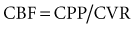31 Advanced Bedside Neuromonitoring
 Monitoring Neurologic Status
Monitoring Neurologic Status
The analytical approach to a patient with a neurologic problem is a process that requires the physician to have a specialized anatomic and physiologic knowledge of the nervous system. Daily evaluation of neurologic and mental status should be included in the neuromonitoring protocol. Function of pyramidal and extrapyramidal systems, status of cranial nerves, function of cerebellum and spinal cord whenever possible, and any trend in change of neurologic status should be recorded for every patient as part of neuromonitoring. In critically ill patients, however, such a complete neurologic evaluation can sometimes be unreliable or impossible owing to the use of sedatives and the need for intubation and ventilatory support as part of the medical treatment of the neurologic problem. Along with the neurologic examination, information about vital signs and key laboratory values should be available at the bedside in a 24-hour record sheet. A handheld pupillometer (ForSite NeurOptics Automated Infrared Pupillometer, NeurOptics Inc., Irvine, California) is a new technology that may reduce observer variability in the neurologic examination. Infrared quantitative pupillometry can produce accurate, reproducible pupillary measurements which are clearly superior to those obtained manually at the patient’s bedside by even an experienced nurse or physician.1 An important limitation of this device is that assessment is quite challenging in patients with altered mental status, in patients with periorbital or scleral edema, and in uncooperative patients. Ambient light and physiologic factors may also affect the measured pupillary characteristics.2 A recent study using this device reported good reliability when correlating the pupillary constriction velocity as a predictor of intracranial pressure (ICP) elevation in neurosurgical patients. More clinical experience is needed before including the pupillometer as a standard of care.3
The Glasgow Coma Scale (GCS) is used as a standardized scale for recording neurologic status in the ICU. The Glasgow outcome scale has been the standard outcome tool for neurocritical care. New tools such as the Neurological Outcome Scale for TBI (NOS-TBI) have been adapted for traumatic brain injury (TBI) patients from the National Institutes of Health Stroke Scale (NIHSS) and potentially could serve as a tool for initial stratification of injury severity and prediction of long-term outcome.4
 Intracranial Pressure and Cerebral Perfusion Pressure
Intracranial Pressure and Cerebral Perfusion Pressure
Normal resting ICP in an adult is less than 10 mm Hg. A sustained ICP value greater than 20 mm Hg is considered clearly abnormal. Mild to moderate intracranial hypertension is considered to be present when ICP is between 20 and 40 mm Hg, and values greater than 40 mm Hg are considered severe, life-threatening intracranial hypertension. In the 2007 Guidelines for the Management of Severe TBI, an ICP threshold above 20 to 25 mm Hg was adopted as a level III recommendation to initiate treatment to reduce ICP in patients with severe life-threatening head injury.5
Cerebral perfusion pressure (CPP) is the difference between the mean arterial blood pressure (MAP) and ICP. Under normal physiologic conditions, a MAP of 80 to 100 mm Hg and an ICP of 5 to 10 mm Hg generate a CPP of 70 to 85 mm Hg.6
Under normal circumstances, the brain is able to maintain a relatively constant CBF of approximately 50 mL per 100 g/min over a wide range of CPP (60 to 150 mm Hg).7 This process called pressure autoregulation is a complex regulatory mechanism involving both myogenic and metabolic components. Following injury, the ability of the brain to pressure autoregulate can be impaired, and CBF is often dependent on CPP.
The indications and thresholds for monitoring of CPP remain controversial. The current recommendation in TBI is to target CPP values within the range of 50 to 70 mm Hg.8 CPP values less than 50 mm Hg increase the risk of cerebral ischemia and hypoperfusion, while therapies required to maintain CPP values greater than 70 mm Hg have been associated with an increased risk of acute respiratory distress syndrome (ARDS).9 Some recent evidence suggests that the status of cerebral autoregulation should play a role in therapeutic decisions. If pressure autoregulation is intact, a CPP-directed therapy may be used with a greater chance for a favorable outcome. However, if pressure autoregulation is impaired, ICP-guided therapy may be of more benefit.10 The on-line correlation between ICP and MAP (pressure reactivity index [PRx]) or between middle cerebral artery blood flow velocity and MAP (Mx) is used to assess the status of pressure autoregulation and direct management in some critical care units.11,12
Intracranial Pressure Monitoring Devices
The current gold standard for ICP monitoring is the ventriculostomy catheter or external ventricular drain (EVD), which is a catheter inserted in the lateral ventricle, usually via a small right frontal burr hole. This ventricular catheter is connected to a standard pressure transducer via fluid-filled tubing. The external transducer must be maintained at a specific level. The reference point for ICP is the foramen of Monro, although in practical terms, the external auditory meatus is often used as a landmark. EVDs measure global ICP and have some useful advantages over other ICP monitors, including the ability to perform periodic in vivo external calibration and therapeutic CSF drainage and CSF sampling. When intracranial mass lesions or ventricular effacement due to swelling are present, EVD placement may be difficult even for the most experienced neurosurgeon. Intraventricular catheters are also associated with the highest rate of infection among the ICP monitors. Several microtransducer-tipped ICP monitors are now available on the market for clinical use (e.g., Camino ICP monitor, Codman microsensor, and Neurovent-P ICP monitor). These microtransducers can be inserted in the subdural space or directly into the brain parenchyma. Neurovent microsensors incorporating three monitoring variables (ICP, brain tissue oxygen partial pressure, and temperature) are now available; however, current clinical data with this device are limited.13 Although there are fewer risks of infection and intracranial hemorrhage with these catheters, the main disadvantage is that none can be calibrated in vivo; after preinsertion calibration, they may exhibit zero drift (degree of difference relative to zero atmospheres) over time.14
The Spiegelberg ICP monitor, which incorporates pneumatic technology, has been recently introduced. This device uses a small air pouch balloon at the end of a catheter to sense changes in pressure and automatically does in vivo calibration. A novel “lab-on-a-tube” intraventricular catheter was recently developed for multimodal neuromonitoring in patients with TBI; it provides real-time ICP, glucose, oxygen, and temperature monitoring and in situ therapeutic CSF drainage.15
Noninvasive ICP monitoring methods have been developed with the aim to reduce the risks associated with invasive monitors. Displacement of the tympanic membrane has been used to determine temporal changes in ICP.16 Recent data suggest that optical detection of cerebral edema using either broadband halogen illumination or a single-wavelength near-infrared (NIR) laser diode may allow earlier detection of cerebral edema compared with the traditional ICP monitors.17 Transcranial Doppler (TCD) pulsatility index and magnetic resonance imaging (MRI) of the optic nerve sheath have been used to provide a noninvasive estimate of ICP.18–21 Measurement of peripapillary retinal nerve fiber layer thickness with optical coherence tomography is a noninvasive quantitative technique to monitor evolution of papilledema as a predictor of intracranial hypertension.22 So far, none of these methods have provided accuracy sufficient to replace invasive ICP monitors.
ICP Waveforms
Typically, the normal ICP waveform consists of three arterial components superimposed on the respiratory rhythm. The first arterial wave is the percussion wave, which reflects the ejection of blood from the heart transmitted through the choroid plexus in the ventricles. The second wave is the tidal wave, which reflects brain compliance; and finally, the third wave is the dicrotic wave that reflects aortic valve closure. Under physiologic conditions, the percussion wave is the tallest, with the tidal and dicrotic waves having progressively smaller amplitudes. When intracranial hypertension is present, cerebral compliance is diminished. This is reflected by an increase in the peak of the tidal and dicrotic waves exceeding that of the percussion wave (Figure 31-1).
Complications
The incidence of infection for ICP devices is reported to be 1% to 27%,23 depending on the type of ICP monitoring device. A recent study investigated the complications with use of an ICP fiberoptic device (Camino) alone and in combination with an external ventricular drain (EVD) catheter; the infection rate was 1.8% and 7.9%, respectively.24 Several other factors have been identified that may affect the risk of EVD infection: use of prophylactic parenteral antibiotics; presence of other concurrent systemic infections; presence of intraventricular or subarachnoid hemorrhage; duration of monitoring; open skull fracture, including basilar skull fractures with CSF leak; leakage around the ventriculostomy catheter; and repeated flushing of the EVD. Routine exchange of ventricular catheters and prophylactic antibiotic use for EVD placement is not recommended to reduce infection rate.25 However, placement of ICP monitors should be done under the most sterile possible conditions, minimizing excessive manipulation and flushing. Although there is evidence that antibiotic-impregnated catheters may decrease rates of infection, more trials should be conducted to evaluate the beneficial effect on clinical outcome.26
 Jugular Venous Oxygen Saturation
Jugular Venous Oxygen Saturation
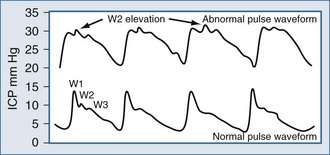
Placement of a jugular venous oxygen saturation (SjvO2) catheter involves retrograde insertion into the internal jugular vein of a catheter equipped with an oxygen sensor at the tip. The internal jugular vein catheter is similar to the type used for CVP monitoring but is directed cephalad into the jugular bulb.27 The tip of the catheter must be placed above the C1-C2 vertebral bodies to avoid contamination with blood coming from the facial vein. Correct positioning of the catheter can be confirmed with a lateral skull x-ray (Figure 31-2). The incidence of complications related to the SjvO2 catheter is low but includes carotid artery puncture, hematoma formation, infection, thrombosis, and increase in ICP that may arise during catheter insertion or due to prolonged monitoring.
The development of in vivo reflectance oximetry using fiberoptic catheters has allowed continuous monitoring of SjvO2 without the need of continuous blood sampling, except for calibration purposes.28 Changes in SjvO2 should be confirmed by measuring the oxygen saturation in a blood sample withdrawn from the jugular venous catheter, and the catheter should be recalibrated if the difference is more than 4% to increase the duration of good-quality records.27,29 Risks of intravascular catheters and possible contamination with noncerebral blood being monitored might be eliminated in the future with a promising noninvasive technique of monitoring the superior sagittal sinus. The technique detects ultrasound waves generated in tissue as pulsed NIR radiation is absorbed, resulting in thermoelastic expansion of the irradiated volume.30
Side of Jugular Catheterization
The choice of side for jugular bulb monitoring remains a debate.31,32 The jugular venous catheter can be placed on the side with the worst pathology or the side where the internal jugular vein circulation is dominant. The dominant internal jugular vein is the side with the largest vein by ultrasound imaging or by ICP response to venous compression. If the strategy is to use SjvO2 as a monitor of global oxygenation, then cannulating the dominant jugular vein is logical because it is most representative of the whole brain. However, if the strategy is to identify the most abnormal oxygen saturation, then the side with the most severe injury should be cannulated.33
Normal Jugular Venous Oxygen Saturation
SjvO2 reflects the global balance between cerebral oxygen delivery (supply) and the cerebral metabolic rate of oxygen (demand). When arterial oxygen saturation, hemoglobin concentration, and the hemoglobin dissociation curve remain stable, SjvO2 generally parallels changes in CBF. Values defining the normal range of SjvO2 are still debated but are usually considered to be 50% to 54% for the lower range and 75% for the upper range.28,34,35 Multiple pathologic clinical scenarios may cause an increase or decrease in SjvO2 values (Table 31-1). A large number of studies have assessed the role of jugular venous saturation monitoring in patients with severe TBI. In 1992, Sheinberg et al. demonstrated that single or multiple episodes of jugular venous desaturation were associated with a higher mortality rate.36 However, high SjvO2 values indicating low cerebral oxygen extraction have also been associated with poor outcome,35 and an elevated mean arteriojugular oxygen content difference has been associated with a better outcome.37 This apparent discrepancy where increased cerebral oxygen extraction is both associated with a higher mortality rate and a better neurologic outcome can probably be explained by specific circumstances in individual patients. Greater cerebral oxygen extraction means a higher cerebral metabolic rate (and better prognosis) so long as cerebral metabolic requirements are met.38 According to the most recent consensus for brain oxygen monitoring and thresholds, evidence supports a level III (SjvO2 <50%) recommendation for use of jugular venous oxygen saturation, in addition to the standard ICP monitors in the management of patients with TBI.39 Because SjvO2 provides only information of a global state of cerebral oxygenation, focal ischemic areas are not evaluated with this technique.
TABLE 31-1 Clinical Conditions Associated with Alterations in SjvO2 Values
| Increased SjvO2 | Restricted oxygen diffusion or extraction due to neuronal infarction or inflammation Decreased cerebral metabolism Increased systemic oxygen supply due to hyperoxia Hyperemia |
| Decreased SjvO2 | Local or systemic hypoperfusion (e.g., intracranial hypertension, shock or prolonged hypotension, vasospasm) Decreased systemic oxygen supply (e.g., low PaO2) Increased cerebral metabolism or oxygen extraction (e.g., seizures, fever) |
 Local or Regional Monitoring
Local or Regional Monitoring
Transcranial Doppler Flow Velocity and Flow Volume
Cerebral vasospasm is a major cause of disability after subarachnoid hemorrhage (SAH) and TBI, with similar incidence in both groups.40 The incidence of critical regional CBF reductions due to vasospasm are seen progressively when flow velocities above 120 cm/sec are present by TCD examination.41 Angiography remains the gold standard for diagnosing cerebral vasospasm, but TCD ultrasonography gives a noninvasive alternative for daily bedside monitoring of the CBF dynamics. The Lindegaard ratio (middle cerebral artery-to-extracranial internal carotid artery flow velocity ratio) helps in differentiating vasospasm from hyperemia; vasospasm is considered to be present if the Lindegaard index is greater than 3:1.42 In hyperemia, flow velocity for both intracranial and extracranial vessels increases, whereas in vasospasm, high flow velocity is seen only in intracranial vessels, resulting in a high ratio.
TCD studies have high specificity for the confirmation of brain death. Brief systolic forward flow spikes with reversed or absent diastolic flow found bilaterally or in three different arteries are accepted TCD criteria for supporting the diagnosis of brain death.43
Brain Tissue Oxygen Partial Pressure
With recent technological advances, two commercially available sensors have been produced. One sensor measures only PbtO2, using a polarographic Clark-type electrode; the other multiparameter sensor measures PbtO2, carbon dioxide, and pH, using fiberoptic technology. Both of these methods have the ability to measure brain temperature using a thermocouple. Both sensors are approximately 0.5 mm in diameter and can be inserted intraoperatively at the time of a craniotomy or through a specially designed bolt that allows insertion and fixation to the skull in the ICU. The Clark electrode polarographic probe has a semipermeable membrane covering two electrodes. In the presence of dissolved oxygen crossing the membrane, an electric current is generated then transferred to a monitor for interpretation. Temperature is also needed to calculate the oxygen tension. Brain temperature rather than core temperature is preferred for this purpose.44
Normal values for PbtO2 are 20 to 40 mm Hg, and critical levels are 8 to 10 mm Hg. The likelihood of death following a severe TBI increases the longer the PbtO2 remains below 15 mm Hg and with any occurrence of PbtO2 below 6 mm Hg.45 Attempts to identify specific PO2 thresholds for ischemia have been made by different authors using different approaches. Although this threshold is as yet not clearly defined with relation to outcome, there are some reports indicating that PbtO2 values less than 8 to 10 mm Hg represent a high risk of ischemia, although others suggest higher threshold values. Other parameters (PbtH <7.0 and PbtCO2 >60 mm Hg) have been proposed as an increased risk for vasospasm and mortality in stroke and TBI, respectively.
For TBI patients, PbtO2 monitoring has been incorporated into an overall management strategy, along with ICP and other standard monitoring. Decreased mortality in TBI patients managed using a PbtO2-targeted management strategy (maintaining PbtO2 >25 mm Hg) has been reported.46 Narotam et al. also reported an improved 6-month clinical outcome over the standard ICP/CPP-directed therapy when aggressive treatment of cerebral hypoxia with a PbtO2-directed protocol (>20 mm Hg).47
Treating a reduced PbtO2 should be first directed to any underlying causes of inadequate cerebral oxygen delivery. Such corrections might include increasing CPP (reducing ICP, increasing MAP), improving arterial oxygenation, transfusions for a low hemoglobin concentration, reducing fever, or treating subclinical seizures. If an underlying cause for the low PbtO2 is not found, or if PbtO2 remains low after optimizing oxygen delivery, obtaining a follow-up CT scan of the head might be considered to assess whether a delayed hematoma or hemorrhagic contusion has developed. A sustained (>30 min) PbtO2 of 0 mm Hg and unresponsive to oxygen challenge is consistent with brain death,48 although care related to interpretation in this regard is needed depending on the location of the probe or malfunction of the probe.
Near-Infrared Spectroscopy
The principle of near-infrared spectroscopy (NIRS) is based on the fact that light in the near-infrared range (700 to 1000 nm) can pass through skin, bone, and other tissues relatively easily. Oxygenated hemoglobin, deoxygenated hemoglobin, and cytochrome aa3 have different absorption spectra. Changes in the absorbance of near-infrared light as it passes through these compounds can be quantified using a modified Beer-Lambert law, which describes optical attenuation. The main advantage of NIRS is that it is a noninvasive method of estimating regional changes in cerebral oxygenation. However, its clinical use is limited by an inability to differentiate between intracranial and extracranial changes in blood flow and oxygenation. This shortcoming adversely affects the reliability of the readings47 and results in an inconsistent impact for monitoring of decreased oxygenation on neurologic outcome.49
Electroencephalogram
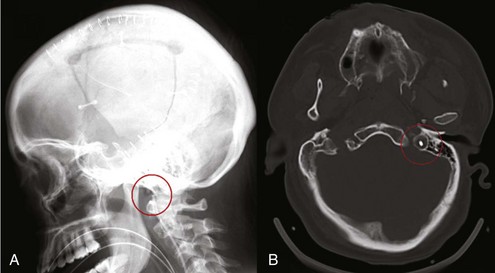
An electroencephalogram (EEG) represents spontaneous electrical activity of the cerebral cortex and is generated mainly by the summation of excitatory and inhibitory postsynaptic potentials of cortical neurons. EEG does not reflect activity in subcortical levels, cranial nerves, or the spinal cord. The electrical signal is amplified, filtered, and then displayed as either 2 (monitoring) or 16 channels (diagnostic) to give a representation of electrical activity of the cortex. EEG activity is usually interpreted in terms of frequency, amplitude, pattern, and symmetry. Indications for continuous EEG (cEEG) include detection of nonconvulsive electrographic seizures (NCSZs); periodic epileptiform discharges (PEDs) or status epilepticus (NCSE) in patients with unexplained fluctuating mental status; better characterization of suspicious tremors, nystagmus, or clonus and inexplicable changes in blood pressure and heart rate; evaluation of level of coma during sedation and burst-suppression management in drug-induced coma (Figure 31-3); uncovering ischemia due to vasospasm or during neurovascular procedures; and for prognostication.
To facilitate continuous EEG monitoring, several other automated EEG processing systems have been developed. Quantitative cEEG (qEEG) allows for evaluation of a large amount of data over long periods of time (raw EEG waveforms) in the form of a summary, many of which were found to correlate with poor prognosis. A recent study confirmed a long-held (but previously unsupported) premise that electrographic seizures are deleterious for TBI patients, resulting in delayed and prolonged increase in ICP and lactate/pyruvate ratio.50 Decreased relative alpha variability may detect the onset of vasospasm up to 2 days before clinical symptoms.51 In patients with acute intracranial hemorrhage, NCSZ was associated with midline shift increase, early hematoma enlargement, and a trend toward poor outcome.52
EEG recordings using depth electrodes are much less frequently contaminated by shivering artifact during induced hypothermia. Studies suggest that epileptiform activity registered with the mini-depth electrode was more sensitive in detecting metabolic crisis confirmed by microdialysis.53 Intracortical EEG can provide high-fidelity intracranial EEG in an ICU setting, can detect ictal discharges not readily obvious on scalp EEG, and can recognize early changes in brain activity caused by secondary neurologic complications.54
The difficulties with implementing cEEG in the ICU setting include easy artifact generation and high costs for EEG equipment and human resources, including EEG technicians to preserve high-quality recordings, electroencephalographers to review the studies, and the need for training nursing and medical staff to recognize basic EEG patterns. Randomized clinical trials comparing EEG-guided therapy with standard medical therapy are warranted, with predetermined endpoints such as neurologic outcomes, ICU and hospital length of stay, and cost-effectiveness.55
Microdialysis
Cerebral microdialysis has been applied to patients in many different clinical situations, including those with TBI, SAH, epilepsy, ischemic stroke, and tumor, as well as during neurosurgery and cardiac surgery. A high lactate/pyruvate ratio (LPR >40) has been classically linked to ischemia/hypoxia and poor prognosis.56 Isolated “nonischemic” elevated LPR due to diminished pyruvate has also been associated with cerebral metabolic derangement.57 Newer semipermeable membranes with a higher limit in size (up to 300 kD) also allow for the passage of polypeptides and proteins from the extracellular space (e.g., cytokines,58 antibiotics, free phenytoin in experimental research).
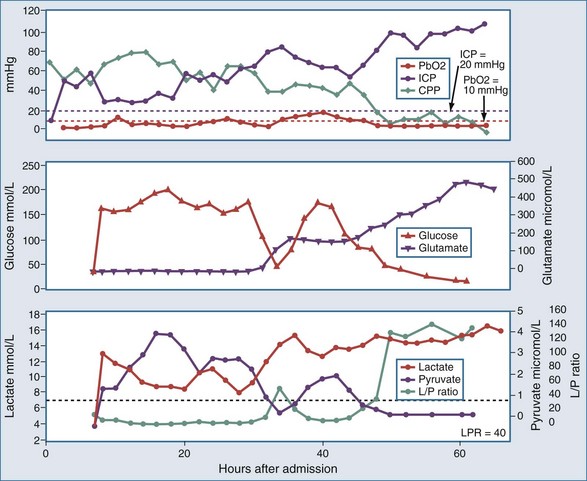
As with PO2 probes, the location of the microdialysis catheter is critical for interpretation of measurements. The catheter can be inserted into areas at risk of ischemia, such as the vascular territory most likely affected by vasospasm or brain regions surrounding a mass lesion, or in a standardized location such as the right frontal lobe in diffuse brain injury. Different patterns of energy substrates have been described at different levels of hypoxia, which could be helpful clinically in assessing effects of treatment and providing prognosis.59 Specific patterns of energy substrates may warn of evolving brain injury after evacuation of subdural hematomas (Figure 31-4).60 Providing nutritional amino acids intravenously in neurointensive care patients does not increase cerebral glutamate.61 Glycerol has also been validated as a marker of cell membrane damage.62 Glutamate levels have been correlated with mortality rate and 6-month functional outcome after severe TBI.63 In patients with SAH where CSF cell count is not helpful, microdialysis may serve as an adjunct criterion for early diagnosis of meningitis (fever + low glucose in microdialysate).64 A new, yet-to-be-characterized small peptide m/z (mass/charge) ≈ 5000 found in the microdialysate after TBI might represent a novel biomarker of metabolic distress.65 Interstitial T-tau levels were higher in microdialysate of TBI patients with mass lesions, whereas Aβ42 levels were found to be higher in TBI patients with diffuse axonal injury.66
Inserting single or multiple microdialysis catheters by using a percutaneous technique has a low complication rate (infection 0%, hemorrhage 3%), but the incidence of technical problems with malfunctioning catheters is high (15%) because of membrane fragility, especially during patient transport.67 In addition, medical staffs find maintaining microdialysis to be cumbersome. High sample storage volume and questionable accuracy of further off-line analysis, owing to thawing/evaporation, are its major disadvantages. Microdialysis may be further innovated by coupling capillary and microchip electrophoresis.68 Currently, microdialysis can only be fruitfully used in combination with other monitoring methods. Evidence of its usefulness is growing, although studies targeting threshold values for metabolites and neurotransmitters are needed.
Key Points
Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care, AANS/CNSJ. Guidelines for the management of severe traumatic brain injury. IX. Cerebral perfusion thresholds. Neurotrauma. 2007;24(Suppl 1):S59-S64.
These guidelines summarize the current clinical applications of CPP-based therapy.
Brady KM, Shaffner DH, Lee JK, et al. Continuous monitoring of cerebrovascular pressure reactivity after traumatic brain injury in children. Pediatrics. 2009;124(6):e1205-e1212.
Andrews PJ, Citerio G, Longhi L, Polderman K, Sahuquillo J, Vajkoczy P, Neuro-Intensive Care and Emergency Medicine (NICEM) Section of the European Society of Intensive Care Medicine. NICEM consensus on neurological monitoring in acute neurological disease. Intensive Care Med. 2008;34(8):1362-1370. Epub 2008 Apr 9
Wartenberg KE, Schmidt JM, Mayer SA. Multimodality monitoring in neurocritical care. Crit Care Clin. 2007;23(3):507-538.
Vespa PM, Miller C, McArthur D, et al. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit Care Med. 2007;35(12):2830-2836.
Claassen J, Jetté N, Chum F, et al. Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology. 2007;69(13):1356-1365.
Marcoux J, McArthur DA, Miller C, et al. Persistent metabolic crisis as measured by elevated cerebral microdialysis lactate-pyruvate ratio predicts chronic frontal lobe brain atrophy after traumatic brain injury. Crit Care Med. 2008;36(10):2871-2877.
1 Meeker M, Du R, Bacchetti P, et al. Pupil examination: validity and clinical utility of an automated pupillometer. J Neurosci Nurs. 2005;37(1):34-40.
2 Boev AN, Fountas KN, Karampelas I, et al. Quantitative pupillometry: normative data in healthy pediatric volunteers. J Neurosurg. 2005;103(6):496-500. Suppl
3 Fountas KN, Kapsalaki EZ, Machinis TG, Boev AN, Robinson JSIII, Troup EC. Clinical Implications of Quantitative Infrared Pupillometry in Neurosurgical Patients. Neurocrit. Care. 2006;05:55-60.
4 Wilde EA, McCauley SR, Kelly TM, et al. Feasibility of the Neurological Outcome Scale for Traumatic Brain Injury (NOS-TBI) in Adults. J Neurotrauma. 2010;27(6):975-981.
5 Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons, Joint Section on Neurotrauma and Critical Care, AANS/CNS. Guidelines for the management of severe traumatic brain injury. VIII. Intracranial pressure thresholds. J Neurotrauma. 2007;24(Suppl 1):S55-S58.
6 Vespa P. What is the optimal threshold for cerebral perfusion pressure following traumatic brain injury? Neurosurg Focus. 2003;15(6):E4.
7 Steiner LA, Andrews PJ. Monitoring the injured brain: ICP and CBF. Br J Anaesth. 2006;97:26-38.
8 Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care, AANS/CNSJ (2007). Guidelines for the management of severe traumatic brain injury. IX. Cerebral perfusion thresholds. Neurotrauma. 2007;24(Suppl 1):S59-S64.
9 Contant CF, Valadka AB, Gopinath SP, Hannay HJ, Robertson CS. Adult respiratory distress syndrome: a complication of induced hypertension after severe head injury. J Neurosurg. 2001;;95:560-568.
10 Howells T, Elf K, Jones PA, et al. Pressure reactivity as a guide in the treatment of cerebral perfusion pressure in patients with brain trauma. J Neurosurg. 2005;102:311-317.
11 Brady KM, Shaffner DH, Lee JK, et al. Continuous monitoring of cerebrovascular pressure reactivity after traumatic brain injury in children. Pediatrics. 2009 Dec;124(6):e1205-e1212.
12 Consonni F, Abate MG, Galli D, Citerio G. Feasibility of a continuous computerized monitoring of cerebral autoregulation in neurointensive care. Neurocrit Care. 2009;10(2):232-240. Epub 2008 Oct 16
13 Citerio G, Piper I, Chambers IR, et al. BrainIT group. Multicenter clinical assessment of the Raumedic Neurovent-P intracranial pressure sensor: a report by the BrainIT group. Invest Ophthalmol Vis Sci. 2009 Nov;50(11):5197-5200. Epub 2008 Nov 14
14 Al-Tamimi YZ, Helmy A, Bavetta S, Price SJ. Assessment of zero drift in the Codman intracranial pressure monitor: a study from 2 neurointensive care units. Neurocrit Care. 2009;10(3):373-386. Epub 2009 Jan 6
15 Li C, Wu PM, Jung W, Ahn CH, Shutter LA, Narayan RK. A novel lab-on-a-tube for multimodality neuromonitoring of patients with traumatic brain injury (TBI). Neurosurgery. 2009 Jan;64(1):94-98. discussion 98-9
16 Shimbles S, Dodd C, Banister K, Mendelow AD, Chambers IR. Clinical comparison of tympanic membrane displacement with invasive ICP measurements. Acta Neurochir Suppl. 2005;95:197-199.
17 Gill AS, Rajneesh KF, Owen CM, Yeh J, Hsu M, Binder DK. Early optical detection of cerebral edema in vivo. Department of Neurological Surgery, University of California, Irvine, California. J Trauma 2009 Dec 24. [Epub ahead of print].
18 Figaji AA, Zwane E, Fieggen AG, Siesjo P, Peter JC. Transcranial Doppler pulsatility index is not a reliable indicator of intracranial pressure in children with severe traumatic brain injury. Division of Neurosurgery, School of Child and Adolescent Health, Red Cross Children’s Hospital, University of Cape Town, Cape Town 7700, South Africa. anthony.figaji@uct.ac.za. Lab Chip. 2009 Jul 21;9(14):1988-1990. Epub 2009 Mar 20
19 Schatlo B, Gläsker S, Zauner A, Thompson BG, Oldfield EH, Pluta RM. Continuous neuromonitoring using transcranial Doppler reflects blood flow during carbon dioxide challenge in primates with global cerebral ischemia. Neurosurgery. 2009 Jun;64(6):1148-1154. discussion 1154
20 Kimberly HH, Noble VE. Using MRI of the optic nerve sheath to detect elevated intracranial pressure. Crit Care. 2008;12(5):181. Epub 2008 Sep 24
21 Geeraerts T, Newcombe VF, Coles JP, et al. Use of T2-weighted magnetic resonance imaging of the optic nerve sheath to detect raised intracranial pressure. Crit Care. 2008;12(5):R114. Epub 2008 Sep 11
22 Rebolleda G, Muñoz-Negrete FJ. Follow-up of mild papilledema in idiopathic intracranial hypertension with optical coherence tomography. Neurosurgery. 2008 Dec;63(6):1152-1158. discussion 1158
23 Lozier AP, Sciacca RR, Romagnoli MF, Connolly ESJr. Ventriculostomy-related infections: a critical review of the literature. Neurosurgery. 2002;51:170-181.
24 Anderson RC, Kan P, Klimo P, Brockmeyer DL, Walker ML, Kestle JR. Complications of intracranial pressure monitoring in children with head trauma. J Neurosurg. 2004 Aug;101(1 Suppl):53-58.
25 Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care; AANS/CNSBratton SL, Chestnut RM, Ghajar J, et al. Guidelines for the management of severe traumatic brain injury. IV. Infection prophylaxis. J Neurotrauma. 2007;24(Suppl 1)):S26-S31.
26 Zambramski JM, Whiting D, Darouiche RO, et al. Efficacy of antimicrobial-impregnated external ventricular drain catheters: a prospective, randomized, controlled trial. Neurosurgery. 2003;98:725-730.
27 Wartenberg KE, Schmidt JM, Mayer SA. Multimodality monitoring in neurocritical care. Crit Care Clin. 2007;23:507-538.
28 Rohlwink UK, Figaji AA. Methods of monitoring brain oxygenation. Childs Nerv Syst. 2010;26:453-464.
29 Gopinath SP, Valadka AB, Uzura M, et al. Comparison of jugular venous oxygen saturation and brain tissue PO2 as monitors of cerebral ischemia after head injury. Crit Care Med. 1999;27(11):2337-2345.
30 Petrova IY, Petrov YY, Esenaliev RO, Deyo DJ, Cicenaite I, Prough DS. Noninvasive monitoring of cerebral blood oxygenation in ovine superior sagittal sinus with novel multi-wavelength optoacoustic system. Opt Express. 2009 Apr 27;17(9):7285-7294.
31 Latronico N, Beindorf AE, Rasulo FA, et al. Limits of intermittent jugular bulb oxygen saturation monitoring in the management of severe head trauma patients. Neurosurgery. 2000;46:1131-1138. discussion 1138-1139
32 Macmillan CS, Andrews PJ. Cerebrovenous oxygen saturation monitoring: practical considerations and clinical relevance. Intensive Care Med. 2000;26:1028-1036.
33 Metz C, Holzschuh M, Bein T, et al. Monitoring of cerebral oxygen metabolism in the jugular bulb: Reliability of unilateral measurements in severe head injury. J Cereb Blood Flow Metab. 1998;18:332-343.
34 Macmillan CS, Andrews PJ, Easton VJ. Increased jugular bulb saturation is associated with poor outcome in traumatic brain injury. J Neurol Neurosurg Psychiatry. 2001;70:101-104.
35 Cormio M, Valadka AB, Robertson CS. Elevated jugular venous oxygen saturation after severe head injury. J Neurosurg. 1999;90:9-15.
36 Sheinberg MS, Kanter MJ, Robertson CS, et al. Continuous monitoring of jugular venous oxygen saturation in head-injured patients. J Neurosurg. 1992;76:212-217.
37 Stocchetti N, Canavesi K, Magnoni S, et al. Arterio-jugular difference of oxygen content and outcome after head injury. Anesth Analg. 2004;99:230-234.
38 Robertson CS, Narayan RK, Gokaslan Z, Pahwa R, Grossman RG. Evaluation of cerebral arteriovenous oxygen difference as an estimate of cerebral blood flow in comatose patients. J Neurosurg. 1989;70:222-230.
39 Bratton SL, Chestnut RM, Ghajar J, et al. Guidelines for the management of severe traumatic brain injury. X. Brain oxygen monitoring and thresholds. Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons, Joint Section on Neurotrauma and Critical Care, AANS/CNS. J Neurotrauma. 2007;24(Suppl 1):S65-S70.
40 Oertel M, Boscardin J, Obrist W, et al. Posttraumatic vasospasm: The epidemiology, severity, and time course of an underestimated phenomenon: a prospective study performed in 299 patients. J Neurosurg. 2005;103:812-824.
41 Chieregato A, Battaglia R, Sabia G, et al. A diagnostic flowchart, including TCD, Xe-CT and angiography, to improve the diagnosis of vasospasm critically affecting cerebral blood flow in patients with subarachnoid haemorrhage, sedated and ventilated. Acta Neurochir Suppl. 2008;104:251-253.
42 Lindegaard KF, Nornes H, Bakke SJ, et al. Cerebral vasospasm after subarachnoid haemorrhage investigated by means of transcranial Doppler ultrasound. Acta Neurochir Suppl (Wien). 1988;42:81-84.
43 Dosemeci L, Dora B, Yilmaz M, et al. Utility of transcranial Doppler ultrasonography for confirmatory diagnosis of brain death: two sides of the coin. Transplantation. 2004;77(1):71-75.
44 Rumana Ch, Gopinath S, Uzura M, Valadka AB, Robertson CS. Brain temperature exceeds rectal temperature in head-injured patients. Crit Care Med. 1998;26:562-567.
45 Valadka A, Gopinath SP, Contant CF, et al. Critical values for brain tissue PO2 to outcome after severe head injury. Crit Care Med. 1998;26:1576-1581.
46 Stiefel MF, Spiotta A, Gracias VH, et al. Reduced mortality rate in patients with severe traumatic brain injury treated with brain tissue oxygen monitoring. J Neurosurg. 2005;103(5):805-811.
47 Narotam P, Morrison J, Nathoo N. Brain tissue monitoring in traumatic brain injury and major trauma: outcome analysis of a brain tissue-directed therapy. J Neurosurg. 2009;111:672-682.
48 Smith M, Counelis G, Maloney-Wilensky E, Stiefel M, Donley K, LeRoux P. Brain tissue oxygen tension in clinical brain death> a case series. Neurol Res. 2007 Oct;29(7):755-759.
49 Murkin J, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth. 2009;103(Suppl. I):i3-i13.
50 Vespa PM, Miller C, McArthur D, et al. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit Care Med. 2007;35(12):2830-2836.
51 Claassen J, Mayer SA, Hirsch LJ. Continuous EEG monitoring in patients with subarachnoid hemorrhage. J Clin Neurophysiol. 2005;22:92-98.
52 Claassen J, Jetté N, Chum F, et al. Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology. 2007;69:1356-1365.
53 Waziri A, Arif H, Oddo M, et al. Early experience with a cortical depth electrode for ICU neurophysiological monitoring. Epilepsia. 2007;48(Suppl 6):208-209.
54 Waziri A, Claassen J, Stuart R, et al. Intracortical electroencephalography in acute brain injury. Ann Neurol. 2009;66:366-377.
55 Kurtz P, Hanafy K, Claassen J. Continuous EEG monitoring: is it ready for prime time? Curr Opin Crit Care. 2009;15:99-109.
56 Marcoux J, McArthur DA, Miller C, et al. Persistent metabolic crisis as measured by elevated cerebral microdialysis lactate-pyruvate ratio predicts chronic frontal lobe brain atrophy after traumatic brain injury. Crit Care Med. 2008;36:2871-2877.
57 Dusick JR, Glenn TC, Lee WN, et al. Increased pentose phosphate pathway flux after clinical traumatic brain injury: a [1,2-13C2]glucose labeling study in humans. J Cereb Blood Flow Metab. 2007;27:1593-1602.
58 Folkersma H, Brevé J, Tilders F, Cherian L, Robertson CS. Cerebral microdialysis of interleukin (IL)-1ß and IL-6: extraction efficiency and production in the acute phase after severe traumatic brain injury in rats. Acta Neurochir. 2008;150:1277-1284.
59 Hlatky R, Valadka A, Goodman J, Contant C, Robertson CS. Patterns of energy substrates during ischemia measured in the brain by microdialysis. J Neurotrauma. 2004 Jul;21(7):894-906.
60 Hlatky R, Valadka AB, Goodman JC, Robertson CS. Evolution of brain tissue injury after evacuation of acute traumatic subdural hematomas. Neurosurgery. 2007 Jul;61(1 Suppl):249-254.
61 Ronne-Engström E, Hillered L, Enblad P, Karlsson T. Cerebral interstitial levels of glutamate and glutamine after intravenous administration of nutritional amino acids in neurointensive care patients. Neurosci Lett. 2005 Aug 12-19;384(1-2):7-10.
62 Merenda A, Gugliotta M, Holloway R, et al. Validation of brain extracellular glycerol as an indicator of cellular membrane damage due to free radical activity after traumatic brain injury. J Neurotrauma. 2008 May;25(5):527-537.
63 Chamoun R, Suki D, Gopinath S, Goodman J, Robertson CS. Role of extracellular glutamate measured by cerebral microdialysis in severe traumatic brain injury. J Neurosurg. 2010 Jan 29. [Epub ahead of print]
64 Schlenk F, Frieler K, Nagel A, Vajkoczy P, Sarrafzadeh AS. Cerebral microdialysis for detection of bacterial meningitis in aneurysmal subarachnoid hemorrhage patients: a cohort study. Crit Care. 2009;13(1):R2. Epub 2009 Jan 20
65 Lakshmanan R, Loo JA, Drake T, et al. Metabolic crisis after traumatic brain injury is associated with a novel microdialysis proteome. Neurocrit Care. 2010 Mar 12. [Epub ahead of print]
66 Marklund N, Blennow K, Zetterberg H, Ronne-Engström E, Enblad P, Hillered L. Monitoring of brain interstitial total tau and beta amyloid proteins by microdialysis in patients with traumatic brain injury. J Neurosurg. 110(6), 2009 Jun. 1227-1223
67 Poca MA, Sahuquillo J, Vilalta A, de los Rios J, Robles A, Exposito L. Percutaneous implantation of cerebral microdialysis catheters by twist-drill craniostomy in neurocritical patients: description of the technique and results of a feasibility study in 97 patients. J Neurotrauma. 2006 Oct;23(10):1510-1517.
68 Guihen E, O’Connor WT. Capillary and microchip electrophoresis in microdialysis: recent applications. Electrophoresis. 2010 Jan;31(1):55-64.