CHAPTER 11 Adjuvant Analgesics for Radicular Pain
PATHOPHYSIOLOGIC BACKGROUND OF RADICULAR PAIN
The pathophysiology of radicular pain remains incompletely understood. It is known that radicular pain is caused by disorders of the nerve root proximal to the dorsal root ganglion (DRG) or at the DRG itself. Proposed mechanisms of radicular pain include: (1) local neuropathic pain originating from lesions of nociceptive sprouts within the degenerated disc, (2) mechanical neuropathic root pain originating from mechanical compression of the nerve root, or (3) inflammatory neuropathic root pain derived from the action of inflammatory mediators originating from the degenerative disc.1 Traditionally, it has been thought that radicular pain arises from mechanical nerve root compression by herniated discs or osteophytes. However, increasing evidence suggests that radicular pain may involve chemical irritation or damage mediated by various agents released from the degenerated nucleus pulposus of herniated discs. These agents may include phospholipase A2,2 TNF-α,3 interleukin (IL)-8,4 IL-6, IL-1β,5 and nitric oxide.6 Chemical damage may lead to a state of hyperexcitability in the injured DRG or nerve root, a phenomenon called peripheral sensitization. The net result is ectopic neuronal firing7 transmitted via sensory neuron-specific, voltage-gated sodium channels. Ectopic neuronal firing also activates N-type voltage-gated calcium channels, permitting calcium influx and the promotion of excitation. As a result, dorsal horn neurons or central neurons innervated by the injured DRG or nerve root undergo dramatic functional changes, including a state of hyperexcitability termed central sensitization.8 Normally, these sensitization events spontaneously resolve as tissue healing occurs and inflammation subsides. Yet, when the primary afferent function is persistently altered by chemical or mechanical injury to the nerve roots, these processes may be highly resistant to treatment.9 Early antiinflammatory intervention (e.g. steroid injection) may reduce nerve root damage, thereby lessening the chance of developing chronic radicular pain. Indeed, this outcome may be the result of treating the peripheral disease, which in turn may eliminate the triggering of central sensitization.10
USE OF ANTIEPILEPTIC DRUGS IN CHRONIC RADICULAR PAIN
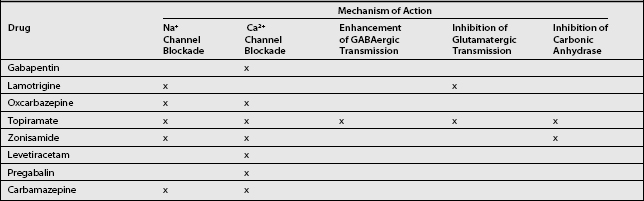
Current first-line analgesic therapies for chronic radicular pain rely mostly on nonsteroidal antiinflammatory drugs (NSAIDs) known to relieve nociceptive pain only. Since the pathophysiology of neuropathic pain and epilepsy are similar, the use of antiepileptic drugs (AEDs) in the treatment of neuropathic pain is also an attractive option.11 Mechanisms of central sensitization and ectopic neuronal firing are common to both epilepsy12 and neuropathic pain.13 Given the similarities between these two disorders, it is reasonable to speculate that the mechanisms of action that may be responsible for the therapeutic efficacy of the newer AEDs in the treatment of epilepsy may also prove beneficial in the treatment of neuropathic pain.14 Several mechanisms of action shared by the newer AEDs relate directly to the pathophysiology of neuropathic pain. Any or all of these actions may account for the effectiveness of these medications: (1) sodium channel blockade,15 (2) calcium channel blockade,15 (3) enhancement of GABAergic transmission,16 (4) inhibition of glutamatergic transmission,16 and (5) inhibition of carbonic anhydrase17 (Table 11.1). The mechanisms of chronic radicular pain may differ somewhat from those of peripheral neuropathic pain, resulting in varying of response to medication.18
Interestingly, a recent survey in the US19 indicated that AEDs are the third most commonly used class of medications for treating radiculopathy, after NSAIDs and opioids. Clinical trials demonstrating the efficacy of AEDs in this indication would be highly valuable, since therapeutic modalities such as AEDs could be a useful alternative for patients who do not respond to NSAIDs.1
NEWER ANTIEPILEPTIC DRUGS
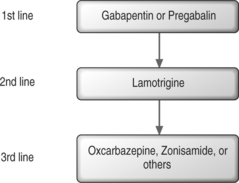
Recommendations for first-line AEDs are based on the positive results from multiple randomized, controlled trials. First-line medications for neuropathic pain include gabapentin, tricyclic antidepressants (TCAs), and tramadol hydrochloride (Fig. 11.1).9
Gabapentin: first-line antiepileptic drugs for chronic radicular pain
Gabapentin is an AED with an unknown mechanism of action apparently dissimilar to that of other antiepileptic agents. This agent also possesses desirable pharmacokinetics: gabapentin is not protein bound and is not metabolized, and does not induce liver enzymes, diminishing the likelihood of interactions with other antiepileptic agents and with other drugs such as oral contraceptives. Although gabapentin is a structural analog of the neurotransmitter gamma-aminobutyric acid (GABA), which does not cross the blood–brain barrier, gabapentin does penetrate into the CNS.20 Evidence suggests that its mechanism of action most likely involves complex synergy between increased GABA synthesis, non-NMDA receptor anatagonism, and binding to the α2δ subunit of voltage-dependent calcium channels. The latter action inhibits the release of excitatory neurotransmitters.21
The most important action of gabapentin appears to be its binding to the α2δ subunit of voltage-dependent calcium channels (see Table 11.1).22 These binding sites are located in the spinal cord, with particularly high density in the superficial laminae of the dorsal horn. The action of gabapentin at these sites may inhibit the release of excitatory neurotransmitters and reduce glutamate availability at NMDA and non-NMDA receptors.23
In chronic radiculopathy, mechanical and chemical nociceptive stimuli lead to the production of ectopic impulses. These ectopic discharges are seen along the length of the nerve root fiber. Ectopic discharges can provide sustained afferent input to the spinal cord from a damaged root or DRG. Gabapentin may inhibit these discharges at the level of the nerve root, DRG,24 spinal cord,21,23 or brain.20
Dosing and titration
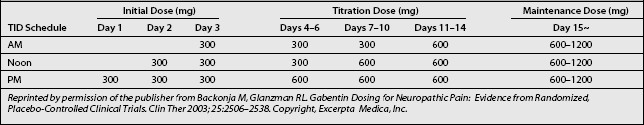
Titration and dosing schedules (Table 11.2) may potentially be efficacious in treating radicular pain and promoting tolerability, particularly in elderly patients, who make up a large proportion of radicular pain patients.25
Initiation
Based on the schedule of treatment initiation used in study protocols, it is reasonable to start gabapentin at 300 mg once daily on day 1, 300 mg twice daily on day 2, and 300 mg thrice daily on day 3 (see Table 11.2). This stepwise escalation was well tolerated in clinical studies and has the advantages of simplicity and rapidity in reaching the goal dosage of 900 mg/day. These factors may contribute to patient compliance. Although infrequent, in cases of intolerance gabapentin should be initiated at lower dosages, such as 100 mg in a single dose at bed time, then titrated daily by 100 mg three times.25
Titration and maintenance
In the reviewed trials, clinically relevant improvements were noted at week 2, during which patients received gabapentin 1800 mg/day. Thereafter, dosages were increased from 1800 to 3600 mg/day as tolerated to achieve better efficacy. In many patients, further dose escalations up to 3600 mg/day may be necessary to reach individualized maximally effective doses that can be maintained without compromising tolerability. Table 11.2 provides a possible schema for stepwise escalations in gabapentin dosing. Dosage increases can be simplified by the availability of 600 and 800 mg gabapentin tablets.
Although drowsiness may occur during the initial titration period, the overall low rate of serious side effects associated with gabapentin treatment in clinical trials indicate that rapid dose escalations are probably safe.20,26,27 In most cases, drowsiness generally resolves within 7–14 days from the initiation of treatment. Contrary to previously published reports, however, some patients may continue to complain of drowsiness of moderate severity even at a daily dose of 900 mg. Based on study findings and the author’s clinical experience, some adverse events may be due to the dose titration process itself. Therefore, patient education about the transient nature of such adverse events may allow early achievement of effective doses and improve patient satisfaction.25 To minimize the problem of persistent drowsiness or other side effects, one can increase the intervals between dosage escalations, based on each patient’s tolerance.
Maximal effective dose
On the basis of reviewed trials, doses of up to 3600 mg/day may be used when required and tolerated, and can be achieved by week 4 of treatment. In an animal model, gabapentin was shown to be more efficacious at lower doses in treating radicular pain than peripheral neuropathic pain.18 However, these findings need to be investigated further in future studies.
Combination treatment with tricyclic antidepressants, and tramadol
Gabapentin, tricyclic antidepressants (TCAs), and tramadol hydrochloride have been used as first-line medications for neuropathic pain. It is common for patients to have a partial response to these medications, and in these cases combination treatment should be considered. No studies have systematically examined the efficacy of various combinations of these three medications, as compared with monotherapy. Despite the lack of controlled data, combinations of two or more of these first-line medications can be recommended when patients have a partial response to monotherapy or at the beginning of treatment. The disadvantage of combination therapy is the difficulty in identifying which medication is responsible for any adverse effects.9
Clinical reports on chronic radiculopathy
Although several published, double-blind, placebo-controlled, randomized clinical trials of gabapentin in the treatment of chronic neuropathic pain have been published,27–30 there are only a few clinical reports designed to study the effect of gabapentin on pathological processes involving the dorsal root ganglion and proximal nerve root. These processes include chronic radiculopathy, arachnoiditis, and epidural fibrosis.
Chronic radiculopathy
A unique randomized, placebo-controlled study evaluated the efficacy of gabapentin monotherapy in patients with chronic radiculopathy over 8 weeks. The results showed significant improvement in the following: pain at rest, straight-leg raise test (SLR), and limitation of spinal flexion. The gabapentin group was treated with doses ranging from a total of 900 mg/day to 3600 mg/day divided into three doses.31
Arachnoiditis
In one open-label study, three patients with arachnoiditis were treated with gabapentin at a maximum of 2700 mg/day. One patient discontinued the study because of adverse effects, and the other two had moderate improvement in their pain.32
Epidural fibrosis
There was one case report in which two patients were treated for radicular pain resulting from epidural fibrosis caused by failed back surgery syndrome. It was reported that functional status improved markedly and pain was significantly diminished with gabapentin regimens of 1500 mg and 2100 mg/day.33
Adverse effects
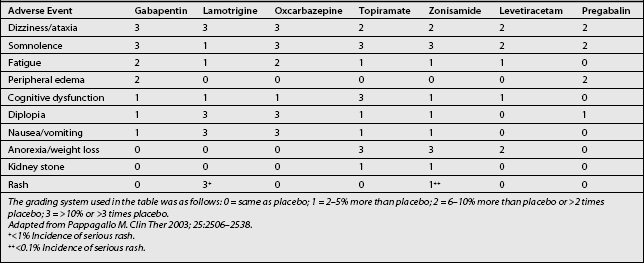
The adverse effects of gabapentin include somnolence, fatigue, dizziness, and, less commonly, mild peripheral edema and gastrointestinal symptoms (Table 11.3). The incidence of mild adverse events has been reported in up to 35–75% of gabapentin recipients, seemingly in a dose-dependent manner.20,26,27 All of these effects require monitoring and dosage adjustment, but usually not discontinuation of the drug. In elderly patients in particular, gabapentin may cause gait and balance problems, as well as cognitive impairment. In addition, dosage adjustment is necessary in patients with renal insufficiency. However, generally excellent tolerability and safety of gabapentin distinguish it from most other oral medications used for the treatment of chronic neuropathic pain. In effect, it is often the first choice for treating many types of neuropathic pain.34 Gabapentin is absorbed orally without interference by food, and it reaches peak serum concentrations after 2–3 hours. No blood monitoring is required with gabapentin therapy because of the absence of toxic effects.20
Lamotrigine
Lamotrigine is a novel AED with at least two antinociceptive properties: it stabilizes the neural membrane through blocking the activation of voltage-sensitive sodium channels, and it inhibits the presynaptic release of glutamate.15 Abnormal neural firing is a principle cause of nerve injury-induced pain,7 and glutamate plays a key role in dorsal horn spinal hyperexcitability by acting at the NMDA receptor (see Table 11.1),35 providing a strong rationale for the use of lamotrigine in the treatment of radicular pain.
Dosing and titration
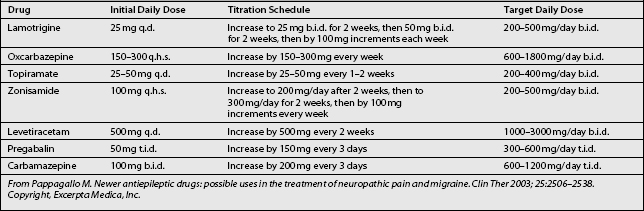
Titration (Table 11.4) is initiated at a dose of 25 mg daily for 2 weeks, increasing to 50 mg/day for 2 weeks. Further increases are by 50 mg b.i.d. for 2 weeks, then by 100 mg/day b.i.d. every week,11,36 until the desired daily dose is achieved. The regimen of gradual dose increments is chosen to avoid the occurrence of adverse drug reactions.20 The analgesic effects of lamotrigine become more profound after prolonged treatment with a steady dose.36
Clinical reports on chronic radiculopathy
Lamotrigine in doses higher than 200–400 mg daily has demonstrated efficacy in relieving pain in patients with trigeminal neuralgia, complex regional pain syndrome type I, chronic neuropathic pain syndrome, painful HIV neuropathy, central post-stroke pain, and incomplete spinal cord lesions.9
A unique open-label study investigated the effect of lamotrigine on chronic radicular pain, and showed that spontaneous pain, pain associated with the straight-leg-raising test), and pain associated with bending the affected side reached a statistically significant level of improvement only at a 400 mg dose. These results suggest that lamotrigine is a potentially effective treatment for painful lumbar radiculopathy, and that it is likely to act in a dose- and plasma concentration-dependent fashion.36 Because of the slow and careful titration required and risk of both severe rash and Stevens-Johnson syndrome associated with its use, lamotrigine should, however, only be considered in cases of chronic radicular pain refractory to gabapentin.
Drug interactions
Lamotrigine is absorbed rapidly and completely from the gastrointestinal tract, and it is approximately 55% bound to plasma protein. The drug is extensively metabolized by conjugation with glucuronic acid. The elimination half-life is 25–30 hours. Because of the wide variability in kinetics caused by interaction with concomitant medications, monitoring serum lamotrigine concentrations could be theoretically useful in clinical practice.34 Plasma lamotrigine levels are reduced by barbiturates, carbamazepine, phenytoin, oxcarbazepine, and steroid oral contraceptives, and they are increased by valproate.37
Adverse effects
Common adverse effects (see Table 11.3) include dizziness, ataxia, somnolence, diplopia, nausea, vomiting, and constipation. A more serious side effect is rash, which, in rare instances, can progress to Stevens-Johnson syndrome. However, the chance of this event is drastically decreased when the drug is titrated slowly. Adverse effects are more pronounced in patients concurrently taking valproate.34
Oxcarbazepine
Oxcarbazepine has been shown to inhibit high-frequency nerve firing without impairing normal impulse conduction. The agent exerts a dual mode of action involving the modulation of both voltage-sensitive sodium channels and high-voltage activated N-type calcium channels (see Table 11.1). As a result, there is a possibility that oxycarbazepine can target certain underlying mechanisms known to be important in the genesis of both peripheral and central sensitization.15,38
Dosing and titration
Since oxcarbazepine does not autoinduce its metabolism, dosage titration and adjustments (see Table 11.4) are relatively simple, and steady-state levels of the drug can be achieved. The recommended adult starting dosage is 150–300 mg/day. The dosage is slowly titrated at weekly intervals, based on clinical response, to a usual maintenance dosage of 600–1800 mg/day. Doses are given twice daily. Some patients with refractory neuropathic pain may require doses as high as 2400 mg/day.11,38
Clinical reports on chronic radiculopathy
An open-label study of adjunctive oxcarbazepine involved 36 consecutive patients with various neuropathic pain disorders that did not respond to treatment with gabapentin. In this study, the addition of oxcarbazepine at dosages of 600–1200 mg/day was helpful in almost two-thirds of patients. Excellent results (>70% improvement in neuropathic symptoms) were reported in 22.2% of patients, good results (51–70% improvement) in 41.7%, and fair or poor results (<50% improvement) in 38.9%. In a subset of patients who presented with radiculopathy (n=18), the proportion of those with excellent or good results was greater than in the entire sample (84% vs. 64%, respectively). Significant improvement was seen in patients with symptoms described as ‘burning’ and allodynia.39
There is growing clinical evidence that oxcarbazepine may be equal to or better than carbamazepine for the treatment of both painful diabetic neuropathy and trigeminal neuralgia. Oxcarbazepine is reported to have a better side effect profile than carbamazepine, and it is now the drug of choice for trigeminal neuralgia in several Western countries.40,41 However, oxcarbazepine still requires further well-designed research and clinical experience. In the interim, it is recommended that this agent be used to treat neuropathic pain only when gabapentin or lamotrigine cannot be used, or when the response to these agents is suboptimal.42
Drug interactions
Oxcarbazepine has predictable, linear pharmacokinetics and minimal drug interactions which facilitate its use in combination therapy. In contrast to carbamazepine, oxcarbazepine does not generally induce hepatic enzymes. However, the agent selectively enhances the metabolism of felodipine. In addition, it induces the CYP3A4 system, affording more rapid metabolism of estrogen and progesterone. Thus, it is recommended that oxcarbazepine recipients not use oral contraceptives.11,42
Adverse effects
The most common side effects of oxcarbazepine (see Table 11.3) include sedation, dizziness, diplopia, nausea, vomiting, vertigo, ataxia, and headache. These effects appear to be dose-dependent. The side effect profile of oxcarbazepine is favorable relative to carbamazepine. Oxcarbazepine is not associated with serious idiosyncratic hematologic or hepatic effects. For this reason, there is no need for routine monitoring of hematologic and hepatic profiles during treatment with the drug. The frequency of hyponatremia with oxycarbazepine has been reported to range from 22% to 73%. Most cases are asymptomatic, but severe cases have been seen occasionally.40,42
Topiramate
Topiramate is a newer AED with several mechanisms of action. It has been shown to modulate voltage-gated sodium and calcium channels, potentiate GABAergic inhibition, block excitatory glutamate activity via the AMPA and kinase receptors, and inhibit carbonic anhydrase (see Table 11.1).43 Based on these mechanisms of action, topiramate should be well suited to inhibit several of the mechanisms putatively involved in neuropathic pain.
Dosing and titration
The recommended starting dosage of topiramate (see Table 11.4) in adults is 25–50 mg/day, followed by titration in 25–50 mg/day increments every 1–2 weeks based on clinical response, to a usual maintenance dosage of 200–400 mg/day (maximum of 600 mg/day). Doses are given twice daily.11,42,43
Clinical reports on chronic radiculopathy
Some small clinical trials showed pain reduction with topiramate therapy at doses up to 400 mg/day in painful diabetic neuropathy, trigeminal neuralgia, intercostal neuralgia, and neuropathic pain after spinal cord injury. Effectiveness in neuropathic pain remains unclear.11,41 Presently, there are no published reports of topiramate’s effect on chronic radicular pain.
Drug interactions
Topiramate induces the CYP3A4 system, inducing more rapid metabolism of most of the steroid hormones in oral contraceptives, and can thus lead to failure of birth control. For that reason, it is recommended that patients taking topiramate not use oral contraceptives. In addition, topiramate enhances the hypoglycemic effect of metformin, although the mechanism by which this occurs is unknown.42
Adverse effects
The most common adverse effects of topiramate (see Table 11.3) are anorexia, nausea, diarrhea, and CNS-related symptoms (including somnolence, dizziness, ataxia, fatigue, impaired cognitive function and concentration, speech disturbance, and digital and perioral paresthesia). The frequency and severity of these effects are increased at higher doses, with more rapid dose escalation, and as part of combination therapy, as opposed to monotherapy. Behavioral events are rarely seen. Nephrolithiasis, particularly calcium phosphate stones, are seen in ≈1.5% of topiramate recipients, yet most stones are very small and are passed spontaneously. In addition, two non-CNS adverse effects are of particular interest. First, the drug may cause acute myopia and secondary angle-closure glaucoma, presumably mediated by carbonic anhydrase inhibition. Symptoms include severe bilateral ocular pain and hyperemia, which usually remit within 24 hours of drug withdrawal. Second, most topiramate recipients experience weight loss, which becomes apparent within the first 3 months of therapy.42
Zonisamide
A newer AED, zonisamide, is believed to exert its effects by inhibiting voltage-dependent sodium and T-type calcium channels, both of which have a pivotal role in membrane excitability. The agent binds but does not modulate GABA receptors and inhibits a carbonic anhydrase (see Table 11.1).17 Thus, the activity profile of zonisamide appears favorable for the treatment of neuropathic pain.
Dosing and titration
The recommended starting dose of zonisamide (see Table 11.4) is 100 mg/day at bedtime, followed by dose titrations every 2 weeks up to 300 mg/day. Dose increases of 100 mg are then made weekly based on response, to a usual maintenance dosage of 200–500 mg/day. Doses are given twice daily.42
Clinical reports on chronic radiculopathy
Although zonisamide has not been evaluated in randomized, controlled trials in patients with neuropathic pain, a number of open-label case series and anecdotal reports have been published. In the largest open-label study,44 zonisamide was added to existing therapy in 40 patients with treatment-refractory neuropathic pain that was primarily associated with cervical or lumbar radiculopathy. At a mean daily dosage of 260 mg/d, daily pain scores were decreased by >60% in 10 patients (25%) and by 30–60% in 8 patients (20%). Only 2 of 40 patients (5%) chose to discontinue the drug because of drowsiness. As a result of starting zonisamide therapy, 18 of 40 patients (45%) were able to discontinue the AED they had originally been taking at the time of entry (gabapentin in 12 of 18 patients, and topiramate in 3 patients). There is also a retrospective study45 that included 142 patients with neuropathic pain who received a prescription for zonisamide. Eighty-six of these patients had a diagnosis of cervical or lumbar radiculopathy, 30 had painful diabetic neuropathy, 19 had fibromyalgia, and 7 had pelvic pain. Only 10 patients (7%) discontinued zonisamide because of adverse effects. At a mean daily dose of 252 mg, 100 of 142 patients (70%) reported at least moderate reduction of pain, and only 9 (6%) felt they received no benefit. One other clinical trial with a smaller study population showed pain reduction with zonisamide therapy at dosages up to 400 mg/day in the setting of various neuropathic pain syndromes.42
Drug interactions
Concurrent use of hepatic enzyme inducers such as phenobarbital, carbamazepine, and phenytoin are known to reduce plasma zonisamide levels.37
Adverse effects
The most common adverse effects of zonisamide (see Table 11.3) are somnolence, ataxia, anorexia, impaired cognitive function, nervousness, fatigue, and dizziness. Clinically significant weight loss may also occur, but is less problematic than with topiramate. In one epilepsy trial, kidney stones were suspected in 4% of patients receiving zonisamide.46 As a member of the sulfonamides, zonisamide poses a risk for serious dermatologic and hematologic reactions. However, the incidence of such adverse effects has been extremely small, and the background rate of rash is reported as very low. Both zonisamide and topiramate are weak carbonic anhydrase inhibitors, and their concomitant use may increase the risk for kidney stones.11,42
Levetiracetam
Levetiracetam inhibits calcium channels and delayed-rectifier potassium currents, and antagonizies negative allosteric modulators of the GABA and glycine responses at nonbenzodiazepine sites (see Table 11.1).37
Dosing and titration
The recommended starting dose of levetiracetam (see Table 11.4) is 500 mg/day, followed by dose titration every 2 weeks based on response, to a usual maintenance dosage of 1000–3000 mg/day. The drug is given twice daily.42
Clinical reports on chronic radiculopathy
A few clinical trials have demonstrated pain reduction in various neuropathic pain syndromes and neoplastic plexopathies with levetiracetam therapy at dosages of 1000–3000 mg/day.42 However, the effectiveness of levetiracetam in neuropathic pain requires further detailed study.
Drug interactions
No clinically significant drug interactions with levetiracetam have been identified to date due to low protein-binding (<10%) and lack of hepatic metabolism.47
Adverse effects
Levetiracetam has a favorable side effect profile (see Table 11.3). The most common adverse effects of levetiracetam occur only occasionally, and include dizziness, headache, fatigue, somnolence, and asthenia. Behavioral effects are rare. Levetiracetam appears to be one of the easiest AEDs to use, since there is no need for dose adjustments in cases of organ dysfunction, and laboratory monitoring is not required. It is one of the best-tolerated agents, and has not been associated with the toxicities seen with oxcarbazepine, topiramate, and zonisamide.42,48
Pregabalin
Pregabalin is an α2δ ligand that has analgesic, anxiolytic, and anticonvulsant activity. The auxiliary protein α2d is associated with voltage-gated calcium channels (see Table 11.1). Pregabalin binds potently to the α2δ subunit,22 which, in turn, reduces calcium influx at nerve terminals. As a result, there is a decrease in the release of several neurotransmitters, including glutamate, noradrenaline, and substance P.49,50 This activity produces the analgesic, anxiolytic, and anticonvulsant activity exhibited by pregabalin. The dual benefits of relieving both pain and anxiety (independent of pain relief) may prove to be highly beneficial clinically.51
Dosing and titration
The recommended starting dose of pregabalin (see Table 11.4) is 150 mg/day (50 mg three times daily), followed by dose titration every 3 days based on response, to a usual maintenance dosage of 300–600 mg/day. The drug is given three times daily.51,52
Clinical reports on chronic radiculopathy
Two randomized, placebo-controlled trials focused on the treatment of postherpetic neuralgia with pregabalin at dosages of 150–600 mg/day. Results showed that pregabalin is safe, efficacious in relieving pain and sleep interference, and associated with greater global improvement than placebo. Pregabalin treatment groups demonstrated significant improvement in pain compared to placebo as early as week 1.51,52 Although no clinical trials have compared pregabalin with gabapentin, it appears that pregabalin has a more rapid onset of action than does gabapentin.51 While this agent shows promise, there has not yet been a report on the effect of pregabalin on chronic radicular pain.
Drug interactions
Pregabalin has a predictable pharmacokinetic profile, few drug interactions, and a rapid onset of action.52
Adverse effects
The most frequent adverse events (see Table 11.3) are dizziness, somnolence, peripheral edema, headache, and dry mouth, all of which are mild to moderate in intensity. These adverse effects are dose dependent. In one study, more patients in the pregabalin treatment groups completed therapy than in the placebo group. This result suggests that the adverse effects associated with pregabalin may be of no clinical significance.51 The frequency of adverse events occurring in patients taking pregabalin seems to be less than in patients taking gabapentin.20,26,27,52
OLDER ANTIEPILEPTIC DRUGS
Carbamazepine
Carbamazepine emerged as a treatment for trigeminal neuralgia before the discovery of much of what is now known about the pathophysiology of nociception and neuropathic pain. Since that time, little progress has been made in the development of medications for neuropathic pain. Carbamazepine is an iminostilbene derivative chemically related to the TCAs. The analgesic effect of carbamazepine is thought possibly to result from its peripheral or central activity. The drug’s ability to block calcium and sodium channels appears to suppress A-delta and C-fiber activity (see Table 11.1) implicated in the genesis of pain.34
Dosing and titration
The recommended starting dose of carbamazepine (see Table 11.4) is 200 mg/day (100 mg twice daily), increased every 3 days based on response to a usual maintenance dosage of 600–1200 mg/day. The drug is given three times daily.16
Clinical reports on chronic radiculopathy
Carbamazepine has a well-established beneficial effect on trigeminal neuralgia,53 and has been approved by the FDA for this indication. Some evidence suggests that carbamazepine is beneficial in the treatment of patients with painful diabetic neuropathy.54 On the basis of clinical trials with AEDs used for chronic neuropathic pain, carbamazepine can be recommended for patients for whom AED treatment is desired, yet who have not responded to an adequate trial of gabapentin. The effect of carbamazepine on chronic radicular pain, however, remains speculative and is supported only by a few case reports and anecdotal experience.
Drug interactions
Carbamazepine is a potent enzyme inducer which causes many drug interactions. This agent reduces plasma levels of lamotrigine, oxcarbazepine, topiramate, and zonisamide.37
Adverse effects
Common adverse effects of carbamazepine (see Table 11.3) include somnolence, dizziness, diplopia, blurred vision, gait disturbance, nausea, and vomiting. In elderly patients, several issues may complicate treatment with carbamazepine, including cardiac disease, water retention, decreased osmolality, and hyponatremia. Hematologic problems, such as agranulocytosis, were of concern during early experiences with carbamazepine, and it is still advisable to monitor patients for this possible complication. Induction of the microsomal enzyme system by carbamazepine may influence drug metabolism.41
Clonazepam
Clonazepam is a GABA agonist that has analgesic properties in the spinal cord and brainstem of animal models.55 In the absence of definitive data, clinical experience suggests clonazepam may relieve trigeminal neuralgia and various neuropathic pain syndromes.56 As of yet, there is no report on the effect of this drug on chronic radicular pain. Clonazepam should probably not be considered a first-line choice even for the above indications, since potential benefits must be weighed against the potential for developing cognitive impairment, physical and psychological dependence, worsening depression, overdose, and other side effects.57 The recommended starting dose of clonazepam is 0.5–1 mg/day, which is increased to a usual maintenance dosage of 4–6 mg/day. The drug is given three times daily.16
Phenytoin
Phenytoin exerts its membrane-stabilizing effect by blocking sodium channels, and it is generally held that it reduces neuronal excitability of pain fibers by this mechanism. Phenytoin was the first drug to be used for trigeminal neuralgia, but there is no randomized, controlled trial on the use of the agent for this condition. Today, phenytoin has limited use for the treatment of chronic neuropathic pain due to its side effects and complicated pharmacokinetic profile. Instead, it has been replaced by drugs such as gabapentin, lamotrigine, oxcarbazepine, and carbamazepine.41
Valproic acid
Valproic acid inhibits sustained neuronal firing in murine cortical and spinal neurons. This effect is mediated by prolonging repolarization of voltage-activated sodium channels. Moreover, the drug increases the amount of GABA in the brain, enhancing the activity of glutamic acid decarboxylase, and inhibiting GABA degradation enzymes.16 In the absence of definitive data, anecdotal experience suggests that valproic acid relieves trigeminal neuralgia and various neuropathic pain syndromes.16 Recently, a randomized, double-blind, placebo-controlled study showed that valproic acid is well tolerated and provides significant subjective improvement in painful diabetic neuropathy.58 However, there was no difference between valproic acid and placebo in regards to the treatment of neuropathic pain after spinal cord injury.59 There are not yet any reports on the effect of valproic acid on chronic radicular pain.
CONCLUSION
Interest in the mechanism and treatment of chronic radicular pain has increased during the past several years, coupled with continued research in the pathophysiology of chronic radicular pain. We will see significant treatment advances in the future. Already, the newer AEDs have safer side effect profiles than the older AEDs, and even though clinical data are lacking, preliminary study results highlight the potential of these newer AEDs to be an important treatment option for chronic radicular pain. Gabapentin, in particular, has emerged as a first-line AED for treating chronic radicular pain. In addition, pregabalin may be expected to have potential promise as a therapy for chronic radicular pain (see Fig. 11.1). Finally, in addition to the adjunctive analgesic drugs discussed, continued research will also lead to better early antiinflammatory interventions for patients susceptible to chronic radicular pain.
1 Baron R, Binder A. Is sciatica neuropathic? The mixed pain concept. Orthopade. 2004;33:568-575.
2 Ozaktay AC, Cavanaugh JM, Blagoev DC, et al. Phospholipase A2-induced electrophysiologic and histologic changes in rabbit dorsal lumbar spine tissues. Spine. 1995;20:2659-2668.
3 Olmarker K, Larsson K. Tumor necrosis factor alpha and nucleus pulposus-induced nerve root injury. Spine. 1998;23:2538-2544.
4 Ahn SH, Cho YW, Ahn MW, et al. mRNA expression of cytokines and chemokines in herniated lumbar intervertebral discs. Spine. 2002;27:911-917.
5 Ozaktay AC, Cavanaugh JM, Asik I, et al. Dorsal root sensitivity to interleukin-1 beta, interleukin-6 and tumor necrosis factor in rats. Eur Spine J. 2002;11:467-475.
6 Brisby H, Byrod G, Olmarker K, et al. Nitric oxide as a mediator of nucleus pulposus-induced effects on spinal nerve roots. J Orthop Res. 2000;18:815-820.
7 Liu CN, Raber P, Ziv-Sefer S, et al. Hyperexcitability in sensory neurons of rats selected for high versus low neuropathic pain phenotype. Neuroscience. 2001;105:265-275.
8 Baba H, Doubell TP, Woolf CJ. Peripheral inflammation facilitates Aβ fiber-mediated synaptic input to the substantia gelatinosa of the adult rat spinal cord. J Neurosci. 1999;19:859-867.
9 Dworkin RH, Backonja M, Rowbotham MC, et al. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch Neurol. 2003;60:1524-1534.
10 Woolf CJ. A new strategy for the treatment of inflammatory pain. Prevention or elimination of central sensitization. Drugs. 1994;47(Suppl 5):1-9.
11 Pappagallo M. Newer antiepileptic drugs: possible uses in the treatment of neuropathic pain and migraine. Clin Ther. 2003;25:2506-2538.
12 Engelborghs S, D’Hooge R, De Deyn PP. Pathophysiology of epilepsy. Acta Neurol Belg. 2000;100:201-213.
13 Chaplan SR, Guo HQ, Lee DH, et al. Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain. J Neurosci. 2003;23:1169-1178.
14 Backonja MM. Anticonvulsants (antineuropathics) for neuropathic pain syndromes. Clin J Pain. 2000;16(Suppl 2):S67-S72.
15 Wallace MS. Calcium and sodium channel antagonists for the treatment of pain. Clin J Pain. 2000;16(Suppl 2):S80-S85.
16 Tremont-Lukats IW, Megeff C, Backonja MM. Anticonvulsants for neuropathic pain syndromes: mechanisms of action and place in therapy. Drugs. 2000;60:1029-1052.
17 Leppik IE. Zonisamide. Epilepsia. 1999;40(Suppl 5):S23-S29.
18 Abe M, Kurihara T, Han W, et al. Changes in expression of voltage-dependent ion channel subunits in dorsal root ganglia of rats with radicular injury and pain. Spine. 2002;27:1517-1524.
19 Cluff R, Mehio AK, Cohen SP, et al. The technical aspects of epidural steroid injections: a national survey. Anesth Analg. 2002;95:403-408.
20 Goa KL, Sorkin EM. Gabapentin. A review of its pharmacological properties and clinical potential in epilepsy. Drugs. 1993;46:409-427.
21 Bennett MI, Simpson KH. Gabapentin in the treatment of neuropathic pain. Palliat Med. 2004;18:5-11.
22 Gee NS, Brown JP, Dissanayake VU, et al. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the α2d subunit of a calcium channel. J Biol Chem. 1996;271:5768-5776.
23 Shimoyama M, Shimoyama N, Hori Y. Gabapentin affects glutamatergic excitatory neurotransmission in the rat dorsal horn. Pain. 2000;85:405-414.
24 Pan HL, Eisenach JC, Chen SR. Gabapentin suppresses ectopic nerve discharges and reverses allodynia in neuropathic rats. J Pharmacol Exp Ther. 1999;288:1026-1030.
25 Backonja M, Glanzman RL. Gabapentin dosing for neuropathic pain: evidence from randomized, placebo-controlled clinical trials. Clin Ther. 2003;25:81-104.
26 Ahn SH, Park HW, Lee BS, et al. Gabapentin effect on neuropathic pain compared among patients with spinal cord injury and different durations of symptoms. Spine. 2003;28:341-346.
27 Levendoglu F, Ogun CO, Ozerbil O, et al. Gabapentin is a first-line drug for the treatment of neuropathic pain in spinal cord injury. Spine. 2004;29:743-751.
28 Backonja M, Beydoun A, Edwards KR, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA. 1998;280:1831-1836.
29 Pandey CK, Bose N, Garg G, et al. Gabapentin for the treatment of pain in Guillain-Barré syndrome: a double-blinded, placebo-controlled, crossover study. Anesth Analg. 2002;95:1719-1723.
30 Rowbotham M, Harden N, Stacey B, et al. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. JAMA. 1998;280:1837-1842.
31 Yildirim K, Sisecioglu M, Karatay S, et al. The effectiveness of gabapentin in patients with chronic radiculopathy. Pain Clin. 2003;15:213-218.
32 Merren MD. Gabapentin for treatment of pain and tremor: a large case series. South Med J. 1998;91:739-744.
33 Braverman DL, Slipman CW, Lenrow DA. Using gabapentin to treat failed back surgery syndrome caused by epidural fibrosis: A report of 2 cases. Arch Phys Med Rehabil. 2001;82:691-693.
34 Backonja M. Neuromodulating drugs for the symptomatic treatment of neuropathic pain. Curr Pain Headache Rep. 2004;8:212-216.
35 Dickenson AH, Sullivan AF. Evidence for a role of the NMDA receptor in the frequency dependent potentiation of deep rat dorsal horn nociceptive neurons following C fibre stimulation. Neuropharmacology. 1987;26:1235-1238.
36 Eisenberg E, Damunni G, Hoffer E, et al. Lamotrigine for intractable sciatica: correlation between dose, plasma concentration and analgesia. Eur J Pain. 2003;7:485-491.
37 Perucca E. Clinical pharmacology and therapeutic use of the new antiepileptic drugs. Fundam Clin Pharmacol. 2001;15:405-417.
38 Carrazana E, Mikoshiba I. Rationale and evidence for the use of oxcarbazepine in neuropathic pain. J Pain Symptom Manage. 2003;25(5 Suppl):S31-S35.
39 Jensen MG, Royal MA, Ward S, et al. An open-label trial of oxcarbazepine in patients with radiculopathy refractory to gabapentin. Pain Clin. 2002;4:11-13.
40 Beydoun A, Kutluay E. Oxcarbazepine. Expert Opin Pharmacother. 2002;3:59-71.
41 Jensen TS. Anticonvulsants in neuropathic pain: rationale and clinical evidence. Eur J Pain. 2002;6(Suppl A):61-68.
42 Guay DRP. Oxcarbazepine, topiramate, zonisamide, and levetiracetam: Potential use in neuropathic pain. Am J Geriatr Pharmacother. 2003;1:18-37.
43 Chong MS, Libretto SE. The rationale and use of topiramate for treating neuropathic pain. Clin J Pain. 2003;19:59-68.
44 Krusz JC. Zonisamide in the treatment of pain and headache disorders. J Neurol Sci. 2001;187(Suppl 1):S107. abstract
45 Kaplan M. Zonisamide – benefits in chronic pain patients. Arch Phys Med Rehabil. 2002;83:1671. (a)
46 Leppik IE. Three new drugs for epilepsy: levetiracetam, oxcarbazepine, and zonisamide. J Child Neurol. 2002;17(Suppl 1):S53-S57.
47 Hachad H, Ragueneau-Majlessi I, Levy RH. New antiepileptic drugs: review on drug interactions. Ther Drug Monit. 2002;24:91-103.
48 Glauser TA, Pellock JM, Bebin EM, et al. Efficacy and safety of levetiracetam in children with partial seizures: an open-label trial. Epilepsia. 2002;43(5):518-524.
49 Fink K, Dooley DJ, Meder WP, et al. Inhibition of neuronal Ca(2+) influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology. 2002;42:229-236.
50 Maneuf YP, Hughes J, McKnight AT. Gabapentin inhibits the substance P-facilitated K(+)-evoked release of [(3)H]glutamate from rat caudal trigeminal nucleus slices. Pain. 2001;93:191-196.
51 Sabatowski R, Galvez R, Cherry DA, et al. Pregabalin reduces pain and improves sleep and mood disturbances in patients with post-herpetic neuralgia: results of a randomized, placebo-controlled clinical trial. Pain. 2004;109:26-35.
52 Dworkin RH, Corbin AE, Young JPJr, et al. Pregabalin for the treatment of postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology. 2003;60:1274-1283.
53 McQuay H, Carroll D, Jadad AR, et al. Anticonvulsant drugs for management of pain: a systematic review. BMJ. 1995;311:1047-1052.
54 Wilton TD. Tegretol in the treatment of diabetic neuropathy. S Afr Med J. 1974;48:869-872.
55 Sawynok J. GABAergic mechanisms of analgesia: an update. Pharmacol Biochem Behav. 1987;26(2):463-474.
56 Bouckoms AJ, Litman RE. Clonazepam in the treatment of neuralgic pain syndrome. Psychosomatics. 1985;26(12):933-936.
57 Reddy S, Patt RB. The benzodiazepines as adjuvant analgesics. J Pain Symptom Manage. 1994;9(8):510-514.
58 Kochar DK, Rawat N, Agrawal RP, et al. Sodium valproate for painful diabetic neuropathy: a randomized double-blind placebo-controlled study. QJM. 2004;97(1):33-38.
59 Drewes AM, Andreasen A, Poulsen LH. Valproate for treatment of chronic central pain after spinal cord injury. A double-blind cross-over study. Paraplegia. 1994;32(8):565-569.