Chapter 6 Adjunctive pharmacotherapy during PCI
 Given the inherent risk of thrombosis following percutaneous coronary intervention, anticoagulant pharmacotherapy is crucial to both the short- and long-term success of these procedures (Table 6.1).
Given the inherent risk of thrombosis following percutaneous coronary intervention, anticoagulant pharmacotherapy is crucial to both the short- and long-term success of these procedures (Table 6.1). All patients without documented allergy who undergo PCI should maintain or initiate aspirin therapy with daily doses between 75 and 150 mg.
All patients without documented allergy who undergo PCI should maintain or initiate aspirin therapy with daily doses between 75 and 150 mg. Patients who receive intracoronary stents should receive clopidogrel as a loading dose of 300–600 mg followed by 75 mg daily.
Patients who receive intracoronary stents should receive clopidogrel as a loading dose of 300–600 mg followed by 75 mg daily. Therapy should be continued for at least one month following deployment of bare metal stents; three months for sirolimus eluting stents and six months for paclitaxel eluting stents with consideration for longer duration therapy if affordable and well-tolerated.
Therapy should be continued for at least one month following deployment of bare metal stents; three months for sirolimus eluting stents and six months for paclitaxel eluting stents with consideration for longer duration therapy if affordable and well-tolerated. The body of clinical evidence suggests that aside from the lowest risk patients, optimal outcomes with PCI are associated with an advanced anticoagulation regimen including either bivalirudin or a heparin plus GP IIb/IIIa antagonist.
The body of clinical evidence suggests that aside from the lowest risk patients, optimal outcomes with PCI are associated with an advanced anticoagulation regimen including either bivalirudin or a heparin plus GP IIb/IIIa antagonist. GP IIb/IIIa inhibition plus a heparin is currently the preferred approach in patients with acute MI and severe ACS although results of ongoing trials with bivalirudin may demonstrate similar efficacy.
GP IIb/IIIa inhibition plus a heparin is currently the preferred approach in patients with acute MI and severe ACS although results of ongoing trials with bivalirudin may demonstrate similar efficacy. For non-emergent interventions, a bivalirudin regimen may be preferred due to reductions in bleeding risk and cost considerations.
For non-emergent interventions, a bivalirudin regimen may be preferred due to reductions in bleeding risk and cost considerations.TABLE 6.1 DOSING SUMMARY OF ADJUNCTIVE MEDICATIONS DURING PCI
| SUMMARY OF ADJUNCTIVE MEDICATIONS | |
|---|---|
| Drug | Dose |
| Antithrombin agents | |
| Unfractionated Heparin (UFH) | With GP IIb/IIIa − 60–70 U/kg IV to target ACT >200–225 sec |
| Without GP IIb/IIIa − 100U/kg IV to target ACT >300 sec | |
| LMWH (enoxaparin) | 0.75 mg/kg IV |
| Bivalirudin (Angiomax®) | 0.75 mg/kg bolus, 1.75 mg/kg·hr infusion |
| Antiplatelet agents | |
| Aspirin | 75–162 mg po qd |
| clopidogrel (Plavix®) | 300–600 mg loading dose |
| 75 mg po qd | |
| Minimum of one month for BMS, 3–6 mo for DES | |
| ticlopidine (Ticlid®) | 250 mg po bid × 2–4 weeks, optional 500 mg po loading dose |
| abciximab (ReoPro®) | 0.25 mg/kg bolus 0.125 mcg/kg·min (max 10 mcg/min) × 12 hrs post PCI |
| eptifibatide (Integrilin®) | 180 mcg/kg bolus, 2.0 mcg/kg·min infusion for 72–96 hrs |
| For PCI, second 180 mcg/kg bolus administered ten minutes after first bolus | |
| tirofiban (Aggrastat®) | 0.4 mcg/kg load × 30 minutes, then 0.1 mcg/kg·min × 48–96 hrs |
| GPIIb/IIIa – glycoprotein IIb/IIIa inhibitor | |
| U – units | |
| IV – intravenous | |
| ACT – activated clotting time | |
| BMS – bare metal stent | |
| DES – drug-eluting stent | |
| mcg – microgram | |
| kg – kilogram | |
| PCI – percutaneous coronary intervention | |
| min – minute | |
| max – maximum | |
ANTIPLATELET THERAPY
Aspirin
The primary antiplatelet mechanism of aspirin is achieved through the irreversible inactivation of cyclooxygenase through acetylation. This inhibition blocks the production of thromboxane A2, a potent platelet aggregator, which is released by platelets in response to numerous activating factors. Aspirin has been extensively evaluated in the setting of myocardial ischemia. For patients undergoing PTCA, a retrospective analysis showed aspirin to produce a robust reduction in the rate of recurrent occlusive intracoronary thrombosis. Prospective data have shown that patients receiving antiplatelet therapy, including aspirin, experience significantly fewer periprocedural ischemic events.1 Based on these and other data, aspirin has become the foundation of interventional antiplatelet therapy.
Patients undergoing percutaneous interventions are likely to be either stabilized on chronic aspirin therapy for secondary prevention in chronic coronary arterial disease or have been recently initiated through the primary treatment of an acute coronary syndrome. The typical dose of 325 mg is likely more than sufficient to completely inhibit platelet thromboxane synthesis. Research has suggested that a dose between 75 and 150 mg is as effective for chronic therapy as higher doses in reducing ischemic events with a lower risk for bleeding events.2
THIENOPYRIDINES
The thienopyridine class includes ticlopidine (Ticlid®) and clopidogrel (Plavix®) which both exert antiplatelet activity through irreversible blockade of the P2Y12 ADP receptor. Initial trials3–7 establishing the additive value of thienopyridines to aspirin were conducted with ticlopidine and demonstrated a reduction in relevant clinical endpoints when compared to warfarin-based therapy. Following its success in the CAPRIE trial,8 clopidogrel was compared to ticlopidine in a head-to-head trial of patients undergoing PCI. This CLASSICS trial9 demonstrated equivalent efficacy in the reduction of cardiac endpoints but that clopidogrel had a significantly superior safety profile with little gastrointestinal intolerance or bone marrow suppression.
Once established as the preferred thienopyridine, investigators pursued the application of clopidogrel in the acute coronary syndromes (ACS), specifically non-ST-segment elevation (NSTE) ACS in the CURE trial.10 As a substudy of CURE, PCI-CURE11 detailed the outcomes of the 2638 patients who underwent PCI following pretreatment with clopidogrel or placebo for a median treatment duration of six days. Patients in the active arm experienced a durable reduction in major adverse cardiac events from the initial 30 days through 12 months of follow-up suggesting that pretreatment with clopidogrel was responsible for the observed difference. As the management of ACS included an increasing number of patients undergoing early PCI, concerns arose about the effect of the duration and dosage of clopidogrel pre-treatment. Compared to the median clopidogrel pre-treatment of six days in PCI-CURE, the CREDO trial12 evaluated the efficacy of pre-treatment 3–24 hours prior to elective PCI. A loading dose of 300 mg more than six hours before stent deployment demonstrated a trend toward lower cardiac endpoints. The loading dose of clopidogrel in PCI has been further investigated given the frequency of early intervention in the NSTE-ACS population. The ARMYDA-2 trial13 recruited 255 patients who were treated with a loading dose of 600 mg (vs. 300 mg) administered 4–8 hours before PCI and demonstrated reduced periprocedural myocardial infarctions without an increased risk of bleeding. The ISAR-REACT14 trial also evaluated the increased 600 mg clopidogrel loading dose (given as late as two hours prior to PCI) and found no benefit of added IIb/IIIa receptor blockade in the setting of low-risk PCI.
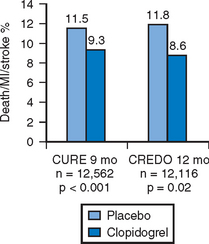
With the introduction of drug eluting stents, there was a concomitant shift in the recommended minimum duration of therapy following stent deployment. Patients who received bare metal stents typically were continued on thienopyridine therapy for a minimum of 30 days – a duration which reflected the time necessary to re-endothelialize the stented arterial segment. In addition to reducing the incidence of neointimal hyperplasia, both sirolimus and paclitaxel eluting stents delay luminal re-endothelialization to varying degrees. There is currently a Class I (Level of Evidence B) recommendation from the ACC/AHA to continue clopidogrel therapy for three months following sirolimus eluting and six months following paclitaxel eluting stents.15 Following the durable benefit seen in CURE and CREDO (Fig. 6.1), many operators advise continuation of dual antiplatelet therapy with aspirin and clopidogrel for 9–12 months or more after stent placement.
GLYCOPROTEIN IIb/IIIa INHIBITORS
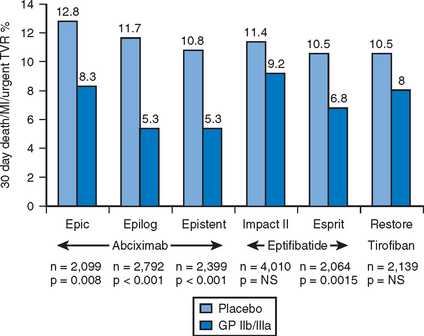
The platelet surface receptor for glycoprotein IIb/IIIa binds either fibrinogen or von Willebrand factor and is the final common pathway to platelet aggregation. There are three approved parenteral inhibitors of GPIIb/IIIa: a chimeric monoclonal antibody fragment abciximab (ReoPro®); a disulfide-linked heptapeptide eptifibatide (Integrilin®); and, a non-peptide derivative of tyrosine tirofiban (Aggrastat®). For patients undergoing PCI, the blockade of GPIIb/IIIa receptors has demonstrated clear benefit with a consistent reduction in acute ischemic events by as much as 50–60% (Fig. 6.2).
The periprocedural use of abciximab was associated with significant reductions in the 30–day rate of death, myocardial infarction or urgent target vessel revascularization for patients undergoing elective percutaneous interventions.16–18 Benefit was seen in these trials across numerous interventional techniques including balloon angioplasty, directional atherectomy and coronary stenting. From a pooled analysis, 30-day mortality trended lower by 29% and was significantly lower at six and twelve months by a similar magnitude.19 Abciximab has been broadly evaluated in the setting of percutaneous revascularization for the primary treatment for ST-segment elevation myocardial infarction (STEMI).20–24 A meta-analysis which included these 3666 patients confirmed the significant reductions in both short and long-term hard ischemic outcomes without increases in major bleeding events for patients.25 However, pretreatment with abciximab was of no benefit for patients receiving medical management of NSTE-ACS.26 The benefits of abciximab have been particularly powerful in high-risk subgroups including patients with diabetes mellitus and unstable angina. Thus, abciximab is efficacious in a variety of clinical settings for patients undergoing percutaneous intervention and confers a durable and robust reduction in important ischemic clinical endpoints including mortality.
For patients undergoing elective percutaneous revascularization, tirofiban and eptifibatide have been evaluated and at lower doses found to be ineffective at reducing ischemic outcomes27,28; only eptifibatide at higher doses was found to produce significant reductions relative to placebo, although it is not clear that the magnitude of benefit was the same as that with abciximab in a similar setting.29 As an adjunct in the management of NSTE-ACS, eptifibatide and tirofiban have been studied in three randomized trials which included over 15 000 patients.30–32 Relative risk reductions in death or myocardial infarction from these trials ranged from 8–27% and were greatest in patients who underwent early PCI. Benefits were seen in both preprocedural stabilization and reduction of ischemic events following stenting. In the low-risk patient population undergoing PCI (absence of recent MI, unstable angina, angiographic thrombus, LV ejection fraction <30%, hemodynamic instability or insulin-dependent diabetes mellitus), glycoprotein IIb/IIIa inhibition with abciximab failed to demonstrate additional benefit for patients treated with 600 mg of clopidogrel at least two hours prior to stent deployment.14
Complications of GPIIb/IIIa inhibition include bleeding and idiopathic thrombocytopenia. Bleeding complications most commonly occur at the site of vascular access and have been reduced in frequency by appropriate weight-based algorithms for both GPIIb/IIIa and antithrombin therapy during PCI. Early removal of vascular sheaths and appropriate care of cannulation sites are important measures which help reduce bleeding complications of these drugs. Life-threatening bleeding can be controlled by discontinuing drug administration and in the case of abciximab, platelet transfusion. Concern exists over the possible increased risks for patients needing to undergo CABG shortly after abciximab infusion. Analysis of trial data17,18 suggests that there was not an increase in perioperative blood loss; however, re-exploration for bleeding was more common but was balanced by a lower frequency of ischemic events. Thrombocytopenia occurs rarely with the GPIIb/IIIa inhibitors but is more frequent with abciximab than tirofiban or eptifibatide and can be extreme in both onset and magnitude
Antithrombin therapy
In addition to platelet activation, rupture of intracoronary plaque during percutaneous intervention is a potent activator of the clotting cascade and the administration of antithrombin therapy is critical to the prevention of periprocedural thrombotic complications. Compounds directed against this pathway include unfractionated heparin (UFH), low-molecular-weight heparins (LMWH) and direct thrombin inhibitors (DTI).
Heparin
Heparin exerts its antithrombotic effect by complexing with anti-thrombin and catalyzing its activity against thrombin and factor Xa. It was widely utilized to prevent abrupt vessel thrombosis during PTCA and has continued to be a mainstay of therapy during PCI. Dosing of heparin is typically monitored through the use of the activated clotting time (ACT) and through early retrospective analyses, patients undergoing PCI with an ACT above 300 seconds appeared to be associated with lower rates of ischemic complications. With the advent of more advanced pharmacologic and mechanical therapies in the catheterization lab additional analyses, have suggested that heparin dosing and ACT targets can be reduced during PCI. When used with glycoprotein IIb/IIIa receptor inhibitors, ACT has been shown to have no impact on efficacy outcomes.33 A more updated analysis confirmed the absence of effect of ACT on efficacy, but did find increases in bleeding complications with increased ACT.34 Heparin has continued as standard antithrombin therapy throughout the increasingly complex pharmacologic environment of the cardiac catheterization lab and operators in the current environment typically aim for lower range heparinization: 70–100U/kg, ACT>250 without GP IIb/IIIa blockade, 50–70 U/kg, ACT > 200 sec with GP IIb/IIIa blockade.
Low molecular weight heparins (LMWH)
Low molecular weight heparins (LMWH) are formed by the chemical or enzymatic depolymerization of UFH and have been evaluated for use in the setting of PCI. Their antithrombotic activity is mediated through a specific pentasaccharide sequence which is more specifically directed to inhibition of factor X than thrombin. Compared with UFH, LMWH are less protein bound, yielding better bioavailability, longer half-life and a more predictable anticoagulant response.35 In most settings, these benefits preclude the need for routine monitoring. Dosing of enoxaparin (Lovenox®), the most widely studied LMWH approved for use in the United States, is typically weight-based and given subcutaneously every 12 hours. In patients undergoing PCI, dosing strategy of enoxaparin was prospectively evaluated and showed that patients can safely undergo intracoronary stenting within eight hours of last enoxaparin without additional antithrombin therapy.36 For patients who undergo PCI between 8 and 12 hours after last dose, a single intravenous bolus of 0.3 mg/kg is sufficient to prevent major thrombotic events.37
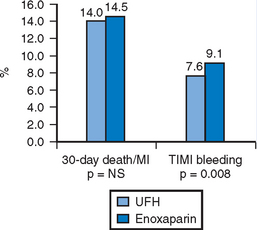
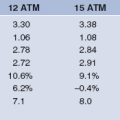
The largest experience with LMWH in the setting of PCI comes from four randomized trials comparing LMWH to UFH in NSTE-ACS.38–41 These large trials complimented small registry data42 which suggested LMWH was as effective as UFH in the setting of PCI. PCI rates in these prospective trials ranged from 15–47% and the total population included over 20 000 patients. The largest trial to be reported was SYNERGY representing almost half the total population and recruited high-risk patients with NSTE-ACS who were to be managed with an early invasive strategy. There was no significant difference in the incidence of 30-day death or MI between the groups treated with enoxaparin or UFH. Enoxaparin did meet criteria for non-inferiority to UFH, but was associated with a significant excess risk of major bleeding (Fig. 6.3). Additional analyses from this trial of a modern ACS paradigm suggest patients who undergo a change in antithrombin therapy choice have worse outcomes compared to those who remain on consistent therapy.
Direct thrombin inhibitors (DTIs)
Thrombin (factor II) plays an integral role in the formation of clot within the damaged vessel. In comparison to the heparins, DTIs are active on both free and clot-bound thrombin, are less protein bound and are less susceptible to circulating inhibitors.43 The direct thrombin inhibitors hirudin (Lepirudin®), a peptide derived from the saliva of the medicinal leech, and bivalirudin (Angiomax®), a peptide analog of hirudin, have been developed and tested for the prevention of thrombosis in several settings including PCI. Following early lessons from investigations of the use of DTIs in ACS44, bivalirudin was approved as a substitute for UFH during PCI. Regulatory approval of this agent was based on trial data from patients undergoing balloon angioplasty for unstable angina45, where bivalirudin was shown to provide somewhat better protection against ischemic events and markedly reduce bleeding complications compared with UFH.
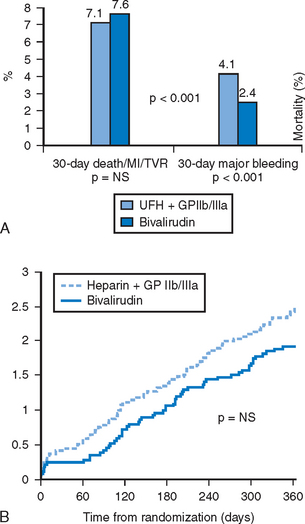
With the advent of newer pharmacologic and percutaneous therapies, bivalirudin was re-evaluated in an updated treatment strategy to include the use of thienopyridines, GPIIb/IIIa inhibitors and intracoronary stents.46 The trial tested the hypothesis that bivalirudin as alternate antithrombin therapy would preclude the need for routine GPIIb/IIIa blockade typically employed with UFH. Patients were randomized to receive a weight-based strategy of bivalirudin and provisional GPIIb/IIIa inhibitor or heparin with planned GPIIb/IIIa inhibitor. The bivalirudin regimen, with only 7.2% of patients requiring provisional GP IIb/IIIa blockade, was associated with similarly low rates of ischemic complications as was heparin with routine use of a GP IIb/IIIa inhibitor. Major bleeding rates were significantly reduced by 41% in patients randomized to bivalirudin when compared to the heparin group. No differences in mortality were observed by 1-year; however, there was a trend that favored bivalirudin therapy (Fig. 6.4A and B). These data confirmed the effectiveness and safety of bivalirudin with discrete use of GPIIb/IIIa blockade as an effective strategy for reducing thrombotic complications of PCI. Patients with high-risk ACS or acute MI were not tested in REPLACE-2, and are under evaluation in other dedicated trials.
1 Schwartz L, Bourassa MG, Lesperance J, et al. Aspirin and dipyridamole in the prevention of restenosis after percutaneous transluminal coronary angioplasty. N Engl J Med. 1988;318:1714-1719.
2 Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. Br Med J. 2002;324:71-86.
3 Leon MB, Baim DS, Popma JJ, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med. 1998;339:1665-1671.
4 Urban P, Macaya C, Rupprecht HJ, et al. Randomized evaluation of anticoagulation versus antiplatelet therapy after coronary stent implantation in high-risk patients: the multicenter aspirin and ticlopidine trial after intracoronary stenting (MATTIS). Circulation. 1998;98:2126-2132.
5 Schuhlen H, Hadamitzky M, Walter H, Ulm K, Schomig A. Major benefit from antiplatelet therapy for patients at high risk for adverse cardiac events after coronary Palmaz-Schatz stent placement: analysis of a prospective risk stratification protocol in the Intracoronary Stenting and Antithrombotic Regimen (ISAR) trial. Circulation. 1997;95:2015-2021.
6 Schomig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med. 1996;334:1084-1089.
7 Bertrand ME, Legrand V, Boland J, et al. Randomized multicenter comparison of conventional anticoagulation versus antiplatelet therapy in unplanned and elective coronary stenting. The full anticoagulation versus aspirin and ticlopidine (fantastic) study. Circulation. 1998;98:1597-1603.
8 A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348:1329-1339.
9 Bertrand ME, Rupprecht HJ, Urban P, Gershlick AH, Investigators FT. Double-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting: the clopidogrel aspirin stent international cooperative study (CLASSICS). Circulation. 2000;102:624-629.
10 Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494-502.
11 Berger PB, Steinhubl S. Clinical implications of percutaneous coronary intervention-clopidogrel in unstable angina to prevent recurrent events (PCI-CURE) study: a US perspective. Circulation. 2002;106:2284-2287.
12 Steinhubl SR, Berger PB, Mann JT3rd, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288:2411-2420.
13 Patti G, Colonna G, Pasceri V, Pepe LL, Montinaro A, Di Sciascio G. Randomized trial of high loading dose of clopidogrel for reduction of periprocedural myocardial infarction in patients undergoing coronary intervention: results from the ARMYDA-2 (Antiplatelet therapy for Reduction of Myocardial Damage during Angioplasty) study. Circulation. 2005;111:2099-2106.
14 Kastrati A, Mehilli J, Schuhlen H, et al. A clinical trial of abciximab in elective percutaneous coronary intervention after pretreatment with clopidogrel. N Engl J Med. 2004;350:232-238.
15 Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction—executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction). J Am Coll Cardiol. 2004;44:671-719.
16 Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high-risk coronary angioplasty. The EPIC Investigation. N Engl J Med. 1994;330:956-961.
17 Platelet glycoprotein IIb/IIIa receptor blockade and low-dose heparin during percutaneous coronary revascularization. The EPILOG Investigators. N Engl J Med. 1997;336:1689-1696.
18 Randomised placebo-controlled and balloon-angioplasty-controlled trial to assess safety of coronary stenting with use of platelet glycoprotein-IIb/IIIa blockade. The EPISTENT Investigators. Evaluation of Platelet IIb/IIIa Inhibitor for Stenting. Lancet. 1998;352:87-92.
19 Kereiakes DJ, Lincoff AM, Anderson KM, et al. Abciximab survival advantage following percutaneous coronary intervention is predicted by clinical risk profile. Am J Cardiol. 2002;90:628-630.
20 Brener SJ, Barr LA, Burchenal JE, et al. Randomized, placebo-controlled trial of platelet glycoprotein IIb/IIIa blockade with primary angioplasty for acute myocardial infarction. ReoPro and Primary PTCA Organization and Randomized Trial (RAPPORT) Investigators. Circulation. 1998;98:734-741.
21 Montalescot G, Barragan P, Wittenberg O, et al. Platelet glycoprotein IIb/IIIa inhibition with coronary stenting for acute myocardial infarction. N Engl J Med. 2001;344:1895-1903.
22 Neumann FJ, Kastrati A, Schmitt C, et al. Effect of glycoprotein IIb/IIIa receptor blockade with abciximab on clinical and angiographic restenosis rate after the placement of coronary stents following acute myocardial infarction. J Am Coll Cardiol. 2000;35:915-921.
23 Antoniucci D, Rodriguez A, Hempel A, et al. A randomized trial comparing primary infarct artery stenting with or without abciximab in acute myocardial infarction. J Am Coll Cardiol. 2003;42:1879-1885.
24 Stone GW, Grines CL, Cox DA, et al. Comparison of angioplasty with stenting, with or without abciximab, in acute myocardial infarction. N Engl J Med. 2002;346:957-966.
25 De Luca G, Suryapranata H, Stone GW, et al. Abciximab as adjunctive therapy to reperfusion in acute ST-segment elevation myocardial infarction. JAMA. 2005;293:1759-1765.
26 Simoons ML. Effect of glycoprotein IIb/IIIa receptor blocker abciximab on outcome in patients with acute coronary syndromes without early coronary revascularisation: the GUSTO IV-ACS randomised trial. Lancet. 2001;357:1915-1924.
27 Randomised placebo-controlled trial of effect of eptifibatide on complications of percutaneous coronary intervention: IMPACT-II. Integrilin to Minimise Platelet Aggregation and Coronary Thrombosis-II. Lancet. 1997;349:1422-1428.
28 Effects of platelet glycoprotein IIb/IIIa blockade with tirofiban on adverse cardiac events in patients with unstable angina or acute myocardial infarction undergoing coronary angioplasty. The RESTORE Investigators. Randomized Efficacy Study of Tirofiban for Outcomes and REstenosis. Circulation. 1997;96:1445-1453.
29 Novel dosing regimen of eptifibatide in planned coronary stent implantation (ESPRIT): a randomised, placebo-controlled trial. Lancet. 2000;356:2037-2044.
30 Inhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromes. The PURSUIT Trial Investigators. Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy. N Engl J Med. 1998;339:436-443.
31 A comparison of aspirin plus tirofiban with aspirin plus heparin for unstable angina. Platelet Receptor Inhibition in Ischemic Syndrome Management (PRISM) Study Investigators. N Engl J Med. 1998;338:1498-1505.
32 Inhibition of the platelet glycoprotein IIb/IIIa receptor with tirofiban in unstable angina and non-Q-wave myocardial infarction. Platelet Receptor Inhibition in Ischemic Syndrome Management in Patients Limited by Unstable Signs and Symptoms (PRISM-PLUS) Study Investigators. N Engl J Med. 1998;338:1488-1497.
33 Chew DP, Bhatt DL, Lincoff AM, et al. Defining the optimal activated clotting time during percutaneous coronary intervention: aggregate results from 6 randomized, controlled trials. Circulation. 2001;103:961-966.
34 Brener SJ, Moliterno DJ, Lincoff AM, Steinhubl SR, Wolski KE, Topol EJ. Relationship between activated clotting time and ischemic or hemorrhagic complications: analysis of 4 recent randomized clinical trials of percutaneous coronary intervention. Circulation. 2004;110:994-998.
35 Weitz JI. Low-molecular-weight heparins. N Engl J Med. 1997;337:688-698.
36 Martin JL, Fry ET, Sanderink GJ, et al. Reliable anticoagulation with enoxaparin in patients undergoing percutaneous coronary intervention: The pharmacokinetics of enoxaparin in PCI (PEPCI) study. Catheter Cardiovasc Interv. 2004;61:163-170.
37 Collet JP, Montalescot G, Lison L, et al. Percutaneous coronary intervention after subcutaneous enoxaparin pretreatment in patients with unstable angina pectoris. Circulation. 2001;103:658-663.
38 Cohen M, Demers C, Gurfinkel EP, et al. A comparison of low-molecular-weight heparin with unfractionated heparin for unstable coronary artery disease. Efficacy and Safety of Subcutaneous Enoxaparin in Non-Q-Wave Coronary Events Study Group. N Engl J Med. 1997;337:447-452.
39 Antman EM, McCabe CH, Gurfinkel EP, et al. Enoxaparin prevents death and cardiac ischemic events in unstable angina/non-Q-wave myocardial infarction. Results of the thrombolysis in myocardial infarction (TIMI) 11B trial. Circulation. 1999;100:1593-1601.
40 Ferguson JJ, Califf RM, Antman EM, et al. Enoxaparin vs unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndromes managed with an intended early invasive strategy: primary results of the SYNERGY randomized trial. JAMA. 2004;292:45-54.
41 Blazing MA, de Lemos JA, White HD, et al. Safety and efficacy of enoxaparin vs unfractionated heparin in patients with non-ST-segment elevation acute coronary syndromes who receive tirofiban and aspirin: a randomized controlled trial. JAMA. 2004;292:55-64.
42 Kereiakes DJ, Grines C, Fry E, et al. Enoxaparin and abciximab adjunctive pharmacotherapy during percutaneous coronary intervention. J Invasive Cardiol. 2001;13:272-278.
43 Lefkovits J, Topol EJ. Direct thrombin inhibitors in cardiovascular medicine. Circulation. 1994;90:1522-1536.
44 Direct thrombin inhibitors in acute coronary syndromes: principal results of a meta-analysis based on individual patients’ data. Lancet. 2002;359:294-302.
45 Bittl JA, Chaitman BR, Feit F, Kimball W, Topol EJ. Bivalirudin versus heparin during coronary angioplasty for unstable or postinfarction angina: Final report reanalysis of the Bivalirudin Angioplasty Study. Am Heart J. 2001;142:952-959.
46 Lincoff AM, Bittl JA, Harrington RA, et al. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA. 2003;289:853-863.