. Venous reflux in patients with previous deep venous thrombosis: correlation with ulceration and other symptoms. J Vasc Surg. 1994;20:20.
166 Labropoulos N, Giannoukas AD, Nicolaides AN, et al. New insights into the pathophysiologic condition of venous ulceration with color-flow duplex imaging: implications for treatment? J Vasc Surg. 1995;22:45.
167 Yamaki T, Nozaki M, Sasaki K. Color duplex ultrasound in the assessment of primary venous leg ulceration. Dermatol Surg. 1998;24:1124.
168 Gooley NA, Sumner DS. Relationship of venous reflux to the site of venous valvular incompetence: implications for venous reconstructive surgery. J Vasc Surg. 1988;7:50.
169 Hanrahan LM, Araki CT, Rodriguez AA, et al. Distribution of valvular incompetence in patients with venous stasis ulceration. J Vasc Surg. 1991;13:805.
170 Hoare MC, Nicolaides AN, Miles CR, et al. The role of primary varicose veins in venous ulceration. Surgery. 1982;92:450.
171 Editorial: Venous ulcers. Lancet. 1977;309:522.
172 Lawrence D, Fish PJ, Kakkar VV. Blood-flow in incompetent perforating veins. Lancet. 1977;309:117.
173 Negus D. Prevention and treatment of venous ulceration. Ann R Coll Surg Engl. 1985;67:144.
174 Koyano K, Sakaguchi S. Ultrasonographic detection and the role of the perforating veins in primary varicose veins. In: Raymond-Martimbeau P, Prescott R, Zummo M, editors. Phlébologie ’92. Paris: John Libbey Eurotext, 1992.
175 Darke SG, Penfold C. Venous ulceration and saphenous ligation. Eur J Vasc Surg. 1992;6:4.
176 Burnand K, Thomas ML, O’Donnell T, Browse NL. Relation between postphlebitic changes in the deep veins and results of surgical treatment of venous ulcers. Lancet. 1976;307:936.
177 Van Rij AM, Solomon C, Christie R. Anatomic and physiologic characteristics of venous ulceration. J Vasc Surg. 1994;20:759.
178 Bergqvist D, Lindholm C, Nelzen O. Chronic leg ulcers: the impact of venous disease. J Vasc Surg. 1999;29:752.
179 Falanga V, Moosa HH, Nemeth AJ, et al. Dermal pericapillary fibrin in venous disease and venous ulceration. Arch Dermatol. 1987;123:620.
180 Lofgren EP. Leg ulcers: symptoms of an underlying disorder. Postgrad Med. 1984;76:51.
181 Lippman HI. Subcutaneous ossification in chronic venous insufficiency, presentation of 23 cases: preliminary report. Angiology. 1957;8:378.
182 Ward WH. Leg ulcers. Australas J Dermatol. 1960;5:145.
183 Lippman HI, Goldin RR. Subcutaneous ossification of the legs in chronic venous insufficiency. Radiology. 1960;74:279.
184 Ceulen RPM, Kessels A, Veraart JCJM, van Neer PAFA. Lateral venous ulceration and incompetence of the small saphenous vein. Dermatol Surg. 2007;33:727.
185 Lloret P, Redono P, Sierra A, Cabrera J. Mixed skin ulcers misdiagnosed as pyoderma gangrenosum and rheumatoid ulcer: successful treatment with ultrasound-guided injection of polidocanol microfoam. Dermatol Surg. 2006;32:749.
186 Michael: Uber der primaren krebs der extremitaten. Tübingen, Germany: Inaug Diss, 1890.
187 Balestrino E, Clerico D, Emmolo I, Bosco MC. Malignant degeneration of chronic venous ulcer (Marjolin’s ulcer). Minerva Chir. 1983;38:211.
188 Phillips TJ, Salman SM, Rogers GS. Nonhealing leg ulcers: a manifestation of basal cell carcinoma. J Am Acad Dermatol. 1991;25:47.
189 Kaufmann R, Klein CE, Sterry W. Neoplastic diseases associated with leg ulcers. In: Raymond-Martimbeau P, Prescott R, Zummo M, editors. Phlébologie ’92. Paris: John Libbey Eurotext, 1992.
190 Igarzabal C, Feisilberg D. Secondary malignant degeneration of chronic leg ulcers. In: Raymond-Martimbeau P, Prescott R, Zummo M, editors. Phlébologie ’92. Paris: John Libbey Eurotext, 1992.
191 Harris B, Eaglstein WH, Falanga V. Basal cell carcinoma arising in venous ulcers and mimicking granulation tissue. J Dermatol Surg Oncol. 1993;19:150.
192 Kofler H, Pichler E, Romani N, et al. Hemangiosarcoma in chronic leg ulcer. Arch Dermatol. 1988;124:1080.
193 Hassan SA, Cheatle TR, Fox JA. Marjolin’s ulcer: a report of three cases and review of the literature. Phlebology. 1993;8:34.
194 Lagattolla NRF, Burnand KG. Chronic venous disease may delay the diagnosis of malignant ulceration of the leg. Phlebology. 1994;9:167.
195 Andrews BT, Stewart JB, Allum WH. Letter to the editor: malignant melanoma occurring in chronic venous ulceration. Dermatol Surg. 1997;23:598.
196 Tenopyr J, Silverman T. The relation of chronic varicose ulcer to epithelioma. Ann Surg. 1932;95:754.
197 Yang D, Stacey MC, Morrison B, Hoskin S. Malignancy in patients presenting with chronic leg ulcers. In: Negus D, editor. Phlebology ’95 1995;1(Suppl). :758.
198 Knox LC. Epithelioma and the chronic varicose ulcer. JAMA. 1925;85:1046.
199 Pannell TC, Hightower F. Malignant changes in post-phlebitic ulcers. South Med J. 1965;58:779.
200 Grusser M. Cancer of leg. Arch Klin Chir. 1923;127:529. JAMA 81:2156, 1923 (abstract)
201 Neumann Z, Ben-Hur N, Shulman J. Trauma and skin cancer: implantation of epidermal elements and possible causes. Plast Reconstr Surg. 1963;32:649.
202 Bostwick J, Prendergast WJ, Vasconez LO. Marjolin’s ulcer: an immunologically privileged tumour. Plast Reconstr Surg. 1975;57:66.
203 Patel NP, Kim SH, Padberg FT. Venous ulceration and basal cell carcinoma: coincident or synergistic? J Vasc Surg. 2006;44:208.
204 Baldursson BT, Hedblad MA, Beitner H, Lindelof B. Squamous cell carcinoma complicating chronic venous leg ulceration: a study of the histopathology, course, and survival in 25 patients. Br J Dermatol. 1999;140:1148.
205 Tretbar LI. Bleeding from varicose veins, treatment with injection sclerotherapy. In: Davy A, Stemmer R, editors. Phlébologie ’89. Montrouge, France: John Libbey Eurotext, 1989.
206 Evans GA, Evans DM, Seal RM, Craven JL. Spontaneous fatal hemorrhage caused by varicose veins. Lancet. 1973;302:1359.
207 Harman RRM. Haemorrhage from varicose veins. Lancet. 1974;303:363.
208 Du Toit DF, Knott-Craig C, Laker L. Bleeding from varicose veins – still potentially fatal. S Afr Med J. 1985;67:303.
209 Jenkins D. Mortality from varicose veins in Australia (1997–2000). Aust N Z J Phlebol. 2003;7:22.
210 Teitelbaum GP, Davis PS. Spontaneous rupture of a lower extremity varix: case report. Cardiovasc Intervent Radiol. 1989;12:101.
211 Bergan JJ. Management of external hemorrhage from varicose veins. Vasc Surg. 1997;31:413.
212 Husni EA, Williams WA. Superficial thrombophlebitis of the lower limbs. Surgery. 1982;91:70.
213 Prountjos P, Bastounis E, Hadjinikolaou L, et al. Superficial venous thrombosis of the lower extremities co-existing with deep venous thrombosis. Int Angiol. 1991;10:63.
214 Jorgensen JO, Hanel KC, Morgan AM, Hunt JM. The incidence of deep venous thrombosis in patients with superficial thrombophlebitis of the lower limbs. J Vasc Surg. 1993;18:70.
215 Zollinger RW, Williams RD, Briggs DO. Problems in the diagnosis and treatment of thrombophlebitis. Arch Surg. 1962;85:18.
216 Unno N, Mitsuoka H, Uchiyama T, et al. Superficial thrombophlebitis of the lower limbs in patients with varicose veins. Surg Today. 2002;32:397.
217 Raso AM, Durando R, Zucchelli A, Sorges L. Studio su 357 casi di flebite degli arti inferiori su due campioni interregionali. Minerva Chir. 1979;34:553.
218 Totten HP. Superficial thrombophlebitis: observations on diagnosis and treatment. Geriatrics. 1967;22:151.
219 Edwards EA. Thrombophlebitis of varicose veins. Gynecol Obstet. 1938;60:236.
220 Gjores JE. Surgical therapy of ascending thrombophlebitis in the saphenous system. Angiology. 1962;13:241.
221 Husni EA, Pena LI, Lenhert AE. Thrombophlebitis in pregnancy. Am J Obstet Gynecol. 1967;97:901.
222 Osius EA. Discussion of Hermann’s paper. AMA Arch Surg. 1952;64:685.
223 Bergqvist D, Jaroszewski H. Deep vein thrombosis in patients with superficial thrombophlebitis of the leg. Br Med J. 1986;292:658.
224 Galloway JMD, Karmody AM, Mavor GE. Thrombophlebitis of the long saphenous vein complicated by pulmonary embolism. Br J Surg. 1969;56:360.
225 Guilmot JL, Wolman F, Lasfargues G. Thromboses veineuses superficielles. Rev Prat. 1988;38:2062.
226 Plate G, Eklof B, Jensen R, Ohlin P. Deep venous thrombosis, pulmonary embolism and acute surgery in thrombophlebitis of the long saphenous vein. Acta Chir Scand. 1985;151:241.
227 Skillman JJ, Kent KC, Porter DH, Kim D. Simultaneous occurrence of superficial and deep thrombophlebitis in the lower extremity. J Vasc Surg. 1990;11:818.
228 Guex JJ. Thrombotic complications of varicose veins: a literature review of the role of superficial venous thrombosis. Dermatol Surg. 1996;22:378.
229 Zollinger RW. Superficial thrombophlebitis. Surg Gynecol Obstet. 1967;124:1077.
230 Hafner CD, Cranley JJ, Krause RJ, Strasser ES. A method of managing superficial thrombophlebitis. Surgery. 1964;55:201.
231 Lofgren EP, Lofgren KA. The surgical treatment of superficial thrombophlebitis. Surgery. 1981;90:49.
232 Mazuch J, Mistuna D, Golian D. Treatment of thrombophlebitis varicose of the lower limbs. Acta Phlebol. 2001;2:7.
233 Mazuch K, Géc L, Machan L, Kovacs V. The surgical treatment of thrombophlebitis of varicose veins of the lower limbs. In: Negus D, editor. Phlebology ’95 1995;1(Suppl). :717.
234 Perrin M, Guex J-J, Gillet JL. Traitement chirurgical des thromboses veineuses superficielles des membres inférieurs. Encycl Méd Chir, Editions médicales et scientifiques, Techniques chirurgicales – Chirurgie vasculaire, 43–165. Paris: Elsevier; 2000.
235 Widmer LK, Stähelin HB, Nissen C, da Silva A. Venen, Arterien-Krankheiten, koronare Herzkrankheit bei Berufstatigen. Bern: Hans Huber; 1981.
236 Stead RB. The hypercoagulable state. In: Goldhaber SZ, editor. Pulmonary embolism and deep venous thrombosis. Philadelphia: Saunders, 1985.
237 Siegel B, Ipsen J, Felix WR. Epidemiology of lower extremity deep venous thrombosis in surgical patients. Ann Surg. 1974;179:278.
238 Hume M, Sevitt S, Thomas DP. Venous thrombosis and pulmonary embolism. Cambridge, Mass: Harvard University Press; 1970.
239 Bick RL. Disseminated intravascular coagulation. In: Bick RL, editor. Disseminated intravascular coagulation and related syndromes. Boca Raton, Fla: CRC, 1983.
240 Falanga V, Bontempo FA, Eaglstein WH. Protein C and protein S plasma levels in patients with lipodermatosclerosis and venous ulceration. Arch Dermatol. 1990;126:1195.
241 Samlaska CP. Protein C and protein S plasma levels in patients with lipodermatosclerosis and venous ulceration. Arch Dermatol. 1991;127:908.
242 Samlaska CP, James WD. Superficial thrombophlebitis. I. Primary hypercoagulable states. J Am Acad Dermatol. 1990;22:975.
243 Samlaska CP, James WD. Superficial thrombophlebitis. II. Secondary hypercoagulable states. J Am Acad Dermatol. 1990;23:1.
244 Barrow DW. The clinical management of varicose veins, 2nd ed. New York: Paul H Hoeber; 1957.
245 Foote RR. Varicose veins. St Louis: CV Mosby; 1949.
246 Clayton JK, Anderson JA, McNicol GP. Preoperative prediction of post-operative deep vein thrombosis. Br Med J. 1976;2:910.
247 Lowe GD, Osborne DH, McArdle BM, et al. Prediction and selective prophylaxis of venous thrombosis in elective gastrointestinal surgery. Lancet. 1982;319:409.
248 Rakoczi I, Chamone D, Collen D, Verstraete M. Prediction of postoperative leg-vein thrombosis in gynecological patients. Lancet. 1978;311:509.
249 Matyas M. The clinical management of varicose veins, 2nd ed. New York: Paul H Hoeber; 1957.
250 Kakkar VV, Howe CT, Nicolaides AN, et al. Deep vein thrombosis of the leg. Is there a ‘high risk’ group? Am J Surg. 1970;120:527.
251 Heyerdale WW, Stalker LK. The management of varicose veins of the lower extremities. Ann Surg. 1941;114:1042.
252 Duffy DM. Small vessel sclerotherapy: an overview. In: Callen JP, Dahl MV, Golitz LE, et al, editors. Advances in dermatology, vol 3. Chicago: Year Book; 1988.