Adult Resuscitation
Perspective
It is estimated that 236,000 to 325,000 patients are treated for out-of-hospital cardiac arrest each year in the United States. The proportion of emergency medical services (EMS)–treated cardiac arrest patients with an initial rhythm of ventricular fibrillation (VF) has declined over time to one third or less in recent U.S. studies.1–4 Furthermore, the number of patients receiving bystander cardiopulmonary resuscitation (CPR) remains low, averaging only 26% in the home and 45% in public locations.5 Bystander automated external defibrillators (AEDs) are applied in 1% of home arrests and 8% of arrests in public settings.5
There is tremendous variability in survival to hospital discharge after EMS-treated cardiac arrest, ranging from 3 to 16.7% with a median of 7.9%.1 For the subset of patients who achieve return of spontaneous circulation (ROSC) long enough to be admitted to the hospital, there is significant interinstitutional variability in survival ranging from 19 to 59%.6 Of patients surviving to hospital discharge, one third have persistent neurologic deficits, and less than half return to prearrest function. In patients meeting the inclusion criteria for clinical therapeutic hypothermia trials, favorable outcome has been reported in approximately 50% of comatose cardiac arrest survivors treated with hypothermia.7,8 Subsequent studies implementing therapeutic hypothermia as part of a goal-directed post–cardiac arrest treatment protocol have reported rates of survival with good neurologic outcome ranging from 44 to 56%.9,10 It is important to recognize that the entire system of care affects patient outcomes, and the variability in outcomes across the country likely reflects variability in how well these systems function.
Principles of Disease
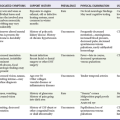
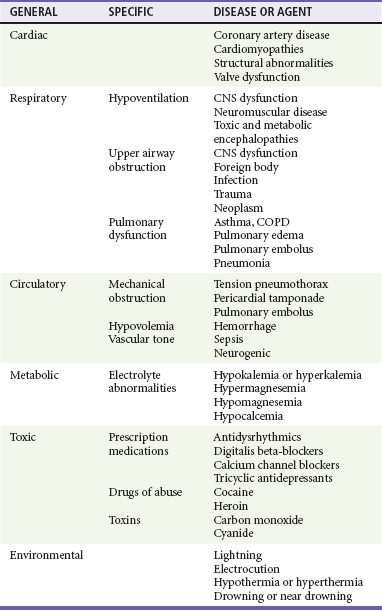
Understanding the causes of cardiac arrest helps direct therapy and diagnostic testing during resuscitation and in the immediate post–cardiac arrest period (Table 9-1). Cardiac arrest from a primary cardiac origin typically manifests as VF or much less often as pulseless ventricular tachycardia (VT). Coronary artery disease is the most common pathologic condition found in patients who die suddenly from VF; autopsy studies show a 75% incidence of previous myocardial infarction (MI) and a 20 to 30% incidence of acute MI.11 Other anatomic abnormalities associated with sudden cardiac death caused by VF or VT include myocardial hypertrophy, cardiomyopathy, and specific structural abnormalities. Pulseless electrical activity (PEA) and asystole are less common presenting rhythms in patients with a cardiac cause of arrest. These rhythms most often occur as a deterioration of VF or VT or develop in response to resuscitation treatments, such as defibrillation.
Clinical Features and Management
Most cardiac arrest cases managed in the emergency department (ED) initially occur outside the hospital. An increasing number of first responders, nontraditional providers, and public venues are being equipped with AEDs. Dramatic resuscitation rates have been achieved when these programs enable providers and the lay public to deliver defibrillation within the first few minutes of arrest.12–15 Programs that fail to enable a significant number of patients to be defibrillated within this critical time window have limited or no effect on survival.16,17
Advanced life support (ALS) units staffed by paramedics often have standing orders to follow advanced protocols. Because quality of CPR and time to defibrillation are the two most important determinants of outcome, there is no evidence to support interrupting properly performed advanced measures to transport a patient who is still in cardiac arrest. In cases of cardiac arrest refractory to properly performed advanced measures, the patient may be pronounced dead at the scene if appropriate protocols have been outlined within the system.18
Simultaneous assessment and management of cardiac arrest occurs in an orchestrated effort by a health care team led by an emergency physician who can make an ongoing assessment and monitor the efficacy and response to therapeutic interventions. It is often difficult or impossible to determine the cause of cardiac arrest at presentation. Although a differential diagnosis can be formulated based on history, physical examination, and ECG rhythm on arrival, key information often is not available or is unreliable.19 The differential diagnosis potentially can be narrowed by the patient’s age, underlying diseases, and medications when known.
History and Physical Examination
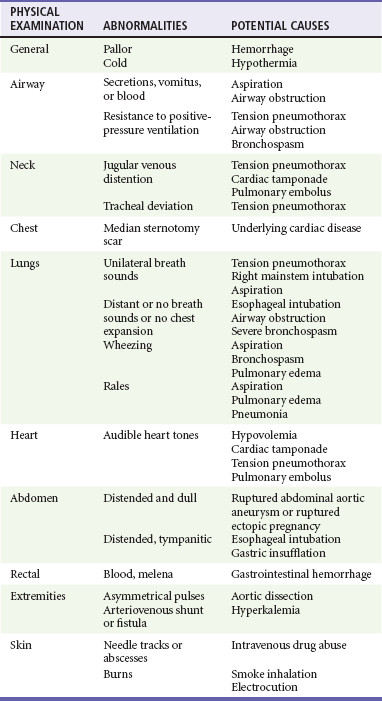
Physical examination of a cardiac arrest patient is necessarily focused on a few key goals: (1) ensure adequacy of airway maintenance and ventilation, (2) confirm the diagnosis of cardiac arrest, (3) find evidence of cause, and (4) monitor for complications of therapeutic interventions. This examination occurs in descending order of importance, simultaneously with therapeutic interventions, and is repeated frequently to assess for response to therapy and occurrence of complications (Table 9-2).
Monitoring
Unfortunately, no ideal monitoring technique provides all the information that might be desired during resuscitation, and even the modalities discussed next are often difficult or impossible to establish or interpret during CPR. A brief overview is provided of CPP, end-tidal carbon dioxide (ETCO2), and central venous oxygen saturation (ScvO2) monitoring, which if available can be used to detect inadequate CPR with high specificity (Table 9-3). In addition, several of these techniques are useful in the immediate post–cardiac arrest period.
Table 9-3
Indicators of Inadequate Blood Flow During Cardiopulmonary Resuscitation
| MONITORING TECHNIQUE | INDICATOR |
| Carotid or femoral pulse | Not palpable |
| CPP | <15 mm Hg |
| Arterial relaxation (diastolic) pressure | <40 mm Hg |
| PETCO2 | <10 mm Hg (before vasopressor) |
| ScvO2 | <40% |
Arterial Blood Pressure and Coronary Perfusion Pressure
Successful resuscitation of the arrested heart depends on generating adequate CPP during CPR, which has been directly correlated with myocardial blood flow.20 Animal and human studies indicate that a minimum CPP of 15 mm Hg is necessary to achieve ROSC if initial defibrillation attempts have failed.20,21 Unfortunately, CPP monitoring is rarely feasible in ED resuscitations of cardiac arrest patients, because it requires both an indwelling arterial pressure catheter and a central venous catheter, both transduced properly to provide simultaneous readings.
Invasive arterial blood pressure monitoring alone can be helpful in guiding resuscitation and should be used when an indwelling arterial pressure catheter is already in place. When adequate personnel are available, it is often feasible to cannulate the femoral artery during CPR, especially with ultrasound guidance. Human studies have shown that radial or femoral arterial relaxation pressures reliably reflect aortic relaxation pressures during CPR.22,23 Researchers have also reported that a CPP of 15 mm Hg or more was required to achieve ROSC and that no patient with an aortic relaxation pressure of less than 17 mm Hg achieved ROSC.21,24 Titrating resuscitation efforts to arterial relaxation (diastolic) pressure is less reliable than CPP because improper CPR (e.g., leaning on chest during CPR diastole and hyperventilation) can cause undetected elevations in right atrial pressure, reducing coronary perfusion. Despite this limitation, it is reasonable to titrate resuscitation efforts to achieve an arterial relaxation (diastolic) pressure of 40 mm Hg or more when invasive arterial pressure monitoring is available. Invasive arterial pressure monitoring during CPR may also be useful to facilitate distinguishing PEA that does or does not result in mechanical heart contraction, to detect ROSC, and to assist in serial arterial blood gas monitoring. Although arterial and central venous catheters are most often placed in the post–cardiac arrest phase of care, a significant number of patients initially achieving ROSC will rearrest in the ED, making these modalities helpful during the patient’s subsequent resuscitation.
End-tidal Carbon Dioxide
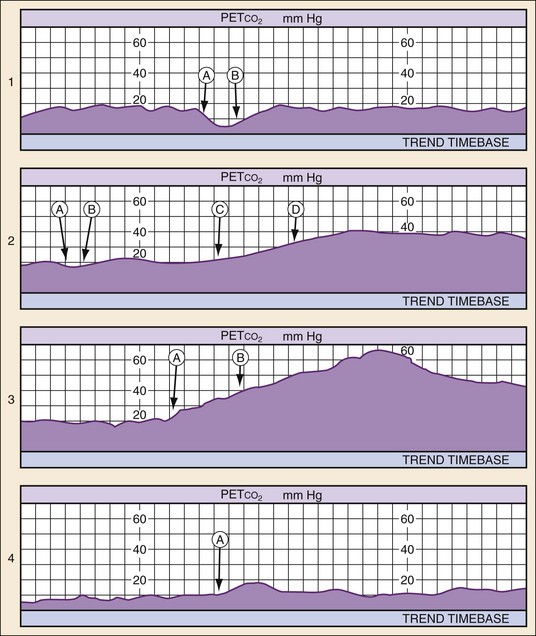
The partial pressure of CO2 in exhaled air at the end of expiration (PETCO2) can be a reliable indicator of cardiac output during CPR. This is most reliably measured through waveform capnography after endotracheal intubation. PETCO2 depends on CO2 production, alveolar ventilation, and pulmonary blood flow (i.e., cardiac output) and correlates well with CPP and cerebral perfusion pressure during CPR.24,25 Therefore when minute ventilation is held constant (a desirable but often unmet goal) and no exogenous CO2 is introduced (e.g., sodium bicarbonate [NaHCO3] administration), only increased cardiac output during CPR and ROSC significantly increases PETCO2. Resuscitation after cardiac arrest is likely to fail if PETCO2 values are less than 10 mm Hg,26,27 and such values should prompt the clinician to enhance the quality of CPR (either rate or force of compression) or consider more invasive maneuvers such as extracorporeal membrane oxygenation (ECMO) if the situation warrants and a good neurologic outcome is believed to be possible.28
PETCO2 monitoring also can aid in the diagnosis and treatment of PEA. Patients in a state of PEA with mechanical heart activity may have pulsatile flow that simply cannot be detected by palpation of a pulse. In such circumstances, PETCO2 levels may be elevated even without compressions. Use of cardiac ultrasound in such cases can prove to be helpful in identifying corresponding cardiac activity. In such cases, volume expansion or the use of vasopressors and inotropes should be considered. PETCO2 monitoring also is useful in rapidly detecting success of tension pneumothorax decompression, pericardiocentesis for pericardial tamponade, and fluid resuscitation for hypovolemia. ROSC causes immediate and significant increases in PETCO2. Therefore PETCO2 monitoring can detect ROSC at any time during the chest compression cycle, providing valuable guidance for pharmacologic therapies and minimizing the need for a pulse check when organized rhythms are detected (Fig. 9-1).
Central Venous Oxygen Saturation
ScvO2, when available, provides an additional method to monitor adequacy of resuscitative measures. The mixed venous blood oxygen saturation in the pulmonary artery (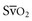 ) represents the oxygen remaining in the blood after systemic extraction. Studies have shown a close correlation between ScvO2 and
) represents the oxygen remaining in the blood after systemic extraction. Studies have shown a close correlation between ScvO2 and 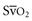 during CPR.29 Because oxygen consumption remains relatively constant during CPR, as do arterial oxygen saturation (SaO2) and hemoglobin, changes in ScvO2 reflect changes in oxygen delivery by means of changes in cardiac output.
during CPR.29 Because oxygen consumption remains relatively constant during CPR, as do arterial oxygen saturation (SaO2) and hemoglobin, changes in ScvO2 reflect changes in oxygen delivery by means of changes in cardiac output.
Although used primarily in the intensive care unit (ICU) setting, multilumen oximetric ScvO2 catheters are placed in the same manner as regular central venous catheters and can be used to monitor ScvO2 continuously in real time. ScvO2 values normally range from 60 to 80%. During cardiac arrest and CPR, these values range from 25 to 35%, indicating greatly enhanced oxygen extraction of tissues owing to the inadequacy of oxygen delivery during CPR. Failure to achieve an ScvO2 of 40% or greater has a negative predictive value for ROSC of almost 100%.29 ScvO2 also helps to rapidly detect ROSC without interruption of chest compressions, as ROSC will result in a rapid increase in Scvo2 as a result of a dramatic increase in oxygen delivery to tissues. ScvO2 monitoring is also useful in the post–cardiac arrest period for hemodynamic optimization and for recognition of any sudden deterioration in the patient’s clinical condition.
Echocardiography
The main usefulness of echocardiography is diagnostic, especially in patients with PEA. Echocardiography distinguishes organized pulseless cardiac activity that does or does not result in mechanical heart contraction. It also may be helpful in diagnosing mechanical causes of PEA, such as tension pneumothorax, pericardial tamponade, and pulmonary embolism.30 Echocardiography also is useful in guiding pericardiocentesis. In the postarrest period, echocardiography could prove to be valuable in determining the need for postarrest cardiac intervention or mechanical assistance of the failing heart.31
Resuscitation
Interventions are performed rapidly and efficiently to maximize the chances of a good neurologic outcome. The quality of CPR is perhaps the most underappreciated component of the resuscitation effort.32 Important quality performance measures include compression rate (at least 100 per minute), compression depth (>5 cm), duty cycle (CPR being performed at >50% of pulseless interval) full relaxation, and minimum pauses, especially before and after defibrillation.33 Furthermore, hyperventilation has been shown to be common and reduces cardiac output during CPR.34,35 A 30 : 2 compression-to-ventilation ratio is currently recommended for health care professionals in all adult resuscitation scenarios until an advanced airway has been established.36 Once the airway is secured, CPR should be performed continuously without pausing, while providing six to eight ventilations every minute.36 As indicated earlier, if PETCO2 is to be helpful as an indicator of cardiac output during CPR, minute ventilation should be relatively constant. Although recent evidence suggests that chest-compression–only CPR is as effective as traditional CPR when performed by bystanders in the out-of-hospital setting, there is inadequate evidence to recommend this as an alternative strategy for heath care professionals. Whereas oxygen uptake from the lungs is low during CPR, oxygen remaining in the functional residual capacity of the lung will reach a critically low value as CPR progresses if not replenished. The exception to this is when inadequate personnel are present to provide compressions, ventilation, and other resuscitative activites.37 Intubation should be performed only when capable personnel are available and without interruption of chest compressions. Use of supraglottic airway adjuncts such as the esophageal tracheal Combitube or laryngeal mask airway may be good alternatives for airway management in the out-of-hospital phase of resuscitation, with the main disadvantage being the inability to use the trachea as a route of drug administration and lack of evidence regarding the reliability of use of PETCO2 to monitor cardiac output during CPR. In addition to monitoring specific CPR performance parameters, physiologic monitoring, if available, can help optimize CPR quality for the individual patient (see Table 9-3). If the inadequacy of CPR is recognized early in the resuscitation despite optimized therapy, the physician in charge may consider more invasive measures such as ECMO if ROSC and a good neurologic outcome are possible. After prolonged arrest, however, clear indications that CPR is inadequate (based on appropriate monitoring techniques) should prompt cessation of resuscitation efforts. Figure 9-2 is an algorithm for management of cardiac arrest. Interventions specific to each rhythm are discussed in the following sections.
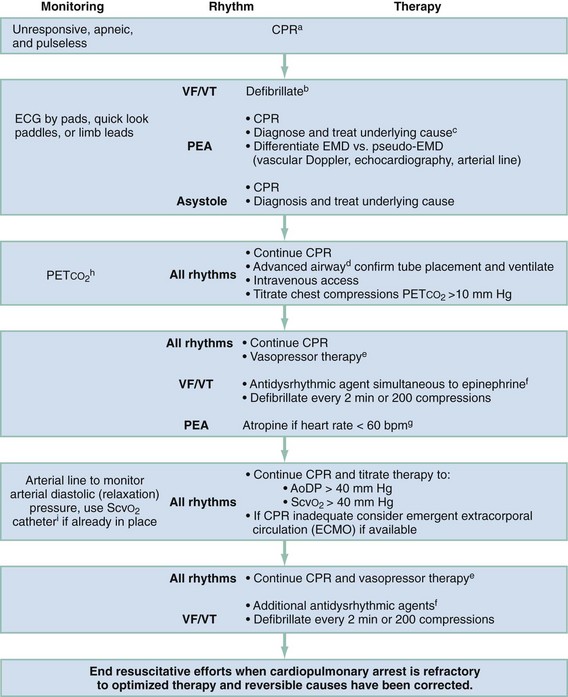
Figure 9-2 Emergency treatment algorithm for treatment of cardiac arrest. aIf arrest is witnessed and known to be of short duration, immediate rhythm assessment and defibrillation or ventricular fibrillation/ventricular tachycardia (VF/VT) precede cardiopulmonary resuscitation (CPR). In cases of prolonged untreated VF/VT, 1 to 2 minutes of CPR before defibrillation may enhance the ability to achieve return of spontaneous circulation. EMD, electromechanical dissociation; PEA, pulseless electrical activity. bBiphasic defibrillation should use manufacturer-recommended energy versus monophasic defibrillation (360 J). cSee Table 9-4. dEndotracheal intubation or supraglottic airway when feasible with minimal interruption in chest compressions. eEpinephrine, initial dose of 1 mg intravenously (IV) or intraosseously (IO) or 2.5 mg by endotracheal tube (ETT). Repeat every 3 to 5 minutes. Subsequent doses may be increased up to 0.1 mg/kg. An alternative to epinephrine is vasopressin, 40 U via intravenous push. Vasopressin is potentially more effective if the presenting rhythm is asystole. The dose (40 U) can be repeated once in 3 minutes, followed by administration of epinephrine every 3 to 5 minutes. fAmiodarone, 300 mg via intravenous push followed by 150 mg every 30 minutes. Lidocaine is an alternative antidysrhythmic if amiodarone is not available. Magnesium sulfate, 1 to 2 g via intravenous push in torsades de pointes or known hypomagnesemia. gAtropine, 1 mg via intravenous push or 2.5 mg by ETT. Repeat dose every 3 to 5 minutes to a total dose of 0.04 mg/kg. AoDP, aortic diastolic pressure; ECG, electrocardiogram; ScvO2, central venous oxygen saturation. hChanges in the partial pressure of end-tidal carbon dioxide (PETCO2) may not be predictive of myocardial blood flow in the setting of high-dose vasopressor therapy. iInvasive monitoring should be performed only if adequate personnel are available and if it would not delay therapeutic interventions.
Ventricular Fibrillation and Pulseless Ventricular Tachycardia
VF and pulseless VT are treated identically because they are generally caused by the same mechanisms and respond to the same interventions. Traditional monophasic defibrillators that use either a monophasic truncated exponential (MTE) or a monophasic dampened sinusoidal (MDS) waveform have almost completely been replaced by defibrillators that use biphasic waveforms. With biphasic defibrillation, the energy required for successful defibrillation, or the “defibrillation threshold,” is less than with monophasic defibrillation. When used, the biphasic waveform increases the likelihood of initial defibrillation success and decreases the likelihood of postcountershock myocardial dysfunction. Despite documented advantages of lower defibrillation threshold and reduced myocardial injury with use of biphasic defibrillation, data are currently inadequate to conclude that any specific waveform (biphasic or monophasic) is superior to any other in achieving ROSC or survival to hospital discharge. New defibrillation technologies have stimulated reevaluation of optimal defibrillation strategies. Current consensus suggests that the most effective strategy is delivery of single countershocks at optimal energy levels with minimal pauses in CPR both before and immediately afterward.38 This is facilitated by placement of defibrillation pads early in the resuscitation sequence, thus not requiring a pause while defibrillation paddles and conducting gel are placed for each shock. Manufacturer-recommended countershock energies range from 120 to 360 J for biphasic defibrillators.38 Health professionals should use the manufacturer-recommended countershock energies for biphasic defibrillator(s). The recommended energy for single monophasic defibrillation is 360 J.36
A patient who develops VF or pulseless VT while on a cardiac monitor may remain conscious for 15 to 30 seconds. The patient should be encouraged to cough vigorously until a defibrillator is available. This “cough CPR” has been demonstrated to keep patients conscious for up to a minute or more. If the patient is unresponsive, chest compressions should be initiated immediately and continued until a defibrillator is available. Defibrillation without antecedent CPR is most likely to result in ROSC when administered in the early minutes of arrest. If the duration of untreated arrest is prolonged (>4-5 minutes), a brief period of chest compressions and ventilations (90-180 seconds) before defibrillation may improve the likelihood of ROSC and survival.34,39,40 The current consensus favors delivering a single countershock with minimal pause in chest compressions before defibrillation.38 Defibrillation is followed immediately by resumption of chest compressions for 2 minutes before a rhythm check and additional defibrillation as appropriate.36
VF and pulseless VT refractory to initial defibrillation should be treated with assisted ventilation and chest compression. Intravenous access and vasopressor therapy (epinephrine or vasopressin) should be administered and repeated every 3 to 5 minutes. Simultaneous administration of epinephrine and vasopressin does not improve outcome relative to epinephrine alone regardless of presenting rhythm.41
Defibrillation attempts should be preceded and followed by minimal interruptions of chest compression.36 Subsequent therapy for refractory VF and pulseless VT includes continued administration of vasopressors and antidysrhythmic agents, followed by repeated countershocks. Antidysrhythmics should be administered up to their maximum loading dose. The use of magnesium sulfate during VF and pulseless VT is of no proven efficacy except in torsades de pointes and possible hypomagnesemia. Beyond the specific indications for NaHCO3 therapy of hyperkalemia and tricyclic antidepressant overdose, there is no evidence to support use of NaHCO3 or other buffers as empirical treatment for metabolic acidosis during cardiac arrest. If a patient is defibrillated into a different pulseless rhythm, such as PEA or asystole, subsequent treatment should be modified to address those specific rhythms.
Pulseless Electrical Activity
Treatment of PEA requires all general resuscitation measures, including CPR, intubation with assisted ventilation, intravenous access, and repeated administration of vasopressors. Initial assessment also should include vascular Doppler ultrasound, echocardiography, or PETCO2 monitoring to distinguish EMD from pseudo-EMD. This is important because volume loading or continuous vasopressor infusion, which is not typically used in routine cardiac arrest resuscitation, may be helpful in cases of pseudo-EMD. In contrast, routine use of atropine during PEA, previously a mainstay of drug therapy, is unlikely to have a therapeutic benefit.38 PEA thought to result from supraventricular tachycardia should be immediately cardioverted. These interventions alone are generally inadequate, unless the underlying cause of PEA is primary respiratory arrest or supraventricular tachycardia. Successful resuscitation of patients with PEA hinges on rapid diagnosis and treatment of the underlying cause. Physical examination may provide valuable clues to the underlying cause (Table 9-4). In hypoxia and hypovolemia, the diagnosis is based on response to empirical therapy, whereas other causes, such as pericardial tamponade, tension pneumothorax, and hypothermia, can be definitively diagnosed during resuscitation. Physical examination and monitoring are used to guide ongoing resuscitation efforts.
Asystole
Treatment of asystole requires all general resuscitation measures, including CPR, intubation with assisted ventilation, intravenous access, and repeated administration of vasopressors. In one randomized prospective out-of-hospital trial, improved survival to hospital admission and discharge was observed in patients in asystole when two doses of vasopressin (40 IU) were given initially during resuscitation compared with standard-dose epinephrine (1 mg), followed by additional epinephrine if needed.42 In a more recent study, simultaneous administration of epinephrine and vasopressin did not improve outcome relative to epinephrine alone, regardless of presenting rhythm.41 Available evidence indicates that routine administration of atropine is not beneficial.36 Extensive research has shown that asystole in the out-of-hospital setting seldom responds to pacing. To be effective, pacing must be initiated within several minutes of arrest.
Post–Cardiac Arrest
Resuscitation of a cardiac arrest victim does not end with ROSC. Management includes rapid diagnosis and treatment of the disorders that caused the arrest and the complications of prolonged global ischemia. Simultaneous management of these two entities makes caring for a post–cardiac arrest patient particularly challenging. A comprehensive goal-driven program of post–cardiac arrest care is most successful in affecting survival and neurologic status.9,10
Induction of prolonged therapeutic hypothermia in comatose survivors of cardiac arrest has been shown to improve survival and functional outcome in two prospective randomized clinical trials.7,8 Both studies enrolled only out-of-hospital patients with witnessed arrest and an initial rhythm of VF. The time to achieve target temperature (<34° C) ranged from less than 2 hours to a median of 8 hours (interquartile range 4-16 hours),7,8 suggesting a broad therapeutic window. Hypothermia was maintained for 12 to 24 hours, followed by gradual rewarming over 12 to 24 hours. Although these parameters provide guidelines within which post–cardiac arrest hypothermia is effective, additional preclinical and clinical data are needed to determine the optimal temperature, time to achieve target temperature, and duration of therapy. In both studies, the rates of complications were not statistically different between groups. Although there are no absolute contraindications, relative contraindications include severe cardiogenic shock, life- threatening dysrhythmias, uncontrolled bleeding, preexisting coagulopathy, pregnancy, another obvious reason for coma (e.g., drug overdose or status epilepticus), known end-stage terminal illness, and a preexisting do-not-resuscitate status. Thrombolytic therapy does not preclude the use of hypothermia.8 Finally, whereas initial studies were limited to patients with witnessed VF out-of-hospital cardiac arrest, evidence is accumulating that induced post–cardiac arrest therapeutic hypothermia is effective in patients with other presenting rhythms including PEA and asystole.43,44
When the decision is made to treat the patient with therapeutic hypothermia, cooling efforts should be initiated as soon as possible. Practical methods of rapidly inducing hypothermia include ice packs (applied to the neck, inguinal areas, and axilla), fan cooling of dampened exposed skin, cooling blankets underneath and on top of the patient, and disabling of ventilator warming circuits. Rapid intravenous infusion of limited volumes (1-2 L) of 4° C saline facilitates rapid cooling, but additional measures are needed to maintain hypothermia as this initial cold fluid bolus will lose its heat transfer properties during circulation.45 A number of automated surface cooling devices are now available that use chest and thigh pads and continuous temperature feedback from bladder or esophageal temperature probes. Although these devices were developed to make cooling less labor intensive and faster, some difficulties inherent with surface cooling remain, including overshooting and maintaining target temperature (SD usually > 1° C),46 uncontrolled rewarming with frequent rebound hyperthermia, and inability to assess skin for cutaneous reactions and injury.47 Automated endovascular cooling systems are also available that require placement of a central venous catheter. Although more invasive, cooling with a heat exchange catheter inserted in the inferior vena cava is the most rapid practical method of cooling and offers the tightest control of temperature at target (SD usually < 0.3° C) and during controlled rewarming.48 Endovascular systems are far less nursing labor-intensive than surface cooling because they monitor core temperature and are automatically controlled through a feedback loop.49 No one cooling strategy or device has been demonstrated to result in superior clinical outcomes.
Shivering, which inhibits cooling, can be prevented with sedation and neuromuscular blockade. If neuromuscular blockade is continued during the maintenance phase of therapeutic hypothermia, continuous electroencephalographic monitoring is strongly encouraged because seizures are common in post–cardiac arrest patients (5-20%).9 Target core body temperature is 32 to 34° C and is best monitored by an indwelling temperature-sensitive bladder catheter or esophageal temperature probe.50 When the patient is stabilized and cooling efforts are initiated, transfer to a critical care unit should occur as soon as possible. Although the optimal duration of post–cardiac arrest hypothermia is unknown and may be related to the total ischemic time,51 target temperatures are typically actively maintained for at least 12 to 24 hours, followed by gradual rewarming over 8 to 12 hours.52 Effective application of therapeutic hypothermia in comatose cardiac arrest survivors requires a coordinated interdisciplinary effort and is best carried out through use of a predetermined goal-directed algorithm developed with input from emergency medicine, cardiology, and critical care physicians and nurses.
A simultaneous immediate concern in a comatose cardiac arrest survivor is whether the patient has an acute coronary syndrome. Diagnosing acute coronary syndrome in an unconscious patient after cardiac arrest presents a unique challenge. A standard 12-lead ECG should be obtained as soon as feasible after ROSC, with additional right-sided and/or posterior leads as indicated. In one study, 50% of patients achieving ROSC after out-of-hospital cardiac arrest were found to have acute coronary occlusion on cardiac catheterization; 10% of them did not have ST segment elevation.19 Subsequent studies have reported that successful immediate percutaneous coronary intervention (PCI) is associated with improved hospital survival in post–cardiac arrest patients with or without ST segment elevation.53
Immediate PCI is indicated in post-ROSC patients with ST segment elevation MI or new left bundle branch block (LBBB), or high clinical suspicion of acute MI, and can be performed during therapeutic hypothermia.54–58 When PCI is indicated but not available, either early transfer of post–cardiac arrest patients to a center capable of PCI or fibrinolytic therapy should be considered.59 Relative exclusion criteria for fibrinolytic therapy unique to the post–cardiac arrest patient include evidence of significant CPR trauma (e.g., pneumothorax, flail chest, pulmonary contusion with hemorrhage), but the effects of therapeutic hypothermia on the efficacy and complications of fibrinolytic therapy in postarrest patients have not been formally studied.
Antiplatelet and anticoagulant therapy should be administered to all patients after ROSC, whether or not therapeutic hypothermia is induced, if no evidence of hemorrhage exists and profound hypertension is absent. The choice of antiplatelet and anticoagulant therapy depends in part on the presence of active ischemia, renal function, and plans for acute angioplasty.60 There is no proven benefit of prophylactic antiarrhythmic therapy or continuous infusion of an antiarrhythmic drug that has been associated with restoration of a stable rhythm during CPR. Concomitant therapies (e.g., nitrates, beta-blockers) are best performed in conjunction with careful hemodynamic monitoring. If indicated, intravenous preparations of nitrates and short-acting beta-blockers (e.g., esmolol) should be used because they have a brief duration of action and are easily titrated. In patients with new LBBB, right bundle branch block with left anterior or posterior hemiblock, second-degree type II block, or third-degree block, transthoracic pacing pads are immediately applied in case they are needed to treat bradycardic rhythms. Placement of transvenous pacing catheters also can be considered but is less often done with the increasing use and demonstrated efficacy of transthoracic cardiac pacing.61
Inadequate oxygen delivery (DO2) causes cells to convert to anaerobic metabolism, resulting in increased lactate production (dysoxia). Continued resuscitation efforts to optimize DO2 may help to stabilize the patient and potentially prevent subsequent multiorgan dysfunction and recurrent arrest. However, supranormal arterial oxygen partial pressure can exacerbate oxidative brain injury during the first minutes to hours after cardiac arrest. Therefore the fraction of inspired oxygen (FIO2) can be titrated to the minimum concentration required to maintain an arterial oxyhemoglobin saturation around 94%. This level of oxygenation avoids detrimental hyperoxia while ensuring appropriate oxygen delivery.62
The use of combined hemodynamic and metabolic endpoints to guide resuscitation in the ED has been shown to improve the outcome of patients with septic shock states.63 Because the post–cardiac arrest condition represents a complex state of cardiovascular shock, the use of such goal-directed therapy is inherently valuable to reduce mismatches of oxygen delivery and consumption that cannot be determined by simple physical examination and vital signs. Use of goal-directed hemodynamic therapy is relatively straightforward and requires only the insertion of a supradiaphragmatic central venous catheter. ScvO2 can be used as a reliable surrogate for 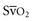 , which eliminates the need for a pulmonary artery catheter.29 If ScvO2 is abnormally low (<65%) but hemoglobin and SaO2 values are normal, cardiac output is insufficient. Central venous pressure (CVP) is used to deduce whether inadequate cardiac output is secondary to hypovolemia or impaired myocardial function. Augmenting CVP to levels between 10 and 15 mm Hg ensures adequate preload in most patients. If CVP is adequate and the patient has a mean arterial pressure of at least 70 mm Hg, therapy with an inotropic agent, such as dobutamine, should be initiated while reperfusion strategies are considered. CVP measurements may decrease rapidly on initiation of dobutamine therapy. Additional volume expansion while the hemoglobin value is kept at least at 10 g/dL should be provided as needed to maintain an adequate CVP.
, which eliminates the need for a pulmonary artery catheter.29 If ScvO2 is abnormally low (<65%) but hemoglobin and SaO2 values are normal, cardiac output is insufficient. Central venous pressure (CVP) is used to deduce whether inadequate cardiac output is secondary to hypovolemia or impaired myocardial function. Augmenting CVP to levels between 10 and 15 mm Hg ensures adequate preload in most patients. If CVP is adequate and the patient has a mean arterial pressure of at least 70 mm Hg, therapy with an inotropic agent, such as dobutamine, should be initiated while reperfusion strategies are considered. CVP measurements may decrease rapidly on initiation of dobutamine therapy. Additional volume expansion while the hemoglobin value is kept at least at 10 g/dL should be provided as needed to maintain an adequate CVP.
The response to DO2-optimizing interventions can be monitored by continuous or serial ScvO2 measurements and serial lactate levels. An increase in ScvO2 coupled with a decrease in lactate levels indicates improved DO2. An unchanging ScvO2 level indicates the need to continue to increase delivery. Persistently elevated lactate levels and low ScvO2 despite maximum pharmacologic support and volume management signal the need for additional interventions to optimize DO2. If this is not done, further accumulation of oxygen debt will occur, which will lead to either death or the development of multisystem organ failure. Options to consider include revascularization or mechanical assistance in the form of intra-aortic balloon pulsation or extracorporeal support. The induction of therapeutic hypothermia may assist in lowering the metabolic demands of tissues in the postarrest state. Figure 9-3 provides a goal-directed guide to care of the postarrest patient. Similar options should be considered in a patient with venous hyperoxia and elevated levels of lactate because the combination of these findings indicates severe microvascular dysfunction, which also leads to the accumulation of oxygen debt incompatible with survival. The incidence of this condition may increase with the increased use of potent vasopressors, such as vasopressin.
References
1. Nichol, G, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–1431.
2. Bobrow, B, et al. Chest compression–only CPR by lay rescuers and survival from out-of-hospital cardiac arrest. JAMA. 2010;304:1447–1454.
3. Rea, T, et al. CPR with chest compression alone or with rescue breathing. N Engl J Med. 2010;363:423–433.
4. Aufderheide, T, et al. Standard cardiopulmonary resuscitation versus active compression-decompression cardiopulmonary resuscitation with augmentation of negative intrathoracic pressure for out-of-hospital cardiac arrest: A randomised trial. Lancet. 2011;377:301–311.
5. Weisfeldt, ML, et al. Ventricular tachyarrhythmias after cardiac arrest in public versus at home. N Engl J Med. 2011;364:313–321.
6. Carr, BG, Kahn, JM, Merchant, RM, Kramer, AA, Neumar, RW. Inter-hospital variability in post–cardiac arrest mortality. Resuscitation. 2009;80:30–34.
7. Bernard, SA, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557.
8. Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556.
9. Sunde, K, et al. Implementation of a standardised treatment protocol for post resuscitation care after out-of-hospital cardiac arrest. Resuscitation. 2007;73:29–39.
10. Gaieski, DF, et al. Early goal-directed hemodynamic optimization combined with therapeutic hypothermia in comatose survivors of out-of-hospital cardiac arrest. Resuscitation. 2009;80:418–424.
11. Myerburg, RJ, Kessler, KM, Castellanos, A. Sudden cardiac death: Structure, function, and time-dependence of risk. Circulation. 1992;85:I2.
12. Caffrey, SL, et al. Public use of automated external defibrillators. N Engl J Med. 2002;347:1242.
13. Hallstrom, AP, et al. Public-access defibrillation and survival after out-of-hospital cardiac arrest. N Engl J Med. 2004;351:637.
14. Page, RL, et al. Use of automated external defibrillators by a U.S. airline. N Engl J Med. 2000;343:1210.
15. Valenzuela, TD, et al. Outcomes of rapid defibrillation by security officers after cardiac arrest in casinos. N Engl J Med. 2000;343:1206.
16. Myerburg, RJ, et al. Impact of community-wide police car deployment of automated external defibrillators on survival from out-of-hospital cardiac arrest. Circulation. 2002;106:1058.
17. van Alem, AP, et al. Use of automated external defibrillator by first responders in out of hospital cardiac arrest: Prospective controlled trial. BMJ. 2003;327:1312.
18. Bonnin, MJ, et al. Distinct criteria for termination of resuscitation in the out-of-hospital setting. JAMA. 1993;270:1457.
19. Spaulding, CM, et al. Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. N Engl J Med. 1997;336:1629.
20. Niemann, JT, et al. Predictive indices of successful cardiac resuscitation after prolonged arrest and experimental cardiopulmonary resuscitation. Ann Emerg Med. 1985;14:521.
21. Paradis, NA, et al. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA. 1990;263:1106.
22. Ornato, JP, et al. The effect of applied chest compression force on systemic arterial pressure and end-tidal carbon dioxide concentration during CPR in human beings. Ann Emerg Med. 1989;18:732–737.
23. Rivers, EP, et al. Simultaneous radial, femoral, and aortic arterial pressures during human cardiopulmonary resuscitation. Crit Care Med. 1993;21:878–883.
24. Lewis, LM, et al. Correlation of end-tidal CO2 to cerebral perfusion during CPR. Ann Emerg Med. 1992;21:1131.
25. Sanders, AB, et al. Expired PCO2 as an index of coronary perfusion pressure. Am J Emerg Med. 1985;3:147.
26. Callaham, M, Barton, C. Prediction of outcome of cardiopulmonary resuscitation from end-tidal carbon dioxide concentration. Crit Care Med. 1990;18:358.
27. Sanders, AB, et al. End-tidal carbon dioxide monitoring during cardiopulmonary resuscitation: A prognostic indicator for survival. JAMA. 1989;262:1347.
28. Kagawa, E, et al. Outcomes of extracorporeal membrane oxygenation in adult patients with refractory shock and cardiac arrest. Circulation. 2010;122:A271.
29. Emerman, CL, et al. A comparison of venous blood gases during cardiac arrest. Am J Emerg Med. 1988;6:580.
30. Hernandez, C, et al. C.A.U.S.E.: Cardiac arrest ultrasound exam—A better approach to managing patients in primary non-arrhythmogenic cardiac arrest. Resuscitation. 2008;76:198–206.
31. Gruenewald, M, et al. Visual evaluation of left ventricular performance predicts volume responsiveness early after resuscitation from cardiac arrest. Resuscitation. 2011;82:1553–1557.
32. Wik, L, et al. Quality of cardiopulmonary resuscitation during out-of-hospital cardiac arrest. JAMA. 2005;293:299.
33. Berg, RA, et al. Part 5: Adult basic life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(Suppl 3):S685–S705.
34. Aufderheide, TP, Lurie, KG. Death by hyperventilation: A common and life-threatening problem during cardiopulmonary resuscitation. Crit Care Med. 2004;32:S345.
35. Aufderheide, TP, et al. Hyperventilation-induced hypotension during cardiopulmonary resuscitation. Circulation. 2004;109:1960.
36. Neumar, RW, et al. Part 8: Adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S729–S767.
37. Sayre, MR, et al. Hands-only (compression-only) cardiopulmonary resuscitation: A call to action for bystander response to adults who experience out-of-hospital sudden cardiac arrest—a science advisory for the public from the American Heart Association Emergency Cardiovascular Care Committee. Circulation. 2008;117:2162.
38. Link, MS, et al. Part 6: Electrical therapies: Automated external defibrillators, defibrillation, cardioversion, and pacing—2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S706–S719.
39. Stiell, IG, et al. Early versus later rhythm analysis in patients with out-of-hospital cardiac arrest. N Engl J Med. 2011;365:787–797.
40. Wik, L, et al. Delaying defibrillation to give basic cardiopulmonary resuscitation to patients with out-of-hospital ventricular fibrillation: A randomized trial. JAMA. 2003;289:1389.
41. Wenzel, V, et al. A comparison of vasopressin and epinephrine for out-of-hospital cardiopulmonary resuscitation. N Engl J Med. 2004;350:105.
42. Gueugniaud, PY, et al. Vasopressin and epinephrine vs epinephrine alone in cardiopulmonary resuscitation. N Engl J Med. 2008;359:21.
43. Arrich, J, European Resuscitation Council Hypothermia After Cardiac Arrest Registry Study Group. Clinical application of mild therapeutic hypothermia after cardiac arrest. Crit Care Med. 2007;35:1041–1047.
44. Derwall, M, Stoppe, C, Brücken, D, Rossaint, R, Fries, M. Changes in S-100 protein serum levels in survivors of out-of-hospital cardiac arrest treated with mild therapeutic hypothermia: A prospective, observational study. Crit Care. 2009;13:R58.
45. Kliegel, A, et al. Cold infusions alone are more effective for induction of therapeutic hypothermia but do not keep patients cool after cardiac arrest. Resuscitation. 2007;73:46–53.
46. Heard, KJ, et al. A randomized controlled trial comparing the Arctic Sun to standard cooling induction of hypothermia after cardiac arrest. Resuscitation. 2010;81:9–14.
47. Merchant, MR, et al. Therapeutic hypothermia after cardiac arrest: Unintentional overcooling is common using ice packs and conventional cooling blankets. Crit Care Med. 2006;34:S490–S494.
48. Gillies, MA, et al. Therapeutic hypothermia after cardiac arrest: A retrospective comparison of surface and endovascular cooling techniques. Resuscitation. 2010;81:1117–1122.
49. Finley Caulfield, A, et al. A comparison of cooling techniques to treat cardiac arrest patients with hypothermia. Stroke Res Treat. 2011;2011:690506.
50. Nolan, JP, et al. Therapeutic hypothermia after cardiac arrest: An advisory statement by the Advancement Life Support Task Force of the International Liaison Committee on Resuscitation. Resuscitation. 2003;57:231.
51. Sawyer, KN, et al. Derivation of a novel hypothermic to ischemic ratio as an indicator for predicting and improving survival from out of hospital cardiac arrest. Circulation. 2011;124:A1.
52. Peberdy, MA, et al. Part 9: Post–cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S768–S786.
53. Dumas, F, et al. Immediate percutaneous coronary intervention is associated with better survival after out-of-hospital cardiac arrest: Insights from the PROCAT (Parisian Region Out of hospital Cardiac ArresT) registry. Circ Cardiovasc Interv. 2010;3:200–207.
54. Garot, P, et al. Six-month outcome of emergency percutaneous coronary intervention in resuscitated patients after cardiac arrest complicating ST-elevation myocardial infarction. Circulation. 2007;115:1354.
55. Keelan, PC, et al. Early direct coronary angioplasty in survivors of out-of-hospital cardiac arrest. Am J Cardiol. 2003;91:1461.
56. Bendz, B, et al. Long-term prognosis after out-of-hospital cardiac arrest and primary percutaneous coronary intervention. Resuscitation. 2004;63:49.
57. Knafelj, R, et al. Primary percutaneous coronary intervention and mild induced hypothermia in comatose survivors of ventricular fibrillation with ST-elevation acute myocardial infarction. Resuscitation. 2007;74:227.
58. O’Connor, RE, et al. Part 10: Acute coronary syndromes: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S787–S817.
59. Peels, HO, et al. Outcome in transferred and nontransferred patients after primary percutaneous coronary intervention for ischaemic out-of-hospital cardiac arrest. Catheter Cardiovasc Interv. 2008;71:147.
60. Anderson, JL, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients with Unstable Angina/Non ST-Elevation Myocardial Infarction): Developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons. Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Circulation. 2007;116:e148.
61. Zimetbaum, PJ, Josephson, ME. Use of the electrocardiogram in acute myocardial infarction. N Engl J Med. 2003;348:933.
62. Neumar, RW. Optimal oxygenation during and after cardiopulmonary resuscitation. Curr Opin Crit Care. 2011;17:236.
63. Rivers, E, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368.

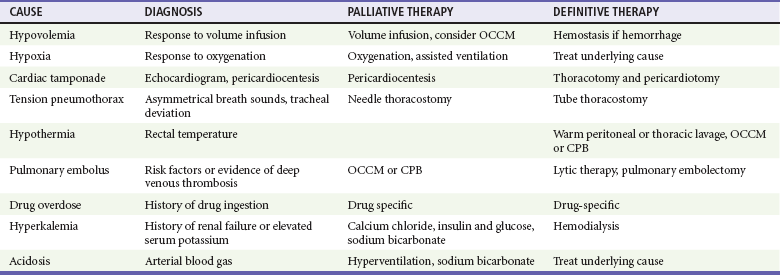
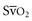 . Low
. Low 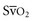 coupled with persistently elevated lactate levels indicates inadequate D
coupled with persistently elevated lactate levels indicates inadequate D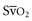 is abnormally high (venous hyperoxia) in the face of inadequate D
is abnormally high (venous hyperoxia) in the face of inadequate D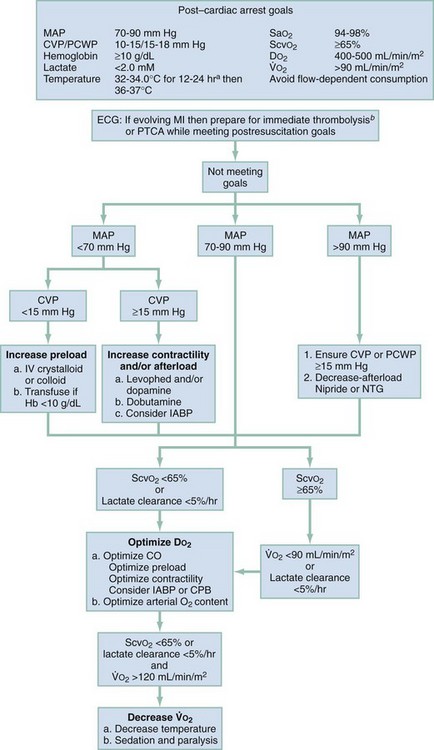
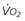 , oxygen consumption.
, oxygen consumption.