Chapter 88 Advances in systemic therapy for hepatocellular carcinoma
Overview
Hepatocellular carcinoma (HCC) is the fifth most common solid tumor and the third most common cause of cancer death worldwide (Abou-Alfa, 2008; see Chapter 80). Its incidence mirrors that of chronic liver injury, most commonly the result of viral infection by both hepatitis B and C (see Chapter 64). Other etiologies that lead to chronic liver injury and cirrhosis include alcohol abuse; nonalcoholic steatohepatitis, commonly associated with morbid obesity and diabetes; and other metabolic diseases, such as hemochromatosis. The highest incidence of HCC remains in Southeast Asia and sub-Saharan Africa (McGlynn et al, 2001); however, concern continues about the rising incidence of HCC in North America (El-Serag & Mason, 1999). A threefold increase in the age-adjusted rates for HCC associated with hepatitis C virus (HCV) infection has been observed, from 2.3 per 100,000 between 1993 and 1995 to 7.0 per 100,000 between 1996 and 1998; this is most likely explained by the increased incidence of HCV in North America during that period.
Hepatocellular Carcinoma and Cirrhosis: two Diseases in One (See Chapter 70A, Chapter 70B )
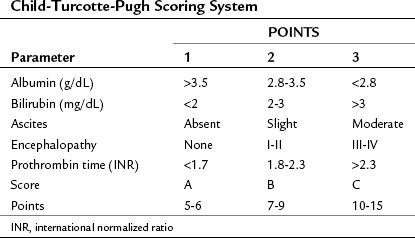
Both cirrhosis and the tumor itself impact the HCC patient’s overall survival (OS), thus the cirrhosis status of patients with liver cancer must be evaluated. A scoring system was originally developed by Child (1964) that consisted of three parameters: jaundice (bilirubin), ascites, and encephalopathy. This scoring system was later updated by Pugh et al (1973), who added an assessment of hepatic synthetic function by evaluating serum albumin levels and prothrombin time (PT) (Table 88.1; see Chapters 2 and 70B). This combined score is known as the Child-Turcotte-Pugh (CTP) score, and it remains the most commonly used scoring system for liver cirrhosis. The pitfall of this classification is its lack of any parameters that relate to the cancer itself, as it was developed in patients who have cirrhosis but no cancer. As such, it is unlikely to be the best predictor of outcome in patients with HCC.
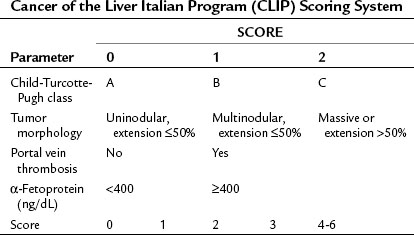
Okuda and colleagues (1985) recognized the need for an HCC staging system that incorporated factors related to both the cirrhosis and the tumor itself and developed what is known as the Okuda staging system, which encompasses four variables: 1) albumin, 2) bilirubin, 3) ascites, and 4) tumor size as a percentage of the size of the liver (greater or less than 50%). The Okuda staging system served as a platform for more advanced and sophisticated prospective scoring systems that used the Cox proportional hazard regression model. The Cancer of the Liver Italian Program (CLIP) developed a scoring system in patients with HCC, with mainly HCV as an etiology (CLIP, 1998, 2000), that found the independent prognostic variables for patients with HCC to be the CTP score plus three additional variables that relate to the tumor itself: tumor morphology, as assessed by the number of lesions and extent of disease in the liver; presence or absence of portal vein thrombosis; and α-fetoprotein (AFP) level (Table 88.2). Patients with a high score (4 to 6) were shown to have a median survival of only 3.2 months.
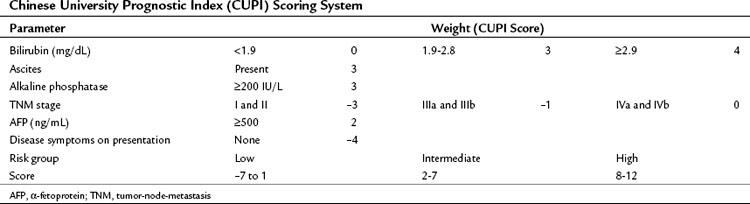
In contrast to the CLIP scoring system, the Chinese University Prognostic Index (CUPI), another scoring system based on multivariate analysis, was developed mainly in patients with hepatitis B virus (HBV)-associated HCC (Leung et al, 2002). This prognostic index identified bilirubin, alkaline phosphatase, and the presence or absence of ascites, in addition to tumor stage based on the tumor-node-metastasis (TNM) staging and AFP levels, as the most important predictors of outcome. In addition, a clinical assessment parameter of presence or absence of symptoms at presentation was incorporated (Table 88.3). An important aspect of the CUPI index is that different weights are attributed to the different parameters. The median survival of the highest risk group was close to 1 month. Other scoring systems include the Groupe d’Étude et de Traitement du Carcinome Hépatocellulaire (GETCH) staging system (Chevret et al, 1999), the Japan Integrated Staging (JIS) Score (Kudo et al, 2003), and the Barcelona Clinic Liver Cancer (BCLC) classification system (Llovet et al, 1999).
In a retrospective analysis of patients with advanced HCC seen by medical oncologists at Memorial Sloan-Kettering Cancer Center (MSKCC) over a period of 5 years, we attempted to identify which of these eight scoring systems would be most valuable in this specific clinical setting (Huitzil-Melendez et al, 2010). By using a concordance index, the GETCH, CLIP, and CUPI were found to be the most informative staging systems in predicting survival in patients with advanced HCC. The BCLC system did not score well in this exercise, which studied a specific niche of patients with advanced HCC, all of whom fell within the single basket of category C of BCLC; therefore it lacks any discriminatory value. Other groups have independently come to the same conclusion regarding the BCLC system (Collette et al, 2007).
Role of Chemotherapy in Hepatocellular Carcinoma
Almost all classes of chemotherapy have been studied in advanced HCC (Nerenstone et al, 1988). Doxorubicin is one of the most studied drugs in this disease, partly as a result of what has thus far been an irreproducible response rate of 79%, originally reported by Olweny and colleagues (1975). It should be noted that this early report from the mid-1970s did not use modern computerized tomography (CT) or magnetic resonance imaging (MRI) methods available and used routinely today. The majority of “responses” were determined either on the basis of physical examinations of the liver or using a colloidal gold liver scan, measuring techniques that would not be acceptable as evidence of a response in clinical trials by current standards. Many subsequent attempts to try to reproduce this high response rate have been made, both with doxorubicin as a single agent (Barbare et al, 1984; Chlebowski et al, 1984; Ihde et al, 1977; Johnson et al, 1978; Sciarrino et al, 1985; Vogel, 1977; Williams & Melia, 1980) and in combination with other chemotherapeutic agents (Choi et al, 1984; Falkson et al, 1978, 1984; Melia et al, 1983; Olweny et al, 1980), but to no avail.
Other older and newer chemotherapeutic agents have also been studied in HCC, including cisplatin (Falkson et al, 1987), etoposide (Melia et al, 1983), mitoxantrone (Falkson et al, 1987), vinblastine (Damrongsak et al, 1973), capecitabine (Patt et al, 2004), gemcitabine (Kubicka et al, 2001), irinotecan (O’Reilly et al, 2001), and paclitaxel (Chao et al, 1998), all with reported dismal response rates and no impact on survival.
Although several combination chemotherapy agents have shown improved response rates, the impact on survival was not noticeable in most cases (Baker et al, 1977; Bobbio-Pallavicini et al, 1997; Porta et al, 1995). As a single agent, interferon α-2b in a trial by the Gastrointestinal Tumor Study Group (GITSG, 1990) showed a limited response rate of only 7%. This disappointingly low response, in addition to increased toxicity as a result of high doses of interferon, led to testing lower doses in combination with chemotherapy. Despite a reported high response rate of 31% in some trials (Ji et al, 1996; Patt et al, 1993), favorable results have not been reproducible (Kardinal et al, 1993; Stuart et al, 1996).
Nevertheless, the preliminary indication of some activity for interferon set the platform for a more intense combination of cisplatin, interferon, doxorubicin, and 5-fluorouracil (5-FU), which became commonly known as PIAF (Patt et al, 1999). PIAF was subsequently modified and tested in the outpatient setting (Leung et al, 1999), and a Phase II trial of 50 patients showed a response rate of 26%. More importantly, nine patients (18%) had their tumor resected after therapy, and of these resected patients, four had a pathologic complete response noted in the resected tumor. Considerable hematologic toxicity was reported in this 50-patient cohort, and two treatment-related deaths resulted from neutropenic fever. It is important to note that this study was conducted before granulocyte-stimulating factors were commonly used for similar intense chemotherapeutic regimens.
These data led to a randomized Phase III trial of doxorubicin versus a combination, PIAF (Yeo et al, 2005). This trial showed the same 21% response rate for PIAF versus 10% for single-agent doxorubicin; however, the study failed to show any survival advantage in favor of the PIAF combination (8.7 vs. 6.8 months; P = .83). Despite this trial having a negative outcome for the primary end point, it did provide several important and useful pieces of information: First, as noted above, it identified what can realistically be considered the true response rate—by current standards of response definition and determination—of doxorubicin in HCC, and that response rate is 10%. Furthermore, although the study failed to provide support for use of PIAF as a routine palliative therapy for advanced disease, it might be considered as a conversion therapy in very carefully selected patients with potentially resectable tumors.
Some of the generally disappointing results are explained by the genetic makeup of HCC, which comprises highly resistant clones of cancer cells (DeVita & Abou-Alfa, 2000). The HCC cells carry a high genetic mutation load, which makes them less amenable to the destructive actions of chemotherapy. HCC cells usually contain a high level of dihydropyrimidine dehydrogenase (DPD), which potentially makes them relatively resistant to 5-FU (Jiang, 1993). HCC cells also overexpress the multidrug resistance gene MDR1 (Chenivesse, 1997) and the gene product P-glycoprotein (P-gp) (Soini et al, 1996). This may help explain the resistance of HCC to paclitaxel (Chao et al, 1998), but it would not necessarily explain the PIAF pathologic response (Leung et al, 1999), the witness to the efficacy that chemotherapy can exert onto HCC, contrary to all attestations that it cannot; this concept will be revisited as part of its evaluation in combination with biologic therapy (Abou-Alfa, 2008).
Several attempts at overcoming this resistance by developing novel cytotoxic agents have been made, although not with any appreciable success. T138067 is an antimicrotubular non–P-gp substrate (Shan et al, 1999; Venook et al, 2004) that showed a modest 9% response in chemotherapy naïve patients (Leung et al, 2002); however, a randomized Phase II/III trial of T138067 versus doxorubicin in patients with advanced HCC was prematurely closed because of lack of survival benefit (Posey et al, 2005). Nolatrexed dihydrochloride is a nonclassic lipophilic inhibitor of thymidylate synthase that is not catabolized by dihydropyrimidine dehydrogenase. It lacks a glutamate side chain and thus has reduced potential transport-associated resistance (Webber et al, 1993). Nolatrexed was studied in two different clinical trials; however, results were disappointing, with an 8% response rate in a Phase II North American study (Stuart, 1999) and no responses in a randomized Phase II study in Hong Kong (Mok et al, 1999). Despite those disappointing results, a randomized Phase III trial that evaluated nolatrexed versus doxorubicin in patients with unresectable HCC was performed. It showed a median OS of 5.6 months for nolatrexed versus 8 months for doxorubicin (P = .0068) (Gish et al, 2007). It is unclear whether nolatrexed conferred a negative survival effect or was simply inactive.
A novel modality of administering floxuridine (FUDR) and dexamethasone via hepatic arterial infusion (HAI) was also studied in HCC (Jarnagin et al, 2009). Among eight patients studied, the response rate was 25%, and the hepatic progression-free survival (PFS) was 9.4 months. Interestingly, patients in the same study with cholangiocarcinoma fared better, with a 53.8% response rate and 11.6 months hepatic PFS. HAI is discussed in detail in Chapter 86.
Novel Biologic Therapies in Hepatocellular Carcinoma
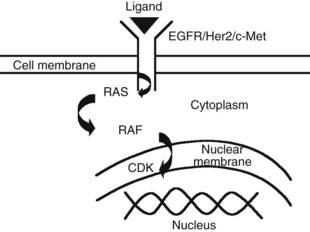
The advent of novel targeted therapeutics and the lack of a consistent standard of care for advanced HCC has led to interest in identifying and evaluating relevant targets in HCC along the signal transduction pathways (Huitzil-Melendez et al, 2009; Fig. 88.1).
Epidermal Growth Factor Receptor, c-MET, and Insulin Growth Factor Receptor
The epidermal growth factor receptor (EGFR) is one of the most thoroughly studied therapeutic targets. Several authors have reported no difference in expression of EGFR between HCC and noncancerous diseased liver tissues (Harada et al, 1999; Kira et al, 1997), whereas others have reported overexpression in 17% of HCC cases (Kiss et al, 1997). Erlotinib, an EGFR-specific receptor tyrosine kinase inhibitor, was tested in HCC as part of a Phase II trial of 38 patients with advanced HCC (Philip et al, 2005). Close to half of the patients had prior therapy, and 71% of all patients had class A cirrhosis. The primary end point was PFS at 6 months, using Response Evaluation Criteria in Solid Tumors (RECIST), and this end point was reached in 12 (32%) of 38 patients, with a median PFS of 3.8 months. In addition, three partial responses (8%) were reported, and median OS was 13 months. Similar to the experience with other tumors, immunohistochemical staining of EFGR expression was not associated with outcome. The most frequent grade 3 and 4 toxicities were skin rash (13%), diarrhea (8%), and fatigue (8%).
Despite having some preclinical activity in HCC (Huether et al, 2005), cetuximab has not been tested in HCC. Lapatinib, an oral agent with dual inhibition of EGFR and tyrosine kinase 1 and 2 (Her2/Neu), has been studied in HCC (Ramanathan et al, 2009) despite the rare expression of Her2/Neu in human HCC tissues (Hsu et al, 2002) and with disappointing results. In a Phase II trial, 40 patients with advanced HCC were treated with lapatinib, and the response rate was 5%, PFS was 2.3 months (95% confidence interval [CI], 1.7 to 5.6), and median survival was 6.2 months. Both fall short of other reported data in the literature, and EGFR genotyping indicates that HCC patients with less than 20 repeats have the worst PFS.
Another potential target is the hepatocyte growth factor (HGF) and its receptor c-Met, which were found to be overexpressed in 33% and 20% of human HCC tissues, respectively (Kiss et al, 1997). In another study, c-Met was found to be overexpressed preferentially in early stage resected HCC, with no association with outcome as measured by OS (Huitzil-Melendez et al, 2009). Certain c-Met inhibitors are already being studied in HCC, such as ARQ 197, which was already evaluated in a Phase I study (Garcia et al, 2007).
Sorafenib
Sorafenib is a novel molecular targeted agent that inhibits the serine/threonine kinase Raf-1 in vitro in addition to the proangiogenic (vascular endothelial growth factor receptor [VEGFR]-1, -2, and -3; platelet-derived growth factor receptor [PDGFR]-β); and tumorigenic receptor tyrosine kinases (RTKs; RET, Flt-3, and c-Kit) (Wilhelm et al, 2004). Sorafenib has been studied extensively in HCC and has now been approved by regulatory agencies worldwide as a standard therapy for unresectable HCC.
HCC is a highly vascular solid tumor with high expression of VEGF, which plays a major role in HCC development and potentiates its metastatic capability in preclinical models (Yoshiji et al, 2004). Proangiogenic platelet-derived growth factor receptor-β (PDGFR-β) also contributes to this increased metastatic potential (Zhang et al, 2005).
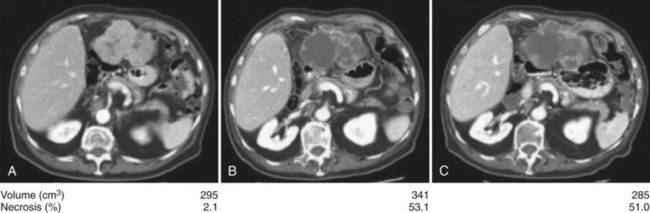
Anti–VEGFR-1, -2, and -3 and PDGFR-β sorafenib are the most studied antiangiogenic drugs in HCC so far; although an initial Phase II trial evaluating response to sorafenib in patients with advanced HCC showed no significant response rate (2%) (Abou-Alfa et al, 2006), 34% of patients had stable disease of a minimum 4 months’ duration. This was commensurate with a median time to progression (TTP) of 4.2 months and median OS of 9.2 months, both of which compare favorably to historic controls (Yeo et al, 2005). The main grade 3 and 4 toxicities were fatigue (9.5%), diarrhea (8%), and hand-foot skin reaction (5.1%), known also as hand-foot syndrome. The high rate of stable disease was associated with an observed phenomenon of central tumor necrosis in many patients in the study whose tumors were evaluated using triphasic CT scans inclusive of an arterial phase (Fig. 88.2). This central tumor necrosis was quantified, and the volume was assessed using a computer algorithm for semiautomated delineation of tumors (Zhao et al, 2006). It was later found that the ratio of the percentage of the described tumor necrosis over the tumor volume correlated with objective response (Abou-Alfa, 2008). This phenomenon is still pending validation and is currently the subject of many prospective correlative studies of ongoing HCC clinical trials.
The suggested improved outcome noted in this Phase II study led to a large, double-blinded, randomized Phase III trial evaluating sorafenib versus placebo in patients with advanced HCC and class A cirrhosis (Llovet et al, 2008) with two primary end points of OS and time to symptomatic progression (TTSP) using the FHSI8-TSP instrument. This Phase III study, known for short as the SHARP trial (Sorafenib HCC Assessment Randomized Protocol), demonstrated a survival of 10.7 months in the sorafenib group versus 7.9 months in the placebo group (P < .001, hazard ratio [HR], 0.69). The second primary end point in evaluating TTSP showed no difference between the two arms (P = .77). This observation is limited by the poor understanding of the validity of the FHS18-TSP instrument in this setting. Add to the fact that a large number of the patients in this study—including 17% with locally advanced tumor, BCLC B patients—had an excellent performance status and lacked any symptoms that the FHS18-TSP instrument would otherwise depend on for its measurements. The toxicity profile was similar to that noted in the Phase II study, with 8% grade 3 to 4 diarrhea and hand-foot syndrome. Rare bleeding events were observed (<1%) that would still force a word of caution considering the antiangiogenic nature of sorafenib, which is true also for other agents in its class, such as bevacizumab and sunitinib, that may cause fatal hemorrhage (Siegel et al, 2008; Faivre et al, 2009).
A second randomized Phase III study to evaluate sorafenib in patients with advanced HCC and class A cirrhosis was conducted in the Asia-Pacific geographical area (Cheng et al, 2009). The study had eligibility criteria similar to the SHARP trial but had two differences in the design: 1) the study had a 2 : 1 randomization, typically to help encourage accrual, and 2) it did not have a predefined primary end point but rather looked at several. Similar to the SHARP trial, the Asia-Pacific study showed an improvement in survival in favor of sorafenib (6.5 months) versus placebo (4.2 months); however, this statistically significant improvement (P = .014) was not of the same magnitude as that of the SHARP trial, despite similar HRs—0.68 and 0.69, respectively—in the Asia-Pacific and SHARP studies. In an attempt to explain the difference in magnitude of the OS, it was argued that in the Asia-Pacific study, patients were more ill at the time of accrual (Abou-Alfa et al, 2009) and had more extensive disease at a more advanced stage. This observation may partly explain the difference in magnitude of benefit from sorafenib between those two populations, and it may suggest, in view of the similar HRs, that the benefit for sorafenib was expressed on the survival curve at two different times in the natural history of the disease: earlier in the SHARP trial and later in the Asia-Pacific study.
In a subgroup analysis of the SHARP trial, it was noted that sorafenib-treated patients with HCV-based HCC (n = 93) had a median survival advantage of 14 months compared with the entire sorafenib-treated group, whose survival was only 10.7 months (Bolondi, 2008); this suggests a possible positive influence of HCV status on the efficacy of sorafenib. The placebo-controlled HCV group did not have any added survival advantage over the placebo population of the study, thus proving the lack of any advantage brought on by the HCV itself. Of note, in HCV infection, the virus core proteins result in high basal activity of Raf-1, which leads to a sustained response to EGFR by hepatocytes and results in an increased possibility of neoplastic transformation (Giambartolomei et al, 2001). A similar observation was noted in a retrospective analysis of the Phase II trial that evaluated sorafenib in patients with advanced HCC (Abou-Alfa et al, 2006). In this analysis, it was noted that patients who were infected with HCV but not HBV (n = 13) had a longer time to progression of 6.5 months, compared with 4 months (P = .05) for the patients infected with HBV (n = 33; Huitzil et al, 2008). A similar trend was noted in survival advantage (P = .29) for the HCV patients (12.4 months) versus HBV patients (7.3 months), with 73% of those accrued in the Asia-Pacific study found to have HBV as an underlying etiology for their HCC, versus only 18% of patients in the SHARP trial; this may offer another explanation, or at least a complementary one, for this difference in the outcome magnitude between the two studies. Of note, the outcome of the 18% of patients with HBV in the SHARP trial for unspecified reasons is still not reported; however, this observation does undermine the antiangiogenic effect of sorafenib, which continues to be indicated for all patients with unresectable HCC irrespective of the etiology of their cancer.
Patients with unresectable HCC and CTP class A cirrhosis who are eligible for sorafenib based on the SHARP trial encompass no more than half of the patients seen by medical oncologists (Huitzil, 2010). The safety and efficacy of sorafenib in patients with class B or C cirrhosis, who make up the other half, remain a subject of discussion. In the Phase II study that evaluated sorafenib in HCC (Abou-Alfa et al, 2006), 28% of patients had class B cirrhosis and pharmacokinetic profiles that included area under the curve (AUC) and maximun drug concentration (Cmax) comparable to patients with class A disease. The class B patients had more frequent worsening of liver function, including elevated total serum bilirubin, worsening ascites, and encephalopathy (Abou-Alfa, 2008). Considering the lack of a control arm, these data cannot help determine whether this deterioration in liver function is part of the expected natural history of class B disease, a result of sorafenib, or a combination of these. The elevated total bilirubin may also be due to sorafenib’s inhibitory effect of uridine diphosphate-glucouronosyl-transferase-1 polypeptide-1 (UGT1A1) and decreased bilirubin glucuronidation; because the study did not collect direct bilirubin measurements, this distinction cannot be made. Median TTP for class A patients was 21 weeks (95% CI, 16 to 25 weeks), and it was 13 weeks for class B patients (95% CI, 9 to 18 weeks); median OS was 41 weeks (95% CI, 36.6 to 63.6 weeks) and 14 weeks (95% CI, 11.6 to 25.7 weeks), respectively. In a phase I study that evaluated two different doses of sorafenib in Japanese patients with advanced HCC (Furuse et al, 2008), geometric means of AUC0-12 and Cmax were slightly lower in patients with class B cirrhosis compared with class A disease despite the lack of any substantial differences in the incidence of adverse events between the two groups.
One additional study evaluated sorafenib in 150 patients with different types of tumors in cohorts of different levels of liver dysfunction, and their results offer some guidance on the use of sorafenib in such patients (Miller et al, 2009). Among all the cohorts with higher than normal total bilirubin, the most commonly reported drug-limiting toxicity (DLT) among patients was further elevation of bilirubin. The study suggested sorafenib doses of 400 mg PO twice per day for bilirubin up to 1.5 times the upper limit of normal (ULN) and 200 mg PO twice per day, or 400 mg PO daily, for bilirubin 1.5 to 3 times the ULN; it was recommended that sorafenib be avoided for patients whose bilirubin is above three times the ULN. These recommendations may at least be used as a guideline, until more data become available regarding the safety and efficacy of sorafenib in patients with HCC and class B or C cirrhosis; this would require a randomized study that allows assessment of the natural history of the disease, and ways of evaluating worsening cirrhosis, in noninvasive ways that are also more predictive than measuring bilirubin levels.
Bevacizumab, an anti-VEGF, has been studied extensively in patients with advanced HCC. Bevacizumab was studied as a single agent in 46 patients with advanced HCC at doses of 5 mg/kg and 10 mg/kg (Siegel et al, 2008). Median PFS was 6.9 months, and median survival was 12.4 months. Grade 3 to 5 hemorrhage occurred in 11% of patients, including one death secondary to a variceal bleed. In the initial phase of this study with 18 patients accrued, 4 had to discontinue therapy because of serious adverse events, including one transient ischemic attack and three serious esophageal bleeding events. The study was thus modified to identify and manage esophageal varices prior to enrollment (Schwartz et al, 2005). Another study that evaluated single-agent bevacizumab in HCC showed similar results (Malka et al, 2007). Among 24 patients evaluable for response, 3 had a partial response and 13 had stable disease.
Sunitinib, a multitargeted receptor tyrosine kinase inhibitor, was evaluated as a single agent in patients with advanced HCC as part of a Phase II study (Zhu, 2009). Of 34 patients treated with sunitinib at a 37.5 mg daily dose, 50% of patients had stable disease, with a median PFS of 3.9 months and an OS of 9.8 months. The most common grade 3 and 4 adverse events included hematologic toxicities, fatigue, and transaminase elevation; in addition, two deaths were reported and attributed to worsening disease and liver failure. A second well-conducted study evaluated sunitinib at a higher dose of 50 mg/day in 37 patients with advanced HCC; however, it did not move to a planned second stage because of a low response rate of 2.7% in the first stage of the study (Faivre et al, 2009). In addition, four deaths were reported that were interpreted as possibly drug related; these included events of hepatic encephalopathy, hematologic toxicities, and a variceal bleed. Despite those findings, enthusiasm continues regarding the potential therapeutic value of sunitinib, which is being studied as part of a Phase III clinical trial to evaluate sunitinib versus sorafenib in patients with advanced HCC.
Brivanib, a dual inhibitor of VEGF and fibroblast growth factor (FGF), was also studied in HCC. In a study that evaluated brivanib as first- and second-line therapy in 96 patients with advanced HCC, limited responses were reported, and OS was 10 months in the treatment-naïve cohort and was not reached in the second-line cohort (Raoul et al, 2009). Although the OS compared favorably to the historic control, PFS was only 2.7 months in the treatment-naïve group, and TTP was only 2 months in the second-line group; however, the drug was well tolerated in the second-line setting (Finn et al, 2009). Another drug recently studied in advanced HCC is ABT-869, a VEGF and PDGF inhibitor. In a recently reported Phase II study of 44 patients, of whom 38 had class A and 6 had class B liver dysfunction, the class A patient outcome was equivalent to historic controls, with a median OS of 9.7 months and TTP of 5.4 months. Despite this similarity in outcome, and with the lack of any apparent advantage over sorafenib (Llovet et al, 2008), both brivanib and ABT-869 are currently being evaluated in large randomized Phase III trials against sorafenib.
Combination Therapy Studies with Antiangiogenics
Bevacizumab is the antiangiogenic most studied in combination with various other agents. Several Phase II studies have evaluated bevacizumab in combination with chemotherapy. Gemcitabine and oxaliplatin plus bevacizumab were examined in a Phase II trial in 30 previously treated HCC patients (Zhu et al, 2006). The objective response rate was 20%, and 27% of patients had stable disease; median PFS was 5.3 months, and median OS was 9.6 months. The most common grade 3 and 4 toxicities included leucopenia and neutropenia, elevation of transaminases, hypertension, and fatigue.
Another Phase II study evaluated the combination of bevacizumab, oxaliplatin, and capecitabine in 30 patients with advanced HCC (Sun et al, 2007). Partial response was noted in 13.3% of the patients, and 76.7% had stable disease. Median PFS and median OS were 4.5 and 10.6 months, respectively. Toxicity was not trivial: 33% of the patients sustained grade 2 or 3 peripheral neuropathy, and 11% experienced grade 2 or 3 hand-foot syndrome. In addition, three bleeding events, one gastrointestinal perforation, and two variceal hemorrhages were reported. Bevacizumab plus capecitabine only was also studied in 25 patients with advanced HCC and yielded parallel results (Hsu et al, 2007), with median PFS of 4.1 months and OS of 10.7 months. The most common treatment-related grade 3 toxicity was hand-foot syndrome, and one gastric ulcer hemorrhage was reported.
Sorafenib has also been evaluated in combination with biologic therapy and chemotherapy in two separate studies: despite the almost inexistent data (Duran et al, 2007) on the combination in HCC, one ongoing randomized Phase III study was initiated to evaluate sorafenib plus erlotinib versus sorafenib alone in patients with advanced HCC; the other study was a randomized Phase II study that evaluated doxorubicin plus sorafenib and doxorubicin plus placebo in 96 patients with advanced HCC and class A cirrhosis (Abou-Alfa, 2008). The primary end point, median TTP, was 9 months for the doxorubicin-sorafenib arm and 5 months for the doxorubicin-placebo arm. An exploratory comparison of OS between the two arms showed a significant difference of 13.7 months in favor of doxorubicin and sorafenib versus 6.5 months for doxorubicin and placebo (P = .0049; HR, 0.45). In both arms of this study, grade 3 and 4 toxicities included fatigue (15%) and neutropenia (50%). In the combination arm, sorafenib-related toxicities included grade 3 and 4 diarrhea (11%) and grade 3 and 4 hand-foot syndrome (9%). In addition, more left ventricular dysfunction was reported in the doxorubicin-sorafenib arm, observed in 19% of the cases (all grades), most of which were subclinical and caught solely on a Multiple Gated Acquisition test (MUGA) or echocardiography evaluation, with only 2% clinical grades 3 or 4.
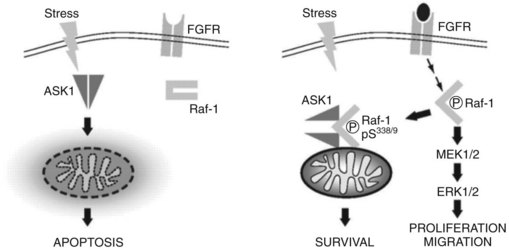
A potential synergistic effect between doxorubicin and sorafenib is suggested that may explain the improved outcome as well as the increased cardiac function. Anthracyclines such as doxorubicin depend on Ask-1 to exert their apoptotic effect. In cancer cells, a basal fibroblast growth factor (bFGF)-mediated activation of Raf-1, a target of sorafenib, may promote a complex between Raf-1 and ASK1 at the mitochondrial level that leads to inhibition of Ask-1 kinase activity and prevention of stress-mediated apoptosis of anthracyclines. Inhibiting RAF kinase activity with sorafenib may release Ask-1 and restore the apoptotic activity of doxorubicin (Alavi et al, 2007; Fig. 88.3). A large, randomized, Phase III trial was recently initiated to evaluate the combination of sorafenib and doxorubicin versus sorafenib alone, and it will answer the question regarding the potential synergy between sorafenib and doxorubicin.
Second-Line Therapies
With the advent of sorafenib as a first-line agent in the treatment of HCC, efforts to study second-line treatments have already been noticed. Brivanib has already been tested in the second-line setting (Finn et al, 2009; Raoul et al, 2009), and it is currently being evaluated against placebo in a large, randomized Phase III trial.
Assessment of Radiologic Response in Hepatocellular Carcinoma
In contrast to early clinical trials conducted in the 1970s, in which assessment of response was mainly dependent on clinical evaluation, the advent of radiologic technologies, such as contrast-enhanced computed tomography (CT) and triphasic CT scanning (see Chapter 16) and magnetic resonance imaging (MRI; see Chapter 17), have helped improve our ability to assess disease extent and tumor response to therapy in a more objective manner. Despite these improved imaging modalities, HCC remains one of the most difficult tumors to evaluate radiologically; this is due in part to the infiltrative nature of these tumors, their poor margination, and their hypervascularity, all of which make it hard to define their exact margins. In addition, there is growing recognition that response can also be assessed through dynamic measures, such as necrosis and blood flow to the tumor.
In the Phase II trial that evaluated sorafenib in patients with advanced HCC, tumor necrosis was noted in the absence of actual tumor regression (Abou-Alfa et al, 2006). This was associated with declines in AFP in several patients and in improvement of symptoms. A subanalysis to evaluate the correlation between tumor necrosis and response followed (Abou-Alfa, 2008). Triphasic CT scans of 12 patients from the study were evaluated; 5 patients were found to have stable disease or stable disease with necrosis, and 7 progressed on therapy. Median survival was based on a landmark analysis computed from the date of the last CT scan to the date of death or last follow-up to ensure that all probability estimates and statistical tests were conditional on the response status of patients at the landmark time (Anderson et al, 1983). The landmark analysis-based median survival was 4.8 months among responders and 3.1 months among nonresponders. The ratio of tumor necrosis and volume (TN/V) was significantly associated with response, with responders having greater increases in the TN/V relative to baseline compared with nonresponders (P = .02). TN/V was not significantly associated with OS and surely needs to be further evaluated prospectively as part of a large clinical trial.
Dynamic contrast-enhanced MRI (DCE-MRI) has been shown to be useful in studying the pathophysiology of tumors (Taylor, 1999; Yuh, 1999) and possibly response (Morgan et al, 2003). In view of the antiangiogenic activity of drugs like sorafenib in HCC, this technology could be helpful in assessing parameters of interest, such as microvascular density, vascular permeability, and the extravascular extracellular space (Knopp et al, 1999). DCE-MRI has also been investigated as an early indicator of response or failure of an agent. Several studies have shown that successful therapies result in early changes (within 48 hours) in DCE-MRI contrast-enhancement parameters, which may prove to be a more accurate early indication of response (Barentsz et al, 1998; Brasch et al, 1997; Pham et al, 1998). In the Phase II study evaluating sunitinib in 34 patients with advanced HCC (Zhu, 2009), DCE-MRI was performed and showed a sunitinib-related rapid decrease in vessel leakiness, and this effect was more pronounced in patients with delayed progression. In the randomized Phase II study that evaluated doxorubicin plus sorafenib and doxorubicin plus placebo in patients with advanced HCC (Abou-Alfa, 2008), DCE-MRI scans obtained on six patients showed no significant association between response and changes in kep, Ktrans, and AFP, but a trend toward significant association was observed between response and AUC180 (P = .07; Abou-Alfa et al, 2009). DCE-MRI as a surrogate of response needs to be better evaluated as part of a large clinical study.
Neoadjuvant and Conversion Therapy
In some instances, surgical resection of a large, localized primary HCC tumor might be feasible, if disease regression was achieved. Conversion therapy as a concept may render some unresectable tumors surgically approachable (see Chapter 87); it may also contribute to better outcome by possibly eradicating microscopic local and systemic disease, in contrast to neoadjuvant therapy, which is potentially given to patients considered resectable a priori. Different modalities have been studied in the context of conversion therapy for HCC, but none thus far has been shown to add any survival benefit over surgery alone. Bridging to transplant may be considered under the same umbrella, in view of its goal of maintaining or reducing a tumor size to fit within the transplant (Milan) criteria (Mazzaferro, 1996).
Transarterial chemoembolization (TACE) has been evaluated in multiple randomized studies. A randomized trial of one to five TACE applications prior to surgery (n = 24) versus surgery alone (n = 28) did not offer a benefit as neoadjuvant therapy (Wu et al, 1995). TACE had no effect on 33% of the patients who had the intervention, and they either remained with stable disease or had progression. No difference was found between the two groups in operative morbidity or mortality rates and pathologic staging. The disease-free survival (DFS) rate in the two groups was similar, but the incidence of extrahepatic cancer recurrence was higher in the neoadjuvant group (57% vs. 23%; P = .03), and 5-year survival was also significantly worse in the TACE group (30% vs. 60%; P = .01).
The experience with transarterial embolization (TAE, without chemotherapy) has not been any different. In a study that randomized 97 patients to TAE plus surgery versus surgery alone, DFS did not differ between the two groups (39 vs. 31.1 months) despite an increased rate of necrosis in the TAE group (Yamasaki et al, 1996).
The experience with yttrium-90 TheraSpheres (MDS Nordion, Kanata, Canada) in the treatment of locally advanced HCC continues to expand (Salem et al, 2002; see Chapter 84A). A new study to evaluate sorafenib with or without 90Y in patients awaiting transplant is underway. In addition, limited experience has been gained with immunoembolization (Lygidakis & Tsiliakos, 1996), radiation (Tang et al, 1995), and chemoradiation (Sitzmann & Abrams, 1993); however, none of these techniques have so far been shown to confer a survival advantage when administered in the neoadjuvant setting.
Chemotherapy-based clinical trials in the conversion therapy setting have lagged because of the discouraging response rates seen in the advanced disease setting. This has changed somewhat with the advent of the more intense chemoimmunotherapeutic combination of cisplatin, interferon, doxorubicin, and 5-FU, also known as PIAF (Patt et al, 1999). In the Phase II study discussed above of 50 patients with unresectable HCC, 13 (26%) had a partial response (Leung et al, 1999). Of those 13 patients, 9 (18%) underwent surgical resection, and 4 had a pathologic complete response, meaning no viable tumor was evident at surgery. Unfortunately, PIAF did not stand up to a randomized Phase III trial (Yeo et al, 2005) against doxorubicin in the advanced disease setting, and it is important to recall that the PIAF combination carries substantial treatment-related morbidity and mortality. The majority of those patients who had excellent performance status had grade 3 or higher hematologic toxicity, and two deaths occurred secondary to neutropenic sepsis. The role of PIAF may therefore be limited to those very thoroughly reviewed cases in a multidisciplinary clinic or conference, where conversion therapy may be valuable to a patient with a potentially resectable tumor.
Adjuvant Therapy
Regardless of surgical techniques, which have improved in the last decade, a high number of patients with resected HCC will have recurrence of their disease (Ziparo et al, 2002). In a recent series, the recurrence rate after surgical resection was 55% after 26 months’ follow-up (Cha et al, 2003). The experience with liver transplantation has been better, with lower recurrence rates; however, these are nonrandomized comparisons, and transplant patients are more carefully selected and in general have a lower volume of disease (Mazzaferro, 1996). Although liver transplantation eliminates the cirrhotic fertile ground for recurrence (Nissen et al, 2004), it unfortunately cannot be the answer for every patient with HCC, not only because of the limited supply of organs but also because of its limited efficacy in anything other than small tumors; thus the question of whether adjuvant therapy reduces the risk of recurrence after surgical resection remains an important and pressing one.
With the lack of a standard, active systemic chemotherapy for advanced disease, chemotherapy has never been seriously considered in the adjuvant setting. One very small, randomized study investigated oral carmofur (1-hexylcarbamoyl-5-fluorouracil) versus no therapy (Yamamoto et al, 1996). The study randomized and stratified 67 patients based on their Liver Cancer Study Group of Japan (LCSGJ) staging (LCSGJ, 1994). The regimen was poorly tolerated and offered no survival advantage for either stage I or II disease. DFS for the stage I patients showed a tangible advantage of 60% versus 30% at 3 years and 50% versus 20% at 5 years (P = .04). This trial was suspended prematurely, because 56% of the treated patients had unacceptable side effects.
Hepatic arterial chemotherapy, with or without systemic chemotherapy, has also been utilized in the adjuvant setting in HCC. An underpowered randomized study that combined hepatic arterial epirubicin and systemic epirubicin and carmofur versus no further therapy (Ono et al, 1997) showed no survival or DFS benefit. Side effects again led to the discontinuation of therapy in 21% of randomized patients. In addition, a randomized trial of hepatic artery epirubicin versus the same plus oral tegafur showed no difference in survival or rate of recurrence between the two groups (Kohno, 2004), and no description of any difference in side effects between the two arms was reported.
TAE, both bland and with chemotherapy, was studied in the adjuvant setting. An improvement in DFS was reported in a randomized study of a bolus hepatic arterial infusion (HAI) of Lipiodol containing doxorubicin and mitomycin C versus no adjuvant therapy after surgical resection (32% vs. 12% at 3 years; P = .02) (Izumi et al, 1994); however, no survival advantage was reported. Another trial randomized patients to a hepatic artery emulsion of iodized oil and cisplatin and intravenous epirubicin (Lai et al, 1998). This trial showed a worse DFS in the treatment group at 3 years (18% vs. 48%; P = .04). A third study of TACE versus no further therapy after surgical resection was able to show a survival advantage in patients defined at risk for residual tumor and thus recurrence (Ren et al, 2004). These risk factors included tumor diameter more than 5 cm, multiple nodules, and vascular invasion; 5-year survival was 44.36% in the adjuvant TACE group versus 37.40% in the control group (P = .0216).
More promising data in the adjuvant setting comes from the use of transarterial 131I-Lipiodol (Lau et al, 1999). In a randomized phase II trial evaluating transarterial 131I-Lipiodol versus no adjuvant therapy after surgical resection, an improvement was seen in DFS in the adjuvant therapy arm of 74% versus 36% in the control arm (P = .037). In addition, 3-year OS improved and was reported in 85% of the treatment group versus 46% of the control group (P = .039). A recent update showed the actuarial 5-year DFS in the treatment and control groups to be 61.9% and 31.8%, respectively (P = .0397), and the actuarial 5-year OS in the treatment and control groups was 66.7% and 36.4%, respectively (P = .0433); however, 7 years after randomization, the DFS and OS benefits lost their statistical significance (Lau et al, 2008). It should be noted that 131I-Lipiodol is not available in the United States.
Immune modulators have also been tested in the adjuvant setting, including an attempt at infusing autologous lymphocytes cultured with interleukin (IL)-2 and antibodies to CD-3 versus no therapy (Takayama et al, 2000), HAI of doxorubicin and IL-2 plus an infusion of lymphokine-activated killer (LAK) cells versus HAI of doxorubicin (Kawata et al, 1995), oral acyclic retinoids versus placebo (Muto et al, 1996), and interferon-α versus no therapy (Kubo, 2001). None of those studies reported a survival advantage with the exception of the retinoid study, which did on further follow-up and 2 years after the study was originally reported (Muto et al, 1999); in addition, 6-year survival was 74% in the acyclic retinoid group versus 46% in the placebo group (P = .04).
Active immunization has also been evaluated in the adjuvant setting for HCC. An HCC vaccine consisting of autologous formalin-fixed tumor tissue fragments, biodegradable microparticles containing human granulocyte macrophage colony-stimulating factor and human IL-2, and tuberculin (autologous formalin-fixed tumor [AFTV]) was tested in a small, randomized trial (Kuang et al, 2004). Nineteen patients received three intradermal vaccinations at 2-week intervals beginning 4 to 6 weeks after hepatic resection, and 22 patients received no further therapy after surgical resection. In a median follow-up of 15 months, the risk of recurrence in the vaccinated patients was reduced by 81% (P = .003). Vaccination significantly prolonged OS by 89% (P = .01). AFTV was most effective in preventing recurrence in patients with small tumors; in addition, 12 vaccinated patients showed a positive delayed-type hypersensitivity response, and 92% of those patients were recurrence-free at the end of the trial. Adverse effects were limited to grade 1 or 2 skin toxicities, such as erythema, dry desquamation, and pruritus. Again, this is a very small trial that is intriguing, but larger confirmatory data are required before the merits of this approach can be fully assessed.
A more aggressive approach was attempted in a very small pilot study of five patients who, after undergoing orthotopic liver transplantation (OLT) for HCC, received a nonmyeloablative preparative regimen of fludarabine combined with total-body irradiation or cyclophosphamide followed byallogeneic peripheral blood stem cell transplant (PBSCT), which was performed 16 to 135 days after OLT with human leukocyte antigen-matched donors (Soderdahl et al, 2003). The aim of the study was to see whether a stable mixed-donor chimerism could be sustained, which was observed in one of two patients with HCC in the study. Chimerism analysis 36 days after PBSCT showed 100% donor T cells and 90% donor myeloid cells in peripheral blood. The patient was reportedly doing well, albeit after only 10 months follow-up after OLT. Sorafenib is also being evaluated in the adjuvant setting.
Abou-Alfa GK. Selection of patients with hepatocellular carcinoma for sorafenib. J Natl Compr Canc Netw. 2009;7(4):397-403.
Abou-Alfa GK, Venook AP. The impact of new data in the treatment of advanced hepatocellular carcinoma. Curr Oncol Rep. 2008;10(3):199-205.
Abou-Alfa GK, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:1-8.
Abou-Alfa GK, et al. Is sorafenib (S) safe and effective in patients (pts) with hepatocellular carcinoma (HCC) and Child-Pugh B (CPB) cirrhosis? J Clin Oncol. 2008;26(Suppl):4518.
Abou-Alfa GK, et al. Tumor necrosis as a correlate for response in subgroup of patients with advanced hepatocellular carcinoma (HCC) treated with sorafenib. 2008.
Abou-Alfa GK, et al. Association of dynamic contrast enhanced-MRI (DCE-MRI) with response in a subgroup of patients with advanced hepatocellular carcinoma (HCC) treated with doxorubicin plus sorafenib (abstract 271), 2009 Gastrointestinal Cancers Symposium, San Francisco, 2009.
Abou-Alfa GK, et al. Phase II randomized, double-blind study of doxorubicin plus sorafenib and doxorubicin plus placebo in patients with advanced hepatocellular carcinoma. JAMA. 2010;304(19):2154-2160.
Alavi AS, et al. Chemoresistance of endothelial cells induced by basic fibroblast growth factor depends on Raf-1-mediated inhibition of the proapoptotic kinase, ASK1. Cancer Res. 2007;67(6):2766-2772.
Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710-719.
Cancer of the Liver Italian Program (CLIP) investigators. A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients. Hepatology. 1998;28(3):751-755.
Cancer of the Liver Italian Program (CLIP) investigators. Prospective validation of the CLIP score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. Hepatology. 2000;31(4):840-845.
Baker LH, et al. Adriamycin and 5-fluorouracil in the treatment of advanced hepatoma: a Southwest Oncology Group study. Cancer Treat Rep. 1977;61(8):1595-1597.
Barbare JC, et al. [Hepatocellular carcinoma with cirrhosis: treatment with doxorubicin—phase II evaluation.]. Bull Cancer. 1984;71(5):442-445.
Barentsz JO, et al. Evaluation of chemotherapy in advanced urinary bladder cancer with fast dynamic contrast-enhanced MR imaging. Radiology. 1998;207(3):791-797.
Bobbio-Pallavicini E, et al. Epirubicin and etoposide combination chemotherapy to treat hepatocellular carcinoma patients: a phase II study. Euro J Cancer. 1997;33(11):1784-1788.
Brasch R, et al. Assessing tumor angiogenesis using macromolecular MR imaging contrast media. J Magn Reson Imaging. 1997;7(1):68-74.
Cha C, et al. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. J Am Coll Surg. 2003;197(5):753-758.
Chao Y, et al. Phase II and pharmacokinetic study of paclitaxel therapy for unresectable hepatocellular carcinoma patients. Br J Cancer. 1998;78(1):34-39.
Cheng AL, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25-34.
Chenivesse X, Franco D, Brechot C. MDR1 (multidrug resistance) gene expression in human primary liver cancer and cirrhosis. J Hepatol. 1993;18(2):168-172.
Chevret S, et al. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma, for the Groupe d’Étude et de Traitement du Carcinome Hépatocellulaire. J Hepatol, 31. 1999;1:133-141.
Child CG. The Liver and Portal Hypertension. Philadelphia: Saunders; 1964. p 50
Chlebowski RT, et al. Doxorubicin (75 mg/m2) for hepatocellular carcinoma: clinical and pharmacokinetic results. Cancer Treat Rep. 1984;68(3):487-491.
Choi TK, Lee NW, Wong J. Chemotherapy for advanced hepatocellular carcinoma: adriamycin versus quadruple chemotherapy. Cancer. 1984;53(3):401-405.
Collette S, et al. Prognosis of hepatocellular carcinoma (HCC): comparison of four staging systems in two French clinical trials. J Clin Oncol ASCO Annual Meeting Proceedings. 2007;25(18S; June 20 Suppl Pt I):4589.
Damrongsak C, et al. Vinblastine in the treatment of carcinoma of liver. J Med Assoc Thailand. 1973;56(6):370-372.
DeVita VTJr, Abou-Alfa GK. Therapeutic implications of the new biology. Cancer J. 2000;6(Suppl 2):S113-S120.
Duran I, et al. Phase I targeted combination trial of sorafenib and erlotinib in patients with advanced solid tumors. Clin Cancer Res. 2007;13(16):4849-4857.
El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340(10):745-750.
Faivre S, et al. Safety and efficacy of sunitinib in patients with advanced hepatocellular carcinoma: an open-label, multicentre, phase II study. Lancet Oncol. 2009;10(8):794-800.
Falkson G, et al. Chemotherapy studies in primary liver cancer: a prospective randomized clinical trial. Cancer. 1978;42(5):2149-2156.
Falkson G, et al. Primary liver cancer: an Eastern Cooperative Oncology Group trial. Cancer. 1984;54(6):970-977.
Falkson G, et al. A random phase II study of mitoxantrone and cisplatin in patients with hepatocellular carcinoma: an ECOG study. Cancer. 1987;60(9):2141-2145.
Finn RS, et al. Phase II, open label study of brivanib alaninate in patients (pts) with hepatocellular carcinoma (HCC) who failed prior antiangiogenic therapy (abstract 200), Gastrointestinal Cancers Symposium, San Francisco, 2009.
Furuse J, et al. Phase I study of sorafenib in Japanese patients with hepatocellular carcinoma. Cancer Sci. 2008;99(1):159-165.
Garcia A, et al. Phase 1 study of ARQ 197, a selective inhibitor of the c-Met RTK in patients with metastatic solid tumors reaches recommended phase 2 dose. J Clin Oncol ASCO Annual Meeting Proceedings. 2007;25(18S; June 20 Suppl Pt 1):3525.
Gastrointestinal Tumor Study Group. A prospective trial of recombinant human interferon alpha 2B in previously untreated patients with hepatocellular carcinoma. Cancer. 1990;66(1):135-139.
Giambartolomei S, et al. Sustained activation of the Raf/MEK/Erk pathway in response to EGF in stable cell lines expressing the hepatitis C virus (HCV) core protein. Oncogene. 2001;20(20):2606-2610.
Gish RG, et al. Phase III randomized controlled trial comparing the survival of patients with unresectable hepatocellular carcinoma treated with nolatrexed or doxorubicin. J Clin Oncol. 2007;25(21):3069-3075.
Harada K, Shiota G, Kawasaki H. Transforming growth factor-alpha and epidermal growth factor receptor in chronic liver disease and hepatocellular carcinoma. Liver. 1999;19(4):318-325.
Hsu C, et al. HER-2/neu overexpression is rare in hepatocellular carcinoma and not predictive of anti-HER-2/neu regulation of cell growth and chemosensitivity. Cancer. 2002;94(2):415-420.
Hsu C, et al. Modified-dose capecitabine + bevacizumab for the treatment of advanced/metastatic hepatocellular carcinoma (HCC): a phase II, single-arm study. J Clin Oncol ASCO Annual Meeting Proceedings. 2007;25(18S; June 20 Suppl Pt 1):15190.
Huether A, et al. EGFR blockade by cetuximab alone or as combination therapy for growth control of hepatocellular carcinoma. Biochem Pharmacol. 2005;70:1568-1578.
Huitzil FD, et al. Expression of the c-Met and HGF in resected hepatocellular carcinoma (rHCC): correlation with clinicopathological features (CP) and overall survival (OS). J Clin Oncol. 2008;26(Suppl):4599.
Huitzil FD, et al. Retrospective analysis of outcome in hepatocellular carcinoma (HCC) patients (pts) with hepatitis C (C+) versus B (B+) treated with Sorafenib (S): program and abstracts of the 2007 Gastrointestinal Cancers Symposium, January 2007, Orlando, FL, 2008.
Huitzil-Melendez FD, Abou-Alfa GK, Morse MA. Novel therapies targeted at signal transduction in liver tumors. In: Clavien, PA, editor. Malignant Liver Tumors: Current and Emerging Therapies. 3rd ed. Sudbury, MA, Jones & Bartlett; 2009:382.
Huitzil-Melendez FD, et al. Advanced hepatocellular carcinoma: which staging systems best predict prognosis? J Clin Oncol. 2010;28(17):2889-2895.
Ihde DC, et al. Adriamycin therapy in American patients with hepatocellular carcinoma. Cancer Treat Rep. 1977;61(7):1385-1387.
Izumi R, et al. Postoperative adjuvant hepatic arterial infusion of Lipiodol containing anticancer drugs in patients with hepatocellular carcinoma. Hepatology. 1994;20(2):295-301.
Jarnagin WR, et al. Regional chemotherapy for unresectable primary liver cancer: results of a phase II clinical trial and assessment of DCE-MRI as a biomarker of survival. Ann Oncol. 2009;20(9):1589-1595.
Ji SK, et al. Combined cis-platinum and alpha interferon therapy of advanced hepatocellular carcinoma. Korean J Intern Med. 1996;11(1):58-68.
Jiang W, et al. Dihydropyrimidine dehydrogenase activity in hepatocellular carcinoma: implication in 5-fluorouracil–based chemotherapy. Clin Cancer Res. 1997;3(3):395-399.
Johnson PJ, et al. Induction of remission in hepatocellular carcinoma with doxorubicin. Lancet. 1978;1(8072):1006-1009.
Kardinal CG, et al. Combined doxorubicin and alpha-interferon therapy of advanced hepatocellular carcinoma. Cancer. 1993;71(7):2187-2190.
Kawata A, et al. Adjuvant chemoimmunotherapy for hepatocellular carcinoma patients: adriamycin, interleukin-2, and lymphokine-activated killer cells versus adriamycin alone. Am J Clin Oncol. 1995;18(3):257-262.
Kira S, et al. Expression of transforming growth factor alpha and epidermal growth factor receptor in human hepatocellular carcinoma. Liver. 1997;17(4):177-182.
Kiss A, et al. Analysis of transforming growth factor (TGF)-alpha/epidermal growth factor receptor, hepatocyte growth factor/c-Met, TGF-beta receptor type II, and p53 expression in human hepatocellular carcinomas. Clin Cancer Res. 1997;3(7):1059-1066.
Knopp MV, et al. Pathophysiologic basis of contrast enhancement in breast tumors. J Magn Reson Imaging. 1999;10(3):260-266.
Kohno H, et al. Effects of long-term postoperative interferon-alpha therapy on intrahepatic recurrence after resection of hepatitis C virus–related hepatocellular carcinoma: a randomized, controlled trial. Ann Int Med. 2001;134(10):963-967.
Kuang M, et al. Phase II randomized trial of autologous formalin-fixed tumor vaccine for postsurgical recurrence of hepatocellular carcinoma. Clin Cancer Res. 2004;10(5):1574-1579.
Kubicka S, et al. Phase II study of systemic gemcitabine chemotherapy for advanced unresectable hepatobiliary carcinomas. Hepatogastroenterology. 2001;48(39):783-789.
Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol. 2003;38(3):207-215.
Lai EC, et al. Postoperative adjuvant chemotherapy after curative resection of hepatocellular carcinoma: a randomized controlled trial. Arch Surg. 1998;133(2):183-188.
Lau WY, et al. Selective internal radiation therapy for nonresectable hepatocellular carcinoma with intraarterial infusion of 90-yttrium microspheres. Int J Radiation Oncol Biol Phys. 1998;40(3):583-592.
Lau WY, et al. Adjuvant intra-arterial iodine-131–labelled Lipiodol for resectable hepatocellular carcinoma: a prospective randomised trial. Lancet. 1999;353(9155):797-801.
Lau WY, et al. Adjuvant intra-arterial iodine-131–labeled Lipiodol for resectable hepatocellular carcinoma: a prospective randomized trial—update on 5-year and 10-year survival. Ann Surg. 2008;247(1):43-48.
Leung TW, et al. Complete pathological remission is possible with systemic combination chemotherapy for inoperable hepatocellular carcinoma. Clin Cancer Res. 1999;5(7):1676-1681.
Leung TW, et al. A phase II study of T138067-sodium in patients (pts) with unresectable hepatocellular carcinoma (HCC). Proc Am Soc Clin Oncol. 21, 2002. 572
Leung TW, et al. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94(6):1760-1769.
Liver Cancer Study Group of Japan. Predictive factors for long-term prognosis after partial hepatectomy for patients with hepatocellular carcinoma in Japan. Cancer. 1994;74(10):2772-2780.
Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liv Dis. 1999;19(3):329-338.
Llovet JM, et al. Sorafenib in advanced hepatocellular carcinoma, for the SHARP Investigators Study Group. N Engl J Med, 359. 2008;4:378-390.
Lygidakis NJ, Tsiliakos S. Multidisciplinary management of hepatocellular carcinoma. Hepatogastroenterology. 1996;43(12):1611-1619.
Malka D, et al. Bevacizumab in patients (pts) with advanced hepatocellular carcinoma (HCC): preliminary results of a phase II study with circulating endothelial cell (CEC) monitoring. J Clin Oncol 2007 ASCO Annual Meeting Proceedings. 2007;25(June 20 Suppl Pt 1):4570. 18S
Mazzaferro V. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693-699.
McGlynn KA, et al. International trends and patterns of primary liver cancer. Int J Cancer. 2001;94(2):290-296.
Melia WM, Johnson PJ, Williams R. Induction of remission in hepatocellular carcinoma: a comparison of VP 16 with adriamycin. Cancer. 1983;51(2):206-210.
Miller AA, et al. Phase I and pharmacokinetic study of sorafenib in patients with hepatic or renal dysfunction: CALGB 60301. J Clin Oncol. 2009;27(11):1800-1805.
Mok TS, et al. A multi-centre randomized phase II study of nolatrexed versus doxorubicin in treatment of Chinese patients with advanced hepatocellular carcinoma. Cancer Chemother Pharmacol. 1999;44(4):307-311.
Morgan B, et al. Dynamic contrast-enhanced magnetic resonance imaging as a biomarker for the pharmacological response of PTK787/ZK 222584, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinases, in patients with advanced colorectal cancer and liver metastases: results from two phase I studies. J Clin Oncol. 2003;21(21):3955-3964.
Muto Y, Moriwaki H, Saito A. Prevention of second primary tumors by an acyclic retinoid in patients with hepatocellular carcinoma. N Engl J Med. 1999;340(13):1046-1047.
Muto Y, et al. Prevention of second primary tumors by an acyclic retinoid, polyprenoic acid, in patients with hepatocellular carcinoma. Hepatoma Prevention Study Group. N Engl J Med. 1996;334(24):1561-1567.
Nerenstone SR, Ihde DC, Friedman MA. Clinical trials in primary hepatocellular carcinoma: current status and future directions. Cancer Treat Rev. 1988;15(1):1-31.
Nissen NN, et al. Emerging role of transplantation for primary liver cancers. Cancer J. 2004;10(2):88-96.
Okuda K, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment: study of 850 patients. Cancer. 1985;56(4):918-928.
Olweny CL, et al. Treatment of hepatocellular carcinoma with adriamycin: preliminary communication. Cancer. 1975;36(4):1250-1257.
Olweny CL, et al. Further experience in treating patients with hepatocellular carcinoma in Uganda. Cancer. 1980;46(12):2717-2722.
Ono T, et al. Adjuvant chemotherapy with epirubicin and carmofur after radical resection of hepatocellular carcinoma: a prospective randomized study. Semin Oncol. 24(2 Suppl 6), 1997. S6-18–S6-25
O’Reilly EM, et al. A phase II study of irinotecan in patients with advanced hepatocellular carcinoma. Cancer. 2001;91(1):101-105.
Patt YZ, et al. Low serum alpha-fetoprotein level in patients with hepatocellular carcinoma as a predictor of response to 5-FU and interferon-alpha-2b. Cancer. 1993;72(9):2574-2582.
Patt YZ, et al. Durable clinical and pathologic response of hepatocellular carcinoma to systemic and hepatic arterial administration of platinol, recombinant interferon alpha 2B, doxorubicin, and 5-fluorouracil: a communication. Am J Clin Oncol. 1999;22(2):209-213.
Patt YZ, et al. Oral capecitabine for the treatment of hepatocellular carcinoma, cholangiocarcinoma, and gallbladder carcinoma. Cancer. 2004;101(3):578-586.
Pham CD, et al. Magnetic resonance imaging detects suppression of tumor vascular permeability after administration of antibody to vascular endothelial growth factor. Cancer Invest. 1998;16(4):225-230.
Philip PA, et al. Phase II study of Erlotinib (OSI-774) in patients with advanced hepatocellular cancer. J Clin Oncol. 2005;23:6657-6663.
Pollak M, Schernhammer E, Hankinson S. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505-518.
Porta C, et al. 5-Fluorouracil and d,l-leucovorin calcium are active to treat unresectable hepatocellular carcinoma patients: preliminary results of a phase II study. Oncology. 1995;52(6):487-491.
Posey J, et al. Results of a phase 2/3 open-label, randomized trial of T138067 versus doxorubicin (DOX) in chemotherapy-naïve, unresectable hepatocellular carcinoma (HCC). J Clin Oncol 2005 ASCO Annual Meeting Proceedings. 2005;23(June 1 Suppl Pt I):4035. 16S
Pugh RN, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646-649.
Ramanathan RK, et al. A phase II study of lapatinib in patients with advanced biliary tree and hepatocellular cancer. Cancer Chemother Pharmacol. 2009;64(4):777-783.
Raoul JL, et al. An open-label phase II study of first- and second-line treatment with brivanib in patients with hepatocellular carcinoma (HCC). J Clin Oncol. 2009;27(15S Suppl):4577.
Ren ZG, et al. Postoperative adjuvant arterial chemoembolization improves survival of hepatocellular carcinoma patients with risk factors for residual tumor: a retrospective control study. World J Gastroenterol. 2004;10(19):2791-2794.
Salem R, et al. Yttrium-90 microspheres: radiation therapy for unresectable liver cancer. J Vasc Interv Radiol. 2002;13:S223-S229.
Schwartz JD, et al. Bevacizumab in hepatocellular carcinoma (HCC) for patients without metastasis and without invasion of the portal vein (abstract 134), 2005 Gastrointestinal Cancer Symposium, 2005.
Sciarrino E, et al. Adriamycin treatment for hepatocellular carcinoma: experience with 109 patients. Cancer. 1985;56(12):2751-2755.
Shan B, et al. Selective, covalent modification of beta-tubulin residue Cys-239 by T138067, an antitumor agent with in vivo efficacy against multidrug-resistant tumors. Proc Natl Acad Sciences U S A. 1999;96(10):5686-5691.
Siegel AB, et al. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol. 2008;26(18):2992-2998.
Sitzmann JV, Abrams R. Improved survival for hepatocellular cancer with combination surgery and multimodality treatment. Ann Surg. 1993;217(2):149-154.
Soderdahl G, et al. Liver transplantation followed by adjuvant nonmyeloablative hemopoietic stem cell transplantation for advanced primary liver cancer in humans. Transplantation. 2003;75(7):1061-1066.
Soini Y, et al. Expression of P-glycoprotein in hepatocellular carcinoma: a potential marker of prognosis. J Clin Pathol. 1996;49(6):470-473.
Stuart K, Tessitore J, Huberman M. 5-Fluorouracil and alpha-interferon in hepatocellular carcinoma. Am J Clin Oncol. 1996;19(2):136-139.
Stuart K, et al. A phase II trial of nolatrexed dihydrochloride in patients with advanced hepatocellular carcinoma. Cancer. 1996;86(3):410-414.
Sun W, et al. Combination of capecitabine, oxaliplatin with bevacizumab in treatment of advanced hepatocellular carcinoma (HCC): a phase II study. J Clin Oncol 2007 ASCO Annual Meeting Proceedings. 2007;25(June 20 Suppl Pt I):4574. 18S
Takayama T, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356(9232):802-807.
Tang ZY, et al. Cytoreduction and sequential resection for surgically verified unresectable hepatocellular carcinoma: evaluation with analysis of 72 patients. World J Surg. 1995;19(6):784-789.
Taylor JS. MR imaging of tumor microcirculation: promise for the new millennium. J Magn Reson Imaging. 1999;10(6):903-907.
Thomas MB, et al. Phase II trial of the combination of bevacizumab and erlotinib in patients who have advanced hepatocellular carcinoma. J Clin Oncol. 1999;27(6):843-850.
Toh H, et al. A phase II study of ABT-869 in hepatocellular carcinoma (HCC): interim analysis. J Clin Oncol. 2009;27(Suppl):4581. 15S
Venook AP, et al. Safety and pharmacokinetics (PK) of T138067 (T67) administered as a weekly 3-hour infusion in subjects with hepatocellular carcinoma (HCC) in a phase 1 study. J Clin Oncol 2004 ASCO Annual Meeting Proceedings (Post-Meeting Edition). 2004;22(14S, July 15 Suppl):4087.
Vogel CL. A phase II study of adriamycin (NSC 123127) in patients with hepatocellular carcinoma from Zambia and the United States. Cancer. 1977;39(5):1923-1929.
Webber SE, et al. Design of thymidylate synthase inhibitors using protein crystal structures: the synthesis and biological evaluation of a novel class of 5-substituted quinazolinones. J Med Chemistry. 1993;36(6):733-746.
Wilhelm SM, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64(19):7099-7109.
Williams R, Melia WM. Liver tumours and their management. Clin Radiol. 1980;31(1):1-11.
Wu CC, et al. Preoperative transcatheter arterial chemoembolization for resectable large hepatocellular carcinoma: a reappraisal. Br J Surg. 1995;82(1):122-126.
Yamamoto M, et al. Adjuvant oral chemotherapy to prevent recurrence after curative resection for hepatocellular carcinoma. Br J Surg. 1996;83(3):336-340.
Yamasaki S, et al. A prospective randomized trial of the preventive effect of pre-operative transcatheter arterial embolization against recurrence of hepatocellular carcinoma. Jpn J Cancer Res. 1996;87(2):206-211.
Yeo W, et al. A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97(20):1532-1538.
Yoshiji H, et al. Halting the interaction between vascular endothelial growth factor and its receptors attenuates liver carcinogenesis in mice. Hepatology. 2004;39:1517-1524.
Yuh WT. An exciting and challenging role for the advanced contrast MR imaging. J Magn Reson Imaging. 1999;10(3):221-222.
Zhang T, et al. Overexpression of platelet-derived growth factor receptor alpha in endothelial cells of hepatocellular carcinoma associated with high metastatic potential. Clin Cancer Res. 2005;11:8557-8563.
Zhao B, et al. Shape-constraint region-growing for delineation of hepatic metastases on contrast-enhanced CT scans. Invest Radiol. 2006;41(10):753-762.
Zhu AX. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J Clin Oncol. 2009;27(18):3027-3035.
Zhu AX, et al. Phase II study of gemcitabine and oxaliplatin in combination with bevacizumab in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:1898-1903.
Ziparo V, et al. Indications and results of resection for hepatocellular carcinoma. Euro J Surg Oncol. 2002;28(7):723-728.