40 Advanced Heart Disease
In contrast to adults, severe advanced heart disease is a rare condition in children.1,2 There are two primary etiologies of pediatric heart failure: cardiomyopathy and congenital heart disease (CHD). The overall incidence of CHD is approximately 8 per 1000 live births,1 and the incidence of cardiomyopathy is estimated at 0.58 per 100,000 children.3,4 Yet only a fraction of children diagnosed with either form of pediatric heart disease eventually progresses to advanced heart disease5 necessitating long-term intensive medical management and/or recurrent hospitalization. Those that do are at high risk for developing end-stage heart disease,5,6 a clinical syndrome characterized by marked reduction in quality of life, functional status, nutritional deficiency, and respiratory distress. Similar to adults, however, the high mortality associated with pediatric advanced heart disease stems from its sequelae, including low cardiac output, respiratory failure, malignant arrhythmias, stroke, thromboembolism, multi-organ dysfunction, and infection.5 As such, children with advanced heart disease represent a diverse group ranging from those cared for at home by their parents and still participating in childhood activities to those in hospice care at the end of life. Regardless of the scenario, there is increasing evidence that advanced heart disease impacts not only the physical health of affected children, but also the psychosocial health and quality of life of the child and his or her family across the illness trajectory. Palliative care provides an opportunity to proactively address physical, psychosocial, and spiritual issues in order to maximize the quality of life children with advanced heart disease and their families, as well as prepare families for the complex end-of-life decision making.
Incidence and Epidemiology of Congenital Heart Disease and Pediatric Advanced Heart Disease
The reported prevalence of CHD varies widely, depending on the method of diagnosis and whether the data reflect prenatally detected CHD or live births of children with CHD. In general, the prevalence of CHD is approximately 3 per 1000 for clinically severe defects, 6 per 1000 when including the more moderate defects, and 9 to 20 per 1000 when including smaller septal defects and mild valve stenosis.1,2 CHD is the leading cause of infant deaths owing to congenital anomalies worldwide.1,2 Between 1940 and 2002, approximately 2 million infants were born with CHD in the United States.1 In those same six decades, enormous achievements in medical and surgical care of these infants and children have resulted in improved long-term survival. Despite dramatically improved short- and long-term outcomes, palliated advanced heart disease remains one of the leading causes of non-accidental death in childhood in the United States. During the past 60 years, surgical procedures have been developed to treat most congenital heart defects, including those that were historically uniformly fatal, such as hypoplastic left heart syndrome.7 During the same time period, major advances have been achieved in intensive care management, ventilatory support, mechanical support, intra-operative management, long-term medical management, and diagnostic imaging.7,8 These improvements are forcing reassessment of the outcomes and impact of CHD. For example, prenatal diagnosis has led to earlier detection, more controlled perinatal transition, and earlier surgical repair as well as more educated and prepared parents. The traditional statistics of prevalence and outcomes are, therefore of historic value, but may be less helpful in determining the longer term needs of children with advanced heart disease.
Improved survival rates of children with CHD is reported over the past two decades. A review9 of the multiple-cause mortality files compiled by the National Center for Health Statistics of the Centers for Disease Control and Prevention from all death certificates filed in the United States found that from 1979 through 1997, mortality from heart defects for all ages declined 39 percent, from 2.5 to 1.5 per 100,000. In the last two years of the study, heart defects contributed to 5822 deaths per year. Of these deaths, 51% were infants and 7% were children 1 to 4 years old. In this series, age at death increased over the decade for every heart defect, as more palliated infants are surviving into adolescence and adulthood.9 Only a small number of children born with CHD will progress to advanced heart disease (Box 40-1). There are no published data on the numbers of children with CHD who meet criteria for advanced heart disease and prognostic indicators to assist with predicting which patients are likely to progress are limited.
Evolution of Palliative Care in Advanced Heart Disease in Children
Because of the previously described advances and improved longevity, pediatric cardiac teams now provide long-term care for children for whom previously the only treatment decision was the location of their death. Early efforts necessarily focused on survival. Over time, the focus expanded to include managing medical morbidity and maximizing the quality of life of children with CHD, including those with advanced heart disease. The evolution of palliative care in children with advanced heart disease has occurred slowly in part due to the perception of heart disease as treatable with the focus on surgical cures. This is similar to the evolution of care in adult advanced heart disease as well.10,11 It is important to recognize that many interactions between families and the healthcare team revolve around the surgical or interventional procedure to fix the child’s heart. Even in complex situations where palliative surgery is planned, death is rarely an immediate outcome. Aggressive, highly technological options and treatments are available and offered even in the final stages of advanced disease. Ongoing successes encourage caretakers and families to pursue continued therapies. Also, the progression of heart failure in children is largely variable, and the point at which there is no possibility for long-term survival is often unclear. Advanced heart disease is often marked by acute decompensations followed by periods of stability. This unpredictable nature often discourages end of life discussions for children and their families.
Because of the trajectory of acute decompensation alternating with periods of stability, many children with advanced heart disease die in the hospital, most often in an intensive care setting as advanced therapies are used even at the end of life. Little data are available for children with advanced heart disease. Fig. 40-1 shows the data from one large Cardiac Intensive Care Unit with approximately 1000 admissions per year. Overall, the ICU mortality is between 2% and 4% each year, which is approximately 30 to 40 deaths yearly. This percentage may vary between institutions and internationally. Although end of life discussions are happening with these families, there are limited data as to when and where these discussions are occurring.
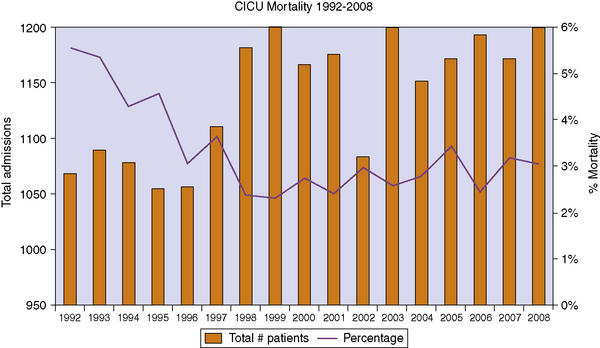
Fig. 40-1 Overall mortality in a large pediatric cardiac intensive care unit.
(Data provided by the authors.)
Symptom management for children with advanced heart disease
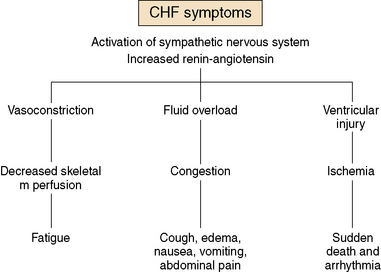
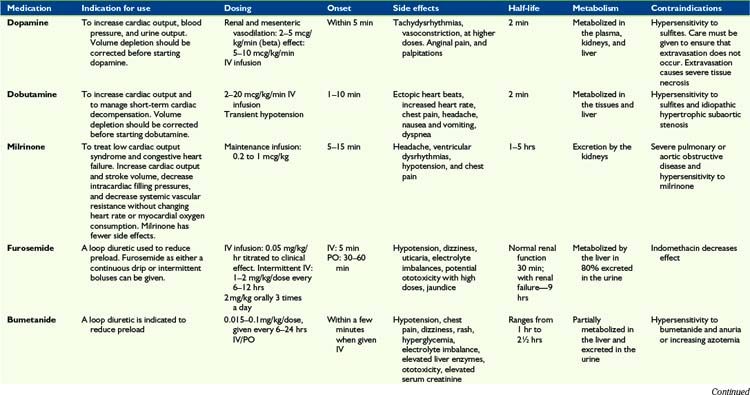
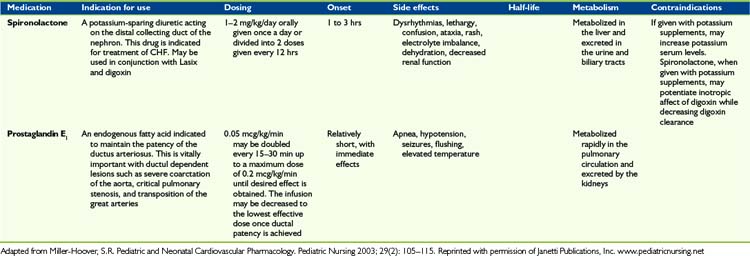
Children with advanced heart disease represent a diverse group with complex cardiac issues that result from either palliated complex CHD or from severe forms of cardiomyopathy or post-transplant care. Symptom management includes a variety of complex medical and psychosocial interventions in order to optimize quality of life. As cardiac function deteriorates, traditional symptoms of heart failure can ensue (Fig. 40-2). Symptom management can be complex and side effects of drugs can worsen symptoms. Some guidelines are offered in Table 40-1.
Fatigue
The activation of both the renin-angiotensin system and the sympathetic nervous system results in vasoconstriction and poor skeletal muscle perfusion. This often leads to overwhelming fatigue, sometimes out of proportion to the cardiac dysfunction. For patients in the early phases of advanced heart disease, fatigue can be particularly difficult to sort out, as it can often be confused with laziness or depression. Modified physical therapy and cardiac rehabilitation programs can be useful to avoid deconditioning and maintaining muscle tone. In addition, modified exercise programs have been shown to be beneficial to outlook and endurance in patients with CHD.12 For the more advanced heart disease patients, systemic vasodilators, such as milrinone, can help lower systemic vascular resistance and result in temporary improvement of fatigue. Many children and young adults describe “something lifting off my chest” after a few hours of a milronone infusion. There is some anecdotal evidence that a short infusion holiday of 3 to 5 days can have an improved effect lasting for several weeks. Some patients may benefit from this therapy either intermittently or as a continuous infusion.
Psychosocial health promotion for children with advanced heart disease
Because the trajectory of advanced heart disease is marked by episodes of acute decompensation alternating with periods of stability, children with advanced heart disease may be community dwelling and attending school, community dwelling, and unable to attend school, hospitalized, or in hospice care. Health promotion is necessarily individualized to each child. However, acknowledging this, awareness of the pervasive impact of CHD on the child and family is critical. Children with CHD may have long-term difficulties with physical growth, gross motor development, exercise capacity, behavioral abnormalities, psychiatric abnormalities, school performance, and quality of life.13 Parents may be dealing with both the uncertainty of their child’s illness and the stress of managing complicated feeding regimens, multiple medications, outpatient visits, and diagnostic procedures. Though evidence is limited, this may result in physical and mental health problems in parents or siblings, attenuated education for mothers, and a negative impact on parent employment.14,15
Psychosocial care for children with advanced heart disease and their families
As with any life-threatening chronic illness, CHD impacts the psychosocial health of affected children and families. The exact nature of the risk is difficult to pinpoint given the complexity of adaptation to chronic illness and inconsistencies in both research methods and findings. However, a body of research is emerging indicating that children with CHD may be at risk for a range of psychosocial issues, including behavioral problems, neurodevelopmental abnormalities, difficulty with social functioning, and psychiatric disorders.13,16 As healthcare team members evaluate children with CHD, each of these areas should be considered in order to identify areas for intervention to maximize the child’s quality of life.
Behavioral Problems
A systematic review of psychological adjustment of children with CHD described parental reports of behavioral difficulties ranging from 5% to 41% and at rates in excess of those occurring in normative samples or control groups.17 Both internalizing and externalizing problems are described in the literature.16–19 Further, school-age children who required surgery for CHD in the newborn period were 3 to 4 times more likely to achieve clinically significant scores for inattention and hyperactivity than normative samples.20 Disease severity was not significantly related to behavior problems in a recent meta-analysis.17 However, there is evidence that older age,17,19 deep hypothermic circulatory arrest, cyanosis, older age at repair, and multiple surgical procedures21 may be risk factors for long-term behavior problems. Despite the technological advances, behavioral outcomes of children repaired recently are not statistically different than those of children repaired in previous eras.22 Family characteristics have been associated with behavioral problems in children with CHD, including maternal worry and distress, maternal mental health, parenting style, and single parent household.17,23 Importantly, in a 2007 study,23 59% of the variance in behavioral outcomes was explained by parenting style, maternal worry, marital status, maternal mental health, and cyanotic status. Behavioral problems may affect many areas of quality of life, including family dynamics, school success, and social interactions, and are a key area for assessment and intervention when identified.
Neurodevelopmental Abnormalities
Children with CHD have, in general, higher rates of neurodevelopmental abnormalities than healthy peers13 with a reported incidence as high as 46% of children who require intervention for CHD.24 These abnormalities are multifactorial, including genetic abnormalities, brain abnormalities, and peri-operative factors such as deep hypothermic circulator arrest.13,25 Specific areas of concern include developmental and cognitive delays, sensorimotor abnormalities, and social skills delays.23,24,26–28 Much like behavioral problems, careful evaluation of the neurodevelopment status of children with CHD is very important along with early referral for special services when abnormalities are identified.
Social Functioning
The social milieu of children with CHD is much like that of other children, with an initial focus on the home and family and broadening to an ever-increasing focus on school and peers. Children with CHD and behavioral and/or neurodevelopmental abnormalities may be at particular risk socially because those abnormalities may accentuate differences from their peers. Further, limited exercise capacity may preclude participation in activities with their peers. These factors are critically important because adolescents and young adults with CHD often describe feeling different from their peers29,30 and report low self-esteem.31 School-age children who had undergone cardiac transplantation, most of whom had CHD, described the impact of scars, the need for medication, activity restrictions, and decreased physical endurance on their self-perception.32 Further, they described problems with being bullied and teased by their peers.32 Children with CHD may be more at risk for social difficulties as they mature, given the increasing importance of peer relationships with age. This may be particularly challenging for children with advanced heart failure because fatigue will decrease their ability to participate in social and recreational activities with peers and accentuate differences from peers.
Family Psychosocial Impact
Life-threatening CHD affects the child’s family as well. Parents of children with CHD have higher rates of psychosocial morbidity than parents of either healthy children or those with other chronic medical conditions.33 Research has identified areas of risk for parents, including high care-giving demand, psychological distress, and psychiatric disorders.13 Parents describe high care-giving demands, particularly related to feeding and medication regimens.34 However, they identify psychological stress as their most significant problem15 and at rates in excess of normative samples.35 Sources of stress include medical and surgical procedures, activity restrictions, uncertainty, the child’s prognosis, the child’s behavior, decisions about disclosing medical details to the child, transitioning older children to self-management, and perceptions that their child is different.34–38 Parents of children with hypoplastic left heart syndrome may be at particular risk18 as are parents of children with advanced heart failure. Parents also experience significant rates of psychiatric comorbidities, including depression, anxiety, and somatization, and there is evidence that these are chronic in nature.39 Further, parents of children with CHD may also be at risk for post-traumatic stress disorder given the known incidence in parents of children with life-threatening illness.40,41 Risk factors for psychiatric comorbidities include the burden of care giving, social isolation, financial issues, and dissatisfaction with medical care.39 Further, mothers of children with CHD may be at risk for attenuated education and employment.15
Less is known about the psychosocial impact of CHD on siblings. However, there is evidence that healthy siblings of children with CHD have an increased incidence of behavior, school and emotional problems, and depression.16,42 In a 2005 study,43 30% of parents indicated that CHD affected their healthy children in a variety of ways including anxiety, depression, anger, jealousy, recreational opportunities, feeling left out, and perceiving that the child with CHD lived under different rules. Parents of transplanted children and those with cyanotic CHD were more likely to report negative sibling effects.43 Given the described impact of CHD on the psychosocial well-being of the affected child and his or her parents and siblings, palliative care for children with CHD involves attention to the psychosocial health of the entire family.
Promoting Quality of Life for Children and Families
Much of the research describing the quality of life of children with CHD indicates that it is good and/or equivalent to that of healthy children, yet describe abnormalities on both the physical and psychosocial domains of quality of life.13,17,44,45 Little is known about the quality of life of children with advanced heart failure. However, in a 2008 study, 20% of children with CHD reported significantly impaired quality of life, with similar rates for both mild and severe CHD.44 In children and adolescents with CHD, parents and healthcare providers were asked to identify quality of life concerns, all groups more frequently identified physical dimensions, most often physical limitations.46 This is critically important in the subset of children with advanced heart failure who lack the physical stamina to participate in many developmentally appropriate activities. Scarring, medication regimens, receiving special treatment, school issues, and social issues were identified by school-age children and/or their parents as negatively affecting the children’s quality of life.46 While medication regimens were also identified by adolescents and their parents, adolescents also noted the negative impact of feeling different from peers and recognition of their own mortality.46 Adolescents also noted positive aspects of CHD, including increased strength as a person and not taking life for granted.46 Research with children with cancer and after heart transplant has demonstrated the utility of asking children a few simple questions to assess quality of life: what makes a good day for you, what makes a bad day for you, and are there some things you would like to do that you cannot?32,47 Although less is known about the quality of life of parents and siblings of children with CHD, in a 2008 study, parents of children with CHD reported significantly worse quality of life than control parents on all dimensions.48
Decision making in children with advanced heart disease
As summarized previously, many children and families with advanced heart disease have long-standing and complex behavioral, psychosocial, and neurocognitive issues that must be clearly understood and evaluated before or in parallel with the decision-making process. There are, however, limited data surrounding the end-of-life care in pediatric cardiology. There are several issues that are specific to children with advanced heart disease that need to be understood in order to help families with decision-making around end of life. The overall level of palliative care needs is most likely similar between cardiac and oncology patients; that is need for symptom relief, and communication and decision-making issues with intense family support. This is complicated by the highly variable trajectory of advanced heart disease with many patients experiencing acute exacerbations followed by periods of stability. The trigger point to discuss palliative decision making versus curative therapy is often hazy, and caretakers do not always agree on that point in time. Care of children with CHD is interdisciplinary from the beginning. However, the shift from a curative approach to palliative care is a critical time for the interdisciplinary team to work together to address symptom relief and end of life options. While the roles of many team members overlap, such as providing support to the child and family, and communicating with the child, family, and other team members, each team member makes a valued contribution to the palliative care of children with advanced heart disease (Table 40-2).
TABLE 40-2 Interdisciplinary Team Approach to Children with Advanced Heart Disease
| Team member | Contribution to care |
|---|---|
| Child and family |
High Technological Interventions and Advanced Planning
Because of the advanced nature of the heart disease of children who require ECMO or ventricular assist device support, there may be instances when withdrawing mechanical support is a consideration. Because both means of support require anticoagulation, there is a risk of hemorrhagic stroke. Children may develop multi-system organ failure that precludes transplantation. In those settings, many families elect to withdraw support. The difficulties for families with a ventricular assist device (VAD) often revolve around the patient, who may be cognitively aware and neurologically intact, with the heart beating, with severe end-organ dysfunction. This type of withdrawal by terminating the pump function feels different to the medical caretakers and families than withdrawal of an endotracheal tube. In a review of caretakers following the withdrawal of intervention support in two patients, medical caretakers identified significant psychological distress regarding discontinuation of VAD support.49 Formal institutional policy for VAD patients at end of life may help improve medical caretakers and family stress around this issue. These issues will need to be addressed with the intensive care unit teams and families together, in a sensitive and proactive manner.
1 Hoffman J.I., Kaplan S., Liberthson R.R. Prevalence of congenital heart disease. Am Heart J. 2004;147:425-439.
2 Keane J., Lock J., Fyler D., editors. Nadas’ pediatric cardiology. Philadelphia: Saunders Elsevier, 2006.
3 Nugent A.W., Daubeney P.E., Chondros P., et al. The epidemiology of childhood cardiomyopathy in Australia. N Engl J Med. 2003;348(17):1639-1646.
4 Lipshultz S.E., Sleeper L.A., Towbin J.A., et al. The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med. 2003;348(17):1647-1655.
5 Rosenthal D., Chrisant M.R., Edens E., et al. International Society for Heart and Lung Transplantation: Practice guidelines for management of heart failure in children. J Heart Lung Transplant. 2004;23(12):1313-1333.
6 Canter C.E., Shaddy R.E., Bernstein D., et al. Indications for heart transplantation in pediatric heart disease: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the young; the Councils on Clinical Cardiology, Cardiovascular Nursing, and Cardiovascular Surgery and Anesthesia; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;115(5):658-676.
7 Noonan J.A. A History of Pediatric Specialties: The development of pediatric cardiology. Pediatr Res. 2004;56(2):298-306.
8 Freedom R.M., Lock J., Bricker J.T. Pediatric cardiology and cardiovascular surgery: 1950–2000. Circulation. 2000;102:IV-58-IV-68.
9 Boneva R.S., Botto L.D., Moore C.A., Yang Q., Correa A., Erickson J.D. Mortality associated with congenital heart defects in the United States: trends and racial disparities, 1979–1997. Circulation. 2001;103(19):2376-2381.
10 O’Leary N. The comparative palliative care needs of those with heart failure and cancer patients. Curr Opin Support Palliat Care. 2009;3(4):241-246.
11 Hupcey J.E., Penrod J., Fensternmacher K. A model of palliative care for heart failure. Am J Hospice Palliat Care Med. 2009;26(5):399-404.
12 Rhodes J., Curran T.J., Camil L., Rabideau N.C., Fulton D.R., Gauthier N.S., Gauvreau K., Jenkins K.J. Impact of cardiac rehabilitation upon the exercise function of children with serious congenital heart disease. Pediatrics. 2005;116:1339-1345.
13 Green A. Outcomes of congenital heart disease: a review. Pediatr Nurs. 2004;30(4):280-284.
14 Bjornstad P.G., Spurkland I., Lindberg H.I. The impact of severe congenital heart disease on physical and psychosocial functioning in adolescents. Cardiol Young. 1995;5:56-62.
15 Samenek M. Congenital heart malformations: prevalence, severity, survival, and quality of life. Cardiol Young. 2000;10:179-185.
16 Mussatto K.A. Psychological and social aspects of paediatric heart disease. In Anderson R.H., editor: Paediatric cardiology, ed 3, Philadelphia: Elsevier, 2009.
17 Latal B., Helfricht S., Fischer J.E., et al. Psychological adjustment and quality of life in children and adolescents following open-heart surgery for congenital heart disease: a systematic review. BMC Pediatr. 2009;9:6.
18 Brosig C.L., Mussatto K.A., Kuhn E.M., et al. Psychosocial outcomes for preschool children and families after surgery for complex congenital heart disease. Pediatr Cardiol. 2007;28:255-262.
19 Karsdrop P.A., Everaerd W., Kindt M., et al. Psychological and cognitive functioning in children and adolescents with congenital heart disease: a meta-analysis. J Pediatr Psychol. 2007;32(5):527-554.
20 Shillingford A.J., Glanzman M.M., Ittenback R.F., et al. Inattention, hyperactivity, and school performance in a population of school-age children with complex congenital heart disease. Pediatrics. 2008;121(4):e759-e767.
21 Utens E.M., Verhults F.C., Duivenvoorden H.J., et al. Prediction of behavioural and emotional problems in children and adolescents with congenital heart disease. Eur Heart J. 1998;19:801-807.
22 Spijkerboer A.W., Utens E.M., Bogers A.J., et al. A historical comparison of long-term behavioral and emotional outcomes in children and adolescents after invasive treatment for congenital heart disease. J Pediatr Surg. 2008;43:534-539.
23 McCusker C.G., Doherty N.N., Molloy B., et al. Determinants of neuropsychological and behavioural outcomes in early childhood survivors of congenital heart disease. Arch Dis Child. 2007;92:137-141.
24 Weinberg S., Kern J., Weiss K., et al. Developmental screening of children diagnosed with congenital heart defects. Clin Pediatr (Phila). 2001;40:49-51.
25 Newburger J.W. Neurodevelopmental outcomes after heart surgery in children. In Allen H.D., editor: Moss and Adams’ heart disease in infants, children, and adolescents: including the fetus and young adults, ed 7, Philadelphia: Lippincott Williams & Wilkins, 2008.
26 Mahle W.T. Neurologic and cognitive outcomes in children with congenital heart disease. Curr Opin Pediatr. 2001;13:482-486.
27 Forbess J.M., Visconti K.J., Hancock-Friessen C., et al. Neurodevelopmental outcome after congenital heart surgery: Results from an institutional registry. Circulation. 2002;106(Suppl 1):I93-I102.
28 Limperopoulos C., Mahnemer A., Shevell M.J., et al. Predictors of developmental disabilities after open-heart surgery in young children with congenital heart defects. J Pediatr. 2002;141(1):51-58.
29 Horner T., Liberthson R., Jellinek M.S. Psychosocial profile of adults with complex congenital heart disease. Mayo Clin Proc. 2000;75(1):31-36.
30 Tong E.M., Sparacino P.S., Messias D.K., et al. Growing up with congenital heart disease: the dilemmas of adolescents and young adults. Cardiol Young. 1998;8:303-309.
31 Salzer-Muhar U., Herle M., Floquet P., et al. Self-concept in male and female adolescents with congenital heart disease. Clin Pediatr (Phila). 2002;41:17-24.
32 Green A., McSweeney J., Ainley K., et al. In my shoes: children’s quality of life after heart transplant. Prog Transplant. 2008;17(3):199-207.
33 Lawoko S. Factors Influencing satisfaction and well-being among parents of congenital heart disease children: development of a conceptual model based upon a literature review. Scand J Caring Sci. 2007;21:106-117.
34 Morelius E., Lundh U., Nelson N. Parental stress in relation to severity of congenital heart disease in offspring. Pediatr Nurs. 2002;28(1):28-32.
35 Uzark K., Jones K. Parenting stress with congenital heart disease. J Pediatr Health Care. 2003;17:163-168.
36 Gudmundsdottir M., Gillis C.L., Sparacino P.S., et al. Congenital heart defects and parent adolescent coping. Fam Syst Health. 1996;14(2):245-255.
37 Tak Y.R., McCubbin M. Family Stress, Perceived social support, and coping following the diagnosis of a child’s congenital heart disease. J Adv Nurs. 2002;39(3):190-198.
38 Sparacino P.SA., Tong E.M., Messias D.KH., et al. The dilemmas of parents of adolescents and young adults with congenital heart disease. Heart Lung. 1997;26(3):187-195.
39 Lawoko S., Soares J.JF. Psychosocial morbidity among parents of children with congenital heart disease: a longitudinal study. Heart Lung. 2006;35:301-314.
40 Santacroce S.J. Parental uncertainty and post-traumatic stress in serious childhood illness. J Nurs Scholarsh. 2003;35(1):45-51.
41 Farley L.M., DeMaso D.R., D’Angela E., et al. Parenting stress and parental post-traumatic stress disorder in families after pediatric heart transplantation. J Heart Lung Transplant. 2007;26(2):120-126.
42 Bellin M.H., Kovacs P.J. Fostering resilience in siblings of youths with a chronic condition: a review of the literature. Health Soc Work. 2006;31(3):209-216.
43 Wray J., Maynard L. Living with congenital or acquired heart disease in childhood: maternal perceptions of the impact on the child and family. Cardiol Young. 2005;15:133-140.
44 Uzark K., Jones K., Slusher J., et al. Quality of life in children with heart disease as perceived by children and families. Pediatrics. 2008;121(5):e1060-e1067.
45 Landolt M.A., Blechel E.R., Latal B. Health-related quality of life in children and adolescents after open-heart surgery. J Pediatr. 2008;152:349-355.
46 Marino B.S., Tomlinson R.S., Drotar D., et al. Quality-of-life concerns differ among patients, parents, and medical providers in children and adolescents with congenital and acquired heart disease. Pediatrics. 2009;123:e708-e715.
47 Hinds P.S., Gattuso J.S., Fletcher A., et al. Quality of life as conveyed by pediatric patients with cancer. Qual Life Res. 2004;13:761-772.
48 Arafa M.A., Zaher S.R., El-Dowaty A.A., et al. Quality of life among parents of children with heart disease. Health Qual Life Outcomes. 2008;6:91.
49. Thiagarajan R: Personal communication, abstract in submission.