Chapter 3 Adult Neurogenic Communication Disorders
A complex array of communication disorders co-occur with the various physical and sensory deficits after many neurologic disorders seen by the physiatrist. As noted by Brookshire,12 the prefix neuro means “related to nerves or the nervous system,” while the suffix genic means “resulting from” or “caused by.” This chapter reviews the major acquired neurogenic communication disorders seen in adults as a result of a disturbance of the neurologic system.
Types of Communication Disorders Caused by Nervous System Pathology
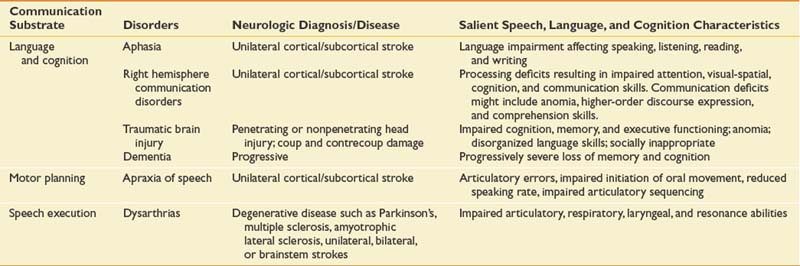
Cognitive-communicative disorders as a result of brain injury, disease, or pathology might include aphasia and related neurobehavioral disorders; communicative-cognitive disorders secondary to right hemisphere stroke, traumatic brain injury, and dementia; and the motor speech disorders including the dysarthrias and apraxia of speech. Table 3-1 presents the major neurogenic communication disorders, neurologic diagnosis or disease, and salient speech-language and cognitive characteristics.
Brain Plasticity and Neurorehabilitation
Research in the basic neurosciences over the past 30 years has provided strong evidence regarding the dynamic aspects of the brain to change. While most of the research to date has been in animal models, human translational studies have begun to emerge. The term brain plasticity refers to the capacity and resiliency of the brain to undergo structural and functional changes.19 The ability of the brain to adapt and restructure is particularly evident after injury and has important implications for rehabilitation and recovery of function. Ideally, the rehabilitation team will work collaboratively to determine the best intervention at the optimal time to take advantage of the brain’s malleability. For an individual patient this can include the selection of the type and intensity of behavioral treatment (the external stimulation). At times this might be paired with administration of adjunctive brain stimulation such as a neuropharmacologic agent, electrical cortical stimulation, and/or transcortical magnetic stimulation.
In animals, the type of the external stimulation has been shown to be very important. A large body of research demonstrates that the type of input affects brain reorganization.36,51,57 This has been termed use-dependent,51 or more recently learning-dependent, activity.57 This refers to the specificity of the experience after brain injury being critical in determining what brain and behavioral changes will occur. Nudo et al.57 found that motor maps are altered by motor skill acquisition versus repetitive use alone, and brain plasticity coincided with the reacquisition of motor skills in lesioned animals and the acquisition of new motor skills in intact animals. These data imply that at critical recovery periods in humans, restorative treatments should target the most complex behaviors that a patient can produce. It could be that focusing only on compensatory activities rather than restorative functions has costs in terms of ultimate recovery of function.
Neuromodulation can be done with certain pharmacologic agents. When paired with behavioral treatment, some agents, particularly those affecting the catecholamine system (dextroamphetamine, methylphenidate, levodopa), have been found to enhance outcome after both focal6,22,35,69 and diffuse46,71 cortical experimental injury. It should be noted that this does not extend to all behaviors.66 Explorations of pharmacologic modulation have been done in humans to facilitate recovery from poststroke deficits such as aphasia and hemiplegia30,65,77,78 and to enhance recovery of cognitive deficits subsequent to traumatic brain injury.38,81 Physiologic events after brain injury can complicate the timing for administration of various agents. Drugs that are effective in the very acute or subacute period after injury may be ineffective or even detrimental at later recovery periods.28 Our experiences and that of others are encouraging regarding the use of certain agents to accelerate recovery; however, not all clinical studies report positive findings.26,59
Aphasia and Related Neurobehavioral Disorders
Aphasia
Aphasia in adults occurs as a result of acquired brain damage to the language-dominant hemisphere, usually the left, and shares common neurophysiologic features with other stroke consequences. Chapey and Hallowell16 provide a straightforward definition: “Aphasia is an acquired communication disorder caused by brain damage, characterized by an impairment of language modalities: speaking, listening, reading, and writing; it is not the result of a sensory deficit, a general intellectual deficit, or a psychiatric disorder.” The language impairments can range from mild with some word-finding problems (anomic aphasia) to severe with very little ability to speak, understand, read, or write (global aphasia). In the United States today, more than 1 million people are living with aphasia,45 and 80,000 new patients with aphasia will be treated each year.56
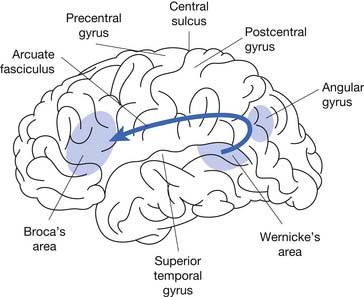
Since the time of Broca,11 aphasia has probably been the most studied neurogenic communication disorder. Because of the nature of the injury and the critical left hemisphere language association areas (Figure 3-1), aphasia has been classified in terms of the characteristics of the linguistic deficits and the location of the lesion.
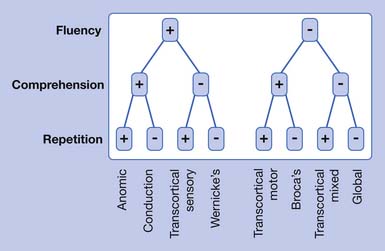
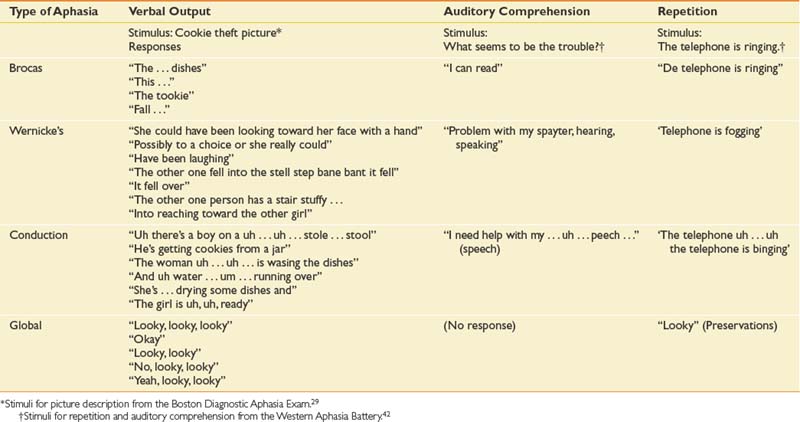
The traditional aphasia classification system9,43 is based on clusters of language symptoms and contrasts the characteristics of verbal output, auditory comprehension, and repetition ability (Table 3-2). This framework forms the basis for two of the most frequently employed formal assessments used by speech-language pathologists: the Boston Diagnostic Aphasia Exam29 and the Western Aphasia Battery.42
Table 3-2 The Aphasias: Comparisons of Verbal Output, Repetition, Auditory Comprehension, Associated Signs, and Region Affected
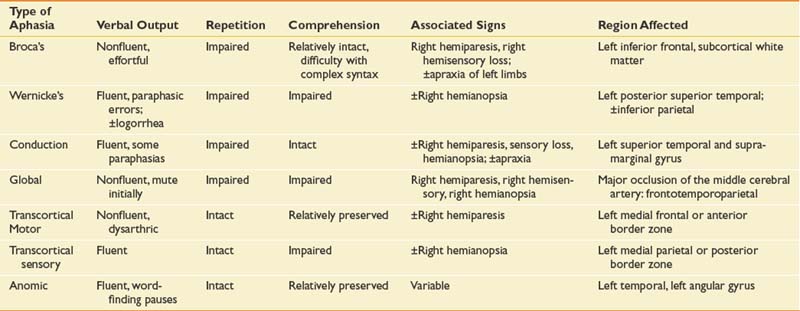
Modern imaging studies and research in psycholinguistics have shown limitations with the traditional classification scheme. In particular, the roles of Wernicke’s and Broca’s area are not as clear as they first appeared.18 A variety of other left hemisphere regions, both cortical and subcortical, have been found to be involved in language processing. While it is now recognized that the processing of language requires a large network of interacting brain areas, it is also the case that certain linguistic behaviors group together and are often predictable depending on the anterior or posterior location of the lesion. Anterior aphasias include Broca’s and transcortical motor aphasia. Posterior aphasias include Wernicke’s, conduction, and transcortical sensory aphasias. Global and anomic aphasias are not as localized. Aphasias limited to strictly subcortical pathology have also been described.55 Whether speech output is nonfluent versus fluent, the degree of auditory comprehension deficit, and the ability to repeat can be checked by the physiatrist at the bedside (Figure 3-2) to get an estimate of the type of aphasia.
Broca’s Aphasia
This classic nonfluent aphasia is characterized by effortful speech and misarticulations (see Table 3-3 for speech samples of the primary aphasia types). Verbal language (i.e., vocabulary and syntax) is restricted, resulting in anomia and reduced utterance length (i.e., typically one to four words in length). Auditory comprehension is impaired but relatively better than verbal language. The reading deficit in Broca’s aphasia resulting from frontal pathology is variable and is well described.9 Written language is usually as severely impaired as verbal language. Lesions causing Broca’s aphasia are most often in the left posterior inferior frontal cortex and underlying structures.
Wernicke’s Aphasia
In 1874, Karl Wernicke described an aphasia syndrome very different from Broca’s aphasia. Patients with Wernicke’s aphasia have fluent speech output with normal prosody (vocal pitch and stress) and close to normal grammar. However, their speech is filled with literal and verbal paraphasic errors. (Literal paraphasias are sound substitutions within a word; for example, “binging” for “ringing.” Semantic paraphasias are whole word substitutions; for example, “mother” for “sister.”) These paraphasias and other made-up words (neologisms) cause the speech of the Wernicke patient to be empty, although the sentence length can be normal. Another characteristic of speech output in Wernicke’s aphasia can be an inability to stop speaking (logorrhea) and press of speech (rapid, compressed utterances). Patients with Wernicke’s aphasia have severely impaired auditory comprehension, sometimes to the point of understanding no spoken language, and are often unaware of their own deficits.43
To be classified as Wernicke’s aphasia, there must be a repetition deficit. Wernicke’s aphasia usually occurs from damage to the left superior temporal region. It might also occur after damage in the inferior parietal cortex involving the supramarginal and angular gyri.43
Conduction Aphasia
Conduction aphasia is relatively uncommon and occurs in only about 10% of patients with aphasia. In this type of aphasia, the speech output is fluent (near-normal utterance length) but with considerable word-finding difficulties (anomia), relatively preserved auditory comprehension, and significant difficulty with verbal repetition. The speech of patients with conduction aphasia is characterized by literal paraphasias and numerous self-corrections as they search for the right word. This self-correction can cause the speech of the individual with conduction aphasia to have numerous pauses and filled pauses (“ah … a … ah … ah”). Reading and writing deficits are variable depending on the specific site of the lesion. Brain damage in conduction aphasia is in the left superior temporal area, the supramarginal gyrus of the parietal lobe, or both.
Global Aphasia
Patients with global aphasia have severe impairments in all language modalities (speaking, listening, reading, and writing). Global aphasia is characterized by severely impaired auditory comprehension and very limited speech output. Individuals with global aphasia produce few understandable utterances, and their speech is marked by perseverative utterances used repeatedly. It should not be overlooked, however, that many patients with global aphasia have some islands of spared intact function, and these must be found to utilize for communication. A type of global aphasia has been described with severe communication deficits but an absence of hemiplegia.75 Brain damage causing global aphasia is usually massive (frontotemporoparietal), caused by complete occlusion of the middle cerebral artery with dense right hemiplegia of both arm and leg.
Transcortical Motor Aphasia
Patients with transcortical motor aphasia have some similarities to those with Broca’s aphasia but with intact repetition. The transcortical aphasias refer to aphasias occurring from lesions in the border zone outside the perisylvian language areas. Individuals with transcortical motor aphasia have nonfluent, limited speech output that sometimes has a dysarthric quality. There are often long pauses between utterances. The patient with transcortical motor aphasia does not have as much difficulty with syntax as the patient with Broca-type aphasia. Auditory comprehension and reading comprehension are generally well preserved. Writing deficits can mirror those seen in spoken language. Transcortical motor aphasias occur as a result of occlusion of the anterior cerebral artery or damage to border zone areas in the frontal lobe superior or anterior to Broca’s area.43
Anomic Aphasia
Anomic aphasia can often be the residual of good recovery from other aphasia syndromes. Patients in the acute stage who are classified as having anomic aphasia have the best prognosis for recovery of any of the aphasias. The primary difficulty in anomic aphasia is word finding and naming. Speech output is fluent with numerous pauses, filled pauses, and circumlocutions (describing and/or defining a function of an object for which a name cannot be retrieved; for example, “you brush your teeth with it”). Verbal repetition, auditory comprehension, reading, and writing are relatively preserved. Although anomic aphasia is the least localized of all the aphasias, it often occurs from focal damage to left temporal and parietal areas.9
Crossed Aphasia
Rarely (incidence between 1% and 11%) a classic aphasia occurs in a strongly right-handed person from a lesion on the right side of the brain. When this occurs it is referred to as a crossed aphasia. The language characteristics in this case can be classic, paralleling the types of aphasias seen from left hemisphere lesions, with almost reversed mirror-image specialization of the two hemispheres.79 Other patients have anomalous hemispheric specialization with both language functions and visual spatial functions in the right hemisphere.2
Primary Progressive Aphasia
A specific primary progressive aphasia (PPA) is now recognized.21,44,50 This disorder blurs the lines between focal and diffuse disease. In contrast to aphasia caused by an acute event such as a stroke, PPA has an insidious onset. Although the family or patient might claim there was a specific event, careful history will reveal a progressive onset with the patient being aware of the language deficits before family members. The most frequent presenting complaint is word-finding difficulty. During the first 2 years, the patient with PPA often has symptoms that appear to be localized, similar to those of an aphasia subsequent to a stroke. After the early course of the disease, PPA usually progresses to dementia with the characteristic cognitive disorders of other dementias. A diagnosis of PPA requires a minimum 2-year history of language decline, relative sparing of other mental functions, independence in activities of daily living, a neurologic workup excluding other causes of aphasia, and a neuropsychological workup that supports the complaints.50
Management of Aphasia
Treatment by the speech-language pathologist is based on a careful assessment of all communication modalities: speaking, listening, reading, and writing. The patient’s deficit areas and relative strengths and weaknesses are determined. Assessments of both impairment and activity/participation levels are ideally done as defined by the World Health Organization.83 The focus of speech-language treatment in the acute and subacute recovery period is restoration of speech and language abilities, and treatment is individualized. Education and counseling with the family are also important.5
Numerous therapy approaches specific to the complex speech-language behaviors exhibited by patients with aphasia are available and have been demonstrated to be effective.23 Metaanalyses63,64 of the outcomes of therapy for aphasia have shown that language therapy for aphasia has a significant positive impact on recovery in the acute and chronic phases, and the amount of speech-language treatment is a critical factor for establishing effective and long-lasting improvements.7,10,17,52 Intensive aphasia therapy (on average 98 hours) appeared to be a requirement for positive outcomes,10,72 and shorter amounts of treatment (on average 44 hours or less) are not effective.7,10,73 Evidence-based practice is not the least expensive use of rehabilitation dollars but is the better investment of resources if significant improvement is expected.
Related Neurobehavioral Disorders
Often co-occurring with aphasia are a number of related neurobehavioral disorders, and it is important to differentiate them from the communication disorder. Only apraxia and agnosia are reviewed here. The physiatrist should consult a more detailed mental status examination such as Strub and Black70 and/or a neuropsychologist for a differential diagnosis of these higher-order motor and sensory processing disorders.
Apraxia
Apraxia is an acquired disorder of learned skilled, sequential motor movements that cannot be accounted for by elementary disturbances of strength, coordination, sensation, or lack of comprehension or attention.25 Apraxia is not a low-level motor disturbance but a deficit in motor planning that involves the integrative steps that precede skilled or learned movements.70 Apraxias occur more often as a result of left hemisphere lesions. Because adequate verbal comprehension is a prerequisite to valid praxis (motor integration needed for execution of complex learned movements) testing, it is important for the speech-language pathologist to be consulted regarding auditory comprehension abilities when a motor planning problem is suspected. It is also important that patients with a motor planning deficit not receive a diagnosis of comprehension difficulties, because the motor planning disorder prevents their making an adequate response to comprehension testing.
Ideamotor apraxia is the most common type of apraxia. Patients with this form of apraxia fail to perform previously learned motor acts accurately. Impairments can be seen in buccofacial, limb, or whole-body musculature. Ideational apraxia is a disturbance of complex motor planning of a higher order than is seen in ideamotor apraxia. It is a breakdown in the performance of a task that involves a series of related steps.70 Brief screening by commands can help the physiatrist to differentiate a motor planning disorder from a true language disorder (Table 3-4).
| Commands | Errors |
|---|---|
| Buccofacial | |
| “Show me how to—”: | |
| 1. “Blow out a match.” | Difficulty giving short, controlled exhalation; saying “blow”; inhaling; difficulty maintaining appropriate mouth posture |
| 2. “Protrude your tongue.” | Inability to stick out tongue; tongue moving in mouth but tending to push against front teeth and not protruding |
| 3. “Drink through a straw.” | Inability to sustain a pucker; blowing instead of drawing through the straw; random mouthing movements |
| Limb | |
| “Show me how to—”: | |
| 1. “Salute.” | Hand over head; hand waving; improper position of hand |
| 2. “Use a toothbrush.” | Failure to show any proper grip; failure to open mouth; grossly missing the mouth; using finger to pick teeth; not allowing adequate distance for shaft of toothbrush; using the finger as a toothbrush |
| 3. “Flip a coin.” | Movements miming tossing the coin into the air with an open hand; supinating or pronating the hand as though turning a doorknob; flexing the arm without flipping thumb against finger |
| 4. “Hammer a nail.” | Moving hand back and forth horizontally; pounding with fist |
| 5. “Comb your hair.” | Using fingers as teeth of comb; smoothing the hair; making inexact hand movements |
| 6. “Snap your fingers.” | Extension of fingers with patting movements; tapping of finger on thumb; sliding finger off thumb with insufficient force |
| 7. “Kick a ball.” | Stamping foot; pushing foot along floor; moving foot laterally |
| 8. “Crush out a cigarette.” | Stamping foot; kicking foot on floor |
| Whole body | |
| “Show me how to—”: | |
| 1. “Stand like a boxer.” | Awkward arm position; hands at side |
| 2. “Swing a baseball bat.” | Difficulty in placing both hands together; chopping movements |
| 3. “Bow” (for a man) or “Curtsy” (for a woman) | Any inappropriate truncal movement |
(Modified from Strub RL, Black FW: The mental status examination in neurology, ed 4, Philadelphia, 2000, FA Davis, with permission of FA Davis.)
Agnosia
Agnosias are acquired complex disorders of recognition in some sensory modality (i.e., visual, auditory, and tactile). Agnosia can also be specific for a particular class within a modality, such as agnosia for objects, agnosia for pictures, agnosia for faces (prosopagnosia), or agnosia for colors.70 Most agnosias are caused from bilateral lesions, although there are exceptions to this.
Just as in the case of the apraxias, it is important to differentiate agnosia from aphasia. Visual agnosia is a complex disorder in which the patient is unable to recognize objects or pictures of objects presented visually, even though visual acuity is adequate. Patients with auditory agnosia can have complete cortical deafness to partial deficits of recognition of specific types of sound.70 Differentiating auditory agnosia from aphasia is complex and requires assessment by a speech-language pathologist and neuropsychologist. Patients with auditory agnosia can hear noises (e.g., a vacuum cleaner, a doorbell ring) but not recognize their meanings. Many patients with auditory agnosia cannot recognize any speech but can respond to the same questions in written form. Tactile agnosias occur from parietal lesions and contribute to a range of sensory disorders. These include astereognosis (inability to identify objects palpated by the opposite hand) or agraphesthesia (inability to recognize numbers or letters written on the opposite side of the body).70 Some consider these deficits part of cortical sensory loss rather than a true agnosia; others call them apperceptive tactile agnosias.70
Right Hemisphere Communication Disorders
Patients with stroke of the nondominant or right hemisphere present a very different profile from those with left hemisphere lesions and aphasia (Table 3-5). In the right hemisphere patient, the communication disorder is often a secondary consequence of significant cognitive and neurobehavioral deficits. Mesulam49 provided one of the earliest descriptions of the complex deficits resulting from right hemisphere damage. He suggested four cardinal signs of right hemisphere involvement: constructional deficits, left-sided unilateral or hemispatial neglect, dressing apraxia, and denial or indifference. Numerous studies of non–brain-damaged as well as brain-damaged adults show the right hemisphere to be specialized for certain aspects of attention, visual-spatial skills, sensory integration, face recognition, memory, affective (emotional) expression and interpretation, nonverbal expression and interpretation, and problem solving. Because of the complexities of the neurobehavioral deficits, right hemisphere–damaged patients might have a few or many of the salient features.
Table 3-5 Comparison of Communication and Neurobehavioral Deficits Between Aphasia and Right Hemisphere Communication Disorders
| Aphasia | Right Hemisphere Disorder |
|---|---|
| Pure linguistic deficits dominant | Linguistic deficits not dominant |
| More severe problems in naming, fluency, auditory and comprehension, reading and writing | Only mild problems |
| No left-sided neglect | Left-sided neglect |
| No denial of illness | Denial of illness |
| Speech generally relevant | Speech often irrelevant, rambling |
| Generally normal affect | Often lacks affect |
| Recognizes familiar faces | May not recognize familiar faces |
| Simplification of drawings | Rotation and left-sided neglect of drawings |
| No significant prosodic defect | Significant prosodic defect |
| Appropriate humor | Inappropriate humor |
| May retell the essence of a story | May retell only nonessential, isolated details |
| May understand implied meanings | Understands only literal meanings |
There is currently little information about lesion localization and a specific type of right hemisphere communication disorder. This is undoubtedly because many of the neurobehavioral abilities of the right hemisphere, which can affect communication, are more diffusely organized. Not all individuals with right hemisphere damage have communication deficits.53,54 The attentional deficits seen in right hemisphere–damaged patients either as a primary deficit or as a consequence of left-sided hemispatial neglect can affect reading and writing ability. Patients with right hemisphere communication disorders miss the “gist” in a communication message because of difficulties in processing emotional and prosodic input. This can affect their ability to interpret implied meanings, nonverbal signals, and/or intonation patterns that signal a question or sarcasm. Individuals with right hemisphere communication disorders often have difficulty conversing with others because they tend to be verbose, digressive, and tangential and convey little relevant information.53,74
Management of Right Hemisphere Communication Deficits
Patients with right hemisphere communication disorders should have both a neuropsychological assessment and an evaluation by the speech-language pathologist to assess the cognitive and communicative profile. Several screening and diagnostic tests have been developed to assist the speech-language pathologist in determining a plan of treatment. These include the Mini Inventory of Right Brain Injury58 and the Burns Brief Inventory of Communication and Cognition13 for screening. The Rehabilitation Institute of Chicago’s Clinical Management of Right Hemisphere Dysfunction32 can be used for more in-depth evaluation and treatment planning. Current practice suggests that treatments for right hemisphere communication disorders should be designed to compensate for deficits. This is accomplished by improving underlying attention deficits, targeting tasks to improve problem-solving abilities, improving task-oriented functional communication, and referring for counseling as needed.
Cognitive Communication Disorders of Traumatic Brain Injury
There are multiple neurobehavioral and cognitive disorders and stages of recovery resulting from traumatic brain injury (TBI) that either directly or indirectly affect communicative function. The primary causes of TBI are motor vehicle and pedestrian accidents, falls, assaults, and alcohol use.15 There are two main types of TBI: penetrating and closed head injuries. Penetrating injuries, such as a gunshot wound, usually result in focal damage. Closed head injuries generally result in diffuse, bilateral damage as a result of several co-occurring factors. These factors include the following:
The result to brain tissue can be diffuse axonal damage, loss of myelin, and small hemorrhages.
Speech and language disorders typically associated with TBI include dysarthria; deficits in naming, auditory and reading comprehension, writing, discourse cohesion, social language skills, and nonverbal communication; and impaired attention and information processing.1,84 Focal deficits can have communication deficits similar to stroke, depending on the site of the damage, with the added burden of problems with memory. Diffuse brain injury results in communication deficits caused by general attention, information processing, cognition, and memory deficits.84 Individuals who sustain TBI can have severe attention deficits characterized by perseveration, distractibility, impulsivity, and disinhibition.67
Management of Communication Disorders Resulting From Traumatic Brain Injury
The young age of the typical patient with TBI (15 to 24 years) presents a societal problem, requiring the expertise of all members of the rehabilitation team. Obviously, the patient’s stage of recovery determines the targeted intervention goals set by the speech-language pathologist. Numerous scales to assess cognitive functioning and to rate the disability37 have been developed.37,62 The Rancho Los Amigos Scale of Cognitive Levels31 provides a set of eight categories to assess TBI according to the cognitive and behavioral characteristics and is widely used by speech-language pathologists (Table 3-6). After a patient has entered a focused rehabilitation program, intervention is usually geared toward community reentry.84 The speech-language pathologist can use a variety of screening tools such as the Behavior Rating Inventory of Executive Function (BRIEF),27 the American Speech Language Hearing Association Functional Assessment of Communication Skills in Adults (ASHA-FACS),24 the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS),61 and the Test of Language Competence—Extended (TLC-E)82 to determine current cognitive-communicative function.76 Treatment programs for patients with TBI can include a wide range of targets including attention training, management of memory impairments,68 social skills and behavior regulation management,39,85 and executive function deficits, as well as the use of amplifiers and vocal programs. (See Chapter 49 for additional information on TBI.)
| Level | Definition |
|---|---|
| 1. No response | No response to pain, touch, sound, or sight. |
| 2. Generalized response | Inconsistent, nonpurposeful, nonspecific responses to intense stimuli. Responds to pain but response might be delayed. |
| 3. Localized response | Blinks to strong light, turns toward or away from sound, responds to physical discomfort. Inconsistent responses to some commands. |
| 4. Confused agitated | Alert, very active with aggressive and/or bizarre behaviors. Attention span is short. Behavior is nonpurposeful, and patient is disoriented and unaware of present events. |
| 5. Confused nonagitated | Exhibits gross attention to environment. Is highly distractible, requires continual redirection to keep on task. Is alert and responds to simple commands. Performs previously learned tasks but has great difficulty learning new ones. Becomes agitated by too much stimulation. Might engage in social conversation but with inappropriate verbalizations. |
| 6. Confused appropriate | Behavior is goal-directed with assistance. Inconsistent orientation to time and place. Retention span and recent memory are impaired. Consistently follows simple directions. |
| 7. Automatic appropriate | Performs daily routine in highly familiar environments without confusion but in an automatic, robot-like manner. Is oriented to setting but insight, judgment, and problem-solving are poor. |
| 8. Purposeful appropriate | Responds appropriately in most situations. Can generalize new learning across situations. Does not require daily supervision. Might have poor tolerance for stress and might exhibit some abstract reasoning disabilities. |
From Hagen C, Malkamus D: Interaction strategies for language disorders secondary to head trauma, Atlanta, 1979. Presented at the annual convention of the American Speech-Language-Hearing Association, with permission.
Communicative and Cognitive Deficits Associated With Dementia
While the physiatrist might not associate language as a major aspect of the early cognitive deficit of the dementias, the original case described by Alzheimer3 revealed a clear description of a fluent aphasia.44 The communicative-cognitive difficulties associated with dementia are multifaceted depending on the etiology of the disease (i.e., Alzheimer’s, vascular disease, Lewy body disease, Parkinson’s disease). The Diagnostic and Statistical Manual of Mental Disorders (fourth edition)4 specifies the criteria required for a diagnosis of dementia. A patient must have multiple cognitive deficits that include both of the following:
Bayles8 describes the complexity of separating the cognitive problems from language difficulties in dementia. Patients might fail a naming task not because of a language deficit but because the demands on attention or other cognitive processes are too great. The memory deficits that define the syndrome of dementia devastate the patient’s ability to communicate normally.8 Patients can also have serious memory problems because of depression. One of the first screens for the physician who works with the elderly patient is to distinguish dementia from pseudodementia (which is really depression). Table 3-7 shows the clear contrasts between these two disorders.
Table 3-7 Differential Features of Pseudodementia and Dementia
| Pseudodementia | Dementia | |
|---|---|---|
| Clinical course and history |
From Strub RL, Black FW: The mental status examination in neurology, ed 4, Philadelphia, 2000, FA Davis, with permission of FA Davis.
Management of Communicative and Cognitive Disorders Resulting From Dementia
Current evidence for the treatment of speech and language deficits subsequent to dementia includes family and staff education, compensatory skills, and direct intervention including spaced retrieval treatment (memory intervention with systematically lengthened intervals between recall opportunities).34 Early in the disease course, maintenance and compensatory activities for speech and cognitive problems are merited. Treatment of the dysarthrias for many of the progressive neurologic motor diseases focuses on compensatory speech and voice techniques and administration of drugs. Treatments for the cognitive deficits focus on reducing demands on memory.33,47 This type of treatment would capitalize on preserved recognition memory and avoid free-recall situations. Quayhagen et al.60 have shown preliminary evidence that intensive cognitive therapy can slow the general cognitive and behavioral decline associated with dementia. A major role of the speech-language pathologist is to work with the families of patients with dementia in terms of education, behavioral management, and approaches that might ease frustration and enhance communication with their family member(s). Evidence-based approaches indicate the necessity of at least four educationally oriented sessions with a focus on describing the dementia and its impact on communication, demonstrating verbal and nonverbal communication strategies to improve communication with individuals with dementia, and practicing use of communicative strategies.89
Motor Speech Disorders
Apraxia of speech (AOS) and dysarthria are motor speech disorders associated with both acute and progressive neurologic disease. The differential diagnosis of motor speech disorders is based on a motor speech assessment that includes a medical history, an oral mechanism examination, a perceptual speech characteristics assessment, a speech intelligibility rating, and an acoustic and physiologic analyses.20
AOS is a motor planning and programming disorder. It is characterized by articulation errors, impaired initiation of oral movement, reduced speaking rate, and prosodic errors.20,48 Automatic speech (reciting the days of the week) can be relatively unimpaired compared with purposeful, propositional speech (describing an illness). AOS often results from damage to the dominant hemisphere, usually the left, in the perisylvian and insular areas and subcortical structures. The typical neurologic diagnosis of disease is a unilateral cortical or subcortical stroke.
Dysarthria is a collective term for a variety of distinct sensorimotor speech execution disorders.20 Overall, dysarthria is characterized by impairments to the articulatory, respiratory, laryngeal, and resonance subsystems of speech.20 It results from damage to the central and/or peripheral nervous system, including the cerebrum, cerebellum, basal ganglia, brainstem, and cranial nerves. Depending on the underlying neurologic disease, its onset can be sudden or gradual and evolve in a recovering, stable, degenerative, or exacerbating-remitting course.20,40,41
The more common causes of dysarthria include unilateral, bilateral, or brainstem stroke, Parkinson’s disease, multiple sclerosis, and amyotrophic lateral sclerosis. Accurate diagnosis of the type of dysarthria is crucial to adequate management and treatment. Table 3-8 outlines the defining characteristics of each type of dysarthria and the typical neurologic diagnoses.
Table 3-8 Various Types of Dysarthria, Neurologic Diagnosis, Onset, and Course, and Salient Speech, Language, and Cognition Characteristics
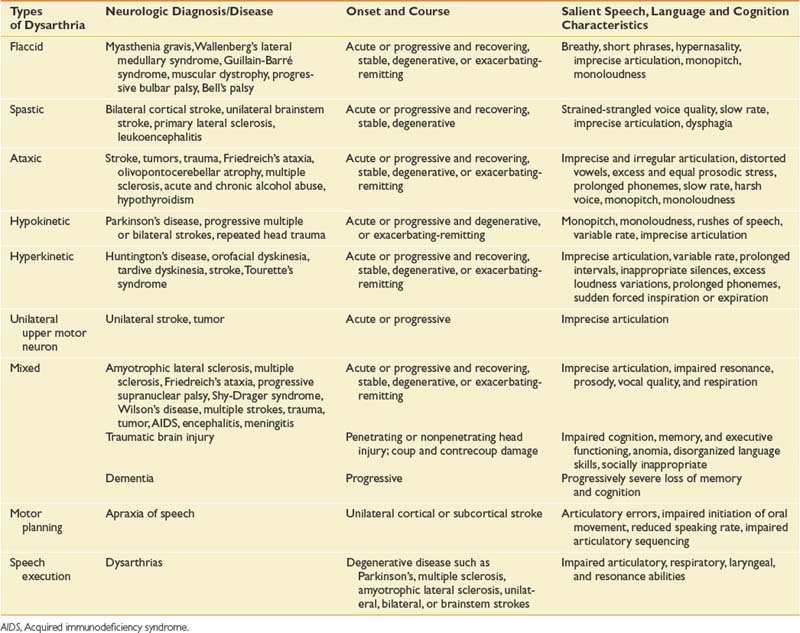
Flaccid dysarthria results from lower motor neuron lesions. The salient speech characteristics include breathy vocal quality, short phrase length, hypernasality, imprecise articulation, monopitch, and monoloudness. The presence of these characteristics depends on the site of damage; for example, Bell’s palsy can cause imprecise articulation, but vocal quality and prosody is unimpaired. The confirming signs of flaccid damage are hypotonic muscles, hyporeflexia, diminished reflexes, muscle atrophy, and fasciculations.20
Spastic dysarthria results from upper motor neuron lesions. The salient speech characteristics are a strained-strangled voice quality, slow speaking rate, and imprecise articulation.20
Ataxic dysarthria results from damage to the cerebellum. It is characterized by imprecise and irregular articulation breakdown, distorted vowels, excess and equal prosodic stress, prolonged phonemes, slow speaking rate, harsh voice quality, monopitch, and monoloudness quality. Confirming signs of ataxic dysarthria include ataxia, dysmetria, disordered stance and gait, and oculomotor abnormalities.20
Hyperkinetic dysarthria also is associated with damage to the basal ganglia. The salient speech characteristics differ from those of hypokinetic dysarthria and include imprecise articulation, variable speaking rate, inappropriate silences, excess loudness variations, prolonged phonemes, and sudden forced inspiration or expiration. Confirming signs of hyperkinetic damage are dyskinesia, tics, chorea, ballism, athetosis, dystonia, spasm, and essential tremor.20
Unilateral upper motor neuron dysarthria is a relatively new diagnostic subtype. It is characterized by a primary articulatory disorder and is caused by a unilateral stroke or tumor affecting the upper motor neuron system.20 It differs from spastic dysarthria because of its lack of respiratory, laryngeal, and resonance impairments. It differs from AOS because of its lack of initiation and sequencing error.
Mixed dysarthrias result from multiple motor system damage that can occur in the central and peripheral nervous system. These mixed dysarthrias are characterized by imprecise articulation and impaired resonance (hyponasal or hypernasal quality), prosody (fast or slow speaking rate), vocal quality (breathy or strained-strangled or harsh), and respiration (short rushes of speech or excessive loudness).20 The specific characteristics depend on which motor systems are damaged, but the most common types of mixed dysarthria are spastic-flaccid resulting from amyotrophic lateral sclerosis, and spastic-ataxic resulting from stroke.
Management of Motor Speech Disorders
Management of motor speech disorders can include medical (e.g., pharmacologic), prosthetic (e.g., augmentative device), and/or behavioral interventions86 (e.g., improving speech intelligibility) with the primary goal of improving communication function.87 Reported potentially effective treatments for AOS include articulatory kinematics (e.g., sound production accuracy and sequencing), speech rate and rhythm control (e.g., metronomic pacing), and prosthetic (e.g., use of pictures or words to communicate).80 Effective treatments for respiratory and phonatory impairments resulting from dysarthria include modification of loudness in individuals with Parkinson’s disease87; biofeedback of subglottal air pressure, excursion of the abdomen and rib cage, and loudness; and use of devices such as delayed auditory feedback and amplifiers.87,88
1. Adamovich B.L.B. Traumatic brain injury. In LaPointe L.L., editor: Aphasia and related neurogenic language disorders, ed 2, New York: Thieme, 1997.
2. Alexander M.P., Fischette M.R., Fischer R.S. Crossed aphasias can be mirror image or anomalous. Brain. 1989;112:953-973.
3. Alzheimer A. Uber eine eigenartige erkrankung der kirnrinde. Allgem Z Psychiatr Psych-Gerich Med 64:146-148, 1907. In: Rottenberg D.A., Hochberg F.H., editors. Neurological classics in modern translation. New York: Hafner Press, 1977.
4. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, ed 4. Washington, DC: The Association; 1994.
5. Avent J., Glista S., Wallace S., et al. Family information needs about aphasia. Aphasiology. 2005;19:365-375.
6. Barbay S., Zoubina E.V., Dancause N., et al. A single injection of d-amphetamine facilitates improvements in motor training following a focal cortical infarct in squirrel monkeys. Neurorehabil Neural Repair. 2006;20:455-458.
7. Basso A. How intensive/prolonged should an intensive/prolonged treatment be? Aphasiology. 2005;19(10/11):975-984.
8. Bayles K.A. Language in aging and dementia. In: Kirshner H.S., editor. Handbook of neurological speech and language disorders. New York: Marcel Dekker, 1995.
9. Benson D.F. Aphasia, alexia and agraphia. New York: Churchill Livingstone; 1979.
10. Bhogal S.K., Teasell R., Speechley Ml. Intensity of aphasia therapy, impact on recovery. Stroke. 2003;34:987-993. published online before print as doi:10.1161/01.STR.0000062343.64383.D0 Available at: http://stroke.ahajournals.org. Accessed February 16, 2009.
11. Broca P. Remarques sur le siege de la faculte du langage articule, suivies d’une observation d’aphemie (perte de la parole). Bull Soc Anat Paris 1861:6330-6357. In: Rottenberg D.A., Hochberg F.H., editors. Neurological classics in modern translation. New York: Hafner Press., 1977.
12. Brookshire R.H. Introduction to neurogenic communication disorders. St Louis: Mosby; 2007.
13. Burns M.S. Burns Brief Inventory of Communication and Cognition. San Antonio: Psychological Corp; 1997.
14. Canter G.J. Syndromes of aphasia in relation to cerebral connectionism. South Bend, IN: Short course presented to the Indiana Speech and Hearing Association; 1979.
15. Centers for Disease Control and Prevention. Traumatic brain injury in the United States. A report to Congress. Available at: http://www.cdc.gov. Accessed January 16, 2009.
16. Chapey R., Hallowell B. Introduction to language intervention strategies in adult aphasia. In Chapey R., editor: Language intervention strategies in aphasia and related neurogenic communication disorders, ed 5, Philadelphia: Lippincott Williams & Wilkins, 2008.
17. Cherney L.R., Patterson J.P., Raymer A., et al. Evidence-based systematic review: effects of intensity of treatment and constraint-induced language therapy for individuals with stroke-induced aphasia. J Speech Lang Hear Res. 2008;51:1282-1299.
18. Dronkers N.F., Pinker S., Damasio A. Language and the aphasias. In Kandel E.R., Schwartz J.H., Jessel T.M., editors: Principles of neural science, ed 4, New York: McGraw-Hill, 2000.
19. Druback D.A., Makley M.D., Dodd M.L. Manipulation of central nervous system plasticity: a new dimension in the care of neurologically impaired patients. Mayo Clin Proc. 2004;79:796-800.
20. Duffy J.R. Motor speech disorders. St Louis: Mosby; 1995.
21. Duffy J.R., Peterson R.C. Primary progressive aphasia. Aphasiology. 1992;6:1-15.
22. Feeney D.M., Gonzales A., Law W. Amphetamine, haloperidol and experience interact to affect rate of recovery after motor cortex injury. Science. 1982;217:855-857.
23. Frattali C., Bayles K., Beeson P., et al. Development of evidence-based practice guidelines: committee update. J Med Speech Lang Pathol. 2003;11(3):ix-xviii.
24. Frattali C., Thompson C., Holland A., et al. American Speech Language Hearing Association functional assessment of communication skills for adults. Rockville, Md: American Speech Language Hearing Association; 1995.
25. Geschwind N. The apraxias: neural mechanisms of disorders of learned movement. Am Sci. 1975;63:188.
26. Gladstone D.J., Danells C.J., Armesto A., et al. Physiotherapy coupled with dextroamphetamine for rehabilitation after hemiparetic stroke: a randomized, double-blind, placebo-controlled trial. Stroke. 2006;37:179-185.
27. Gioia G.A., Isquith P.K., Guy S.C., et al. Behavior Rating Inventory of Executive Function. Odessa, Fla: Psychological Assessment Resources; 2000.
28. Goldstein L.B. Potential impact of drugs on post stroke motor recovery. In: Goldstein L.B., editor. Restorative neurology: advances in pharmacotherapy for recovery after stroke. Armonk: Futura Publishing, 1998.
29. Goodglass H., Kaplan E. The Boston Diagnostic Aphasia Examination, ed 3. Philadelphia: Lippincott Williams & Wilkins; 2001.
30. Grade C., Redford B., Chrostowski J., et al. Methylphenidate in early post stroke recovery: a double-blind, placebo controlled study. Arch Phys Med Rehabil. 1999;79:1047-1050.
31. Hagen C., Malkamus D. Interaction strategies for language disorders secondary to head trauma. Atlanta: Presented at the annual convention of the American Speech-Language-Hearing Association; 1979.
32. Halper A.S., Cherney L.R., Burns M.S. Clinical management of right hemisphere dysfunction, ed 2. Gaithersburg: Aspen Publishers; 1996.
33. Hopper R., Bayles K.A. Management of neurogenic communication disorders associated with dementia. In Chapey R., editor: Language intervention strategies in aphasia and related neurogenic communication disorders, ed 5, Philadelphia: Lippincott Williams & Wilkins, 2008.
34. Hopper T., Mahendra N., Kim E., et al. Evidence-based practice recommendations for working with individuals with dementia: spaced-retrieval training. J Med Speech Lang Pathol. 2005;13(4):xxvii-xxxiv.
35. Hurwitz B.E., Dietrich W.D., McCabe P.M., et al. Amphetamine promotes recovery from sensory-motor integration deficit after thrombotic infarction of the primary somatosensory rat cortex. Stroke. 1991;22:648-654.
36. Jenkins W.M., Merzenich M.M., Ochs M.T., et al. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol. 1990;63:82-104.
37. Jennett B., Bond M. Assessment of outcome after severe brain damage: a practical scale. Lancet. 1972;1:480-484.
38. Karli D.C., Burke D.T., Kim H.J., et al. Effects of dopaminergic combination therapy for frontal lobe dysfunction in traumatic brain injury rehabilitation. Brain Inj. 1999;13:63-68.
39. Kennedy M.R.T., Coelho C. Self-regulation after traumatic brain injury: a framework for intervention of memory and problem solving. Semin Speech Lang. 2005;26:242-255.
40. Kent R.D. Models of speech motor control: implications from recent developments in neurophysiological and neurobehavioral science. In: Maassen B., Kent R., Peters H., et al, editors. Speech motor control in normal and disordered speech. Oxford: Oxford University Press, 2004.
41. Kent R.D., Rosen K. Motor control perspectives on motor speech disorders. In: Maassen B., Kent R., Peters H., et al, editors. Speech motor control in normal and disordered speech. Oxford: Oxford University Press, 2004.
42. Kertesz A. The Western Aphasia Battery. Orlando: Grune & Stratton; 1982.
43. Kirshner H.S. Classical aphasia syndromes. In: Kirshner H.S., editor. Handbook of neurological speech and language disorders. New York: Marcel Dekker, 1995.
44. Kirshner H.S. Primary progressive aphasia syndrome. In: Kirshner H.S., editor. Handbook of neurological speech and language disorders. New York: Marcel Dekker, 1995.
45. Klein K. Aphasia community group manual. New York: National Aphasia Association; 1995.
46. Kline A.E., Yan H.Q., Bao J., et al. Chronic methylphenidate treatment enhances water maze performance following traumatic brain injury in rats. Neurosci Lett. 2000;280:163-166.
47. Mahendra N., Kim E., Bayles K., et al. Evidence-based practice recommendations for working with individuals with dementia: computer-assisted cognitive interventions (CACIs). J Med Speech Lang Pathol. 2006;13(4):xxxv-xliv.
48. McNeil M.R., Pratt S.R., Fossett T.R.D. The differential diagnosis of apraxia of speech. In: Maassen B., Kent R., Peters H., et al, editors. Speech motor control in normal and disordered speech. Oxford: Oxford University Press, 2004.
49. Mesulam M.M. A cortical network for directed attention and unilateral neglect. Ann Neurol. 1981;10:307-325.
50. Mesalum M.M. Primary progressive aphasia: a language-based dementia. N Engl J Med. 2003;349:1535-1547.
51. Merzenich M.M., Kaas J.H., Wall J. Topographic reorganization of somatosensory cortical areas 3B and 1 in adult monkeys following restricted deafferentation. Neuroscience. 1983;8:33-55.
52. Moss A., Nicholas M. Language rehabilitation in chronic aphasia and time postonset: a review of single-subject data. Stroke. 2006;37:3043-3051. published online before print as doi:10.1161/01.STR.0000249427.74970.15
53. Myers P.S.: Toward a definition of RHD syndrome, Aphasiology Available at: http://stroke.ahajournals.org. Accessed February 16, 2009.
54. Myers P.S. communication disorders associated with right-hemisphere damage. In: Chapey R., editor. Language intervention strategies in aphasia and related neurogenic communication disorders. Philadelphia: Lippincott Williams & Wilkins, 2008.
55. Nadeau S.F., Crosson B. Subcortical aphasia. Brain Lang. 1997;58:436-458.
56. National Institute on Neurological Disorders and Stroke. Aphasia hope through research. Bethesda: National Institute on Neurological Disorders and Stroke; 1990. Publication No. 990-391
57. Nudo R.J., Plautz E.J., Milliken G.W. Adaptive plasticity in primate motor cortex as a consequent of behavioral experience and neuronal injury. Semin Neurosci. 1997;9:13-23.
58. Pimental P.A., Kingsbury N.A. Mini Inventory of Right Brain Injury. Austin: Pro-Ed; 1989.
59. Platz T, Kim JH, Engel U, et al. Amphetamine fails to facilitate motor performance and to enhance motor recovery among stroke patient with mild arm paresis: interim analysis and termination of a double blind, randomized, placebo controlled trial. Restor Neurol Neurosci 23:271-280, 2005.
60. Quayhagen M.P., Quayhagen M., Corbeil R.R., et al. A dyadic remediation program for care recipients with dementia. Nurs Res. 1995;44:153-159.
61. Randolph C. Repeatable Battery for the Assessment of Neuropsychological Status, ed 1. San Antonio: Psychological Corp; 2001.
62. Rappaport M., Hall K.M., Hopkins K., et al. Disability rating scale for severe head trauma: coma to community. Arch Phys Med Rehabil. 1982;63:118-123.
63. Robey R.R. The efficacy of treatment for aphasic persons: a meta-analysis. Brain Lang. 1994;47:582-608.
64. Robey R.R. A meta-analysis of clinical outcomes in the treatment of aphasia. J Speech Lang Hear Res. 1998;41:172-187.
65. Scheidtmann K., Fries W., Muller F., et al. Effect of levodopa in combination with physiotherapy on functional motor recovery after stroke: a prospective, randomized, double-blind study. Lancet. 2001;358:787-790.
66. Schmanke T.D., Avery R.A., Barth T.M. The effects of amphetamine on recovery of function after cortical damage in the rat depends on the behavioral requirement of the task. J Neurotrauma. 1996;13:293.
67. Sohlberg M., Avery J., Kennedy M.R.T., et al. Practice guidelines for direct attention training. J Med Speech Lang Pathol. 2003;11(3):xix-xxxix.
68. Sohlberg M.M., Kennedy M.R.T., Avery J., et al. Evidence-based practice for the use of external aids as a memory rehabilitation technique. J Med Speech Lang Pathol. 2007;15(1):xv-li.
69. Stroemer R.P., Kent T.A., Hulsebosch C.E. Enhanced neocortical neural sprouting, synaptogenesis and behavioral recovery with d-amphetamine therapy after neocortical infarction in rats. Stroke. 1998;29:2381-2395.
70. Strub R.L., Black F.W. The mental status examination in neurology, ed 4. Philadelphia: FA Davis; 2000.
71. Sutton R.L., Feeney D.M. α-Noradrenergic agonists and antagonists affect recovery and maintenance of beam-walking ability after sensorimotor cortex ablation in the rat. Restor Neurol Neurosci. 1992;4:1-11.
72. Teasell R.W., Foley N.C., Bhogal S.K., et al. An evidence-based review of stroke rehabilitation. Top Stroke Rehabil. 2003;10:29-58.
73. Teasell R.W., Jutai J.W., Bhogal S.K., et al. Research gaps in stroke rehabilitation. Top Stroke Rehabil. 2003;10:59-70.
74. Tompkins C.A. Right hemisphere communication disorders: theory and management. San Diego: Singular Publishing Group; 1995.
75. Tranel D., Biller J., Damasio H., et al. Global aphasia without hemiparesis. Arch Neurol. 1987;44:304-308.
76. Turkstra L., Ylvisaker M., Coelho C., et al. Practice guidelines for standardized assessment for persons with traumatic brain injury. J Med Speech Lang Pathol. 13(2), 2005.
77. Walker-Batson D., Curtis S., Rajeshwari N., et al. A double-blind, placebo-controlled study of the use of amphetamine in the treatment of aphasia. Stroke. 2001;32(9):2093-2097.
78. Walker-Batson D., Smith P., Curtis S., et al. Amphetamine paired with physical therapy accelerates recovery from stroke: further evidence. Stroke. 1995;26:2254-2259.
79. Walker-Batson D., Wendt J.S., Devous M., et al. A long-term follow-up case study of crossed aphasia assessed by single-photon emission tomography (SPECT), language, and neuropsychological testing. Brain Lang. 1988;33:311-322.
80. Wambaugh J., Duffy J., McNeil M., et al. Treatment guidelines for acquired apraxia of speech: treatment descriptions and recommendations. J Med Speech Lang Pathol. 2006;14(2):xxxv-lxvii.
81. Whyte J., Hart T., Schuster K., et al. Effects of methylphenidate on attentional function after traumatic brain injury: a randomized, placebo-controlled trial. Am J Phys Med Rehabil. 1997;76:440-450.
82. Wiig E., Secord W. Test of language competence—expanded edition. San Antonio: Psychological Corp; 1989.
83. World Health Organization. International classification of functioning, disability and health: ICF. Geneva: WHO; 2001.
84. Ylvisaker M., Szekeres S.F., Fenney T. Communication disorders associated with traumatic brain injury. In Chapey R., editor: Language intervention strategies in aphasia and related neurogenic communication disorders, ed 5, Philadelphia: Lippincott Williams & Wilkins, 2008.
85. Ylvisaker M., Turkstra L., Coelho C., et al. Brain injury, behavioural interventions for children and adults with behaviour disorders after TBI: a systematic review of the evidence. J Med Speech Lang Pathol. 2007;21(8):769-805.
86. Yorkston K.M., Beukelman D.R., Strand E.A., et al. Management of motor speech disorders in children and adults, ed 2. Austin: Pro-Ed; 1999.
87. Yorkston K.M., Hakel M., Beukelman D.R., et al. Evidence for effectiveness of treatment of loudness, rate or prosody in dysarthria: a systematic review. J Med Speech Lang Pathol. 2007;15(2):xi-xxxvi.
88. Yorkston K.M., Spencer K.A., Duffy J.R. Behavioral management of respiratory/phonatory dysfunction from dysarthria: a systematic review of the evidence. J Med Speech Lang Pathol. 2003;11(2):xiii-xxxviii.
89. Zientz J., Rackley A., Chapman S., et al. Evidence-based practice recommendations: educating caregivers on Alzheimer’s disease and training communication strategies. J Med Speech Lang Pathol. 2007;15(1):liii-lxiv.