Adrenal insufficiency
1. What is adrenal insufficiency, and how is it categorized?
“Adrenal insufficiency” is the term used to describe inadequate production of glucocorticoids, mineralocorticoids, or both by the adrenal glands. It can occur because of dysfunction or complete destruction of the adrenal cortex (primary adrenal insufficiency), inadequate adrenocorticotropic hormone (ACTH) production by the pituitary (secondary adrenal insufficiency), or inadequate corticotropin-releasing hormone (CRH) production by the hypothalamus (tertiary adrenal insufficiency).
2. What are common causes of adrenal insufficiency?
Autoimmune adrenalitis (Addison’s disease) is the most common cause of primary adrenal insufficiency and is associated with increased levels of 21-hydroxylase antibodies. Addison’s disease can occur in isolation or in combination with other endocrine deficiencies as part of an autoimmune polyglandular syndrome. The most common cause of central (secondary/tertiary) adrenal insufficiency is withdrawal of glucocorticoids after long-term use. Central adrenal insufficiency can also occur as part of panhypopituitarism from large pituitary tumors or their treatment with surgery and/or radiation therapy. See Table 30-1 for other causes of adrenal insufficiency.
TABLE 30-1.
CAUSES OF ADRENAL INSUFFICIENCY
| Primary | Autoimmune |
| Bilateral adrenal hemorrhage or thrombosis: coagulopathy, meningococcal sepsis | |
| Metastases: lymphoma, lung, breast, renal, gastrointestinal | |
| Infectious: tuberculosis, human immunodeficiency virus, cytomegalovirus, fungal (Histoplasma, Coccidioides) | |
| Adrenoleukodystrophy and other congenital disorders | |
| After adrenalectomy | |
| Infiltrative: hemochromatosis, amyloidosis | |
| Congenital adrenal hyperplasia | |
| Adrenal enzyme deficiency | |
| Drugs (see text) | |
| Secondary | Withdrawal of long-term suppressive glucocorticoid therapy |
| Pituitary tumors including craniopharyngioma | |
| Metastases to the pituitary | |
| Pituitary surgery or irradiation | |
| Lymphocytic hypophysitis | |
| Infiltrative diseases: hemochromatosis, sarcoidosis, histiocytosis X | |
| Infection (e.g., tuberculosis, histoplasmosis) | |
| Sheehan syndrome (massive blood loss leading to shock in the peripartum period) | |
| Severe head trauma disrupting the pituitary stalk or otherwise affecting the pituitary | |
| Tertiary | Withdrawal of long-term suppressive glucocorticoid therapy |
| Hypothalamic tumors | |
| Metastases to the hypothalamus | |
| Infiltrative diseases affecting the hypothalamus | |
| Cranial irradiation | |
| Trauma | |
| Infections (e.g., tuberculosis) |
3. What are common symptoms of adrenal insufficiency?
Most patients report nonspecific symptoms such as weakness, fatigue, and anorexia. Many also complain of gastrointestinal symptoms such as nausea, vomiting, vague abdominal pain, and constipation. Psychiatric symptoms and symptoms of orthostatic hypotension, arthralgias, myalgias, and salt craving are also reported.
4. How does adrenal insufficiency usually present?
Weight loss is a common presenting sign. Hyperpigmentation, particularly of the buccal mucosa and gums, is noted in most patients with primary adrenal insufficiency. Patients should be examined for darkening of the palmar creases, nail beds, and scars forming after onset of ACTH excess. Hyperpigmentation occurs because production of proopiomelanocortin (POMC), a prohormone that is cleaved into ACTH, melanocyte-stimulating hormone (MSH), and other hormones is increased and leads to increased melanin production. Orthostasis is common in both primary and central adrenal insufficiency.
5. What laboratory abnormalities can be found in adrenal insufficiency?
The classic laboratory abnormalities are hyponatremia and hyperkalemia. The hyperkalemia is due to mineralocorticoid deficiency, whereas the hyponatremia occurs mainly because of glucocorticoid deficiency. Hyponatremia is the result of elevated vasopressin values with free water retention, shift of extracellular sodium into cells, and decreased delivery of filtrate to the diluting segments of the nephron due to decreased glomerular filtration rate. Azotemia can be seen because of hypovolemia. Patients often demonstrate a normocytic normochromic anemia and may have eosinophilia and lymphocytosis. Mild to moderate hypercalcemia may occur. Fasting blood glucose is usually low-normal, but occasionally patients can have fasting or postprandial hypoglycemia. Patients with coexisting type 1 diabetes mellitus and adrenal insufficiency may experience greater frequency and severity of hypoglycemic episodes.
6. How do the clinical presentations of primary and central forms of adrenal insufficiency differ?
Hyperpigmentation and hyperkalemia are not observed in secondary/tertiary adrenal insufficiency. Otherwise, the clinical presentations are similar.
7. How is adrenal insufficiency usually diagnosed biochemically?
In the outpatient setting, a low morning cortisol value (< 3 μg/dL) is sufficient to diagnose adrenal insufficiency, and a high morning cortisol value (> 20 μg/dL) excludes the diagnosis. In most instances, a dynamic test, the cosyntropin stimulation test, is also performed. This test determines whether the adrenals are able to respond to maximal stimulation by synthetic ACTH. This test can also be used in the diagnosis of central adrenal insufficiency, as long as sufficient time has elapsed for the adrenal cortex to atrophy in response to lack of ACTH stimulation.
The standard cosyntropin test is performed by collecting a specimen for measurement of a baseline serum cortisol level, administration of 250 μg of cosyntropin (brand name Cortrosyn, Synacthen) intravenously (IV) or intramuscularly (IM), and then collecting specimens for serum cortisol measurement 30 and 60 minutes later. An abnormal result is defined as a stimulated cortisol level at either 30 or 60 minutes of less than 18 to 20 μg/dL (< 450-500 nmol/L). This test can be performed at any time during the day. If an individual is receiving glucocorticoid therapy, the dose should be withheld (12 hours for hydrocortisone, 24 hours for prednisone) before the test is performed to avoid detection of synthetic glucocorticoids in the cortisol assay.
Other dynamic testing includes the insulin tolerance test, metyrapone test, glucagon stimulation test, and CRH stimulation test. The insulin tolerance test evaluates the hypothalamic-pituitary-adrenal (HPA) axis in response to insulin-induced hypoglycemia (blood glucose level < 40 mg/dL). This test should be performed in experienced centers only by trained staff, and should not be performed if the individual has significant coronary artery disease or an uncontrolled seizure disorder.
8. What about the low-dose cosyntropin stimulation test?
It has been argued that mild cases of primary adrenal insufficiency may be missed with the standard-dose cosyntropin stimulation test because the dose of ACTH administered in this test is quite supraphysiologic. Data from studies examining the potential role of low-dose cosyntropin stimulation testing, in which 1 μg cosyntropin is administered, do not clearly establish that the low-dose test is better than the standard test. There are several potential problems with performing the test, including false-positive results because of inaccurate or irreproducible dilution of cosyntropin, the need for IV administration, and the need for carefully timed sampling for serum cortisol levels. It is unclear whether abnormal results from this test are clinically relevant. Therefore the standard-dose test should be used in most instances.
9. What testing can be used to distinguish primary from central adrenal insufficiency?
In primary adrenal insufficiency, ACTH is elevated, whereas ACTH is “abnormally normal” (i.e., not elevated in response to low cortisol) or frankly low in central adrenal insufficiency.
10. When can the results of the ACTH stimulation test be misleading?
Partial ACTH deficiency and recent ACTH deficiency are situations that may lead to false-negative results of the cosyntropin stimulation test. Insulin-induced hypoglycemia (insulin tolerance testing) or metyrapone testing may be used in these situations.
11. When are imaging tests appropriate?
After the biochemical diagnosis of adrenal insufficiency, imaging may be performed in certain instances to help determine the cause. In cases of central adrenal insufficiency, magnetic resonance imaging (MRI) of the pituitary and hypothalamus is indicated if exogenous glucocorticoids have not been implicated. If a primary adrenal process is suspected, abdominal computed tomography (CT) can be performed with thin slices through the adrenals. Imaging should not be performed before a biochemical diagnosis is made because of the high incidence of incidental imaging findings that are without clinical significance.
12. When should the diagnosis of adrenal crisis be considered?
Adrenal crisis should be suspected in patients with unexplained catecholamine-resistant hypotension or other severe signs or symptoms consistent with adrenal insufficiency. Symptoms of adrenal crisis are often nonspecific—weakness, fatigue, nausea, vomiting, abdominal pain, fever, and altered mental status. Acute adrenal hemorrhage should be suspected if there is a constellation of abdominal/flank pain, hypotension/shock, fever, and hypoglycemia in a deteriorating patient. Adrenal crisis is more common in primary than in central adrenal insufficiency.
13. How is adrenal crisis managed?
If adrenal crisis is suspected, it should be treated aggressively because, if left untreated, adrenal crisis is fatal. A formal diagnosis of adrenal insufficiency can be performed later. The patient can receive a dose of dexamethasone initially (4 mg IV) while the basal cortisol measurement and the cosyntropin stimulation test are performed; empiric treatment with IV hydrocortisone (100 mg IV q8h, with rapid tapering) can then be initiated. In addition, treatment should include intravenous saline and glucose to correct volume depletion, dehydration, and hypoglycemia. Patients often need intensive care unit (ICU)–level supportive care. A search for precipitating factors and the underlying cause needs to be performed.
14. How is adrenal insufficiency diagnosed in the critical care setting?
Because the diurnal rhythm of ACTH and cortisol secretion is disrupted in acute illness, and because severe stress should stimulate cortisol production, a random cortisol specimen can be drawn to diagnose complete or relative adrenal insufficiency in the critical care setting. In patients who (1) are hemodynamically unstable and unresponsive to vasopressors despite adequate fluid resuscitation or (2) have signs or symptoms suggestive of adrenal insufficiency, random cortisol specimen should be collected, and a cosyntropin stimulation test performed immediately afterward.
The cortisol level at which adrenal insufficiency should be diagnosed (a random level of < 20 μg/dL, some other value such as < 25 μg/dL, and/or an increment of 9 μg/dL after cosyntropin administration) is controversial. The reasons are concerns about the existence of a cortisol-resistant state in critically ill patients due to inflammatory cytokines, reduction in binding affinity to cortisol-binding globulin, and proinflammatory transcription factors. Some authorities in this field believe that a cortisol level that is adequate in an ambulatory setting may not be adequate in the setting of severe stress or prolonged or complicated surgical procedures; this latter inadequacy is referred to as relative adrenal insufficiency.
15. When and how should glucocorticoids be used in the critical care setting?
There is considerable debate about the most appropriate use of glucocorticoids in the critical care setting. Although the results of clinical trials of glucocorticoid treatment for sepsis without proven adrenal insufficiency have been mixed, a systematic review showed that 5 or more days of 300 mg/day or less of hydrocortisone or its equivalent resulted in a significant reduction in 28-day all-cause mortality and hospital mortality. However, there is marked heterogeneity among trial results, and the Corticosteroid Therapy of Septic Shock (CORTICUS) trial failed to show benefit for empiric glucocorticoid treatment.
Some groups advocate using stress-dose steroids empirically in critically ill patients with resistant hypotension, testing for adrenal insufficiency with a random cortisol measurement and cosyntropin stimulation testing, and then stopping stress-dose steroids if the tests for adrenal insufficiency are normal. On the other hand, the American College of Critical Care Medicine, in its Surviving Sepsis Campaign, does not advocate formal testing for adrenal insufficiency, but instead recommends use of glucocorticoids in selected groups of patients: those with vasopressor-dependent septic shock and those with early severe acute respiratory distress syndrome.
If steroids are used, typical hydrocortisone doses in the critical care setting are 50 mg IV every 6 hours or 100 mg IV every 8 hours. These dosages should be tapered quickly as the patient’s clinical status improves and the underlying illness resolves.
16. How do I manage chronic adrenal insufficiency, and when should I consider prescribing fludrocortisone?
All patients with chronic adrenal insufficiency require replacement with glucocorticoids, and occasionally with mineralocorticoids. Hydrocortisone is frequently used in primary adrenal insufficiency because it has some mineralocorticoid activity. The usual dosage of hydrocortisone is 10 to 15 mg every morning and 5 to 10 mg in the afternoon. When prednisone is used, typical doses are 2.5 to 5 mg daily. If additional mineralocorticoid effect is necessary for persistent hyperkalemia and/or orthostatic hypotension, fludrocortisone 0.05 to 0.2 mg once a day may be added.
17. What are some deficiencies in the current approach to treating adrenal insufficiency?
Many individuals with adrenal insufficiency experience significantly reduced subjective health status and quality of life, increased fatigue, and depression. The reason for some of these effects may be that current treatment regimens do not replicate the physiologic diurnal cortisol profile or because most female patients do not receive replacement of adrenal androgens. Work is being done to develop sustained-release glucocorticoid formulations that better approximate the natural diurnal cortisol pattern.
18. Should I recommend dehydroepiandrosterone replacement for my adrenally insufficient patient?
Dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEA-S) are the main androgens produced by the adrenals. Both are weak androgens, but they are converted to the more potent androgens, testosterone and 5α-dihydrotestosterone (DHT), peripherally. This peripheral conversion is a significant source of androgens in women. Oral DHEA supplementation using 25 to 50 mg/day normalizes circulating levels of androgens in women with adrenal insufficiency. A metaanalysis of 10 randomized, placebo-controlled trials showed a small improvement in health-related quality of life and depression after treatment with DHEA, with no significant improvement in anxiety or sexual well-being. The data are insufficient to recommend DHEA therapy for all women with adrenal insufficiency, but it may be tried in women who continue to have significantly impaired well-being despite optimal glucocorticoid and mineralocorticoid treatment. In the United States, DHEA is classified as a dietary supplement and therefore is not subject to the same quality control as medications.
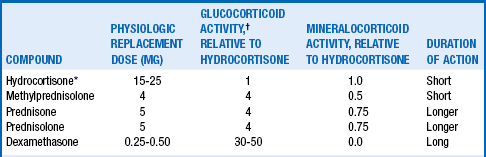
19. What are the relative potencies of available glucocorticoids?
20. How is treatment for chronic adrenal insufficiency monitored?
Adequate treatment for chronic adrenal insufficiency is monitored by taking a focused history regarding overall well-being and symptoms suggestive of orthostasis and obtaining blood pressure, weight, and electrolytes. It is important to avoid the use of excessive dosages of replacement glucocorticoids, which lead to iatrogenic Cushing syndrome, which in turn can result in needless weight gain, osteoporosis, hypertension, hyperglycemia, glaucoma, or avascular necrosis. The goal should be to use the smallest replacement dosage of glucocorticoids possible that maintains normal electrolyte levels and good quality of life. Serum cortisol and plasma ACTH levels are not useful for monitoring treatment of adrenal insufficiency.
21. When do individuals with chronic adrenal insufficiency require “stress-dose” glucocorticoids, and what doses should be used?
Any medical stress, including febrile illnesses, trauma, labor and delivery, and diagnostic or surgical procedures, can precipitate an acute adrenal crisis in the patient with chronic adrenal insufficiency. Supplemental steroids should be used to prevent adrenal crisis, but care should be taken to avoid unnecessary supplemental doses of glucocorticoids. Typically, the usual replacement dose is doubled or tripled for mild to moderate infections and during labor and delivery. Doses should also be doubled or tripled for approximately 24 hours for dental surgery, minor surgery (cataract, laparoscopic), and invasive diagnostic procedures. For moderate surgical stress, patients should be given doses equivalent to hydrocortisone 50 to 75 mg/day in divided doses for 1 to 2 days. For major surgical procedures, severe infections, and severe acute illnesses, patients are typically given hydrocortisone 200 to 300 mg/day for 2 to 3 days in divided doses every 6 to 8 hours.
Patients with adrenal insufficiency should wear a medical alert bracelet or necklace identifying them as individuals with adrenal insufficiency, in case they are incapable of providing an adequate history. An alternative form of hydrocortisone or dexamethasone can be provided so that patients are still able to receive glucocorticoids intramuscularly (hydrocortisone or dexamethasone) or per rectum (hydrocortisone) in an emergency situation.
22. What drugs can cause adrenal insufficiency?
The most common cause of central adrenal insufficiency is glucocorticoid therapy. Glucocorticoids can cause exogenous Cushing syndrome, leading to suppression of the HPA axis. Patients may then be unable to mount an adequate cortisol response to stress, or adrenal insufficiency may develop if the steroid dose is abruptly stopped or tapered. Exogenous Cushing syndrome can result from oral, ocular, inhaled, transdermal, rectal, or parenteral glucocorticoids. Some injected glucocorticoids for musculoskeletal disorders can last for weeks to months. Glucocorticoids are also found in some herbal or complementary/alternative therapies. Protease inhibitors and other drugs slow metabolism of glucocorticoids via interactions with the CYP3A4 enzyme. Thus, when protease inhibitors and glucocorticoids are used to together, exogenous Cushing’s disease with HPA suppression can result even at low glucocorticoid doses.
High-dose progestins, such as megestrol acetate and medroxyprogesterone acetate, have enough glucocorticoid activity to cause Cushing syndrome. Opioids can also suppress the HPA axis. Drugs that can cause primary adrenal sufficiency include the azole antifungal agents, the anesthetic etomidate, the antiparasitic suramin, and steroid synthesis inhibitors such as aminoglutethimide, metyrapone, and mitotane. Mifepristone, a progesterone antagonist, is a glucocorticoid receptor antagonist.
23. How should steroid dosage be tapered in patients taking pharmacologic doses of steroids to treat nonadrenal diseases?
Patients may be started on glucocorticoids to treat a variety of autoimmune, neoplastic, or inflammatory disorders. Discontinuing glucocorticoid therapy can be challenging because of (1) worsening of the disorder for which the glucocorticoid is being used, (2) suppression of the HPA axis with resulting secondary adrenal insufficiency upon discontinuation of the glucocorticoid, and (3) steroid withdrawal syndrome.
The initial tapering of glucocorticoids from pharmacologic to physiologic doses depends on the underlying illness for which the steroids are being used. If the illness worsens during this period of tapering, the dosage needs to be increased and the higher dosage continued until the symptoms stabilize before another attempt at more gradual tapering. When the patient is taking a near-physiologic dosage, she or he can be switched to a shorter-acting glucocorticoid such as hydrocortisone, and tapering of the dosage can be continued to below physiologic dosages or to alternate-day therapy in certain instances.
Testing should be performed when patients have been receiving physiologic or lower doses for at least 1 month, to ensure that adrenal suppression has resolved and that normal responsiveness of the HPA axis has returned. A morning cortisol specimen should be collected 12 to 24 hours after the last dose of glucocorticoid (12 hours for short-acting synthetic glucocorticoids such as hydrocortisone, and 24 hours for longer-acting ones such as prednisone). A plasma cortisol level less than 3 μg/dL is consistent with adrenal insufficiency, so the glucocorticoid should be continued for 4 to 6 weeks before retesting. A level greater than 20 μg/dL is consistent with return of adrenal function, and glucocorticoids can be discontinued. A level between 3 and 20 μg/dL is equivocal, and further testing is needed, usually with a cosyntropin stimulation test. It may take months for the HPA axis to respond normally to ACTH.
Central adrenal insufficiency should be suspected in individuals who have a clinical presentation suggestive of adrenal insufficiency and who have received the equivalent of 20 mg prednisone for 5 days or physiologic dosages of glucocorticoid for at least 30 days in the past 12 months. These patients should receive stress doses of glucocorticoids during moderate to severe illness or surgery.
Alkatib, AA, Cosma, M, Elamin, MB, et al. A systemic review and meta-analysis of randomized placebo-controlled trials of DHEA treatment effects on quality of life in women with adrenal insufficiency. J Clin Endocrinol Metab. 2009;94:3676–3681.
Annane, D, Bellissant, E, Bollaert, P-E, et al, Corticosteroids in the treatment of severe sepsis and septic shock in adults. a systematic review. JAMA 2009;301:2362–2375.
Arafah, BM, Hypothalamic pituitary adrenal function during critical illness. limitations of current assessment methods. J Clin Endocrinol Metab 2006;91:3725–3745.
Axelrod, L. Perioperative management of patients treated with glucocorticoids. Endocrinol Metab Clin N Am. 2003;32:367–383.
Bornstein, SR. Predisposing factors for adrenal insufficiency. N Engl J Medicine. 2009;360:2328–2339.
Bouillon, R. Acute adrenal insufficiency. Endocrinol Metab Clin N Am. 2006;35:767–775.
Carroll, TB, Aron, DC, Findling, JW, et al. Glucocorticoids and adrenal androgens. In: Gardner DG, Shoback D, eds. Greenspan’s basic and clinical endocrinology. 9th ed. Chicago: McGraw-Hill Medical; 2011:285.
Dorin, RI, Qualls, CR, Crapo, LM. Diagnosis of adrenal insufficiency. Ann Intern Med. 2003;139:194–204.
Grossman, AB. The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010;95:4855–4863.
Hahner, S, Allolio, B. Therapeutic management of adrenal insufficiency. Best Pract Res Clin Endocrinol Metab. 2009;23:167–179.
Hopkins, RL, Leinung, MC. Exogenous Cushing’s syndrome and glucocorticoid withdrawal. Endocrinol Metab Clin N Am. 2005;34:371–384.
Marik, PE, Pastores, SM, Annane, D, et al, Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients. consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med 2008;36:1949–1973.
Meikle, AQ, Tyler, FH, Potency and duration of action of glucocorticoids. effects of hydrocortisone, prednisone and dexamethasone on human pituitary-adrenal function. Am J Med 1977;63:200–207.
Neary, N, Nieman, L, Adrenal insufficiency. etiology, diagnosis and treatment. Curr Opin Endocrinol Diabetes Obes 2010;17:217–223.
Sprung, CL, Annane, D, Keh, D, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111–124.
Stewart, PM, Krone, NP, et al. The adrenal cortex. In: Melmed S, Polonsky KS, Larsen PR, eds. Williams textbook of endocrinology. 12th ed. Philadelphia: Saunders; 2011:495–496.