Chapter 15 Adrenal exhaustion
AETIOLOGY
Stress
Hans Selye, in 1935, was the first to develop a theory of stress. When he imposed different types of physical stressors on rats he discovered that, regardless of the kind of stressor, the same physiological response was the result: hypertrophied adrenal glands, atrophied lymphatic organs (lymph nodes, spleen and thymus) and bleeding gastric ulcers. These symptoms developed over time. He called this the ‘general adaptation syndrome’ (GAS), stating ‘stress is the nonspecific response of the body to any demand made upon it’.1 This requires adjustment or adaptation to a new situation.
From his observations he hypothesised three stages: alarm, resistance and exhaustion. The alarm stage was characterised by hormonal changes such as increased sympathetic nervous system (SNS) activity, with its typical fight or flight response and noradrenaline secretions, and up-regulated cortisol. In the resistance stage the body had adapted to the stressor, the above symptoms had disappeared and body metabolism had returned to normal. In the exhaustion stage the stress triad of hypertrophied adrenals, atrophied lymph organs and gastric ulcers were noticed, together with an initial increase in cortisol, which later declined to below normal. Only in this stage, with severe stress over prolonged periods of time, did the body lose its ability to cope and eventually death ensued. However, in general the physiological changes mentioned above were thought of as being protective to ensure the animal’s survival.1,2 Therefore, stress is actually a positive occurrence needed for protection (readiness for action when in danger, increased immune particles when injured). It becomes pathological only if it is protracted or uncontrolled.3–5
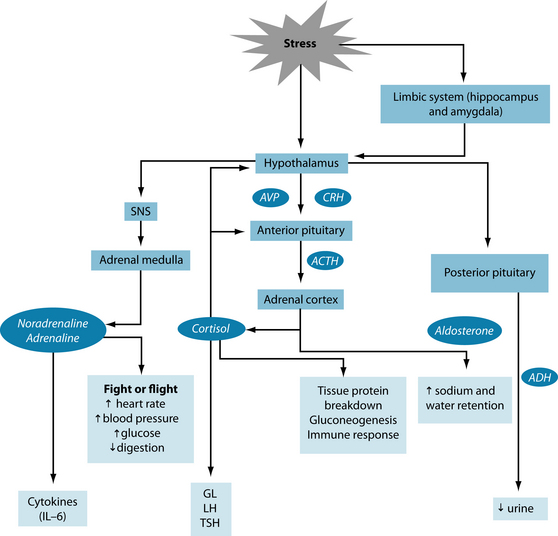
Physiologically, a stressor causes disruptions in homeostasis, leading to neural and endocrine changes known as the ‘stress response’ or ‘stress cascade’ (Figure 15.1).6,7 Mental and emotional stressors stimulate the hypothalamus via the limbic system (the hippocampus and amygdala),6–8 whereas physical and physiological processes, such as injury or hypoglycaemia, can stimulate the hypothalamus directly.
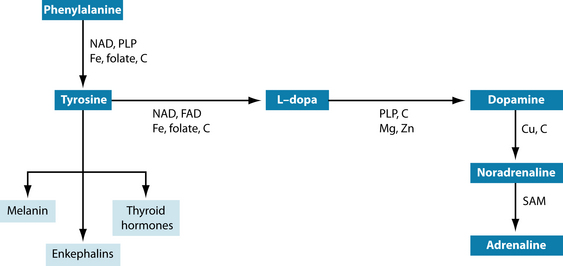
The first (and immediate) reaction to a stressor is caused by imbalances in the central nervous system (CNS) through overstimulation of the SNS and suppression of the parasympathetic nervous system (PNS). The SNS stimulates the adrenal medulla to secrete the catecholamines noradrenaline and adrenaline. They are produced from phenylalanine and hence tyrosine (Figure 15.2). These hormones lead to heightened alertness to quickly judge a situation regarding its potential danger. Blood pressure, breathing and heart rate are accelerated for fight or flight, for which glycogenolysis (release of glycogen from the liver) provides the extra fuel. Endorphins are released to dampen pain from a potential injury. Digestion, relaxation and sleep are suppressed. In other words, the body is prepared for swift action needed for survival.3,6,9,10
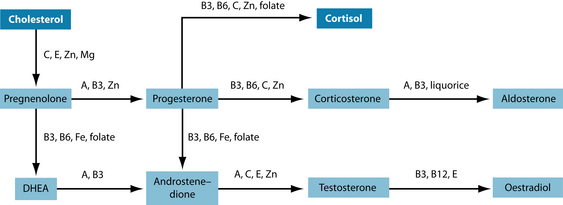
Stimulation of the anterior pituitary gland is achieved through secreting corticotrophin releasing hormone (CRH), and to a lesser extent arginine vasopressin (AVP), which leads to the release of adrenocorticotropic hormone (ACTH). ACTH triggers the release of vast amounts of cortisol glucocorticoid and moderate amounts of aldosterone a mineralo corticoid from the adrenal cortex. Both these hormones are made (a minerale certicoid) from cholesterol (Figure 15.3).
Under normal circumstances cortisol levels are highest in the morning and lowest in the evening. Its physiological actions control carbohydrate, protein and fat metabolism, and it inhibits prostaglandin synthesis and contributes to emotional stability.
In stress, however, cortisol levels in blood are elevated, triggering an increase in protein breakdown and mobilisation of fatty acids (gluconeogenesis) in order to provide glucose for the fight or flight response. This results in a hyperglycaemic state in the liver with peripheral hypoglycaemia, temporarily leading to moderate insulin resistance. High cortisol also decreases lymphocyte and eosinophil counts, effectively dampening any inflammation or immune response.2,6,7 Moreover, elevated glucocorticoids influence reproduction, growth and thyroid functions by inhibiting gonadotropin-releasing hormone (GnRH) and luteinising hormone (LH), growth hormone (GH) and thyroid-stimulating hormone (TSH), respectively.6 As a result, these functions are suppressed because the body deems them to be of minor importance in the face of an acute stressor.6,11–14
Cortisol, adrenaline and glucagon all have the ability to raise blood glucose levels. Due to their hyperglycaemic action they have a catabolic effect on the body. Cortisol is involved in replenishing depleted energy stores; hence it converts food into glycogen and fat, and initiates hunger. Adrenaline increases mental alertness, blood pressure, breathing and heart rate, muscle tone, glycogenolysis and the release of endorphins. Simultaneously, it down-regulates appetite, digestion, elimination, relaxation and sleep.3 Glucagon opposes insulin by mediating the release of glucose from storage.
The second hormone released by the adrenal cortex—aldosterone—retains sodium and water in the body. In addition, stimulation of the posterior pituitary gland by the hypothalamus results in antidiuretic hormone (ADH) secretion. The combined result of these actions is fluid retention and increased blood volume, which can lead to increased blood pressure.2
The secretion and interplay of the stress hormones will vary, depending on the type, intensity and duration of the stressor as well as its hormonal regulation.15,16 High amounts of circulating cortisol will, via negative feedback loops, shut off the release of hormones from the hypothalamus and anterior pituitary glands. Therefore, once the acute stressor has subsided, the stress cascade abates and the physiology of the organism returns to normal. The individual becomes more resilient as a result of successful adaptation to a new situation in which wear and tear are minimised.17 This memory is stored in the hippocampus and readily accessible when a similar stressful situation arises, so the learning from the first event can guide the (re)actions when it recurs. The role of the amygdala is to retain the emotional impact of the stressor and, together with the hippocampus, will ensure a better memory of an emotionally charged event. Both cortisol and adrenaline are needed for this memory to happen.3 Thus mind and body can both be strengthened from a stressful experience, becoming more resilient to future stressors.
Distress
If, however, stress is chronic or intense and exceeds the person’s mental and physical resources, it becomes distress. This is the case in adrenal exhaustion which corresponds to the final stage of Selye’s GAS. The circulating hormones will not return to their normal levels and initially stay in a state of hyperarousal.6,8 As a result, CRH, AVP and ACTH are no longer inhibited via negative feedback (leading to dysregulation of the HPA axis), target organs become overstimulated, receptors possibly become desensitised and tissue damage ensues.4 This ‘wear and tear’ or ‘cost’ of adaptation or allostasis has been termed ‘allostatic load’. It is implicated in numerous disease processes4,18–20 and has been associated with an energy-deficiency state of the body.14 It is mediated by adrenaline and cortisol. Both hormones actually serve to imprint the stressful event into long-term memory, but prolonged action will cause damage to the part of the brain that should shut them off. This in turn leads to higher levels of these hormones circulating in the blood (‘cortisol resistance’), which can do more damage to the brain, especially the hippocampus. High levels of cortisol have been linked to the conditions outlined in Table 15.1.
| HORMONAL DYSREGULATION | DISORDER |
|---|---|
| General effects | |
| Lowered serotonin levels | Anxiety, panic disorder and melancholic depression5,6,25–27 |
| High cytokines leading to oxidative stress | |
| Suppression of immune function | Infections31,32 |
| ↑ bone demineralisation | Reduction in bone mass and osteoporosis6,27 |
| ↑ storage of fat around abdomen → ↑ gluconeogenesis from protein (loss of muscle mass) to meet energy demands → ↑ insulin → ↑ cortisol → ↑ eating energy-dense foods → ↑ storage of fat | |
| Shrivelling of dendrites → destruction of neurons → ↓ neurogenesis → cerebral ischaemia → ↓ hippocampus size | Impaired memory and loss of cognitive function, acceleration of ageing28 |
| Impaired conversion of T4 to T3 | Thyroid dysfunction6 |
| Dysregulation of reproductive hormones37 | Hormonal disturbances6,13 |
However, with time there is a blunted response before cortisol levels will decline or the diurnal rhythm will flatten.20 With lowered cortisol levels, endogenous glucose production is compromised, and sugar and stimulant cravings are likely as a consequence of the resultant hypoglycaemia. If untreated, this can lead to adrenal burnout and chronic fatigue.34 The results of low cortisol are shown in Table 15.2.
Table 15.2 Chronic diseases caused by low cortisol (insufficient HPA response to stress)3
| HORMONAL DYSREGULATION | DISORDER |
|---|---|
| General | |
| Unresponsive HPA, low (exhausted) cortisol levels and blunted response to exercise, disturbances in serotonergic neurotransmission and AVP | |
| Flat cortisol rhythm | |
| Burnout → loss of regulation in limbic system → brain damage and atrophy | |
| Depleted adrenaline | |
| Hypoarousal | Non-melancholic depression3 |
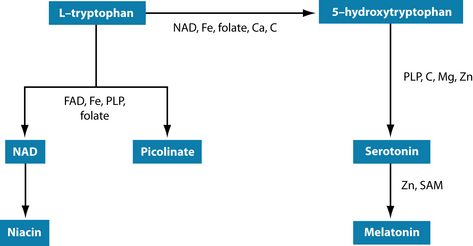
The flow-on effect of these disturbances in neurotransmitters and stress hormones can lead to exhausted serotonin levels as well, potentially resulting in anxiety and sleep disturbances. The precursor of serotonin is tryptophan (Figure 15.4). The established link between carbohydrate cravings and depression is thought to be due to its tryptophan-increasing properties,42,43 with mood-elevating results.44
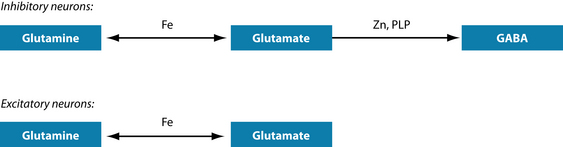
Agitation and anxiety can also be caused by glutamatergic activation. Note that the precursor for both the inhibitory and the excitatory pathways is the same amino acid: glutamine (Figure 15.5). If zinc or vitamin B6 are in short supply then adequate amounts of GABA, the inhibitory neurotransmitter, cannot be formed. Instead, glutamate, the excitatory neurotransmitter, accumulates, leading to the above-mentioned symptoms.45
Testing for adrenal exhaustion
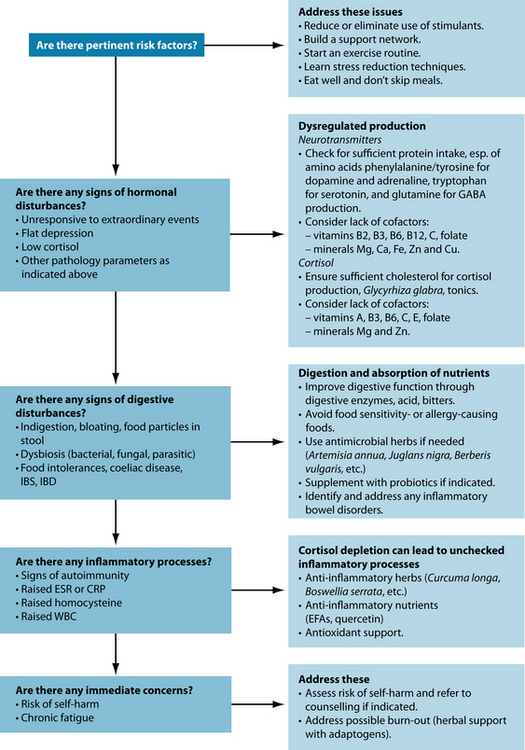
Adrenal exhaustion is a vague term that is not generally used in medicine. ‘Adrenal fatigue’ has been used to describe hypoadrenia. It is therefore important to assess HPA axis dysregulation before treatment is instigated.46 The tests in Table 15.3 have been shown to be useful in diagnosing HPA axis dysregulation. The information gleaned from these tests will indicate not only the level of cortisol excess or depletion but also any concomitant health conditions that may have developed as a result of stress, such as insulin resistance, hypopituitarism and inflammation.
| TEST | TISSUE | COMMENTS |
|---|---|---|
| Cortisol | Serum | Cortisol is diurnal—it is highest in the morning at 6 to 8 a.m. and drops throughout the day, with lowest levels occurring around midnight. Therefore, tests should be performed at around 8 a.m. and again at 4 p.m., with the morning readings close to the maximum and the afternoon values closer to the lower end of the reference range. |
| Urine | 24-hour readings indicate whether adequate amounts of cortisol have been produced overall. | |
| Saliva | Several readings can be taken throughout the day to determine the diurnal variation. | |
| ACTH | Serum | ACTH needs to be present to stimulate cortisol release and is therefore following the same diurnal variation as cortisol. If both cortisol and ACTH are low, an ACTH stimulation test should be performed to determine whether cortisol is low because of lack of ACTH. |
| ACTH stimulation | Serum |
A synthetic form of ACTH (cosyntropin) is injected after taking a blood sample for baseline cortisol measurements. Blood samples are taken in half-hourly intervals for the following 1–2 hours.
|
RISK FACTORS
While the SNS is responsible for arousal, for fight and flight, the PNS facilitates rest, relaxation and healing (repair), it slows heart rate and promotes digestion and elimination. Neurotransmitters activated by the SNS include adrenaline and noradrenaline, whereas acetylcholine is the predominant neurotransmitter of the PNS. In a healthy state there is balance between the SNS and PNS. However, during stress the SNS is dominant, suppressing the actions of the PNS. This can lead to digestive and sleep disturbances and agitation.49 Repercussions of this may include reduced absorption of nutrients through diminished production of digestive juices, anxiety and further drain on energy through lack of restful sleep.
The use of stimulants such as coffee increases the stress response and adrenal output, as shown by elevated catecholamines, notably adrenaline, in urine.50,51 If coffee is used as a pick-me-up without having an effect, leading to increasingly greater consumption, it begs the question as to whether adrenaline production ability has been exhausted. This kind of stimulation may therefore hasten the decline in adrenal function, thus being a stressor in its own right. Adrenal exhaustion can be the result of multiple stressors, each of them not being enough to cause HPA dysfunction. However, the additive effects (if not addressed) can weaken the system to such an extent that other health problems could arise, such as chronic fatigue, clinical depression, hypothyroidism, inflammatory and autoimmune conditions, and hormonal disturbances, as outlined in Table 15.2.
CONVENTIONAL TREATMENT
The diagnosis of adrenal exhaustion is not often made in conventional medicine, and if it is made, it is from a different perspective to what naturopaths consider. Primary hypoadrenalism (Addison’s disease) is rare. If it is part of an autoimmune endocrine disease,53 where adrenal function has been maintained through endogenous up-regulation of corticotropic hormone stimulation, treatment may not be required. If secondary hypoadrenalism has been diagnosed, glucocorticoids are the drugs of choice,54,55 with mineralocorticoids if needed.56 DHEA has been trialled, with mixed results.56,57 Apart from the removal of the adrenals like in Cushing’s syndrome, hypoadrenalism is mainly recognised in the medical literature as being the result of brain injury,58 tumours, endocrine disorders or critical illness,59,60 where adrenal crisis has either happened or may be imminent.61 In any case, the diagnosis seems to be thought of only at a very late stage.53 Where naturopaths use the diagnosis of adrenal exhaustion, conventional treatment would therefore focus on the symptoms, with antidepressants being the most likely prescription.62–64 Other interventions may include beta-blockers65,66 and glutamatergic agents.63 Novelty treatments trialled are glucocorticoid receptor antagonists and atrial natriuretic peptide receptor agonists.64
KEY TREATMENT PROTOCOLS
The aim of treatment is to repair suboptimally functioning cells, thus increasing the energy and wellbeing in the patient. Since the HPA axis is stimulated by mental and emotional as well as physical events, giving the patient resources requires not only support with nutrients but also teaching better coping strategies.46
Modulation of the HPA axis
The HPA axis activates and is inactivated by cortisol (Figure 15.1).67 In the case of low cortisol, the negative feedback is impaired, leading to dysregulation of other stress hormones. Diet, lifestyle and thought patterns have a key influence on the HPA axis. Adjusting these are the primary concerns as they will need to be the first line of defence for future stressors. Herbs have been used traditionally to modulate the nervous system and the stress response.
Diet
Reduction or avoidance of stimulants such as tea and coffee, so commonly used to keep up alertness and functioning in today’s hectic life, will prevent further drain on the adrenals. These beverages can be replaced with decaffeinated coffee or tea, herb teas and filtered water. The change is best done slowly to reduce the risk of caffeine withdrawal symptoms such as headache and further fatigue.51
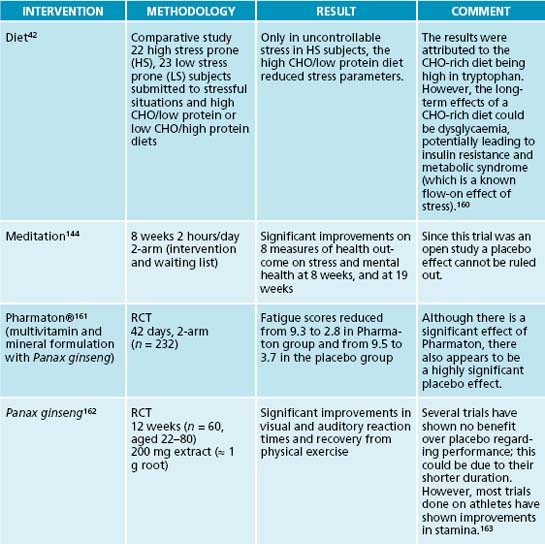
Stress is often accompanied by carbohydrate cravings, which can lead to blood sugar imbalances, especially if they are high in sugar and refined starches. Fruit is best limited to two pieces a day as it may increase glycaemic load. Whole grains are preferable due to their fibre content, thus slowing down the release of sugar,68 and their B vitamin content. B vitamins are essential in the Krebs cycle for energy production.45 Carbohydrates are commonly craved during stress due to their tryptophan-serotonin enhancing qualities.42,43 This partially explains the weight gain experienced by some people when under stress.43 High cortisol is also known to lead to weight increases and potentially to metabolic syndrome,69,70 although in adrenal exhaustion cortisol is usually low (depleted). High-protein foods and snacks for amino acids, especially fish as the latter also contributes to a favourable EFA balance, may decrease carbohydrate cravings while at the same time providing the necessary amino acids as precursors for neurotransmitter synthesis. Protein powders can be used in smoothies to provide additional amino acids for neurotransmitter production and to help reduce hypoglycaemic episodes.
Lifestyle
Balance of work, relaxation and sleep, socialising, physical activities and daily chores is difficult to achieve in modern life. However, it is important to be aware of a person’s commitments and how these influence their life. Chronic stress has been linked to premature death,71 and social support networks are related to positive states of health and reduced disease burden.72
The importance of physical activity cannot be underestimated. Since the body is geared up for action when under stress (‘fight or flight’), exercise is a potent tool to bring stress hormones back under control.73 Regular physical activity has been shown to help in reducing stress, depression and anxiety.74
Herbal medicines
One of the most important herbal remedies acting on cortisol is Glycyrrhiza glabra. G. glabra, especially its active constituent glycyrrhetinic acid, is a potent inhibitor of 11 beta-hydroxysteroid dehydrogenase (which converts active cortisol to inactive cortisone),75,76 thus increasing the amount of circulating cortisol.77–79 It is therefore ideally suited to cases of low cortisol, such as in adrenal exhaustion although contraindicated in the ‘alarm’ or ‘adaptation’ stress phase. However, care needs to be taken as G. glabra can lead to pseudoaldosteronism by binding to mineralocorticoid receptors, thus promoting sodium and fluid retention as well as potassium loss, potentially leading to hypertension.80 It is therefore not recommended in liver and kidney disease. A diet high in potassium and low in sodium is recommended if G. glabra is used long-term.81
Further, herbs with anxiolytic, sedative, adaptogenic and memory-enhancing properties82–84 may be indicated in HPA-axis dysregulation. In order to treat specific diseases arising from allostatic load, many more herbs (such as anti-inflammatory and immune-modulating herbs) are available and need to be carefully selected to match the condition of the patient. The information on the following herbs has been taken from several textbooks81-83,85-89 as well as some additional references stated in the text below.
Anxiolytics and sedative herbs
For mild forms of anxiety and restlessness, Matricaria recutita could be useful.81 It is a mild sedative and generally safe. However, it should not be taken at the same time as iron supplements. Care should be taken when a patient is on anticoagulants. In rare cases allergies have been reported. Alternatively, Melissa officinalis, a safe herb with a subtle lemon taste, could be used as a mild nervine and antispasmodic.81 Both herbs are suitable for children.
If a stronger action is desirable, Valeriana spp. should be considered. Valeriana officinalis and Valeriana edulis are both used, with the former having higher levels of terpenes and the latter higher levels of valepotriates.81 They improve sleep quality without morning drowsiness or impairment of concentration or reaction time and are therefore preferable to drug treatment.90 In a blinded, randomised crossover study with V. officinalis, V. edulis has shown stronger effects.91 Both have nervine and soporific actions and combine well with Humulus lupulus M. recutita and M. officinales for sleep disorders with anxiety and restlessness.90 These herbs are safe, but long-term use of valerian can lead to insomnia. On rare occasions, valerian can have a stimulating effect in some people and should therefore be avoided.
Passiflora incarnata has been used for any kind of nervous system disorders, from headaches, irritability and restlessness to insomnia.90 It has similar actions to hops and valerian and could be used in their place or combined with them. Alternatively, Scutellaria lateriflora could be prescribed due to its trophorestorative and nervine properties.
Piper methysticum is an excellent remedy for sleeplessness. It has anxiolytic, antispasmodic and soporific qualities. In recent years alcohol and acetone extracts of kava have been withdrawn in some countries (including Australia) due to severe liver damage in rare cases,92 possibly due to mitochondrial toxicity.93 Traditionally, watery extracts were used with no adverse effects,94 and P. methysticum produced in the traditional way is now available again in tablet form. Nonetheless, caution is indicated in patients with liver disease. Due to its possible dopamine antagonism it should not be used for patients with Parkinson’s disease. Other contraindications include the use of non-steroidal anti-inflammatory drugs and alcohol; it should not be given to children or patients with endogenous depression.
In the Chinese tradition Zizyphus jujuba var. spinosa has been used for Yin deficiency and as a Qi tonic. Apart from its hypnotic and sedative qualities it is prescribed for irritability and to prevent sweating, especially at night. In addition, it can reduce heart palpitations and hypertension.90 It may augment the effects of corticosteroids.
Adaptogens
Adaptogens have been defined as ‘herbal preparations used to increase attention and endurance during fatigue and to reduce or prevent stress-induced impairments of neuroendocrine or immune function’.95 Three categories of adaptogenic activity have been identified: regulating the stress response via the HPA axis, antioxidant action and modulating the central nervous system.11 Several herbs, as shown below, have increased physical and mental stamina.11,96 This class of herbs is therefore ideally suited to treat adrenal exhaustion.
Perhaps the strongest of the adaptogenic herbs is Panax ginseng. It has a long tradition as a tonic in China, Japan, Korea and Russia. In the West, it is used to combat mental or physical fatigue and stress, and to enhance energy, wellbeing and performance.79,97–99 It is used as an adrenal tonic, antidepressant, adaptogen and immunomodulator. It is not recommended for diabeties as it may potentiate the actions of oral hypoglycaemic drugs and insulin. Further, it may decrease the effectiveness of warfarin or cause symptoms of oestrogen excess. Long-term use or high amounts may cause overstimulation.
Due to the limited resources of P. ginseng, Russians have investigated Eleutherococcus senticosus and found it to be the most important substitute for P. ginseng.100 E. senticosus has shown non-specific body resistance to stress and fatigue.101 It has similar properties to P. senticosus, in that it is an adaptogenic, a tonic and an immune modulator, and has anabolic qualities. It has been suggested that a threshold exists: when stress hormones are high E. senticosus will have a lowering effect, and if stress hormones are low they will be enhanced.12 This herb is generally safe but may give falsely elevated test results for digoxin and hypertension.
Another adaptogen from the ginseng family is Panax quinquefolium. It has tonic, immune-modulating and anti-inflammatory properties, combats stress and enhances the nervous and immune systems.102 Both P. quinquefolium and P. ginseng can elevate cortisol levels due to its effect on the HPA axis.103 Research in mice has resulted in normalising dopamine, noradrenaline and 5-hydroxytryptophan after chronic unpredictable stress when P. quinquefolium was given at an oral dose of 200 mg/kg, but not at 100 mg/kg.104
A much gentler, yet just as patent, adaptogen is Withania somnifera. Its many qualities include a regulatory effect on the HPA axis by exerting a positive influence on the endocrine, nervous and cardiovascular systems. Main actions are adaptogenic, mildly sedative, anti-stress, immunomodulatory, trophorestorative, anti-inflammatory, antioxidant and rejuvenating.11,84,97,105,106 Application includes convalescing after illness, fatigue and weakness.79
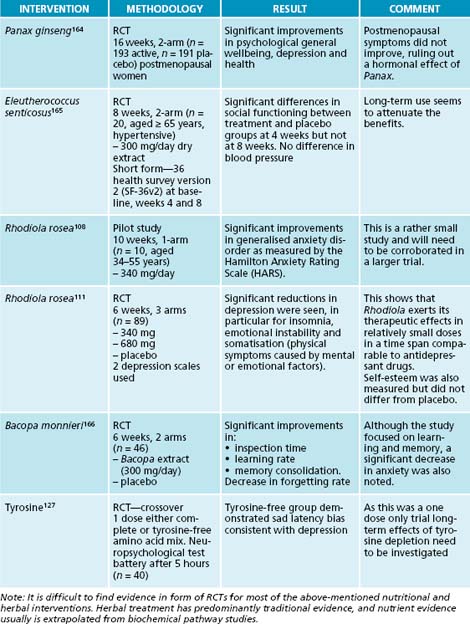
The Russian herb Rhodiola rosea belongs to the key adaptogenic herbs.11,84,96,107 It has been used traditionally to alleviate symptoms of anxiety, insomnia and depression. Clinical trials have corroborated these findings.108–111 R. rosea has been found to relieve fatigue,112 increase work and exercise performance,96,113 increase physical fitness and decrease mental fatigue in stressful situations.114 It has therefore been used in asthenic conditions (conditions of weakness and debility, decline in function)109 and chronic stress.115 Apart from the actions described here there are many more benefits to be gained from this herb,107,116 but they are beyond the scope of this chapter.
Other herbs with adaptogenic activity and stress control include Rehmannia glutinosa,81 Ocimum sanctum,84,117 Ginkgo biloba118 and Bacopa monnieri.119 The last two are also used for increasing cognitive function and concentration.81
Homoeopathy
In naturopathy in general and homoeopathy in particular, stress is not necessarily deemed ‘bad’, but only becomes ‘negative’ if it dominates our lives.120,121 Homoeopathy has been used to treat stress and adrenal exhaustion, but remedies depend on the personal characteristics of the patient. The remedies are prescribed singly or in combination, and methods of determining the remedy include questionnaires, repertorisation and muscle testing.122 The following remedies have been used in anxiety, where the symptom picture agrees: Arsenicum Album (Ars), Gelsemium (Gels), Argentum Nitricum (Arg-n), Lycopodium (Lyc) and Sulphur (Sulph).123
Nourishing depleted adrenals and reducing strain on HPA axis
Nutritional supplements
To support depleted adrenal glands, nutrients such as B vitamins and vitamin C are essential. B vitamins and coenzyme Q10 are cofactors in the Krebs cycle and oxidative phosphorylation (electron transport chain), respectively, which can be compromised (as is seen particularly in chronic fatigue syndrome). These nutrients may therefore be needed to increase energy production. Magnesium is a cofactor in energy production and needed for glucose regulation. It is best given with an organic ligand such as citrate for optimal bioavailability.124,125
Phenylalanine and tyrosine are precursors for dopamine, noradrenaline and adrenaline synthesis. Reduced dopamine levels have been implicated in impaired reward/punishment processing.126,127 Precursors for serotonin and melatonin synthesis are tryptophan or 5-hydroxytryptophan (5HTP).128 Glutamine is needed for GABA production.45 Vitamins B2, B3 and B6 (ideally in the form of pyridoxyl 5-phosphate—PLP), folate, vitamins B12 and C, and minerals iron, copper, calcium and zinc are needed as cofactors for neurotransmitter synthesis (see Figures 15.2, 15.4 and 15.5). In depression, it may well be the synergy of these neurotransmitters and their cofactors that is unbalanced.126 Ensuring their optimal supply in cellular metabolism seems mandatory for resilience to stress.
At a dose of 1500 mg/day vitamin C has reduced adrenaline, cortisol and anti-inflammatory peptides in stress,129 thus showing a lowered stress response and allostatic load. Most animals (except primates, guinea pigs, fruit bats and some birds) produce their own vitamin C from glucose in response to a stressor, indicating that this vitamin is indeed essential for mounting an effective stress response.45
With low digestive capacity, vitamin B12 absorption will also be affected due to insufficient or absent intrinsic factor.130 It can be administered either as sublingual troches or by intramuscular injection by a health-care provider qualified to do so. Vitamin B12 plays an essential role in lowering homocysteine and providing methyl groups for neurotransmitter functioning.131 Lack of vitamin B12 and folate have been linked to depression,131 with vitamin B12 termed the ‘master key’ as it influences so many different systems. It may be needed even when serum levels appear normal.132 Both nutrients are involved in S-adenosyl methionine (SAM or SAMe—SAMe is the brand name for SAM) production and are therefore crucial for methyl transfer. SAMe has been used quite successfully for depression.128 Endogenously, it is made from homocysteine and requires vitamin B12, folate and adenosine as cofactors. If homocysteine is low methionine or SAMe need to be given as a substrate for methyl groups. SAM is the universal methyl donor and is needed for a range of metabolic reactions, including neurotransmitter production (see Figures 15.2 and 15.4, last step).
Omega-3 fatty acids have been indicated in depression128 and are needed to support a healthy nervous system and brain function. Due to their anti-inflammatory properties they also dampen stress-induced cytokine activation.133 Further, antioxidants such as vitamins A, C and E, the minerals selenium and zinc, as well as bioflavonoids, coenzyme Q10 and glutathione are required in higher amounts to combat free-radical damage and oxidative stress.134–136
Coenzyme Q10 (CoQ10) tends to be low when cortisol is low.137 (Abnormal thyroid function can have an influence on CoQ10 levels and should be ruled out.)
The amino acids L-lysine and L-arginine have been shown to enhance ACTH, cortisol, adrenaline and noradrenaline levels in response to psychosocial stress and anxiety.138
INTEGRATIVE MEDICAL CONSIDERATIONS
Mindfulness meditation141,142 attempts to foster greater awareness of thought processes in the ‘here and now’ in order to increase coping ability. A meta-analysis has found that mindfulness-based stress reduction is indeed able to assist patients in dealing with both mental and physical stressors.143 Other forms of meditation can be powerful adjuncts to positive thinking, as well as being able to reduce anxiety and pain, and to promote healing.144
Cognitive-behavioural therapy has been investigated and found to be a powerful instrument in stress-related health problems such as anxiety, depression, phobias and panic, anorexia and distress. Effects seen were comparable or superior to antidepressants and similar to other behavioural therapies.145
Other means to help with problem solving include imagery and creative visualisation.73 Art and music therapy146 have been shown to reduce stress, anxiety and pain. Spiritual or pastoral counselling may be needed if the stress is manifesting on the spiritual level.147 Light therapy has been used successfully for depression,128 especially for seasonal affective disorders.3
Acupuncture has been evaluated for its effect on stress modulation in connection with endothelial dysfunction. It was found that highly hypnotisable subjects responded much better to the procedure than less hypnotisable ones. The researchers concluded that the psychological state of the patient needs to be taken into account when choosing a relaxation method.148 However, acupuncture may be beneficial to restore energy flows to depleted systems, notably the adrenals and the nervous system.
Massage (with or without aromatherapy),149,150 reiki151 and others152 are means to reduce tension in the musculoskeletal as well as the nervous system due to stress. Touch is one of the most ancient ways of healing and has shown beneficial effects for anxiety and depression.150 Progressive muscular relaxation, where sequential muscle groups are first tensed and then relaxed, is used to invoke a relaxation response.73
CORTISOL EXCESS
Confirming the diagnosis
This patient has been exposed to a number of stress factors, including a demanding job in the past, a shift to another state and loss of her personal support system. She has used stimulants, such as coffee, and carbohydrate-rich foods in order to increase adrenal output and energy levels, in an attempt to feel better. At the time of the consultation these means of ‘self-medication’ did not have the desired effect any more.
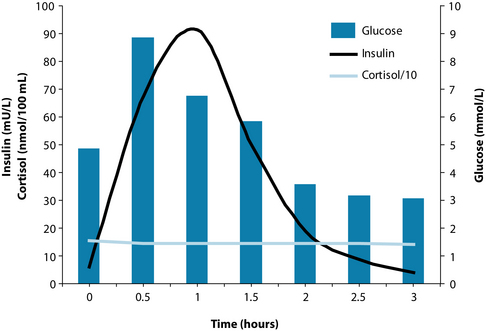
The diagnosis of adrenal exhaustion was confirmed by low urea (indicative of reduced protein intake and/or absorption), low cortisol both morning and afternoon, high to normal homocysteine and low to normal vitamin B12 (indicative of impaired methylation), high cholesterol and borderline high triglycerides. GTT revealed elevated insulin with delayed response to glucose (indicative of mild insulin resistance), followed by hypoglycaemia. Cortisol was low with no noticeable change throughout and unresponsive to a below normal glucose level (Figure 15.7). All other parameters were normal.
Example treatment
The following options were discussed with the patient:
At the first consultation, the patient was given advice on diet changes, as outlined above. In order to increase omega-3 fatty acids she was advised to have five fish meals a week. A smoothie with protein powder, to be consumed once a day in addition to her regular meals, was recommended. Emphasis was on low glycaemic load and additional protein for better glucose sugar balance and increased nutrients, especially amino acids as precursors for neurotransmitter synthesis.
Herbal treatment
To strengthen her system overall, with particular focus on the adrenal glands and low moods, a herbal formula was prescribed containing Rhodiola rosea to increase mental and physical stamina,107,115 Glycyrrhiza glabra to increase cortisol and adrenal output,77,80 Hypericum perforatum to decrease the reuptake of dopamine and noradrenaline153 (see also the section on the nervous system) and Bacopa monnieri to increase concentration and memory.119,154
Nutritional treatment
Additionally, nutritional supplements were prescribed to replenish cellular nutrient depletion. Tyrosine was given to increase dopamine–adrenaline synthesis,45 and to improve mood and the ability to experience pleasure.126 B vitamins with PLP and high amounts of folate and vitamin B12 were given to increase Krebs cycle cofactors for energy production45 and to provide essential nutrients for methylation.131,155–157 Further, CoQ10 was added for energy production in the electron transport chain,45,137 and magnesium (as citrate)124 due to its role in the Krebs cycle45 and for blood glucose regulation.158
Expected outcomes and follow-up protocols
As can be seen from the list of possible treatments (detailed under the ‘Key treatment protocols’ section), both herbally and nutritionally, there is scope for changing the treatment should the current prescription fail to produce the desired results (improved mood, energy and coping ability). In cases like the one described here, both physical and mental/emotional symptoms are present and need to be taken into consideration in the design of a treatment plan. The connection between psychological, neurological and endocrine symptoms is now well established in disciplines such as psychoneuroendocrinology.159 This patient should respond very favourably to treatment, due to her own commitment and her husband’s support. Neither the dietary changes, nor the prescriptions nor the increased social activities on their own would have resulted in the great improvement seen in this case. Willingness to change both attitudes and habits has been the recipe for success. However, in longer-standing problems (as is the case in chronic fatigue), cellular metabolism is further compromised with potential oxidative damage to the brain, as well as the development of a range of chronic diseases.5,17 Treatment in that case would be more comprehensive and extended over a longer time span.
Allostatic load can be modified and even reversed. People who can ward off allostatic load best tend to have the following attributes: a positive mental attitude, acceptance of themselves and others, fulfilling relationships yet are able to retain their own autonomy, beliefs and principles which they defend, the ability to create and shape their own environment conducive to their needs for health and happiness, a purpose in life and a sense of personal growth. They can pursue their goals and realise their potential.3 They have good nutritional status and a balanced lifestyle. In short, they are well resourced mentally and physically to withstand stress.
KEY POINTS
1. Selye H. Stress without distress. London: Hodder and Stoughton; 1974. 27
2. Thibodeau G., Patton K. Anatomy and physiology, 5th edn. St Louis: Mosby; 2003. Chapter 22
3. McEwen B.S. The end of stress as we know it. Washington: The Dana Press (Joseph Henry Press); 2002.
4. McEwen B.S. Interacting mediators of allostasis and allostatic load: towards an understanding of resilience in aging. Metabolism. 2003;52(10 Suppl 2):10-16.
5. McEwen B.S. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci. 2004;1032:1-7.
6. Tsigos C., Chrousos G.P. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53(4):865-871.
7. Miller D.B., O’Callaghan J.P. Neuroendocrine aspects of the response to stress. Metabolism. 2002;51(6 Suppl 1):5-10.
8. Vanitallie T.B. Stress: a risk factor for serious illness. Metabolism. 2002;51(6 Suppl 1):40-45.
9. Haddad J.J., et al. Cytokines and neuro-immune-endocrine interactions: a role for the hypothalamic-pituitary-adrenal revolving axis. J Neuroimmunol. 2002;133(1–2):1-19.
10. Eskandari F., Sternberg E.M. Neural-immune interactions in health and disease. Ann N Y Acad Sci. 2002;966:20-27.
11. Wilson L. Review of adaptogenic mechanisms: Eleutherococcus senticosus, Panax ginseng, Rhodiola rosea, Schisandra chinensis and Withania somnifera. Australian Journal of Medical Herbalism. 2007;19(3):126-138.
12. Gaffney B.T., et al. The effects of Eleutherococcus senticosus and Panax ginseng on steroidal hormone indices of stress and lymphocyte subset numbers in endurance athletes. Life Sci. 2001;70(4):431-442.
13. Cutolo M., et al. Hypothalamic-pituitary-adrenocortical and gonadal functions in rheumatoid arthritis. Ann N Y Acad Sci. 2003;992:107-117.
14. Loucks A.B., Redman L.M. The effect of stress on menstrual function. Trends Endocrinol Metab. 2004;15(10):466-471.
15. Gilad G.M., Gilad V.H. Overview of the brain polyamine-stress-response: regulation, development, and modulation by lithium and role in cell survival. Cell Mol Neurobiol. 2003;23(4–5):637-649.
16. Goldstein D.S., McEwen B. Allostasis, homeostasis, and the nature of stress. Stress. 2002;5(1):55-58.
17. McEwen B.S. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol Aging. 2002;23(5):921-939.
18. McEwen B.S. Stressed or stressed out: what is the difference? J Psychiatry Neurosci. 2005;30(5):315-318.
19. McEwen B., Lasley E.N. Allostatic load: when protection gives way to damage. Adv Mind Body Med. 2003;19(1):28-33.
20. Abercrombie H.C., et al. Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology. 2004;29(8):1082-1092.
21. King A., et al. Attenuated cortisol response to alcohol in heavy social drinkers. Int J Psychophysiol. 2006;59(3):203-209.
22. Meyer G., et al. Casino gambling increases heart rate and salivary cortisol in regular gamblers. Biol Psychiatry. 2000;48(9):948-953.
23. Lovallo W.R. Cortisol secretion patterns in addiction and addiction risk. Int J Psychophysiol. 2006;59(3):195-202.
24. Hellhammer J., et al. Allostatic load, perceived stress, and health: a prospective study in two age groups. Ann N Y Acad Sci. 2004;1032:8-13.
25. McEwen B.S. Early life influences on life-long patterns of behavior and health. Ment Retard Dev Disabil Res Rev. 2003;9(3):149-154.
26. Korte S.M., et al. The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci Biobehav Rev. 2005;29(1):3-38.
27. McEwen B.S. Mood disorders and allostatic load. Biol Psychiatry. 2003;54(3):200-207.
28. Carroll B.J. Ageing, stress and the brain. Novartis Found Symp. 2002;242:26-36. discussion –45
29. Weaver J.D., et al. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 2002;59(3):371-378.
30. Angeli A., et al. The overtraining syndrome in athletes: a stress-related disorder. J Endocrinol Invest. 2004;27(6):603-612.
31. Jefferies W.M. Cortisol and immunity. Med Hypotheses. 1991;34(3):198-208.
32. Butcher S.K., et al. Raised cortisol:DHEAS ratios in the elderly after injury: potential impact upon neutrophil function and immunity. Aging Cell. 2005;4(6):319-324.
33. Mechanick J.I. Metabolic mechanisms of stress hyperglycemia. J Parenter Enteral Nutr. 2006;30(2):157-163.
34. Maloney E.M., et al. Chronic fatigue syndrome and high allostatic load. Pharmacogenomics. 2006;7(3):467-473.
35. Björntorp P., Rosmond R. The metabolic syndrome—a neuroendocrine disorder? Br J Nutr. 2000;83(Suppl 1):S49-S57.
36. Nieuwenhuizen A.G., Rutters F. The hypothalamic-pituitary-adrenal-axis in the regulation of energy balance. Physiol Behav. 2008;94(2):169-177.
37. Kalantaridou S.N., et al. Stress and the female reproductive system. J Reprod Immunol. 2004;62(1–2):61-68.
38. Anderson K.N., editor. Mosby’s medical, nursing and allied health dictionary, 6th edn, St Louis, London: CV Mosby, 2002.
39. Demitrack M.A., Crofford L.J. Evidence for and pathophysiologic implications of hypothalamic-pituitary-adrenal axis dysregulation in fibromyalgia and chronic fatigue syndrome. Ann N Y Acad Sci. 1998;840:684-697.
40. Harbuz M. Neuroendocrine function and chronic inflammatory stress. Exp Physiol. 2002;87(5):519-525.
41. Berridge K.C. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81(2):179-209.
42. Markus R., et al. Effects of food on cortisol and mood in vulnerable subjects under controllable and uncontrollable stress. Physiol Behav. 2000;70(3–4):333-342.
43. Takeda E., et al. Stress control and human nutrition. J Med Invest. 2004;51(3–4):139-145.
44. Christensen L., Brooks A. Changing food preference as a function of mood. J Psychol. 2006;140(4):293-306.
45. Gropper S., et al. Advanced nutrition and human metabolism, 5th edn. Australia: Wadsworth: Cengage Learning; 2009.
46. Anderson D.C. Assessment and nutraceutical management of stress-induced adrenal dysfunction. Integrative Medicine: A Clinician’s Journal. 2008;7(5):18-25.
47. Pagana K.D., Pagana T.J. Mosby’s manual of diagnostic and laboratory tests, 3rd edn. USA: CV Mosby; 2006.
48. Giordano R., et al. Hypothalamus-pituitary-adrenal axis evaluation in patients with hypothalamo-pituitary disorders: comparison of different provocative tests. Clin Endocrinol. 2008;68(6):935-941.
49. Thibodeau G., Patton K. Anatomy and physiology, 5th edn. St Louis, London: CV Mosby; 2003.
50. Papadelis C., et al. Effects of mental workload and caffeine on catecholamines and blood pressure compared to performance variations. Brain Cogni. 2003;51(1):143-154.
51. Lane J.D., et al. Caffeine affects cardiovascular and neuroendocrine activation at work and home. Psychosom Med. 2002;64(4):595-603.
52. Crowley L.V. An introduction to human disease: pathology and pathophysiology correlations, 6th edn. Sudbury: Jones and Bartlett Publishers; 2004.
53. Giordano R., et al. Corticotrope hypersecretion coupled with cortisol hypo-responsiveness to stimuli is present in patients with autoimmune endocrine diseases: evidence for subclinical primary hypoadrenalism? Eur J Endocrinol. 2006;155(3):421-428.
54. Debono M., Ross R.J. Doses and steroids to be used in primary and central hypoadrenalism. Ann Endocrinol (Paris). 2007;68(4):265-267.
55. Barbetta L., et al. Comparison of different regimens of glucocorticoid replacement therapy in patients with hypoadrenalism. J Endocrinol Invest. 2005;28(7):632-637.
56. Libè R., et al. Effects of dehydroepiandrosterone (DHEA) supplementation on hormonal, metabolic and behavioral status in patients with hypoadrenalism. J Endocrinol Invest. 2004;27(8):736-741.
57. Bhagra S., et al. Dehydroepiandrosterone in adrenal insufficiency and ageing. Curr Opin Endocrinol Diabetes Obes. 2008;15(3):239-243.
58. Giordano G., et al. Variations of pituitary function over time after brain injuries: the lesson from a prospective study. Pituitary. 2005;8(3–4):227-231.
59. Beishuizen A., Thijs L.G. The immunoneuroendocrine axis in critical illness: beneficial adaptation or neuroendocrine exhaustion? Curr Opin Crit Care. 2004;10(6):461-467.
60. Marik P.E. Adrenal-exhaustion syndrome in patients with liver disease. Intensive Care Med. 2006;32(2):275-280.
61. Omori K., et al. Risk factors for adrenal crisis in patients with adrenal insufficiency. Endocr J. 2003;50(6):745-752.
62. Ströhle A. The neuroendocrinology of stress and the pathophysiology and therapy of depression and anxiety. Nervenarzt. 2003;74(3):279-291. quiz 292
63. Cortese B.M., Phan K.L. The role of glutamate in anxiety and related disorders. CNS Spectr. 2005;10(10):820-830.
64. StrÖhle A., Holsboer F. Stress responsive neurohormones in depression and anxiety. Pharmacopsychiatry. 2003;36(Suppl 3):S207-S214.
65. Chierichetti S.M., et al. Beta-blockers and psychic stress: a double-blind, placebo-controlled study of bopindolol vs lorazepam and butalbital in surgical patients. Int J Clin Pharmacol Ther Toxicol. 1985;23(9):510-514.
66. Schweizer R., et al. Effect of two beta-blockers on stress during mental arithmetic. Psychopharmacology. 1991;105(4):573-577.
67. De Kloet E.R., Derijk R. Signaling pathways in brain involved in predisposition and pathogenesis of stress-related disease: genetic and kinetic factors affecting the MR/GR balance. Ann N YAcad Sci. 2004;1032:14-34.
68. Mukherjee A. Fight stress with food. McClatchy-Tribune Business News. 2008.
69. Golub M.S. The adrenal and the metabolic syndrome. Curr Hypertens Rep. 2001;3(2):117-120.
70. Epel E., et al. Are stress eaters at risk for the metabolic syndrome? Ann N Y Acad Sci. 2004;1032:208-210.
71. Kopp M.S., Rethelyi J. Where psychology meets physiology: chronic stress and premature mortality—the Central-Eastern European health paradox. Brain Res Bull. 2004;62(5):351-367.
72. Singer B., et al. Protective environments and health status: cross-talk between human and animal studies. Neurobiol Aging. 2005;26(Suppl 1):113-118.
73. Eliopoulus C. Invitation to holistic health. Boston: Jones and Bartlett Publishers; 2004.
74. Stear S. Health and fitness series—1. The importance of physical activity for health. J Fam Health Care. 2003;13(1):10-13.
75. Duax W.L., et al. Steroid dehydrogenase structures, mechanism of action, and disease. Vitam Horm. 2000;58:121-148.
76. Kohlmeier M. Nutrient metabolism. Amsterdam: Academic Press; 2003.
77. Braun L., Cohen M. Herbs and natural supplements, 2nd edn. Sydney: Churchill Livingstone; 2007.
78. Kato H., et al. 3-monoglucuronyl-glycyrrhetinic acid is a major metabolite that causes licorice-induced pseudoaldosteronism. J Clin Endocrinol Metab. 1995;80(6):1929-1933.
79. Morgan M., Bone K. Herbs with tonic, adaptogenic, adrenal tonic and nervine activity. Phytotherapist Perspectiv. 2005:58.
80. Armanini D., et al. History of the endocrine effects of licorice. Exp Clin Endocrinol Diabetes. 2002;110(6):257-261.
81. Bone K. A clinical guide to blending liquid herbs. USA: Elsevier; 2003.
82. Blumenthal M. The ABC clinical guide to herbs. Austin: American Botanical Council; 2003.
83. Mills S., Bone K. Principles and practice of phytotherapy. Sydney: Churchill Livingstone; 2000.
84. Rege N.N., et al. Adaptogenic properties of six rasayana herbs used in Ayurvedic medicine. Phytother Res. 1999;13(4):275-291.
85. Mills S., Bone K. The essential guide to herbal safety. USA: Elsevier; 2005.
86. Kraft K., Hobbs C. Pocket guide to herbal medicine. New York: Thieme; 2004.
87. Basch E.M., Ulbricht C.E. Natural standard herb and supplement handbook. Boston: Elsevier; 2005.
88. Harkness R., Bratman S. Mosby’s handbook of drug-herb and drug-supplement interactions. Sydney: CV Mosby; 2003.
89. Blumenthal M. The complete German Commission E monographs: therapeutic guide to herbal medicines [CD-ROM]. Austin, Texas: American Botanical Council; 1999.
90. Burgoyne B. Herbal treatment of insomnia. Modern Phytotherapist. 2002;7(1):12-21.
91. Herrera-Arellano A., et al. Polysomnographic evaluation of the hypnotic effect of Valeriana edulis standardized extract in patients suffering from insomnia. Planta Med. 2001;67(8):695-699.
92. Teschke R., et al. Kava hepatotoxicity: a European view. N Z Med J. 2008;121(1283):90-98.
93. Lüde S., et al. Hepatocellular toxicity of kava leaf and root extracts. Phytomedicine. 2008;15(1–2):120-131.
94. Loew D., Gaus W. Kava-Kava: TragÖdie einer Fehlbeurteilung. Erfahrungsheilkunde. 2003;52(6):386.
95. Bone K. Rhodiola. Clinical Monitor. 2008;23:1-2.
96. Panossian A., Wagner H. Stimulating effect of adaptogens: an overview with particular reference to their efficacy following single dose administration. Phytother Res. 2005;19(10):819-838.
97. Morgan M. Withania, ginseng: gentle tonic and adaptogenic. Phytotherapist Perspective. 2005:59.
98. Beyer I., Rimpler M. Ginseng: Adaptogenitat zur Umstimmungstherapie—Teil 2. Biologische Medizin. 1996;25(4):151.
99. Beyer I., Rimpler M. Ginseng: Adaptogenitat zur Umstimmungstherapie—Teil 1. Biologische Medizin. 1996;25(3):98.
100. Baranov A.I. Medicinal uses of ginseng and related plants in the Soviet Union: recent trends in the Soviet literature. J Ethnopharmacol. 1982;6(3):339-353.
101. Kimura Y., Sumiyoshi M. Effects of various Eleutherococcus senticosus cortex on swimming time, natural killer activity and corticosterone level in forced swimming stressed mice. J Ethnopharmacol. 2004;95(2–3):447-453.
102. Kitts D., Hu C. Efficacy and safety of ginseng. Public Health Nutr. 2000;3(4A):473-485.
103. Nocerino E., et al. The aphrodisiac and adaptogenic properties of ginseng. Fitoterapia. 2000;71(Suppl 1):S1-S5.
104. Rasheed N., et al. Involvement of monoamines and proinflammatory cytokines in mediating the anti-stress effects of Panax quinquefolium. J Ethnopharmacol. 2008;117(2):257-262.
105. Lindner S. Withania somnifera: winter cherry, Indian ginseng, ashwagandha. Australian Journal of Medical Herbalism. 1996;8(3):78.
106. Mishra L.C., et al. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Altern Med Rev. 2000;5(4):334-346.
107. Morgan M., Bone K. Rhodiola rosea—rhodiola. Mediherb Newsletter. 2005:47.
108. Bystritsky A., et al. A pilot study of Rhodiola rosea (Rhodax) for generalized anxiety disorder (GAD). J Altern Complement Med. 2008;14(2):175-180.
109. Kelly G.S. Rhodiola rosea: a possible plant adaptogen. Altern Med Rev. 2001;6(3):293-302.
110. Sarris J. Herbal medicines in the treatment of psychiatric disorders: a systematic review. Phytother Res. 2007;21(8):703-716.
111. Darbinyan V., et al. Clinical trial of Rhodiola rosea L. extract SHR-5 in the treatment of mild to moderate depression. Nord J Psychiatry. 2007;61(5):343-348.
112. Shevtsov V.A., et al. A randomized trial of two different doses of a SHR-5 Rhodiola rosea extract versus placebo and control of capacity for mental work. Phytomedicine. 2003;10(2–3):95-105.
113. De Bock K., et al. Acute Rhodiola rosea intake can improve endurance exercise performance. Int J Sport Nutr Exerc Metab. 2004;14(3):298-307.
114. Spasov A.A., et al. A double-blind, placebo-controlled pilot study of the stimulating and adaptogenic effect of Rhodiola rosea SHR-5 extract on the fatigue of students caused by stress during an examination period with a repeated low-dose regimen. Phytomedicine. 2000;7(2):85-89.
115. Rhodiola rosea. Monograph. Altern Med Rev. 2002;7(5):421-423.
116. Brown R.P., et al. Rhodiola rosea: a phytomedicinal overview. HerbalGram. 2002;56:40-52.
117. Morgan M. Holy Basil. Phytotherapist Perspective. 2001;19:1-3.
118. Shirai M., et al. Approach to novel functional foods for stress control 5. Antioxidant activity profiles of antidepressant herbs and their active components. J Med Invest. 2005;52(Suppl):249-251.
119. Rai D., et al. Adaptogenic effect of Bacopa monniera (brahmi). Pharmacol Biochem Behav. 2003;75(4):823-830.
120. Dew J.M. Homoeopathy and the effects of stress. Homoeopathy. 1996;46(3):50.
121. Duelli N..Suffer less from stress: homeopathy can help. Alive: Canadian Journal of Health and Nutrition. 2005(271):72-73.
122. King F. Homeopathy. Exploring the hypoadrenia-homeopathy link. Chiropr J. 1991;5(11):40.
123. Davidson J., et al. Multivariate analysis of five homoeopathic medicines in a psychiatric population. Br Homoeopath J. 1995;84(4):195.
124. Lindberg J.S., et al. Magnesium bioavailability from magnesium citrate and magnesium oxide. J Am Coll Nutr. 1990;9(1):48-55.
125. Walker A.F., et al. Mg citrate found more bioavailable than other Mg preparations in a randomised, double-blind study. Magnes Res. 2003;16(3):183-191.
126. Roiser J.P., et al. The subjective and cognitive effects of acute phenylalanine and tyrosine depletion in patients recovered from depression. Neuropsychopharmacology. 2005;30(4):775-785.
127. McLean A., et al. The effects of tyrosine depletion in normal healthy volunteers: implications for unipolar depression. Psychopharmacology. 2004;171(3):286-297.
128. Freeman M.P., et al. Selected integrative medicine treatments for depression: considerations for women. J Am Med Women’s Assoc. 2004;59(3):216-224.
129. Peters E.M., et al. Vitamin C supplementation attenuates the increases in circulating cortisol, adrenaline and anti-inflammatory polypeptides following ultramarathon running. Int J Sports Med. 2001;22(7):537-543.
130. Rufenacht P., et al. [Vitamin B12 deficiency: a challenging diagnosis and treatment]. Rev Méd Suisse. 2008;4(175):2212.
131. Coppen A., Bolander-Gouaille C. Treatment of depression: time to consider folic acid and vitamin B12. J Psychopharmacol. 2005;19(1):59-65.
132. Volkov I., et al. Vitamin B12 could be a ‘master key’ in the regulation of multiple pathological processes. J Nippon Med Sch. 2006;73(2):65-69.
133. Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8(3):223-246.
134. Urso M.L., Clarkson P.M. Oxidative stress, exercise, and antioxidant supplementation. Toxicology. 2003;189(1–2):41-54.
135. Mayne S.T. Antioxidant nutrients and chronic disease: use of biomarkers of exposure and oxidative stress status in epidemiologic research. J Nutr. 2003;133(Suppl 3):933S-940S.
136. Dhanasekaran M., Ren J. The emerging role of coenzyme Q-10 in aging, neurodegeneration, cardiovascular disease, cancer and diabetes mellitus. Curr Neurovasc Res. 2005;2(5):447-459.
137. Mancini A., et al. Coenzyme Q10 evaluation in pituitary-adrenal axis disease: preliminary data. Biofactors. 2005;25(1–4):197-199.
138. Jezova D., et al. Subchronic treatment with amino acid mixture of L-lysine and L-arginine modifies neuroendocrine activation during psychosocial stress in subjects with high trait anxiety. Nutr Neurosci. 2005;8(3):155-160.
139. Putignano P., et al. Tissue-specific dysregulation of 11 beta-hydroxysteroid dehydrogenase type 1 and pathogenesis of the metabolic syndrome. J Endocrinol Invest. 2004;27(10):969-974.
140. Tode T., et al. Effect of Korean red ginseng on psychological functions in patients with severe climacteric syndromes. Int J Gynaecol Obstet. 1999;67(3):169-174.
141. Adelman E.M. Mind-body intelligence: a new perspective integrating eastern and western healing traditions. Holist Nurs Pract. 2006;20(3):147-151.
142. Shigaki C.L., et al. Mindfulness-based stress reduction in medical settings. J Clin Psychol Med Settings. 2006;13(3):209-216.
143. Grossman P., et al. Mindfulness-based stress reduction and health benefits—a meta-analysis. J Psychosom Res. 2004;57(1):35-43.
144. Oman D., et al. Passage meditation reduces perceived stress in health professionals: a randomized, controlled trial. J Consult Clin Psychol. 2006;74(4):714-719.
145. Butler A.C., et al. The empirical status of cognitive-behavioral therapy: a review of meta-analyses. Clin Psychol Rev. 2006;26(1):17-31.
146. Pratt R.R. Art, dance, and music therapy. Phys Med Rehabil Clin N Am. 2004;15(4):827-841.
147. Pronk K. Role of the doctor in relieving spiritual distress at the end of life. Am J Hosp Palliat Care. 2005;22(6):419-425.
148. Jambrik Z., et al. Traditional acupuncture does not modulate the endothelial dysfunction induced by mental stress. Int J Cardiovasc Imaging. 2004;20(5):357-362.
149. Murakami S., et al. Aromatherapy for outpatients with menopausal symptoms in obstetrics and gynecology. J Altern Complement Med. 2005;11(3):491-494.
150. Robson T. An introduction to complementary medicine. Sydney: Allen & Unwin; 2003.
151. Burden B., et al. The increasing use of reiki as a complementary therapy in specialist palliative care. Int J Palliat Nurs. 2005;11(5):248-253.
152. Long L., et al. Which complementary and alternative therapies benefit which conditions? A survey of the opinions of 223 professional organizations. Complement Ther Med. 2001;9(3):178-185.
153. Rodríguez-Landa J.F., Contreras C.M. A review of clinical and experimental observations about antidepressant actions and side effects produced by Hypericum perforatum extracts. Phytomedicine. 2003;10(8):688-699.
154. Kidd P.M. A review of nutrients and botanicals in the integrative management of cognitive dysfunction. Altern Med Rev. 1999;4(3):144-161.
155. Miller A.L. The methylation, neurotransmitter, and antioxidant connections between folate and depression. Altern Med Rev. 2008;13(3):216.
156. Bottiglieri T. Homocysteine and folate metabolism in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(7):1103-1112.
157. Abou-Saleh M.T., Coppen A. Folic acid and the treatment of depression. J Psychosom Res. 2006;61(3):285-287.
158. Werbach M.R. Nutritional strategies for treating chronic fatigue syndrome. Altern Med Rev. 2000;5(2):93-108.
159. Vitetta L., et al. Mind-body medicine: stress and its impact on overall health and longevity. Ann N Y Acad Sci. 2005;1057:492-505.
160. Brunner E.J., et al. Adrenocortical, autonomic, and inflammatory causes of the metabolic syndrome: nested case-control study. Circulation. 2002;106(21):2659-2665.
161. Le Gal M., et al. Pharmaton capsules in the treatment of functional fatigue: a double-blind study versus placebo evaluated by a new methodology. Phytother Res. 1996;10(1):49-53.
162. Dorling E., et al. Do ginsenosides influence the performance? Notabene Med. 1980;10(5):241-246.
163. Bone K. Ginseng—the regal herb part 2. Mediherb Professional Review. 1998;63:1-5.
164. Wiklund I.K., et al. Effects of a standardized ginseng extract on quality of life and physiological parameters in symptomatic postmenopausal women: a double-blind, placebo-controlled trial. Swedish Alternative Medicine Group. Int J Clin Pharmacol Res. 1999;19(3):89-99.
165. Cicero A.F., et al.Effects of Siberian ginseng (Eleutherococcus senticosus Maxim.) on elderly quality of life: a randomized clinical trial. Arch Gerontol Geriatr Suppl. 2004(9):69-73.
166. Stough C., et al. The chronic effects of an extract of Bacopa monniera (brahmi) on cognitive function in healthy human subjects. Psychopharmacology. 2001;156(4):481-484.