Chapter 10 Adverse circulatory effects of exercise
SYNCOPE
In an upright individual, the gravitational field between heart and brain means that cerebral perfusion pressure is always lower than blood pressure at the level of the arterial baroreceptors. In consequence, cerebral perfusion varies more dramatically with variations in cardiac ejection and peripheral resistance than does perfusion to other organs, and situations that result in substantially reduced blood pressure may, in the upright posture, result in fainting (syncope) due to inadequate blood flow to the forebrain.
Causes of syncope
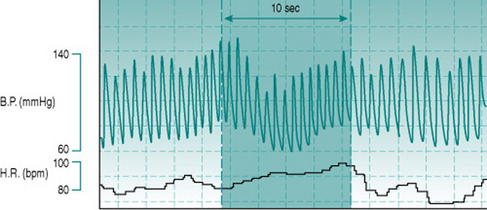
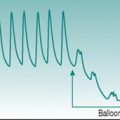
Such reductions in blood pressure could result from reduced venous return and cardiac filling or from reduced baroreflex capacity to induce peripheral vasoconstriction. In the context of exercise, the typical primary effect is obstructed systemic venous return due to elevation of intrathoracic pressure. This pressure increase can be induced voluntarily by forced expiration against a closed glottis and is known as the Valsalva manoeuvre (Fig. 10.1). It occurs naturally during any activity that involves thoracic muscle activation and breath holding, such as carrying heavy suitcases, weight lifting or straining on the lavatory.
All of these additional factors may be associated with athletic performance of specific types. In competitions that involve weight divisions, many performers restrict their fluid intake dramatically in order to make weight, with resulting depletion of plasma volume. Intense, long-term dynamic training has been reported to cause increases in compliance of the large veins, predisposing these athletes to increased venous pooling and to hypotension and fainting during rapid postural change, even in the absence of a Valsalva manoeuvre (Hernandez & Franke 2004). Finally, the elevation of plasma adrenaline (epinephrine) and noradrenaline (norepinephrine) due to the generalized sympathetic discharge associated with central command is enhanced by the additional sympathetic activation due to the stress of competition. Although the cerebral vasculature itself receives only a sparse sympathetic innervation, these circulating catecholamines exert a moderate vasoconstrictor effect on the cerebral precapillary resistance vessels and so reduce brain perfusion at any given level of pressure gradient. An example of Valsalva-induced syncope in a competitive athlete appears in the Case history below.
HYPERTHERMIA
Dependence on evaporative cooling
As discussed in Chapter 9 (p. 106), the internal heat generated by even intense prolonged exercise produces only moderate hyperthermia, so long as there is effective evaporative cooling by sweat. By definition, therefore, anything that interferes with heat loss is likely to result in excessive elevation of body temperature. If this rises to more than 41° C (106° F) then central nervous function begins to be impaired, with permanent brain damage or death occurring at only slightly higher temperatures.
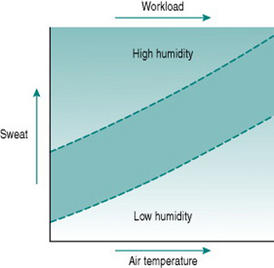
Sweat is a highly effective source of heat loss when air humidity is low. This is illustrated dramatically by our capacity to maintain a near-normal core temperature in sauna baths, where the temperature is routinely set at around 100° C (212° F), but relative humidity is around 10% or less. However, the efficiency of evaporative heat loss is highly dependent on absolute relative humidity, as witnessed by the greatly increased sweat production produced by quite small increments in sauna humidity when one pours water on the coals. If environmental relative humidity is 90% or more, as is often the case in many tropical climates, then virtually no sweat evaporates and there is consequently almost no benefit for heat loss, despite the fact that the volume of sweat secreted for a given workload and ambient temperature is much greater than under less humid conditions (Fig. 10.2). Under these circumstances, heat loss depends almost entirely on radiation and convection, which are far less efficient than evaporation.
Heat exhaustion
In the absence of fluid replacement, plasma volume falls during exercise in line with the volume of sweat produced. Below a critical volume, it becomes impossible to maintain blood pressure in the face of the overall low peripheral resistance imposed by dilatation in muscle and cutaneous beds. As a result, cerebral perfusion is prejudiced in the upright posture and the individual collapses. The time to onset of this state of heat exhaustion will be determined primarily by the rate of sweating and, therefore, depends on workload and on environmental temperature and humidity. Under competitive conditions, it can also be prolonged by motivation, to the extent that collapse does not occur until fluid depletion is so great that sweat production has begun to decline.
Tissue damage due to fluid depletion
Plasma volume depletion equivalent to reduction of total blood volume by 1.5 l or more is sufficient to reduce mean blood pressure even when sympathetic drive is maximal. In these circumstances, the consequent fall in tissue perfusion may have deleterious outcomes unless effective fluid replacement is achieved within 1–2 h. The problems occur mainly in regions of the circulation where microcirculatory counter-current exchange leads to local hypoxia (see Chapter 5, p. 58). In the kidney, the fall in medullary blood flow can cause necrotic damage to the loops of Henle and the collecting ducts, resulting in loss of urinary concentrating power and further fluid loss. In the small intestine, the fall in perfusion of the villi can result in sloughing of the villar epithelium, leading to exposure of the underlying tissue to intestinal proteolytic enzymes and to potential entry into the bloodstream of Gram-negative bacteria that are normally restricted to the gut lumen.
If blood pressure is low enough to prevent effective capillary perfusion through some areas of peripheral tissues, then blood cells begin to fall out of suspension and form clumps within the downstream venules (see Chapter 5, p. 60). Once this has occurred, even restoration of blood volume sufficient to reduce peripheral resistance to normal may not restore effective perfusion in these areas, because the venular cell clumps create a resistance sufficient to redirect blood flow to other, lower resistance, regions of the tissue.
Drug-induced hyperthermia
The 3,4-methylenedioxymethamphetamine molecule, commonly known as ecstasy, is widely used as a recreational drug and has been the cause of a number of deaths due to hyperthermia. Ecstasy has several pharmacological properties that collectively predispose to elevated core temperature (Mills et al 2004). First, it increases central sympathetic drive, with resulting cutaneous vasoconstriction. Second, it acts in a variety of tissues to stimulate uncoupling proteins. These uncouple mitochondrial respiration from ATP synthesis and so generate additional metabolic heat. Third, it appears to act on amine-receptive synapses in the hypothalamus to reset the central thermostat to a higher operating point.
Exertional rhabdomyolysis
Rhabdomyolysis has as well been reported as a consequence of ecstasy intoxication, presumably in individuals with similar genotypic predispositions. Because the abnormal calcium channel function appears to only occur during prolonged muscle activation, short bouts of even intense exercise do not generate excessive heat and so are not associated with risk of hyperthermic damage (Muldoon et al 2004).
SUDDEN CARDIAC DEATH
There are two main causes of sudden cardiac death (Catanzaro et al 2006, Corrado et al 2006). One is a genetically determined type of cardiac muscle cell membrane abnormality (hypertrophic cardiomyopathy), characterized by increased capacity to generate arrhythmias. The second is developmental abnormality of the coronary arteries. The left and right coronary arteries normally arise from the left and right sides of the aortic sinus respectively and run from there to the left and right myocardium. Occasionally, however, both arteries arise from the same side of the aortic sinus and so one must travel between the aorta and the pulmonary artery in order to reach the appropriate side of the heart. When cardiac output rises during exercise, aorta and pulmonary arteries become distended and the aberrant coronary artery may be compressed, resulting in hypoxia to that side of the heart.
Much debate has gone on as to whether routine screening programmes should be put in place to exclude individuals with one of these abnormalities from participating in sport (Corrado et al 2006). In practice, this would be extremely difficult and the benefits would be uncertain. Retrospective analysis indicates that only a minority of individuals who sustain sudden cardiac death during exercise have any suggestive signs, including identifiable ECG abnormalities. Finding those at risk would, therefore, require complex and expensive investigations, with, even then, no guarantee that all such individuals would be identified. Against this approach is the certainty that participation in sports programmes has proven advantages for health in general and that many people involved in sports would be likely to choose to continue their involvement in spite of a known risk.
On balance, a sensible approach seems to be as follows. Identify by careful screening the people at obvious risk (history of fainting or dizziness, ECG evidence of atrial fibrillation, ventricular extrasystoles or ischaemia) and exclude them from experimental exercise studies. Ensure that individuals in this category are investigated further before being accepted into competitive sports programmes. But also recognize that a tiny number of unidentifiable people will remain at risk and in whom it is entirely uncertain whether they might die as a result of exercise.
Catanzaro JN, Makaryus AN, Catanese C. Sudden cardiac death associated with an extremely rare coronary anomaly of the left and right coronary arteries arising exclusively from the posterior (noncoronary) sinus of Valsalva. Clinical Cardiology. 2006;28:542-544.
Corrado D, Basso C, Pavei A, et al. Trends in sudden cardiovascular death in young competitive athletes after implementation of a preparticipation screening program. Journal of the American Medical Association. 2006;296:1593-1601.
Hernandez JP, Franke WD. Age- and fitness-related differences in limb venous compliance do not affect tolerance to maximal lower body negative pressure in men and women. Journal of Applied Physiology. 2004;97:925-929.
Muldoon S, Deuster P, Brandom B, Bunger R. Is there a link between malignant hyperthermia and exertional heat illness? Exercise and Sport Science Reviews. 2004;32:174-179.
Mills EM, Rusyniak DE, Sprague JE. The role of the sympathetic nervous system and uncoupling proteins in the thermogenesis induced by 3,4-methylenedioxymethamphetamine. Journal of Molecular Medicine. 2004;82:787-799.