CHAPTER 3 Adjuvant Therapy and Breast Reconstruction
Key Points
Systemic Therapy
Cytotoxic chemotherapy
Cytotoxic chemotherapy generally causes myelosuppression, which may interfere with wound healing and increase the risk of infections. Several studies have evaluated the efficacy of breast reconstruction in patients who are scheduled to receive adjuvant chemotherapy. Wound healing problems tend to occur when drugs are delivered during the 2 weeks before reconstruction or during the first week following reconstruction. As the interval between reconstruction and chemotherapy increases, the risk of developing wound healing complications decreases.1–3
Neoadjuvant chemotherapy
Although it has not been shown to provide survival benefit compared with postoperative adjuvant chemotherapy, neoadjuvant chemotherapy has become a widely accepted treatment option for breast cancer patients. Neoadjuvant chemotherapy has been shown to reduce tumor size in some patients with large tumors, thereby facilitating breast-conservation surgery and enabling in vivo assessment of the tumor’s response to chemotherapy.4–6 Many studies have shown that neoadjuvant chemotherapy followed by mastectomy and immediate breast reconstruction is safe and viable, does not delay other adjuvant treatment, and can be used to identify patients who do not respond to chemotherapy, which enables oncologists to modify post-surgical treatment.7–9 In addition, studies have shown that the complication rate after implant- or autologous tissue-based reconstruction is not significantly higher in patients who have undergone neoadjuvant chemotherapy, nor are the local recurrence and disease-free survival rates affected.7,9–13
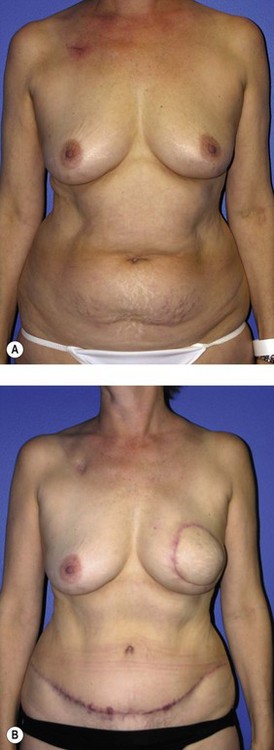
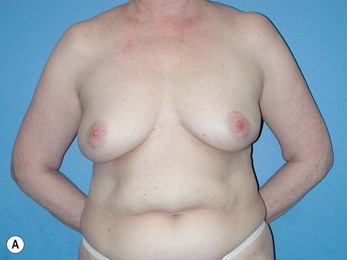
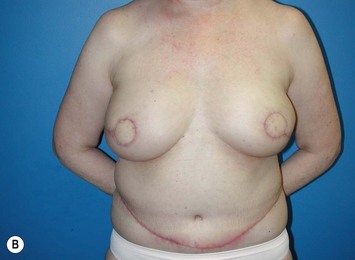
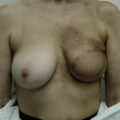
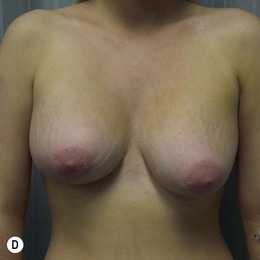
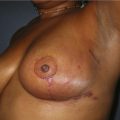
Generally, neoadjuvant chemotherapy is not a contraindication to immediate breast reconstruction and does not increase the complication rate or significantly delay further adjuvant therapy. At our institution, we recommend delaying reconstruction for 3–4 weeks following neoadjuvant chemotherapy to allow the immunosuppressive effects of the chemotherapy to resolve (Fig. 3.1A, B).
Adjuvant chemotherapy
Postoperative chemotherapy is a common adjuvant treatment for breast cancer because it reduces the risk of local and systemic recurrence due to occult micrometastatic disease. Randomized trials and meta-analyses have shown that post-mastectomy adjuvant chemotherapy reduces recurrence and death rates in breast cancer patients.14–16
Many researchers have studied whether immediate breast reconstruction affects the timely delivery of adjuvant chemotherapy and whether adjuvant chemotherapy after reconstruction affects wound healing. Allweiss et al compared 49 patients who underwent mastectomy, immediate breast reconstruction with various autologous tissue techniques, chemotherapy with 308 patients who underwent mastectomy alone followed by chemotherapy and found that the type of reconstruction did not significantly delay chemotherapy and that the time to chemotherapy was in fact significantly longer in patients who did not undergo immediate reconstruction.17 In addition, Wilson et al compared patients who had undergone mastectomy with immediate reconstruction, mastectomy with no reconstruction, or breast-conservation surgery, all followed by adjuvant chemotherapy, and found no significant differences in the time to chemotherapy among the three cohorts.18 At our institution, Mortenson et al found that patients who underwent immediate breast reconstruction followed by adjuvant chemotherapy had a higher incidence of wound complications (22.3%) than patients who did not undergo immediate reconstruction (8.3%) but did not find that immediate breast reconstruction delayed postoperative chemotherapy.19
With regard to implant-based reconstruction, most researchers have found that adjuvant chemotherapy typically does not increase implant infection or complication rates or affect cosmetic outcomes.2,20–22 In addition, no significant delays in receiving chemotherapy or changes in dose intensity have been observed.3,20 Based on these studies and our experience with implant-based reconstruction and tissue expansion during chemotherapy, we recommend that patients undergo tissue expansion and implant placement before starting chemotherapy. If tissue expansion is not complete before a patient starts chemotherapy, absolute neutrophil counts should be assessed to make certain that the patient’s immune system can fight the bacteria that are introduced during the expansion process.
Although whether delaying adjuvant chemotherapy affects cancer-related outcomes is not yet definitively known, most oncologists prefer to initiate therapy 4–6 weeks after mastectomy or breast-conservation surgery given concerns that longer periods may increase recurrence or diminish survival.23 Immediate breast reconstruction may increase the risk of complications as a result of the additional surgical procedures performed, but it does not seem to delay adjuvant chemotherapy or affect overall survival and recurrence rates.
Hormone therapy
Selective estrogen receptor modulators
Tamoxifen has been shown to reduce the risk of recurrence and death in women with early-stage, hormone receptor-positive invasive breast cancer; reduce the risk of invasive and non-invasive recurrences in women who undergo breast-conserving surgery for ductal carcinoma in situ; and reduce the risk of breast cancer in women who have a high risk for the disease because of personal characteristics or family history.16,24 Despite tamoxifen’s benefits, 1–2% of patients may experience thromboembolic events such as deep vein thromboses, pulmonary embolisms, and cerebrovascular thrombi. Although the mechanisms by which tamoxifen causes thromboembolic events are not totally understood, the events are thought to be related to tamoxifen’s reduction of levels of antithrombin III, and factor V, and protein C.25–27
Because tamoxifen presents a theoretical risk of thrombosis, it may be appropriate to have the patient stop tamoxifen therapy 10–14 days prior to undergoing free-flap reconstruction and restart the therapy after breast reconstruction. (This time frame is based on the pharmacokinetics and the 9–14-day terminal half-life of tamoxifen’s major metabolite.28) However, we recommend consulting with the patient’s medical and surgical oncologists to confirm that tamoxifen therapy can be stopped safely without negatively affecting the patient’s cancer treatment.
Aromatase inhibitors
The most common aromatase inhibitors studied in breast cancer patients are anastrozole and letrozole. In comparison trials, anastrozole was associated with higher disease-free survival rates, lower breast cancer event rates, and fewer incidences of contralateral breast cancer than tamoxifen. Also, significantly fewer venous thrombolic events occurred in the anastrozole group than in the tamoxifen group.29 We currently do not routinely stop aromatase inhibitor therapy prior to breast reconstruction.
Biological therapy
Trastuzumab, a humanized monoclonal antibody directed against the HER-2 receptor, has been shown to significantly improve survival rates in metastatic breast cancer patients when used alone or in combination with chemotherapy. Trastuzumab also has been shown to reduce recurrence rates and improve survival in early-stage breast cancer patients.30
Patients who receive trastuzumab alone or in combination with other chemotherapy may experience neutropenia and an increased incidence of infections. An increase in the incidence of thrombotic events has also been reported.31 Because of these potential complications, we recommend that patients complete trastuzumab therapy and undergo immune status evaluation before undergoing breast reconstruction. And as always, we recommend consulting with the patient’s medical and surgical oncologists.
Radiotherapy
Giving adjuvant radiotherapy following mastectomy has been shown to significantly reduce the risk of locoregional recurrence in early-stage breast cancer patients with positive lymph nodes.32,33 Furthermore, recent prospective trials have demonstrated improved locoregional control, disease-free survival, and overall survival rates in node-positive breast cancer patients who receive adjuvant radiotherapy in addition to mastectomy and chemotherapy.34,35 Currently, post-mastectomy adjuvant radiotherapy is recommended for patients with locally advanced tumors or four or more involved lymph nodes.36,37 However, many institutions are evaluating the efficacy of radiotherapy in patients who have T1 or T2 disease and one to three involved nodes.33
Breast reconstruction in a previously irradiated breast
The negative effects of radiation on implant-based breast reconstruction have been particularly well documented; studies of implant-based reconstruction of previously irradiated breasts have reported complications including infection, extrusion, capsular contracture, and failed reconstruction in 20–60% of cases. Thus, most prefer to use autologous tissue only for breast reconstruction in a previously irradiated breast. However, recent reports may support that when autologous tissue flap and implant are used together in a previously irradiated breast for breast reconstruction, an autologous tissue flap may protect the implant from the negative effects of radiotherapy. Spear et al evaluated 28 patients with previously irradiated breasts who underwent latissimus dorsi (LD) flap/implant breast reconstruction and found a 14% implant-related complication rate with a mean cosmetic satisfaction rating of 8.5 of 10 and mean overall satisfaction rating of 8.8 of 10. The authors concluded that although breast reconstruction with autologous tissue alone may be the best choice following radiotherapy, LD flap/implant reconstructions provide cosmetically acceptable results and acceptable complication rates.38 In a study from our institution, of patients who received radiotherapy prior to mastectomy and reconstruction, significantly fewer reconstructions failed in patients with LD flap/implant reconstructions (15%) or transverse rectus abdominis myocutaneous (TRAM) flap/implant reconstructions (10%) than in patients with expander/implant-only reconstructions (42%; p < 0.03).39
Immediate breast reconstruction followed by radiotherapy
The mechanisms and clinical effects of radiation on the reconstructed breast depend on the radiation dose, the use of compensating filters and wedges, the use of bolus dosing to the skin, and the boost to the radiation bed.40 The variability among radiation oncologists and centers makes it difficult to predict the effects radiotherapy will have on a reconstructed breast. Incompletely understood intrinsic patient factors can also alter the effects of radiotherapy. Immediate breast reconstruction can create technical challenges in administering adjuvant radiotherapy and may increase radiation exposure in the mediastinum. A flat chest – that is, one without a reconstructed breast – allows for beam angulations that minimize radiation doses to the heart and lungs while treating the internal mammary lymph nodes; however, the sloped contour of a reconstructed breast contributes to less precise geometric matching of the lateral and medial radiation fields, resulting in greater radiation doses in the mediastinum.33,41–43
Autologous tissue-based reconstruction followed by radiotherapy
Most authors agree that autologous tissue-based reconstruction tolerates radiation better with more pleasing aesthetic outcomes and fewer complications than implant-based reconstruction.44,45 Jhaveri et al performed a retrospective analysis of the long-term complication rates and cosmetic results in 69 patients who underwent immediate tissue expander/implant-based reconstruction with adjuvant radiotherapy and 23 patients who underwent autologous tissue-based reconstruction with adjuvant radiotherapy. The authors found that the tissue expander/implant group had a significantly higher rate of severe complications – those that required surgical intervention or removal and/or replacement of the implants (33.3%) – than the group who underwent autologous tissue-based reconstruction (0%). The autologous tissue group also had a higher rate of acceptable cosmesis (83%) than the tissue expander/implant group (51%).46
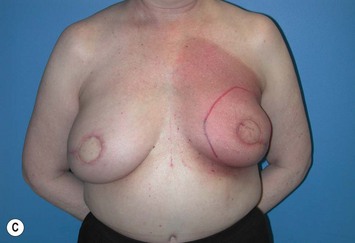
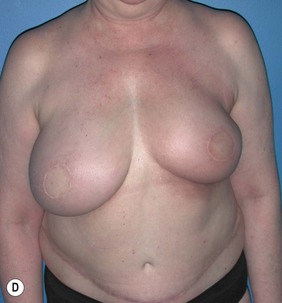
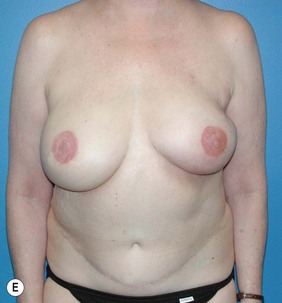
However, many feel that irradiating a reconstructed breast may diminish the aesthetic outcome and thus advocate delay reconstruction in patients who require adjuvant radiotherapy. Tran et al reported on a series of 41 patients undergoing immediate TRAM flap reconstruction (9 pedicled and 32 free) followed by radiation. No flap loss was reported; however, 10 patients (24%) required an additional flap to correct contracture, and only 9 patients (22%) maintained normal breast volumes. Hyperpigmentation occurred in 15 patients (37%), palpable fat necrosis in 14 patients (34%), and a loss of symmetry in 32 patients (78%).47 In a later study, Tran et al evaluated 32 patients who underwent immediate reconstruction followed by radiotherapy and 70 patients who underwent reconstruction after radiotherapy. No significant difference was found in early flap complications between the two groups but significantly higher (87.5% versus 8.6%) rates of late complications (fat necrosis, flap volume loss, and flap contracture) were noted in patients who received immediate reconstruction prior to radiotherapy, with 28% requiring an additional flap to correct deformities (Fig. 3.2A–E).48 Other studies have found similar results with higher rates of fat necrosis and necessary additional surgeries or flaps due to volume loss in patients who undergo autologous tissue-based reconstructions before adjuvant radiotherapy.49–52
Meanwhile, for some surgeons, immediate reconstruction with autologous tissue remains the preferred approach in patients who require adjuvant radiotherapy. In a series of 25 patients, Soong et al found that radiotherapy did not increase the rate of complications after immediate reconstruction with TRAM flaps. The authors concluded that radiotherapy after reconstruction with TRAM flaps is well tolerated and that cosmetic outcome and local control of the breast cancer are satisfactory.53 Other authors have reported similar results with good to excellent cosmetic outcomes and minimal flap loss and complications.54–57
Implant-based breast reconstruction followed by radiotherapy
Among patients who had immediate implant-based breast reconstruction followed by radiotherapy, Cordeiro et al found that 68% experienced capsular contracture after radiotherapy, a rate significantly higher than in patients who did not receive radiotherapy. Also, the patients who did not receive radiotherapy had a higher percentage of very good to excellent outcomes of the reconstructed breast than patients who did receive radiotherapy. Nevertheless, the authors concluded that immediate reconstruction with expanders and implants is an acceptable option when adjuvant radiotherapy is necessary.58 Hazard et al reached the same conclusion in a retrospective study in which 85% of patients had good or excellent cosmetic outcomes, with an acceptable rate of capsular contracture.59
Other authors have reported higher complication, reoperation, and capsular contracture rates in patients with implant-based reconstructions followed by adjuvant radiotherapy, resulting in poor cosmetic outcome. Spear et al evaluated outcomes in 40 patients undergoing two-stage reconstruction with saline implants and adjuvant radiotherapy with 40 patients undergoing two-stage reconstruction without radiation and found that the group who received radiation had significantly more complications (52.5% versus 10.0%), capsular contractures (32% versus 0%), and additional procedures (47% versus 10%).40 Other authors have come to the same conclusion and even consider the need for radiotherapy to be a relative contraindication to immediate breast reconstruction with tissue expanders and implants.60–66
Delayed-immediate breast reconstruction
Because of this dilemma, Kronowitz et al described the ‘delayed immediate’ technique, in which a completely filled textured saline tissue expander is placed during mastectomy to preserve the skin envelope.42 Once the final pathology results are received, patients can undergo delayed reconstruction after they complete radiotherapy or immediate reconstruction if radiotherapy is unnecessary. The advantage of this technique is that it preserves the skin to achieve superior aesthetic outcomes in immediate reconstruction and it enables delay of final reconstruction in patients who are found to need postmastectomy radiation, thereby avoiding most effects of irradiating a reconstructed breast.
1 Drake DB, Oishi SN. Wound healing considerations in chemotherapy and radiation therapy. Clin Plast Surg. 1995;22:31-37.
2 Furey PC, Macgillivray DC, Castiglione CL, et al. Wound complications in patients receiving adjuvant chemotherapy after mastectomy and immediate breast reconstruction for breast cancer. J Surg Oncol. 1994;55:194-197.
3 Rey P, Martinelli G, Petit JY, et al. Immediate breast reconstruction and high-dose chemotherapy. Ann Plast Surg. 2005;55:250-254.
4 Boughey JC, Peintinger F, Meric-Bernstam F, et al. Impact of preoperative versus postoperative chemotherapy on the extent and number of surgical procedures in patients treated in randomized clinical trials for breast cancer. Ann Surg. 2006;244:464-470.
5 Fisher ER, Wang J, Bryant J, et al. Pathobiology of preoperative chemotherapy: findings from the National Surgical Adjuvant Breast and Bowel (NSABP) protocol B-18. Cancer. 2002;95:681-695.
6 Wolmark N, Wang J, Mamounas E, et al. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001:96-102.
7 Godfrey PM, Godfrey NV, Romita MC. Immediate autogenous breast reconstruction in clinically advanced disease. Plast Reconstr Surg. 1995;95:1039-1044.
8 Gouy S, Rouzier R, Missana MC, et al. Immediate reconstruction after neoadjuvant chemotherapy: effect on adjuvant treatment starting and survival. Ann Surg Oncol. 2005;12:161-166.
9 Sultan MR, Smith ML, Estabrook A, et al. Immediate breast reconstruction in patients with locally advanced disease. Ann Plast Surg. 1997;38:345-349.
10 Banic A, Boeckx W, Greulich M, et al. Late results of breast reconstruction with free TRAM flaps: a prospective multicentric study. Plast Reconstr Surg. 1995;95:1195-1204.
11 Deutsch MF, Smith M, Wang B, et al. Immediate breast reconstruction with the TRAM flap after neoadjuvant therapy. Ann Plast Surg. 1999;42:240-244.
12 Forouhi P, Dixon JM, Leonard RC, et al. Prospective randomized study of surgical morbidity following primary systemic therapy for breast cancer. Br J Surg. 1995;82:79-82.
13 Jacobsen WM, Meland NB, Woods JE. Autologous breast reconstruction with use of transverse rectus abdominis musculocutaneous flap: Mayo Clinic experience with 147 cases. Mayo Clin Proc. 1994;69:635-640.
14 Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;351:1451-1467.
15 Polychemotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;352:930-942.
16 Liu M, Isaacs C. Adjuvant systemic therapy. associate editors. Spear S, Willey SC, Robb GL, et al, editors. Surgery of the breast: principles and art, 2nd ed. Baltimore: Lippincott Williams & Wilkins. 2006: 277-291.
17 Allweis TM, Boisvert ME, Otero SE, et al. Immediate reconstruction after mastectomy for breast cancer does not prolong the time to starting adjuvant chemotherapy. Am J Surg. 2002;183:218-221.
18 Wilson CR, Brown IM, Weiller-Mithoff E, et al. Immediate breast reconstruction does not lead to a delay in the delivery of adjuvant chemotherapy. Eur J Surg Oncol. 2004;30:624-627.
19 Mortenson MM, Schneider PD, Khatri VP, et al. Immediate breast reconstruction after mastectomy increases wound complications: however, initiation of adjuvant chemotherapy is not delayed. Arch Surg. 2004;139:988-991.
20 Caffo O, Cazzolli D, Scalet A, et al. Concurrent adjuvant chemotherapy and immediate breast reconstruction with skin expanders after mastectomy for breast cancer. Breast Cancer Res Treat. 2000;60:267-275.
21 Vandeweyer E, Deraemaecker R, Nogaret JM, et al. Immediate breast reconstruction with implants and adjuvant chemotherapy: a good option? Acta Chir Belg. 2003;103:98-101.
22 Yule GJ, Concannon MJ, Croll G, et al. Is there liability with chemotherapy following immediate breast construction? Plast Reconstr Surg. 1996;97:969-973.
23 Buzdar AU, Smith TL, Powell KC, et al. Effect of timing of initiation of adjuvant chemotherapy on disease-free survival in breast cancer. Breast Cancer Res Treat. 1982;2:163-169.
24 Fisher B, Jeong JH, Dignam J, et al. Findings from recent National Surgical Adjuvant Breast and Bowel Project adjuvant studies in stage I breast cancer. J Natl Cancer Inst Monogr. 2001:62-66.
25 Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125-137.
26 Cushman M, Costantino JP, Bovill EG, et al. Effect of tamoxifen on venous thrombosis risk factors in women without cancer: the Breast Cancer Prevention Trial. Br J Haematol. 2003;120:109-116.
27 Decensi A, Maisonneuve P, Rotmensz N, et al. Effect of tamoxifen on venous thromboembolic events in a breast cancer prevention trial. Circulation. 2005;111:650-656.
28 McEvoy G. AHFS Drug information 2006. Bethesda, Maryland; 2006.
29 Baum M, Buzdar A, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer: results of the ATAC (Arimidex, Tamoxifen Alone or in Combination) trial efficacy and safety update analyses. Cancer. 2003;98:1802-1810.
30 Lin A, Rugo HS. The role of trastuzumab in early stage breast cancer: current data and treatment recommendations. Curr Treat Options Oncol. 2007;8:47-60.
31 Trastuzumab. The complete drug reference MICROMEDEX: thomson micromedex editorial board. Greenwood Village, CO; 2006.
32 Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 2000;355:1757-1770.
33 Kronowitz SJ, Robb GL. Breast reconstruction with postmastectomy radiation therapy: current issues. Plast Reconstr Surg. 2004;114:950-960.
34 Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337:949-955.
35 Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337:956-962.
36 Harris JR, Halpin-Murphy P, McNeese M, et al. Consensus Statement on postmastectomy radiation therapy. Int J Radiat Oncol Biol Phys. 1999;44:989-990.
37 Recht A, Edge SB, Solin LJ, et al. Postmastectomy radiotherapy: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19:1539-1569.
38 Spear SL, Boehmler JH, Taylor NS, et al. The role of the latissimus dorsi flap in reconstruction of the irradiated breast. Plast Reconstr Surg. 2007;119:1-9.
39 Chang D, Barnea Y, Robb G. Effects of an autologous flap combined with an implant for breast reconstruction: an evaluation of 1000 consecutive reconstructions of previously irradiated breasts. Plast Reconstr Surg. 2008;122:356-362.
40 Spear SL, Onyewu C. Staged breast reconstruction with saline-filled implants in the irradiated breast: recent trends and therapeutic implications. Plast Reconstr Surg. 2000;105:930-942.
41 Buchholz TA, Kronowitz SJ, Kuerer HM. Immediate breast reconstruction after skin-sparing mastectomy for the treatment of advanced breast cancer: radiation oncology considerations. Ann Surg Oncol. 2002;9:820-821.
42 Kronowitz SJ, Hunt KK, Kuerer HM, et al. Delayed-immediate breast reconstruction. Plast Reconstr Surg. 2004;113:1617-1628.
43 Strom E. Radiation therapy for early and advanced breast disease. New York: Springer-Verlag; 2001.
44 Anderson PR, Hanlon AL, Fowble BL, et al. Low complication rates are achievable after postmastectomy breast reconstruction and radiation therapy. Int J Radiat Oncol Biol Phys. 2004;59:1080-1087.
45 Chawla AK, Kachnic LA, Taghian AG, et al. Radiotherapy and breast reconstruction: complications and cosmesis with TRAM versus tissue expander/implant. Int J Radiat Oncol Biol Phys. 2002;54:520-526.
46 Jhaveri JD, Rush SC, Kostroff K, et al. Clinical outcomes of postmastectomy radiation therapy after immediate breast reconstruction. Int J Radiat Oncol Biol Phys. 2008;72(3):859-865.
47 Tran NV, Evans GR, Kroll SS, et al. Postoperative adjuvant irradiation: effects on tranverse rectus abdominis muscle flap breast reconstruction. Plast Reconstr Surg. 2000;106:313-317.
48 Tran NV, Chang DW, Gupta A, et al. Comparison of immediate and delayed free TRAM flap breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg. 2001;108:78-82.
49 Halyard MY, McCombs KE, Wong WW, et al. Acute and chronic results of adjuvant radiotherapy after mastectomy and transverse rectus abdominis myocutaneous (TRAM) flap reconstruction for breast cancer. Am J Clin Oncol. 2004;27:389-394.
50 Rogers NE, Allen RJ. Radiation effects on breast reconstruction with the deep inferior epigastric perforator flap. Plast Reconstr Surg. 2002;109:1919-1924.
51 Spear SL, Ducic I, Low M, et al. The effect of radiation on pedicled TRAM flap breast reconstruction: outcomes and implications. Plast Reconstr Surg. 2005;115:84-95.
52 Williams JK, Carlson GW, Bostwick J3rd, et al. The effects of radiation treatment after TRAM flap breast reconstruction. Plast Reconstr Surg. 1997;100:1153-1160.
53 Soong IS, Yau TK, Ho CM, et al. Post-mastectomy radiotherapy after immediate autologous breast reconstruction in primary treatment of breast cancers. Clin Oncol (R Coll Radiol). 2004;16:283-289.
54 Hanks SH, Lyons JA, Crowe J, et al. The acute effects of postoperative radiation therapy on the transverse rectus abdominis myocutaneous flap used in immediate breast reconstruction. Int J Radiat Oncol Biol Phys. 2000;47:1185-1190.
55 Mehta VK, Goffinet D. Postmastectomy radiation therapy after TRAM flap breast reconstruction. Breast J. 2004;10:118-122.
56 Proulx GM, Loree T, Edge S, et al. Outcome with postmastectomy radiation with transverse rectus abdominis musculocutaneous flap breast reconstruction. Am Surg. 2002;68:410-413.
57 Zimmerman RP, Mark RJ, Kim AI, et al. Radiation tolerance of transverse rectus abdominis myocutaneous-free flaps used in immediate breast reconstruction. Am J Clin Oncol. 1998;21:381-385.
58 Cordeiro PG, Pusic AL, Disa JJ, et al. Irradiation after immediate tissue expander/implant breast reconstruction: outcomes, complications, aesthetic results, and satisfaction among 156 patients. Plast Reconstr Surg. 2004;113:877-881.
59 Hazard L, Miercort C, Gaffney D, et al. Local-regional radiation therapy after breast reconstruction: what is the appropriate target volume? A case–control study of patients treated with electron arc radiotherapy and review of the literature. Am J Clin Oncol. 2004;27:555-564.
60 Ascherman JA, Hanasono MM, Newman MI, et al. Implant reconstruction in breast cancer patients treated with radiation therapy. Plast Reconstr Surg. 2006;117:359-365.
61 Evans GR, Schusterman MA, Kroll SS, et al. Reconstruction and the radiated breast: is there a role for implants? Plast Reconstr Surg. 1995;96:1111-1115.
62 Kraemer O, Andersen M, Siim E. Breast reconstruction and tissue expansion in irradiated versus not irradiated women after mastectomy. Scand J Plast Reconstr Surg Hand Surg. 1996;30:201-206.
63 Krueger EA, Wilkins EG, Strawderman M, et al. Complications and patient satisfaction following expander/implant breast reconstruction with and without radiotherapy. Int J Radiat Oncol Biol Phys. 2001;49:713-721.
64 McCarthy CM, Pusic AL, Disa JJ, et al. Unilateral postoperative chest wall radiotherapy in bilateral tissue expander/implant reconstruction patients: a prospective outcomes analysis. Plast Reconstr Surg. 2005;116:1642-1647.
65 Tallet AV, Salem N, Moutardier V, et al. Radiotherapy and immediate two-stage breast reconstruction with a tissue expander and implant: complications and esthetic results. Int J Radiat Oncol Biol Phys. 2003;57:136-142.
66 Vandeweyer E, Deraemaecker R. Radiation therapy after immediate breast reconstruction with implants. Plast Reconstr Surg. 2000;106:56-58.