Chapter 48 Acute Relapsing Pancreatitis
Introduction
Acute pancreatitis is caused by acute or chronic alcohol intake or cholelithiasis in 80% of cases.1,2 In the absence of alcohol or gallstones, numerous established and putative etiologies must be considered, any one of which can cause recurrent attacks of acute pancreatitis.3 In instances in which the underlying etiology eludes detection and leads to a second attack, the term acute relapsing pancreatitis (ARP) is applied. Table 48.1 lists the etiologies of ARP categorized by entities typically managed medically versus entities that respond to endoscopic therapy to prevent recurrences. This chapter focuses on the etiologies of ARP responding to endoscopic therapy. We also provide a brief update on the newly discovered and expanding body of knowledge of genetic causes of ARP, autoimmune ARP, and celiac-associated ARP. Endoscopic management for the genetic conditions is usually reserved for complications that develop from chronic pancreatitis (CP) and is discussed in Chapter 49. For a summary of all etiologies of ARP, readers are directed to the comprehensive review of Somogyi and coworkers.4
Table 48.1 Putative Etiology of Acute Relapsing Pancreatitis
| Medical Management | Endoscopic or Surgical Management |
|---|---|
| Alcohol | Annular pancreas |
| Autoimmune | Biliary stones or microlithiasis |
| Celiac disease | Choledochocyst and choledochocele |
| Drug-induced | Pancreas divisum |
| Genetic | Pancreatic and ampullary neoplasms |
| Hereditary pancreatitis CFTR mutations SPINK mutations Tropical pancreatitis | Periampullary diverticulum |
| Sphincter of Oddi dysfunction | |
| Hypercalcemia | |
| Hyperlipidemia | |
| Infectious | |
| Vascular |
An initial complete history and physical examination, routine blood work including liver function tests and corrected or ionized calcium and triglyceride levels, and transabdominal ultrasound or computed tomography (CT) scan reveal an etiology in 70% to 90% of cases of pancreatitis.2,5–8 In younger patients (<40 years old), transabdominal ultrasound may suffice, but in older patients, a CT scan of the abdomen is advised because a pancreatic or ampullary neoplasm may manifest with ARP.9,10 Without an adequate initial work-up and directed therapy, more than half of patients with an initial attack of acute pancreatitis experience recurrent attacks or develop CP.5,11 In patients with gallstone pancreatitis, treatment of the index attack and prevention of further attacks may involve endoscopic sphincterotomy (ES) with bile duct stone extraction and laparoscopic cholecystectomy to remove the stone reservoir.6,7 Patients who remain untreated have a 33% to 66% chance of a recurrent attack.8,11–13
Work-up of Acute Relapsing Pancreatitis
After an initial unrevealing evaluation for acute pancreatitis, ERCP reveals an etiology in approximately 70% of patients.5,14 Although some experts advocate performing ERCP in all patients after a single attack of pancreatitis, most agree that ERCP is warranted only after a severe attack in which the etiology is not obvious or after recurrent attacks.15,16 The utility of this test lies in its unique ability to diagnose and treat biliary microlithiasis, sphincter of Oddi dysfunction (SOD), and pancreas divisum, the most commonly encountered diagnoses in the work-up of ARP. Less often, pancreatic and ampullary cancers; duodenal diverticulum; pancreatic duct strictures or stones; and congenital malformations such as choledochocele, annular pancreas, and anomalous pancreaticobiliary junction may be encountered. ERCP is risky because it causes acute pancreatitis in 3% to 20% of patients17,18 depending on the indication and maneuver performed. Acute pancreatitis is more frequent when ERCP is performed for diagnostic purposes, particularly when coupled with treatment of SOD, compared with other indications, most notably bile duct stones.19 Other risk factors include multiple or high-pressure injections of contrast agent into the pancreatic duct, therapeutic intervention, a past history of pancreatitis, and operator inexperience.20 In a referral center treating 279 patients with acute pancreatitis over a 5-year period, ERCP was the causal factor in 4% of cases.21 However, 3 of 11 patients in the subgroup with ERCP-related pancreatitis died.
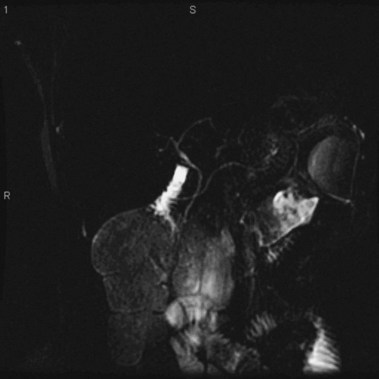
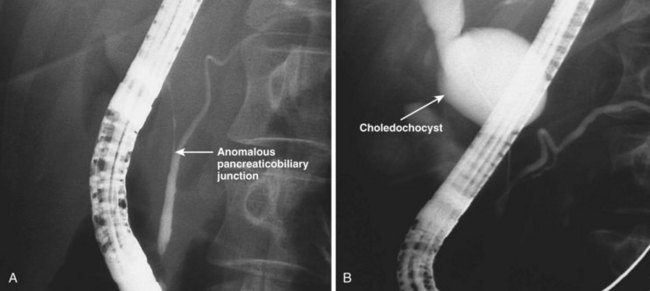
MRCP is replacing diagnostic ERCP in many centers because of ERCP-related complications.22 With heavily T2-weighted images, fluid within the bile and pancreatic ducts produces an image akin to an endoscopically generated cholangiopancreatogram. MRCP is accurate in detecting common bile duct stones23,24; its role in the evaluation of ARP includes the identification of anatomic abnormalities such as pancreas divisum (Fig. 48.1), choledochocysts, annular pancreas, and anomalous pancreaticobiliary junction.24–27 However, ERCP continues to be used in the diagnostic evaluation of ARP because of the ability to visualize the ampulla, to sample tissue and bile, and to perform sphincter of Oddi manometry.
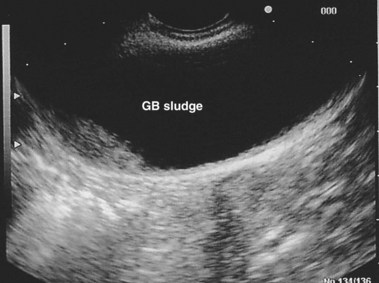
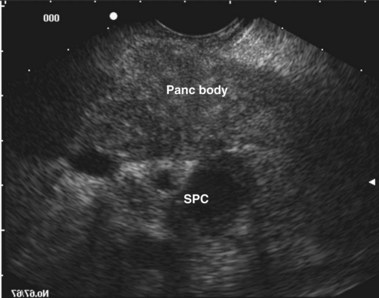
EUS uses higher frequencies than conventional abdominal ultrasound, and the image quality is not compromised by intestinal gas, providing a higher sensitivity and specificity for detecting cholelithiasis than conventional ultrasound.28–30 It has been shown to be as accurate as ERCP in the diagnosis of choledocholithiasis,31 and the positive predictive value for biliary tract disease including microlithiasis in the gallbladder and biliary sludge (Fig. 48.2)32,33 is about 98%.14 It remains the endoscopic procedure of choice for visualizing the pancreas,34–38 and it is the most accurate technique for the detection and local staging of pancreatic carcinoma.39,40 EUS is also useful in detecting changes in the pancreatic parenchyma and ducts.38,39
In patients with ARP, EUS correctly identified a cause of acute pancreatitis in 155 of 168 patients in whom a cause was found by a multidisciplinary diagnostic approach, involving ERCP, bile crystal analysis, surgery, and medical follow-up. EUS may also be useful in the detection of pancreas divisum,41 SOD,42 and anomalous pancreaticobiliary junction,43 but more data are required before the performance characteristics of EUS for these diagnoses is known. Given the high yield and lower complication rate compared with ERCP, EUS is being used earlier in the work-up of ARP. However, a consensus has not been reached so far regarding the exact place for EUS in the diagnostic algorithm for ARP. Increasingly, EUS evaluation of the pancreas, ampulla, and biliary system is being used to detect potentially diagnosable and treatable causes of ARP before use of ERCP.
Pancreas Divisum
Introduction
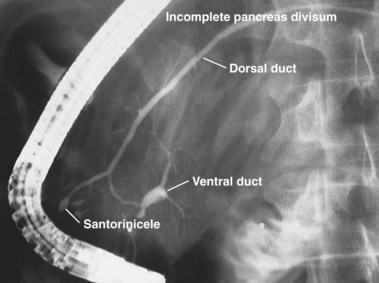
The term pancreas divisum refers to two pancreatic ductal systems that do not unite during embryologic organogenesis and drain separately via the two duodenal papillae—the dominant dorsal system through the minor papilla and the smaller ventral system through the major papilla (see Fig. 48.1). Incomplete pancreas divisum is a threadlike communication between dorsal and ventral pancreatic ducts and, when symptomatic, is treated similar to complete pancreas divisum (Fig. 48.3).
Epidemiology
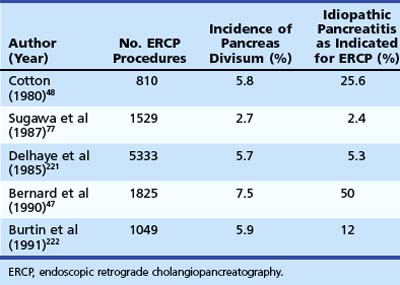
Pancreas divisum is the most common congenital anomaly of the pancreas and may be found in 7% to 14% of autopsy series.44–46 The frequency of pancreas divisum among ERCP series varies greatly (2.7% to 7.5%) and depends on the population studied and the diligence with which complete pancreatography is pursued. Pancreas divisum is reported to occur less often in Asians (1% to 2%)47 and blacks (2%).48 The clinical significance of pancreas divisum is controversial. Although estimates reveal that less than 5% of the population with pancreas divisum ever develops pancreatic symptoms, authorities recognize its association with ARP, CP, and abdominal pain.49–51 Patients undergoing pancreatography for documented pancreatitis are substantially more likely to have pancreas divisum than patients who have incidental pancreatograms during ERCP or for unexplained chronic abdominal pain.52 Table 48.2 summarizes the larger ERCP series of patients with pancreas divisum and pancreatitis. Caution should be exercised when assigning causation to pancreas divisum in patients with ARP given its prevalence in the population, and other known causes of pancreatitis should be sought for and excluded.
Pathogenesis
Because most exocrine flow is routed through the minor papilla in this ductal anomaly, it is hypothesized that in some patients an increased resistance to flow across this small orifice results in dorsal duct hypertension and clinical symptoms.53–56 The resulting increased dorsal duct pressure may also make the pancreas more prone to injury from alcohol and drugs.57,58 Surgical and endoscopic procedures aimed at decreasing resistance to flow across the minor papilla have been reported with varying success. Another mechanism by which pancreas divisum may lead to ARP is a decrease in the cystic fibrosis transmembrane conductance regulator protein (CFTR) function.59 The association between loss of CFTR function and ARP is discussed later in the section on genetic causes.
Diagnosis
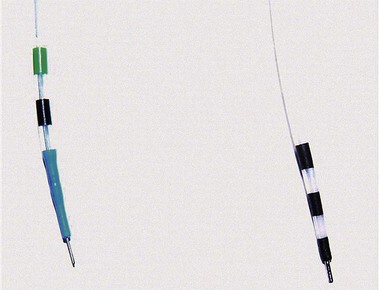
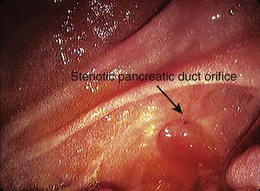
ERCP remains the “gold standard” for diagnosing pancreas divisum. A characteristic ventral pancreatogram with an attenuated duct that arborizes strongly suggests pancreas divisum. Caution must be exercised in patients with an obstructed pancreatic duct that may look like pancreas divisum. Pancreas divisum is confirmed by locating the minor papilla and injecting contrast agent into the dorsal duct (see Video). The minor papilla is usually located 2 cm proximal and 2 cm medial to the major papilla. It is best seen with the duodenoscope passed in a long position, without reducing the loop along the greater curve of the stomach. Occasionally, the minor papilla cannot be identified. The use of intravenous secretin (0.2 mcg/kg) during ERCP may stimulate the exocrine pancreas and help identify the minor papilla as a point origin of clear liquid “squirting” into the duodenum.60 The prior application of methylene blue to the periampullary mucosa before intravenous secretin has been suggested to assist in the identification of the minor papilla.61 Cannulation of the papilla may be difficult, and various tapered or metal-tipped catheters and papillotomes have been developed (Fig. 48.4). The success of minor papilla cannulation is optimized when the diagnosis is predetermined; this calls for noninvasive diagnostic modalities to avoid repeat ERCP. MRCP is best suited for this purpose. The use of intravenous secretin improves pancreatic duct visualization during MRCP.62,63 The diagnosis of pancreas divisum may be made by CT (Fig. 48.5) or EUS, but the accuracy of diagnosis of these modalities is unknown.
Clinical Presentation and Treatment
The earliest attempts at treatment for patients with presumed symptomatic pancreas divisum were surgical. The surgical procedures first performed to reduce resistance to exocrine flow consisted of a transduodenal minor and major sphincteroplasty with cholecystectomy. More recently, a transduodenal minor sphincterotomy or sphincteroplasty alone64 has evolved as the surgical treatment of choice. The clinical presentation of ARP, the presence of minor papilla stenosis either intraoperatively or by delayed clearance of dye after dorsal ductography during ERCP, and a positive ultrasound secretin test are the best predictors of outcome after surgical intervention for pancreas divisum.65
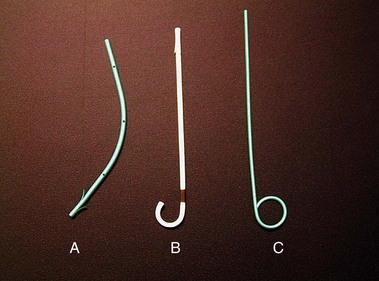
To avoid laparotomy, numerous endoscopic maneuvers have been applied for the management of symptoms related to pancreas divisum. These have included minor papilla dilation, stent placement, and sphincterotomy. The technique of minor papilla stent placement involves free selective cannulation of the dorsal duct, placement of a guidewire, and advancement of a stent specifically designed for use in the pancreas over the guidewire with a pushing cannula (see Video). These stents are plastic and vary in diameter from 3-Fr to 10-Fr (Fig. 48.6). Larger stents (7-Fr and 10-Fr) are reserved for patients with dilated pancreatic ducts or CP. Pancreatic stents 5-Fr and larger have multiple side holes to permit drainage from side branches and have external flanges or pigtails to prevent inward migration. Some stents have internal barbs to prevent outward migration. The number and need for multiple stent exchanges have not been firmly established; the interval necessary for stent exchanges likewise has not been established, although most authors advocate 4 to 8 weeks for stents 5-Fr to 7-Fr in diameter.
Minor papilla dilation may be accomplished with catheter dilators or hydrostatic balloons. Catheter dilators generally vary in diameter from 5-Fr to 10-Fr and are advanced into the dorsal ducts over a preplaced wire. Although balloons vary in diameter, for the sole purpose of dilating the minor papilla, 4-mm over-the-wire hydrostatic balloons are the smallest commercially available devices. Larger balloons should be used with caution, unless concurrent CP exists with need for additional therapy (e.g., stone extraction or stricture dilation; see Chapter 49).
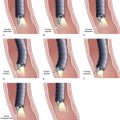
Two techniques have been described for minor papilla sphincterotomy: standard traction papillotomy (see Video) and needle-knife sphincterotomy over a stent. Traction papillotomes with braided or monofilament cutting wires can be used to accomplish traction papillotomy. The papillotome tip in the pancreatic duct usually directs the wire in a 12 o’clock to 2 o’clock direction; the length of the cut depends on the size of the minor papilla mound, usually about 5 mm. Some authors advocate the use of pure cutting current to prevent cautery to the pancreatic parenchyma with resulting stenosis of the outflow tract. It is generally advisable to leave a temporary pancreatic stent, which has been shown to decrease the risk of postprocedure pancreatitis for pancreatic sphincterotomy of the major papilla.66,67 The needle-knife technique involves placing a pancreatic stent into the dorsal duct and using it as a guide to create an approximately 5-mm cut over the stent. The stent may be left in place temporarily to reduce the risk of post-ERCP pancreatitis. Minor papilla dilation alone has not been studied in a controlled fashion but has been used in combination with stent placement.
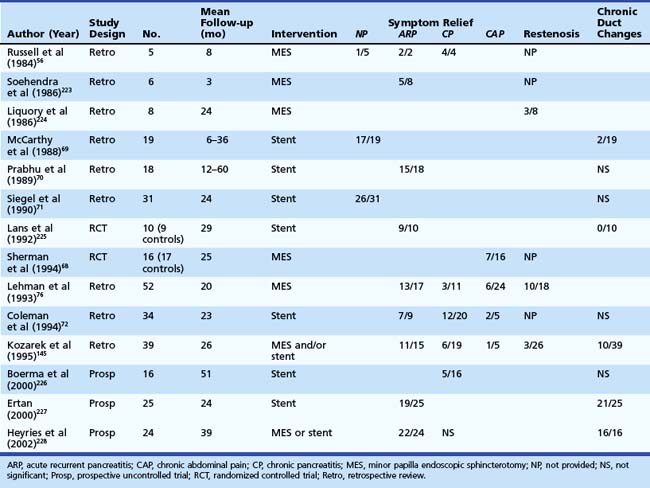
Categorizing patients with symptomatic pancreas divisum may help decide management and predict outcome (Table 48.3). Two prospective, randomized, controlled trials have evaluated endoscopic therapy for patients with pancreas divisum. One trial looked at the role of stent placement in the minor papilla in patients with pancreas divisum and ARP and reported 90% success over a mean 29-month period.64 The second trial evaluated the role of minor papillotomy in treating patients with pancreas divisum and chronic abdominal pain and reported symptomatic relief in 44% of patients.68 Studies looking at dorsal duct stent placement and minor papillotomy summarized in Table 48.369–72 suggest symptomatic improvement or resolution of ARP in 70% to 80% of patients with pancreas divisum; however, these studies are limited by heterogeneous study populations, varied follow-up, and lack of controls. The response in the setting of CP is less satisfactory and, for chronic abdominal pain, suboptimal and ill-advised in our opinion.
Regarding the choice of therapy, most authorities now favor minor papillotomy over dorsal duct stent placement. The overall success rate of endoscopic therapy (stent placement, dilation, or sphincterotomy) is similar to the results of surgical sphincteroplasty. The restenosis rate in the surgical literature appears to be less than for endoscopic minor papillotomy, although reports suggesting that patients who have restenosis after ES also have restenosis after sphincteroplasty.73 Endoscopic techniques seem preferable as a first choice because laparotomy can be avoided. The short-term success rates of minor papilla ES and surgical sphincteroplasty may be similar, but long-term follow-up and comparative trials are needed before firmer recommendations regarding procedure choice and cost-effectiveness can be made.
Complications
Complications of stent therapy include acute pancreatitis; the induction of pancreatic ductal changes, many of which may be irreversible74; stent occlusion or migration; pancreatic duct perforation; and the need for repeated procedures. Complications of minor papilla ES, including bleeding, perforation, and pancreatitis, have been reported and are similar to complications of major papilla ES.75 Lehman and coworkers76 reported a 15% procedural complication rate for minor papilla ES, primarily mild pancreatitis. Frequency of restenosis is reported to be 5% to 10%.77
Biliary Microlithiasis
Introduction
Many terms have been used interchangeably for biliary microlithiasis including biliary sludge and biliary sand. The term biliary microlithiasis typically refers to finding cholesterol monohydrate crystals and calcium bilirubinate granules on light microscopy of an endoscopically acquired centrifuged sample of bile.78 The criteria for differentiating between biliary microliths and small stones are unclear, but generally a gallstone is defined as a particle with a diameter greater than 2 to 3 mm that cannot be crushed by digital compression.78 Biliary sludge is the ultrasound finding of low-level echoes without acoustic shadowing that gravitate toward the dependent portion in the gallbladder79 and move with positioning. Biliary sludge consists of cholesterol monohydrate crystals and calcium bilirubinate granules suspended in gallbladder mucus. Other calcium salts, proteins, and xenobiotics such as ceftriaxone can also be found.80
Epidemiology
Similar to gallstones, the risk of developing biliary microlithiasis is increased in women and in several conditions including pregnancy,81,82 rapid weight loss,83 critical illness,84 prolonged fasting,85 long-term administration of total parenteral nutrition,85–88 ceftriaxone86–91 or octreotide administration,92–94 and bone marrow or solid organ transplantation.95–99 The development of ARP in these clinical situations should prompt an aggressive search for microlithiasis (approximately 31%). Approximately 31% of patients with nonalcoholic pancreatitis have biliary microlithiasis, and 74% of patients with “idiopathic” pancreatitis have been shown to have biliary microlithiasis.11,100 Two prospective studies of consecutive patients with apparently idiopathic pancreatitis found that two-thirds to three-fourths had microlithiasis as the presumed cause, as documented by biliary drainage studies, follow-up ultrasound studies, and ERCP with sphincterotomy or cholecystectomy.11,100
Pathogenesis
The clinical significance of biliary microlithiasis is very controversial. Experts remain divided over whether it is a transient phenomenon or a precursor to gallstones. After chemical dissolution of gallstones, gallbladder sludge is usually seen on ultrasound before gallstone recurrence,101 suggesting that the pathogenesis of sludge is similar to that of gallstones.102–106 Sludge resolves spontaneously in most cases, and gallstones form in only a few individuals with sludge. There are few studies of the pathogenesis and natural history of biliary sludge, and most are limited by insufficient follow-up.101,107,108 Three clinical outcomes were noted in one study, including complete resolution, a waxing and waning course, and gallstone formation.101 Sludge found in patients with abdominal pain seems to disappear spontaneously in about 50% of cases. Asymptomatic persistence is seen in about 20% of cases over 3 years, and symptoms may develop in 10% to 15% of patients. Gallstones develop in 5% to 15%.109
Further support for the role of biliary microlithiasis in producing pancreaticobiliary symptoms comes from the observations that symptomatic patients with gallstones receiving ursodeoxycholic acid had resolution of symptoms in 3 months, although the number and size of gallstones remained unchanged.110 The supposition is that the treatment resolved concurrent biliary microlithiasis, which led to the symptoms. Asymptomatic patients with gallstones who receive shock wave lithotripsy have been reported to develop biliary colic, cholecystitis, or acute pancreatitis.111–115 In this situation, the therapy may have created sludge, which produced the symptoms.
Hypothetically, microlithiasis can lead to pancreatitis through numerous mechanisms. Small stones may transiently impact at the papilla leading to pancreatic duct obstruction and pancreatitis.116 Recurrent passage of stones may lead to papillary stenosis or SOD, both of which are associated with pancreatitis.117
Diagnosis
The diagnosis of biliary sludge is confirmed by ultrasound. The sensitivity of transabdominal ultrasound for sludge is approximately 55%, whereas the sensitivity of EUS is approximately 96% (see Fig. 48.2), compared with duodenal bile collection (67%).118,119 Although clinically less applicable, microscopic examination of gallbladder contents is considered the diagnostic “gold standard” and allows the chemical composition of sludge to be defined. The sensitivity is 83% when bile is obtained directly from the common bile duct during ERCP.120 Bile sampling is indicated only if less invasive studies are negative, the clinical suspicion for microlithiasis is high, and the results would guide management. Techniques vary as to the site of bile collection, cholecystokinin use, sample processing, and criteria for a positive test. The relationship between the quantity of crystals and the clinical outcome remains unproved.
Treatment
Numerous therapeutic options are available to treat symptomatic biliary microlithiasis and include cholecystectomy, ES, and chemical dissolution. The benefit of therapy has been shown by the significant decrease in recurrent episodes of pancreatitis after therapy (<10%) versus a recurrence rate of approximately 66% to 75% in untreated patients.11,121–126
Laparoscopic cholecystectomy offers definitive therapy11,78 and is indicated in good operative candidates of almost any age. Biliary ES is an effective alternative in very elderly patients or patients with significant comorbid illness. The benefits of sphincter ablation124–130 include enhanced gallbladder motility and reduced stasis, which may persist for years.131 Biliary ES also reduces bile lithogenicity by modifying the bile composition.
Although there is no demonstrable difference in the clinical benefit of cholecystectomy or biliary ES for microlithiasis-induced pancreatitis, the safety profile of laparoscopic cholecystectomy outweighs the immediate and late complications of biliary ES for an indication of ARP in most clinical situations. Biliary ES may be used as a temporizing measure in patients who have severe acute pancreatitis secondary to microlithiasis in whom cholecystectomy must be deferred for weeks or months. Few studies have examined ursodeoxycholic acid for the treatment of biliary sludge. In patients with rapid weight loss, ursodeoxycholic acid has been shown to decrease the incidence of gallstones by 50% to 100%.132,133 In patients with idiopathic pancreatitis and sludge,11 maintenance therapy after initial treatment with ursodeoxycholic acid to dissolve cholesterol crystals successfully prevents the recurrence of sludge and pancreatitis. This form of therapy is a reasonable alternative in poor operative candidates and elderly patients.
Sphincter of Oddi Dysfunction
A detailed description of SOD is provided in Chapter 46. Direct evidence of the involvement of the sphincter of Oddi in the pathogenesis of pancreatitis is lacking. However, experts recognize SOD as a condition that may be associated with ARP. Using probes, surgeons have documented narrowing of the sphincter of Oddi in patients undergoing surgery for ARP. Secretin-stimulated ultrasound studies of the pancreatic duct in patients with SOD have revealed prolonged dilation of the pancreatic duct compared with controls, which is associated with a good outcome after surgical sphincteroplasty.134 The morphine-Prostigmin test has been used to show an association between SOD and abdominal pain associated with pancreatitis. Likewise, manometric studies have shown an association between SOD and a proportion of patients with ARP, most commonly an abnormally increased sphincter of Oddi basal pressure135,136; intuitively, surgeons and endoscopists have devised treatment strategies aimed at relieving obstruction at the sphincter of Oddi.
Earlier surgical literature was characterized by inhomogeneous patient populations and mixed results.137,138 More recent literature using sphincter of Oddi manometry to guide selection of patients with ARP for open sphincteroplasty and septoplasty reveals cessation of attacks in greater than 90% of patients with manometric stenosis; however, in patients with ARP, manometric evidence of SOD varies widely. SOD can involve either the biliary or the pancreatic segment or both. Pancreatic SOD has been implicated in causing recurrent pancreatitis in patients with prior biliary sphincter ablation.139,140 More recent literature reveals that pancreatic SOD often coexists with biliary SOD; many centers recommend documentation and ablation of both conditions.141,142
Pancreatic sphincterotomy of the major papilla uses the same techniques as described previously for the minor papilla (see Video). Whether concomitant biliary sphincterotomy is required is controversial and currently under investigation. Leaving a temporary pancreatic stent in place after biliary sphincterotomy alone for SOD or after pancreatic sphincterotomy reduces the incidence and severity of postsphincterotomy pancreatitis.67,143 Most data regarding SOD have evolved from studies of the biliary segment and therapy directed at it. Assumptions regarding manipulation of treatment directed at the pancreatic duct based on such data can be misleading and potentially dangerous. Response of the pancreatic duct to endoscopic stent placement has been used either to treat patients or to select patients for surgery.144,145 However, long-term outcomes are lacking. In a randomized controlled trial, sphincter of Oddi basal pressure in the biliary tree has been shown to predict who will respond to biliary sphincterotomy among patients with type II biliary SOD.146 Corresponding studies for pancreatic duct manometry have not been performed in ARP; the ability of pancreatic duct manometry to discriminate between responders and nonresponders to pancreatic duct sphincterotomy among patients with ARP and pancreatic duct SOD has never been shown.
Circumstantial evidence suggests a higher incidence of post-ERCP pancreatitis in patients with ARP undergoing ERCP and sphincter of Oddi manometry.147 Patients with ARP and a dilated pancreatic duct in the absence of morphologic criteria for CP (type 1 SOD) are considered to have stenosis of the pancreatic sphincter (Fig. 48.7),148 and, analogous to the biliary tract, pancreatic sphincterotomy appears reasonable.
Ampullary and Pancreatic Malignancies
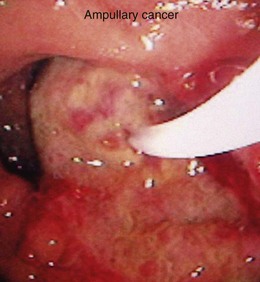
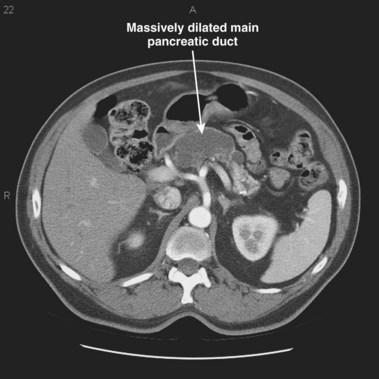
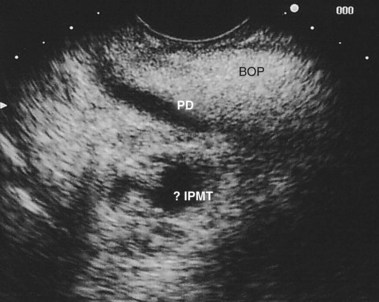
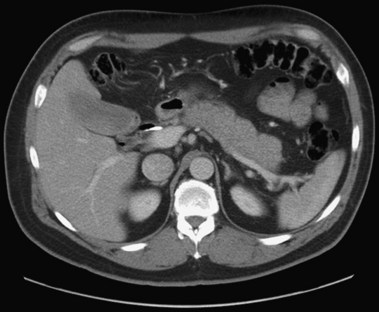
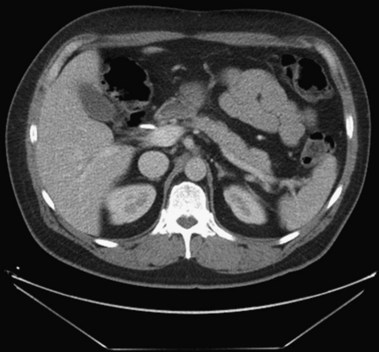
In a small subset of patients, malignant or premalignant obstruction of the pancreatic duct may lead to the development of ARP; this is most commonly due to ductal adenocarcinomas of the pancreas and ampulla but can also be seen with neuroendocrine tumors,149 metastatic tumors, and ampullary adenomas (Fig. 48.8).150 Intraductal papillary mucinous neoplasms (IPMNs) of the pancreas are a rarer form of premalignant or malignant tumors that typically manifest with ARP. Mucin secretion from hyperplastic ductal cells can obstruct the pancreatic duct and cause ARP. CT scans commonly show a dilated main pancreatic duct (Fig. 48.9) or isolated side branch dilation (Fig. 48.10). Associated cystic lesions may also be present. At ERCP, a fish-mouth papilla held open with mucus is pathognomonic (Fig. 48.11). EUS may show a diffusely enlarged main pancreatic duct with thickened walls and side branch dilation forming small cystic spaces.
IPMNs are categorized into three forms based on areas of involvement: main pancreatic duct (MD), side branch (SB), or combined main duct and side branch.151 Both ARP and CP have been observed in patients with MD-IPMN; however, the rate of ARP with SB-IPMN is not well established.152–154 A more recent retrospective study suggested a frequent association (34%) of acute pancreatitis in patients with suspected SB-IPMN.155 However, additional studies are necessary to determine the natural course of SB-IPMN and frequency of ARP. Pancreatic stents have been used to palliate obstructive pain and to treat ARP in patients with pancreatic malignancies who are not candidates for surgery.156 Pancreatic duct ES may allow mucus passage and palliate symptoms in patients with IPMNs who are nonoperative candidates. Patients with ARP secondary to ampullary adenomas can be treated with endoscopic or surgical ampullectomy.157
Choledochocysts
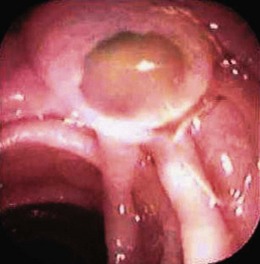
Choledochocysts are congenital anomalies of the biliary tract that are most often diagnosed in children and often associated with pancreaticobiliary malunion (Fig. 48.12). ARP in children with choledochocysts is usually associated with biliary stones or sludge that forms in the native or retained cyst.158 Complete surgical excision is the treatment of choice to prevent pancreaticobiliary symptoms and to prevent the development of biliary cancer, which is increased in the cysts if left in situ. Type 3 choledochocysts (choledochoceles) have been associated with ARP and can be treated with biliary ES through the cyst or via surgical transduodenal marsupialization.159 They may be recognized at endoscopy as a suprapapillary submucosal bulge and confirmed fluoroscopically at ERCP.
Periampullary Duodenal Diverticula
Periampullary diverticula have been indicated in the etiology of ARP. These diverticula are more commonly seen in elderly patients and are more often associated with choledocholithiasis and ARP compared with patients without diverticula.160 Periampullary diverticula are associated with an incompetent sphincter of Oddi with resultant colonization of the bile duct with intestinal bacteria, leading to the generation of mixed pigment bile duct stones.161 Although direct causative evidence linking periampullary diverticula to ARP are lacking, relative outflow obstruction of bile duct with bacterial colonization and microlith formation or outflow obstruction of exocrine pancreas secretions have been postulated in the pathogenesis of ARP, and a biliary sphincterotomy is generally performed.
Annular Pancreas
Annular pancreas is a rare congenital malformation in which the pancreatic parenchyma encircles the duodenal sweep. It most commonly manifests with obstruction of the duodenum and is best treated with a surgical duodenal bypass. Case reports of ARP associated with annular pancreas exist4,162; however, causality has not been confirmed.
Genetic Causes
Hereditary Pancreatitis
Epidemiology
Familial pancreatitis refers to pancreatitis of any etiology occurring in a family with an incidence that is greater than expected by chance. HP specifically refers to unexplained pancreatitis in a member of a family in which a pancreatitis-causing gene mutation is expressed in an autosomal dominant pattern. The phenotypic features of HP encompass the spectrum of pancreatic inflammatory diseases. HP typically manifests as ARP in childhood and progresses to CP by young adulthood in more than half of cases and is associated with an approximately 40% risk of pancreatic cancer by age 71,163,164 which is exaggerated by smoking.165
Pathogenesis
The gene for HP was mapped to chromosome 7q35166,167 and was identified as the cationic trypsinogen gene (protease, serine, 1; PRSS1; OMIM 276000) by Whitcomb and colleagues in 1996.168 Two point mutations, R122H and N29I169 occurring in exon3 and exon2 of cationic trypsinogen, account for most cases. These are the only mutations at the present time for which genetic testing is recommended according to a consensus conference.170,171 The same consensus also provides guidelines for testing. The R122H mutant cationic trypsin is thought to be resistant to autolysis through elimination of the arginine or lysine residues recognized by trypsin, which is critical in initiating trypsin autolysis.172 Trypsin is a proteolytic enzyme that activates most pancreatic digestive enzymes within the intestinal lumen and is maintained in the inactive trypsinogen form within the pancreas. Several mechanisms protect the pancreas from the consequences of prematurely activated trypsinogen. When trypsinogen is activated within the pancreas, the first known protective mechanism is inhibition by pancreatic secretory trypsin inhibitor (PSTI; unigene symbol SPINK1; serine protease inhibitor, Kazal type 1; OMIM 167790), which has the capacity to inhibit approximately 20% of potential trypsin activity.172
If trypsin activity overwhelms the SPINK1/PSTI inhibitory capacity, trypsin inactivation occurs via trypsin autolysis. With mutations that eliminate the autolysis site in HP, the second protective mechanism is lost, and pancreatitis occurs. Studies of families with HP reveal mutations in cationic trypsinogen that enhance autoactivation (N29I), mutations in SPINK1/PSTI that likely diminish inhibition of active trypsinogen (e.g., N34S), and mutations in cationic trypsinogen that prevent autolysis (R122H). One-third of families with HP have no mutation in either cationic trypsinogen or SPINK1/PSTI, suggesting that other genes are also important. For reasons that are unclear, HP disease penetrance is incomplete. A review of large kindred groups worldwide revealed a fairly constant rate of penetrance of 80%.163–176 A study of identical twins with HP171 suggested that the mechanism of disease penetrance is not one of simple genetics and that the protective element is not a modifier gene or obvious environmental factor. SPINK1/PSTI mutations are common in the general population and are not clearly associated with pancreatitis but may enhance the likelihood of pancreatitis related to other etiologies.165,176 The most commonly identified mutation in control populations is N34S (1% allele frequency), and the highest frequency is seen in patients with idiopathic CP.
Approximately 10% of familial pancreatitis kindreds have identifiable SPINK1 mutations. The clinical presentations are varied and include ARP or more commonly CP. The age at first presentation is typically before 20 years. Because less than 1% of patients with a heterozygous SPINK1 mutation alone ever develop pancreatitis, most experts do not recommend genetic testing or screening.170
Tropical Pancreatitis
Tropical pancreatitis is a syndrome of ARP that progresses to CP, which usually develops in children from lower socioeconomic families in tropical regions of Indonesia, Asia, Africa, and South America. There appears to be some variation in presentation; some children have severe calcific CP with late diabetes mellitus, whereas others develop diabetes and pancreatic fibrosis before developing calcification. The etiology of tropical pancreatitis is uncertain, but some clues are beginning to accumulate. First, diet has been eliminated as a cause of this disease. Second, there seems to be genetic susceptibility because a significant minority of patients has mutations in the pancreatic secretory trypsin inhibitor gene SPINK1.177,178 Third, the immune systems and pancreaticobiliary tree of these children are constantly challenged by infectious agents including helminths such as Ascaris lumbricoides that are endemic.179–181 If these infectious agents trigger acute pancreatitis, an altered immune response may drive pancreatic fibrosis in genetically susceptible individuals.182 Current evidence suggests that tropical pancreatitis is a complex condition with strong environmental trigger factors and genetic susceptibility and modifier factors that eventually result in irreversible pancreatic damage.
Cystic Fibrosis
Pathogenesis
CF (OMIM 219700) is the most common lethal autosomal recessive disorder in whites and is caused by a mutation of the CFTR gene (OMIM 602421) located on chromosome 7q32.183 CFTR is a chloride channel located on the luminal surface of the pancreatic duct cell that is tightly linked to bicarbonate secretion.175–187 Major mutations in both alleles leads to loss of CFTR function and the ability to hydrate mucus resulting in inspissated glands. Based on their functional impact, CFTR gene mutations are classified as either severe (class 1 to 3) or mild (class 4 and 5).187,188 Severe mutations yield little or no functional protein, whereas mild mutations diminish CFTR function.
Pulmonary consequences and abnormal sweat chloride occurs with CFTR function less than 5%; however, pancreatic exocrine function may be maintained down to 1% CFTR function. One or two mild CFTR mutations retaining more than 1% CFTR function may not lead to pancreatic insufficiency despite the overall disease status.189,190 Such patients are susceptible to ARP, as may be patients with one severe mutation (e.g., delta F508, the most common mutation [approximately 70%]) and a second milder mutation (e.g., CFTR R117H, R334W, R347W) any time the CFTR function becomes less than 10% of normal.191,192 The pancreas behaves differently from the lung and vas deferens qualitatively depending on the degree of CFTR impairment.
Autoimmune Pancreatitis
Pancreatitis associated with hypergammaglobulinemia was first reported in 1965.193 Since then, evidence for an autoimmune basis for recurrent attacks (but more commonly CP) in a subset of patients has been increasing.194,195
Epidemiology and Diagnosis
The clinical findings in autoimmune pancreatitis (AIP) encompass the clinical findings typical of pancreatitis but often with milder symptoms and usually without acute attacks. Obstructive jaundice seems to be a common feature, and a therapeutic response to steroid therapy has been reported.196 The sex ratio for AIP varies remarkably in the literature, probably because of the small study populations.197,198 In a more recent major U.S. study, the median age at presentation was 62.5 years (range 23 to 86 years) with 65% male and 88% white.199 The most common clinical presentations included new-onset mild abdominal pain (65%), jaundice (65%), and weight loss (42%).
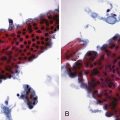
Radiologic findings include diffuse or focal pancreatic parenchymal enlargement with a “sausage-shaped” pancreas on abdominal CT (Fig. 48.13), diffuse or focal main pancreatic duct narrowing and beading, and a characteristic absence of pancreatic calcifications or cysts.200,201 EUS typically shows a diffusely hypoechoic, enlarged pancreas or a focal, irregularly shaped mass (Fig. 48.14).202 In the absence of cytologic evaluation, it may be difficult to discern these findings from a malignant pancreatic neoplasm. EUS-guided fine needle aspiration rarely provides an adequate tissue sample for a definitive diagnosis in cases of AIP. More recently, EUS-guided Tru-Cut needle biopsies have enabled core tissue sampling from the pancreas and other organs.203 Studies have shown that EUS-guided Tru-Cut needle biopsy is a safe and effective technique for obtaining a histologic diagnosis in patients with suspected AIP.204 Classic histologic findings include a lymphoplasmacytic infiltrate with obliterative venulitis and immunostaining positive for IgG4 plasma cells. Patients with AIP may also have an abundant infiltration of IgG4-positive plasma cells at the major duodenal papilla, and mucosal biopsies may provide an alternative method for helping to establish the diagnosis.205 Formal diagnostic criteria have been developed for the diagnosis of AIP.206–208 Strict adherence to these criteria may help to avoid misdiagnosis.
Laboratory findings in AIP may differ between isolated AIP and AIP associated with an autoimmune disorder. Both entities are characterized by elevated serum IgG and the presence of autoantibodies including antinuclear, antilactoferrin, and anti–carbonic anhydrase II and less commonly anti–smooth muscle and rheumatoid factor.201 Elevated IgG4 levels are useful in diagnosing isolated AIP.200,209 IgG4 is the least common of the IgG subclasses and has been considered a noninflammatory protective antibody,210 and its secretion along with IgE is regulated by T helper-2 cytokines such as interleukin-4, interleukin-5, and interleukin-13.1 The antigens driving the elevated IgG4 antibody titers in isolated AIP remain obscure.
Therapy
The finding of elevated IgG4 levels in the appropriate clinical setting may also be useful for deciding on a trial of steroid therapy.210,211 In addition to the improvement in symptoms and radiographic features, patients with AIP and diabetes mellitus display a trend toward normalizing insulin secretion and glycemic control with steroid therapy.211 Other regimens have been reported, including the use of additional immunosuppressive therapy such as azathioprine and mycophenolate mofetil. Repeat abdominal CT scan after immunosuppressive therapy may show dramatic improvement in the pancreas (Fig. 48.15).
Celiac Disease
A known association between celiac disease and pancreatic insufficiency has been recognized for many years,212–214 and reports exist of improvement in pancreatic function in non-CP cases with gluten-free diet. Postulated mechanisms include malnutrition215,216 and reduced gallbladder emptying resulting from impaired cholecystokinin release.217–219 More recently, an association between ARP and celiac disease has been reported, presumably resulting from duodenal inflammation leading to papillary stenosis.220 In 169 patients referred for suspected SOD, celiac disease was diagnosed in 12 (3 men, 9 women); 10 patients had ARP, and 2 had elevated liver function tests associated with abdominal pain. These patients had manometric evidence of papillary stenosis and histologic evidence of periampullary inflammation and celiac disease. Improvement in duodenal inflammation and symptoms was seen on a gluten-free diet; however, all patients had undergone biliary ES, and the data are difficult to interpret.
1 Steer ML. Classification and pathogenesis of pancreatitis. Surg Clin North Am. 1989;69:467-480.
2 Reber HA. Acute pancreatitis: Another piece of the puzzle? N Engl J Med. 1991;325:423-424.
3 Sakorafas GH, Tsiotou AG. Etiology and pathogenesis of acute pancreatitis: Current concepts. J Clin Gastroenterol. 2000;30:343-356.
4 Somogyi L, Martin SP, Venkatesan T, et al. Recurrent acute pancreatitis: An algorithmic approach to identification and elimination of inciting factors. Gastroenterology. 2001;120:708-717.
5 Venu RP, Geenen JE, Hogan W, et al. Idiopathic recurrent pancreatitis: An approach to diagnosis and treatment. Dig Dis Sci. 1989;34:56-60.
6 Glazer G, Mann DV, Working Party of the British Society of Gastroenterology. United Kingdom guidelines for the management of acute pancreatitis. Gut. 1998;42(Suppl 2):S1-S13.
7 Baillie J. Treatment of acute biliary pancreatitis. N Engl J Med. 1997;336:286-287.
8 Goodman AJ, Neoptolemos JP, Carr-Locke DL, et al. Detection of gall stones after acute pancreatitis. Gut. 1985;26:125-132.
9 Lin A, Feller ER. Pancreatic carcinoma as a cause of unexplained pancreatitis: Report of ten cases. Ann Intern Med. 1990;113:166-167.
10 Gutman M, Inbar M, Klausner JM. Metastases-induced acute pancreatitis: A rare presentation of cancer. Eur J Surg Oncol. 1993;19:302-304.
11 Ros E, Navarro S, Bru C, et al. Occult microlithiasis in ‘idiopathic’ acute pancreatitis: Prevention of relapses by cholecystectomy or ursodeoxycholic acid therapy. Gastroenterology. 1991;101:1701-1709.
12 Patti MG, Pellegrini CA. Gallstone pancreatitis. Surg Clin North Am. 1990;70:1277-1295.
13 Lo SK, Chen J. The role of ERCP in choledocholithiasis. Abdom Imaging. 1996;21:120-132.
14 Amouyal P, Amouyal G, Levy P. Diagnosis of choledocholithiasis by endoscopic ultrasonography. Gastroenterology. 1994;42:225-231.
15 Bank S, Indaram A. Causes of acute and recurrent pancreatitis: Clinical considerations and clues to diagnosis. Gastroenterol Clin North Am. 1999;28:571-589.
16 Gregor JC, Ponich TP, Detsky AS. Should ERCP be routine after an episode of “idiopathic” pancreatitis? A cost-utility analysis. Gastrointest Endosc. 1996;44:118-123.
17 Thornton J, Axon A. Towards safer endoscopic retrograde cholangiography. Gut. 1993;34:721-724.
18 Sherman S, Hawes RH, Rathgaber SW, et al. Post-ERCP pancreatitis: Randomized, prospective study comparing a low- and high-osmolality contrast agent. Gastrointest Endosc. 1994;40:422-427.
19 Chen YK, Foliente RL, Santoro MJ, et al. Endoscopic sphincterotomy-induced pancreatitis: Increased risk associated with nondilated bile ducts and sphincter of Oddi dysfunction. Am J Gastroenterol. 1994;89:327-333.
20 Roszler MH, Campbell WL. Post-ERCP pancreatitis: Association with urographic visualization during ERCP. J Radiol. 1985;157:595-598.
21 de Beaux AC, Palmer KR, Carter DC. Factors influencing morbidity and mortality in acute pancreatitis: An analysis of 279 cases. Gut. 1995;37:121-126.
22 Baron TH, Fleischer DE. Past, present, and future of endoscopic retrograde cholangiopancreatography: Perspectives on the National Institutes of Health consensus conference. Mayo Clin Proc. 2002;77:407-412.
23 Soto JA, Barish MA, Yucel EK, et al. Magnetic resonance cholangiography: Comparison with endoscopic retrograde cholangiopancreatography. Gastroenterology. 1996;110:589-597.
24 Barish MA, Yucel EK, Gerrucci JT. Magnetic resonance cholangiopancreatography. N Engl J Med. 1999;341:258-264.
25 Taourel P, Bret PM, Reinhold C, et al. Anatomic variants of the biliary tree: Diagnosis with MR cholangiopancreatography. Radiology. 1996;199:521-527.
26 Barish M, Soto J, Ferrucci J. Magnetic resonance pancreatography. Endoscopy. 1997;29:487-495.
27 Bret PM, Reinhold C, Paourel P, et al. Pancreas divisum: Evaluation with MR cholangiopancreatography. Radiology. 1996;199:99-103.
28 Buscail L, Escourrou J, Moreau J, et al. Endoscopic ultrasonography in chronic pancreatitis: A comparative prospective study with conventional ultrasonography, computed tomography and ERCP. Pancreas. 1995;10:251-257.
29 Chak A, Hawes RH, Cooper GS, et al. Prospective assessment of utility of EUS in the evaluation of gallstone pancreatitis. Gastrointest Endosc. 1999;49:599-604.
30 Liu CL, Lo CM, Chan JK, et al. EUS for detection of occult cholelithiasis in patients with idiopathic pancreatitis. Gastrointest Endosc. 2000;51:28-32.
31 Norton SA, Alderson D. Prospective comparison of endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography in the detection of bile duct stones. Br J Surg. 1997;84:1366-1369.
32 Dahan P, Andat C, Levy P, et al. Prospective evaluation of endoscopic ultrasonography and microscopic examination of duodenal bile in the diagnosis of cholecystolithiasis in 45 patients with normal conventional ultrasonography. Gut. 1996;38:277-281.
33 Dille JE. Symptom resolution or relief after cholecystectomy correlates strongly with positive combined endoscopic ultrasound and stimulated biliary drainage. Endoscopy. 1997;29:646-648.
34 Axon AT, Classen M, Cotton PB, et al. Pancreatography in chronic pancreatitis: International definitions. Gut. 1984;25:1107-1112.
35 Prat F, Amouyal G, Amouyal P, et al. Prospective controlled study of endoscopic ultrasonography and endoscopic retrograde cholangiography in patients with suspected common bile duct lithiasis. Lancet. 1996;347:75-79.
36 Frossard JL, Hadengue A, Amouyal G, et al. Choledocholithiasis: A prospective study of spontaneous common bile duct stone migration. Gastrointest Endosc. 2000;51:175-179.
37 Yasuda K, Mukai H, Fujimoto S, et al. The diagnosis of pancreatic cancer by endoscopic ultrasonography. Gastrointest Endosc. 1988;34:1-8.
38 Rosch T, Lorenz R, Braig C, et al. Endoscopic ultrasound in pancreatic tumor diagnosis. Gastrointest Endosc. 1991;37:347-352.
39 Zuccaro G, Sivak MV. Endoscopic ultrasonography in the diagnosis of chronic pancreatitis. Endoscopy. 1992;24:347-349.
40 Nattermann C, Goldschmidt AJ, Dancygier H. Endosonography in chronic pancreatitis—a comparison between endoscopic retrograde pancreatography and endoscopic ultrasonography. Endoscopy. 1993;24:565-570.
41 Bhutani MS, Hoffman BJ, Hawes RH. Diagnosis of pancreas divisum by endoscopic ultrasonography. Endoscopy. 1999;31:167-169.
42 Di Francesco V, Brunori MP, Rigo L, et al. Comparison of ultrasound-secretin test and sphincter of Oddi manometry in patients with recurrent acute pancreatitis. Dig Dis Sci. 1999;44:336-340.
43 Sugiyama M, Atomi Y. Endoscopic ultrasonography for diagnosing anomalous pancreaticobiliary junction. Gastrointest Endosc. 1997;45:261-267.
44 Varshney S, Johnson CD. Pancreas divisum. Int J Pancreatol. 1999;24:135-141.
45 Narisawa R, Asakura H, Niwa M, et al. Morphological study of pancreas divisum using ERCP in Niigata. Digest Endosc. 1994;6:158-162.
46 Smanio T. Proposed nomenclature and classification of the human pancreatic ducts and duodenal papillae: Study based on 200 post mortems. Int Surg. 1969;52:125-134.
47 Bernard JP, Sahel J, Giovanini M, et al. Pancreas divisum is a probable cause of acute pancreatitis: A report of 137 cases. Pancreas. 1990;5:248-254.
48 Cotton PB. Congenital anomaly of pancreas divisum as cause of obstructive pain and pancreatitis. Gut. 1980;21:105-114.
49 Gregg JA. Pancreas divisum: Its association with pancreatitis. Am J Surg. 1997;134:539-543.
50 Krueger KJ, Wootton FT, Cunningham JT, et al. Unexpected anomalies of the common bile and pancreatic ducts. Am J Gastroenterol. 1992;87:1492-1495.
51 Sahel J, Cros RC, Bourry J, et al. Clinico-pathological conditions associated with pancreas divisum. Digestion. 1982;23:1-8.
52 Richter JM, Schapiro RH, Mulley AG, et al. Association of the pancreas divisum and pancreatitis and its treatment by sphincteroplasty of the accessory ampulla. Gastroenterology. 1981;81:1104-1110.
53 Cotton PB. Congenital anomaly of pancreas divisum as cause of obstructive pain and pancreatic. Gut. 1980;21:104-114.
54 Bernard JP, Sahel J, Giovannini M, et al. Pancreas divisum is a probable cause of pancreatitis: A report of 137 cases. Pancreas. 1990;5:248-254.
55 Warshaw AL, Richter JM, Schapiro RH. The cause and treatment of pancreatitis associated with pancreas divisum. Ann Surg. 1983;198:443-452.
56 Russell RC, Wong NW, Cotton PB. Accessory sphincterotomy (endoscopic and surgical) in patients with pancreas divisum. Br J Surg. 1984;71:954-957.
57 Lowes JR, Rode J, Lees WR, et al. Obstructive pancreatitis: Unusual causes of chronic pancreatitis. Br J Surg. 1988;75:1129-1133.
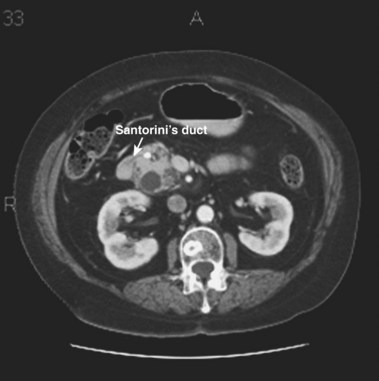
58 Mairose UB, Wurbs D, Classen M. Santorini’s duct an insignificant variant from normal or an important overflow valve? Endoscopy. 1978;10:24-29.
59 Gelrud A, Sheth S, Banerjee S, et al. Analysis of cystic fibrosis gene product (CFTR) function in patients with pancreas divisum and recurrent acute pancreatitis. Am J Gastroenterol. 2004;99:1557-1562.
60 Devereaux BM, Lehman GA, Fein S, et al. Facilitation of pancreatic duct cannulation using a new synthetic porcine secretin. Am J Gastroenterol. 2002;97:2279-2281.
61 Park SH, de Bellis M, McHenry L, et al. Use of methylene blue to identify the minor papilla or its orifice in patients with pancreas divisum. Gastrointest Endosc. 2003;57:358-363.
62 Khalid A, Peterson M, Slivka A. Secretin-stimulated magnetic resonance pancreaticogram to assess pancreatic duct outflow obstruction in evaluation of idiopathic acute recurrent pancreatitis: A pilot study. Dig Dis Sci. 2003;48:1475-1481.
63 Matos C, Metens T, Deviere J, et al. Pancreatic duct morphological and functional evaluation with dynamic MR pancreatography after secretin stimulation. Radiology. 1997;203:435-441.
64 Keith RG. Surgery for pancreas divisum. Gastrointest Clin North Am. 1995;4:171-180.
65 Lans JI, Geenen JE, Johanson JF, et al. Endoscopic therapy in patients with pancreas divisum and acute pancreatitis: A prospective, randomized, controlled clinical trial. Gastrointest Endosc. 1992;38:430-434.
66 Tarnasky PR. Mechanical prevention of post-ERCP pancreatitis by pancreatic stents: Results, techniques, and indications. JOP. 2003;4:58-67.
67 Fazel A, Quadri A, Catalano MF, et al. Does a pancreatic duct stent prevent post-ERCP pancreatitis? A prospective randomized study. Gastrointest Endosc. 2003;57:291-294.
68 Sherman S, Hawes R, Nisi R, et al. Randomized controlled trial of minor papilla sphincterotomy (MiES) in pancreas divisum (Pdiv) patients with pain only [abstract]. Gastrointest Endosc. 1994;40:A125.
69 McCarthy J, Geenen JE, Hogan W. Preliminary experience with endoscopic stent placement in benign diseases. Gastrointest Endosc. 1988;34:16-18.
70 Prabhu M, Geenen JE, Hogan WJ, et al. Role of endoscopic stent placement in the treatment of acute recurrent pancreatitis associated with pancreas divisum: A prospective assessment [abstract]. Gastrointest Endosc. 1989;34:165.
71 Siegel JH, Ben-svi JS, Pullano W, et al. Effectiveness of endoscopic drainage for pancreas divisum. Endoscopy. 1990;22:129-133.
72 Coleman SD, Eisen GM, Troughton AB, et al. Endoscopic treatment in pancreas divisum. Am J Gastroenterol. 1994;89:1152-1155.
73 Kozarek RA. Pancreatic stents can induce ductal changes consistent with chronic pancreatitis. Gastrointest Endosc. 1990;36:93-95.
74 Smith M, Ikenberry S, Uzer M, et al. Alterations in pancreatic duct morphology following pancreatic stent therapy. Gastrointest Endosc. 1996;44:268-275.
75 Lehman GA, Sherman S. Diagnosis and therapy of pancreas divisum. Gastrointest Endosc Clin N Am. 1998;8:55-77.
76 Lehman GA, Sherman S, Nisi R, et al. Pancreas divisum: Results of minor papilla sphincterotomy. Gastrointest Endosc. 1993;39:1-8.
77 Sugawa C, Walt AJ, Nunez DC, et al. Pancreas divisum: Is it a normal anatomic variant? Am J Surg. 1987;153:62-67.
78 Lee SP. Biliary sludge: Curiosity or culprit? [editorial]. Hepatology. 1994;20:523-525.
79 Filly RA, Allen B, Minton MJ, et al. In vitro investigation of the origin of echoes with biliary sludge. J Clin Ultrasound. 1980;8:193-200.
80 Allen B, Bernhoft R, Blackaert N, et al. Sludge is calcium bilirubinate associated with bile stasis. Am J Surg. 1981;141:51-56.
81 Maringhini A, Marceno MP, Lanzarone F, et al. Sludge and stones after pregnancy: Prevalence and risk factors. J Hepatol. 1987;5:218-223.
82 Maringhini A, Ciambra M, Baccelliere P, et al. Biliary sludge and gallstones in pregnancy: Incidence, risk factors, and natural history. Ann Intern Med. 1993;119:116-120.
83 Shiffman ML, Sugerman JH, Kellum JM, et al. Gallstone formation after rapid weight loss: A prospective study in patients undergoing gastric bypass surgery for treatment of morbid obesity. Am J Gastroenterol. 1991;86:1000-1005.
84 Murray FE, Stinchcombe SJ, Hawkey CJ. Development of biliary sludge in patients on intensive care unit: Results of a prospective ultrasonographic study. Gut. 1992;33:1123-1125.
85 Bolondi L, Gaiani S, Testa S, et al. Gallbladder sludge formation during prolonged fasting after gastrointestinal tract surgery. Gut. 1985;26:734-738.
86 Messing B, Bories C, Kuntslinger F, et al. Does total parenteral nutrition induce gallbladder sludge formation and lithiasis? Gastroenterology. 1983;84:1012-1019.
87 Gafa M, Sarli L, Miselli A, et al. Sludge and microlithiasis of the biliary tract after total gastrectomy and postoperative total parenteral nutrition. Surg Gynecol Obstet. 1987;165:413-418.
88 Pitt HA, King W3rd, Mann LL, et al. Increased risk of cholelithiasis with prolonged total parenteral nutrition. Am J Surg. 1983;145:106-112.
89 Schaad UB, Tschappeler H, Lentze MJ. Transient formation of precipitations in the gallbladder associated with ceftriaxone therapy. Pediatr Infect Dis. 1986;5:708-710.
90 Schaad UB, Wedgwood-Krucko J, Tschaeppeler H. Reversible ceftriaxone-associated biliary pseudolithiasis in children. Lancet. 1988;2:1411-1413.
91 Ettestad PJ, Campbell GL, Welbel SF, et al. Biliary complications in the treatment of unsubstantiated Lyme disease. J Infect Dis. 1995;171:356-361.
92 Vance ML, Harris AG. Long-term treatment of 189 acromegalic patients with the somatostatin analog octreotide: Results of the international Multicenter Acromegaly Study Group. Arch Intern Med. 1991;151:1573-1578.
93 Newman CB, Melmed S, Snyder PJ, et al. Safety and efficacy of long-term octreotide therapy of acromegaly: Results of a multicenter trial in 103 patients—a clinical research center study. J Clin Endocrinol Metab. 1995;80:2768-2775.
94 Ezzat S, Snyder PJ, Younge WF, et al. Octreotide treatment of acromegaly: A randomized, multicenter study. Ann Intern Med. 1992;117:711-718.
95 Teefey SA, Hollister MS, Lee SP, et al. Gallbladder sludge formation after bone marrow transplant: Sonographic observations. Abdom Imaging. 1994;19:57-60.
96 Frick MP, Snover DC, Feinberg SB, et al. Sonography of the gallbladder in bone marrow transplant patients. Am J Gastroenterol. 1984;79:122-127.
97 Lorber MI, Van Buren CT, Flechner SM, et al. Hepatobiliary and pancreatic complications of cyclosporine therapy in 466 renal transplant recipients. Transplantation. 1987;43:35-40.
98 Peterseim DS, Pappas TN, Meyers CH, et al. Management of biliary complications after heart transplantation. J Heart Lung Transplant. 1995;14:623-631.
99 Jacobson AF, Teefey SA, Lee SP, et al. Frequent occurrence of new hepatobiliary abnormalities after bone marrow transplantation: Results of a prospective study using scintigraphy and sonography. Am J Gastroenterol. 1993;88:1044-1049.
100 Lee SP, Nicholls JF, Park HZ. Biliary sludge as a cause of acute pancreatitis. N Engl J Med. 1992;326:589-593.
101 Lee SP, Maher K, Nicholls JF. Origin and fate of biliary sludge. Gastroenterology. 1988;94:170-179.
102 Johnston DE, Kaplan MM. Pathogenesis and treatment of gallstones. N Engl J Med. 1993;328:412-421.
103 Paumgartner G, Sauerbruch T. Gallbladder stones: Pathogenesis. Lancet. 1991;338:1117-1124.
104 Carey MC, Cahalane MJ. Whither biliary sludge? Gastroenterology. 1988;95:508-523.
105 Lee SP. Pathogenesis of biliary sludge. Hepatology. 1990;12(3 Pt 2):200S-203S.
106 Cahalane MJ, Neubrand MW, Carey MC. Physical-chemical pathogenesis of pigment gallstones. Semin Liver Dis. 1988;8:317-328.
107 Janowitz P, Kratzer W, Zemmler T, et al. Gallbladder sludge: Spontaneous course and incidence of complications in patients with stones. Hepatology. 1994;20:291-294.
108 Ohara N, Schaefer J. Clinical significance of biliary sludge. J Clin Gastroenterol. 1990;12:291-294.
109 Ko CW, Sekijima JH, Lee SP. Biliary sludge. Ann Intern Med. 1999;130:301-311.
110 Tint GS, Dyrszka H, Sanghavi B, et al. Lithotripsy plus ursodiol is superior to ursodiol alone for cholesterol gallstones. Gastroenterology. 1992;102:2042-2049.
111 Sauerbruch T, Delius M, Paumgartner G, et al. Fragmentation of gallstones by extracorporeal shock waves. N Engl J Med. 1986;314:818-822.
112 Sackmann M, Weber W, Delius M, et al. Extracorporeal shock-wave lithotripsy of gallstones without general anesthesia: First clinical experience. Ann Intern Med. 1987;107:347-348.
113 Sackmann M, Delius M, Sauerbruch T, et al. Shock-wave lithotripsy of gallbladder stones: The first 175 patients. N Engl J Med. 1988;318:393-397.
114 Sackmann M, Pauletzki J, Sauerbruch T, et al. The Munich Gallbladder Lithotripsy Study. Results of the first 5 years with 711 patients. Ann Intern Med. 1991;114:290-296.
115 Opie EL. The etiology of acute hemorrhagic pancreatitis. Bull Johns Hopkins Hosp. 1901;12:182-188.
116 Hernandez CA, Lerch MM. Sphincter stenosis and gallstone migration through the biliary tract. Lancet. 1993;341:1371-1373.
117 Chebli JM, Ferrari Junior AP, Silva MR, et al. Biliary microcrystals in idiopathic acute pancreatitis: Clue for occult underlying biliary etiology [In Portuguese with English abstract]. Arq Gastroenterol. 2000;37:93-101.
118 Dahan P, Andant C, Levy P, et al. Prospective evaluation of endoscopic ultrasonography and microscopic examination of duodenal bile in the diagnosis of cholecystolithiasis in 45 patients with normal conventional ultrasonography. Gut. 1996;38:277-281.
119 Dill JE, Hill S, Berkhouse L. Combined endoscopic ultrasound and stimulated biliary drainage in cholecystitis and microlithiasis—diagnoses and outcomes. Endoscopy. 1995;27:424-427.
120 Buscail L, Escourrou J, Delvaux M, et al. Microscopic examination of bile directly collected during endoscopic cannulation of the papilla: Utility in patients with suspected microlithiasis. Dig Dis Sci. 1992;37:116-120.
121 Kaufman Z, Shpitz B, Dinbar A. Microlithiasis of the cystic duct. Am J Gastroenterol. 1986;81:303-304.
122 Moreau JA, Zinsmeister AR, Melton LJ3rd, et al. Gallstone pancreatitis and the effect of cholecystectomy: A population-based cohort study. Mayo Clin Proc. 1988;63:466-473.
123 Tanaka M, Ikeda S, Yoshimoto H, et al. The long-term fate of the gallbladder after endoscopic sphincterotomy: Complete follow-up study of 122 patients. Am J Surg. 1987;154:505-509.
124 Siegel JH, Veerappan A, Cohen SA, et al. Endoscopic sphincterotomy for biliary pancreatitis: An alternative to cholecystectomy in high-risk patients. Gastrointest Endosc. 1994;40:573-575.
125 Welbourn CR, Beckly DE, Eyre-Brook IA. Endoscopic sphincterotomy without cholecystectomy for gall stone pancreatitis. Gut. 1995;37:119-120.
126 Dalian P, Andant C, Levy P, et al. Prospective evaluation of endoscopic ultrasonography and microscopic examination of duodenal bile in the diagnosis of cholecystolithiasis in 45 patients with normal conventional ultrasonography. Gut. 1996;38:277-281.
127 Sharma BC, Agarwal DK, Baijal SS, et al. Effect of endoscopic sphincterotomy on gall bladder bile lithogenicity and motility. Gut. 1998;42:288-292.
128 Rosseland AR, Solhaug JH. Primary endoscopic papillotomy (EPT) in patients with stones in the common bile duct and the gallbladder in situ: A 5–8–year follow-up study. World J Surg. 1988;12:111-116.
129 Agarwal DK, Sharma BC, Dhiman RK, et al. Effect of endoscopic sphincterotomy on gallbladder motility. Dig Dis Sci. 1997;42:1495-1500.
130 Shiffman ML, Kaplan GD, Brinkman-Kaplan V, et al. Prophylaxis against gallstone formation with ursodeoxycholic acid in patients participating in a very-low-calorie diet program. Ann Intern Med. 1995;122:899-905.
131 Sugiyama M, Atomi Y. Longterm effects of endoscopic sphincterotomy on gall bladder motility. Gut. 1996;39:856-859.
132 Broomfield PH, Chopra R, Scheinbaum RC, et al. Effects of ursodeoxycholic acid and aspirin on the formation of lithogenic bile and gallstones during loss of weight. N Engl J Med. 1988;319:567-572.
133 Warshaw AL, Simeone J, Schapiro RH, et al. Objective evaluation of ampullary stenosis with ultrasonography and pancreatic stimulation. Am J Surg. 1985;149:65-72.
134 Toouli J, Roberts-Thomson IC, Dent J, et al. Sphincter of Oddi motility disorders in patients with idiopathic recurrent pancreatitis. Br J Surg. 1985;72:859-863.
135 Hogan WJ, Geenen JE. Biliary dyskinesia. Endoscopy. 1988;20:179-188.
136 Toouli J, Di Francesco V, Saccone G, et al. Division of the sphincter of Oddi for treatment of dysfunction associated with recurrent pancreatitis. Br J Surg. 1996;83:1205-1210.
137 Nardi GL, Michelassi F, Zannini P. Transduodenal sphincteroplasty: 5–25 year follow of 89 patients. Ann Surg. 1983;198:453-461.
138 Stephens RV, Burdick GE. Microscopic transduodenal sphincteroplasty and transampullary septoplasty for papillary stenosis. Am J Surg. 1986;142:621-627.
139 Gregg JA, Carr-Locke DL. Endoscopic pancreatic and biliary manometry in pancreatic, biliary and papillary disease, and after endoscopic sphincterotomy and surgical sphincteroplasty. Gut. 1984;25:1247-1254.
140 Guelrud M, Siegel JH. Hypertensive pancreatic duct sphincter as a cause of pancreatitis: Successful treatment with hydrostatic balloon dilation. Dig Dis Sci. 1984;29:225-231.
141 Eversman D, Fogel EL, Rusche M, et al. Frequency of abnormal pancreatic and biliary sphincter manometry compared with clinical suspicion of sphincter of Oddi dysfunction. Gastrointest Endosc. 1999;50:637-641.
142 Park SH, Watkins JL, Fogel EL, et al. Long-term outcome of endoscopic dual pancreatobiliary sphincterotomy in patients with manometry-documented sphincter of Oddi dysfunction and normal pancreatogram. Gastrointest Endosc. 2003;57:483-491.
143 Freeman ML. Understanding risk factors and avoiding complications with endoscopic retrograde cholangiopancreatography. Curr Gastroenterol Rep. 2003;5:145-153.
144 McCarthy J, Geenen JE, Hogan WJ. Preliminary experience with endoscopic stent placement in benign pancreatic diseases. Gastrointest Endosc. 1988;34:16-18.
145 Kozarek RA, Ball TJ, Patterson DJ, et al. Endoscopic approach to pancreas divisum. Dig Dis Sci. 1995;40:1974-1981.
146 Geenen JE, Hogan WJ, Dodds WJ, et al. The efficacy of endoscopic sphincterotomy after cholecystectomy in patients with sphincter of Oddi dysfunction. N Engl J Med. 1989;320:82-87.
147 Scicchitano J, Saccone GT, Baker RA, et al. How safe is endoscopic sphincter of Oddi manometry? J Gastroenterol Hepatol. 1995;10:334-336.
148 Khandekar S, Disario JA. Endoscopic therapy for stenosis of the biliary and pancreatic duct orifices. Gastrointest Endosc. 2000;52:500-505.
149 Grino P, Martinez J, Grino E, et al. Acute pancreatitis secondary to pancreatic neuroendocrine tumours. JOP. 2003;4:104-110.
150 Heiskanen I, Kellokumpu I, Jarvinen H. Management of duodenal adenomas in 98 patients with familial adenomatous polyposis. Endoscopy. 1999;46:1959-1962.
151 Ohashi K, Murakami Y, Maruyam M, et al. Four cases of a mucous secreting pancreatic cancer. Prog Dig Endosc. 1982;20:348-351.
152 Salvia R, Bassi C, Falconi M, et al. Intraductal papillary mucinous tumors of the pancreas: Surgical treatment: At what point should we stop? JOP. 2005;6:112-117.
153 Loftus EVJr, Olivares-Pakzad BA, Batts KP, et al. Intraductal papillary mucinous tumors of the pancreas: Clinicopathologic features, outcome, and nomenclature. Members of the Pancreas Clinic, and Pancreatic Surgeons of Mayo Clinic. Gastroenterology. 1996;110:1909-1918.
154 Zamora C, Sahel J, Cantu DG, et al. Intraductal papillary or mucinous tumors (IPMT) of the pancreas: Report of a case series and review of the literature. Am J Gastroenterol. 2001;96:1441-1447.
155 Ringold DA, Shroff P, Sikka SK, et al. Pancreatitis is frequent among patients with side-branch intraductal papillary mucinous neoplasia diagnosed by EUS. Gastrointest Endosc. 2009;70:488-494.
156 Tham TC, Lichtenstein DR, Vandervoort J, et al. Pancreatic duct stents for “obstructive type” pain in pancreatic malignancy. Am J Gastroenterol. 2000;95:956-960.
157 Martin JA, Haber GB. Ampullary adenoma: Clinical manifestations, diagnosis, and treatment. Gastrointest Endosc Clin N Am. 2003;13:649-669.
158 Komuro H, Makino SI, Yasuda Y, et al. Pancreatic complications in choledochal cyst and their surgical outcomes. World J Surg. 2001;25:1519-1523.
159 Martin RF, Biber BP, Bosco JJ, et al. Symptomatic choledochoceles in adults: Endoscopic retrograde cholangiopancreatography recognition and management. Arch Surg. 1992;127:536-538.
160 Uomo G, Manes G, Ragozzino A, et al. Periampullary extraluminal duodenal diverticula and acute pancreatitis: An underestimated etiological association. Am J Gastroenterol. 1996;91:1186-1188.
161 Lobo DN, Balfour TW, Iftikhar SY, et al. Periampullary diverticula and pancreaticobiliary disease. Br J Surg. 1999;86:588-597.
162 Chevillotte G, Sahel J, Raillat A, et al. Annular pancreas: Report of one case associated with acute pancreatitis and diagnosed by endoscopic retrograde pancreatography. Dig Dis Sci. 1984;29:75-77.
163 Sossenheimer MJ, Aston CE, Preston RA, et al. Clinical characteristics of hereditary pancreatitis in a large family based on high-risk haplotype. The Midwest Multicenter Pancreatic Study Group (MMPSG). Am J Gastroenterol. 1997;92:1113-1116.
164 Lowenfels A, Maisonneuve P, DiMagno E, et al. Hereditary pancreatitis and the risk of pancreatic cancer. J Natl Cancer Inst. 1997;89:442-446.
165 Lowenfels AB, Maisonneuve P, Whitcomb DC, et al. Cigarette smoking as a risk factor for pancreatic cancer in patients with hereditary pancreatitis. JAMA. 2001;286:169-170.
166 LeBodic L, Bignon JD, Raguenes O, et al. The hereditary pancreatitis gene maps to long arm of chromosome 7. Hum Mol Genet. 1996;5:549-554.
167 Whitcomb DC, Preston RA, Aston CE, et al. A gene for hereditary pancreatitis maps to chromosome 7q35. Gastroenterology. 1996;110:1975-1980.
168 Whitcomb DC, Gorry MC, Preston RA, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996;14:141-145.
169 Gorry M, Gabbaaizadeh D, Furey W, et al. Multiple mutations in the cationic trypsinogen gene are associated with hereditary pancreatitis. Gastroenterology. 1997;113:1063-1068.
170 Ellis I, Lerch MM, Whitcomb DC. Genetic testing for hereditary pancreatitis: Guidelines for indications, counseling, consent and privacy issues. Pancreatology. 2001;1:405-415.
171 Gates L, Ulrich C, Whitcomb D. Hereditary pancreatitis: Gene defects and their implications. Surg Clin North Am. 1999;79:711-722.
172 Riderknecht H. Activation of pancreatic zymogens: Normal activation, premature intrapancreatic activation, protective mechanisms against inappropriate activation. Dig Dis Sci. 1986;31:314-321.
173 Pfutzer RH, Barmada MM, Brunskill AP. SPINK1/PSTI polymorphisms act as disease modifiers in familial and idiopathic chronic pancreatitis. Gastroenterology. 2000;119:615-623.
174 Witt H, Hennies HC, Becker M. SPINK1 mutations in chronic pancreatitis. Gastroenterology. 2001;120:1060-1061.
175 Witt H, Luck W, Hennies HC, et al. Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nat Genet. 2000;25:213-216.
176 Chen JM, Mercier B, Audrezet MP, et al. Mutational analysis of the human pancreatic secretory trypsin inhibitor (PSTI) gene in hereditary and sporadic chronic pancreatitis. J Med Genet. 2000;37:67-69.
177 Schneider A, Suman A, Rossi L, et al. SPINK1/PSTI mutations are associated with tropical pancreatitis and type II diabetes mellitus in Bangladesh. Gastroenterology. 2002;123:1026-1030.
178 Rossi L, Pfützer RL, Parvin S, et al. SPINK1/PSTI mutations are associated with tropical pancreatitis in Bangladesh: A preliminary report. Pancreatology. 2001;1:242-245.
179 Das S. Pancreatitis in children associated with round worms. Indian Pediatr. 1977;14:81-83.
180 Gilbert MG, Carbonnel ML. Pancreatitis in childhood associated with ascariasis. Pediatrics. 1964;33:589-592.
181 Coelho da Rocha RF, Chapcha P, Aun F. Abdominal complications of ascariasis in children. Prob Gen Surg. 2001;18:92-99.
182 Schneider A, Whitcomb DC. Hereditary pancreatitis: A model for inflammatory diseases of the pancreas. Best Pract Res Clin Gastroenterol. 2002;16:347-363.
183 Lebodic L, Bignon JD, Raguenes O, et al. The hereditary pancreatitis gene maps to long arm of chromosome 7. Hum Mol Genet. 1996;5:549-554.
184 Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science. 1989;245:1066-1073.
185 Marino CR, Matovcik LM, Gorelick FS, et al. Localization of the cystic fibrosis transmembrane conductance regulator in pancreas. J Clin Invest. 1991;88:712-716.
186 Shumaker H, Amlal H, Frizzell R, et al. CFTR drives Na+-nHCO−3 cotransport in pancreatic duct cells: A basis for defective HCO−3 secretion in CF. Am J Physiol. 1999;276:C16-C25.
187 Mickle JE, Cutting GR. Genotype-phenotype relationships in cystic fibrosis. Med Clin North Am. 2000;84:597-607.
188 Zielenski J, Tsui LC. Cystic fibrosis: Genotypic and phenotypic variations. Ann Rev Genet. 1995;29:777-807.
189 Cohn JA, Friedman KJ, Noone PG, et al. Relation between mutations of the cystic fibrosis gene and idiopathic pancreatitis. N Engl J Med. 1998;339:653-658.
190 Sheppard DN, Ostedgaard LS, Winter MC, et al. Mechanism of dysfunction of two nucleotide binding domain mutations in cystic fibrosis transmembrane conductance regulator that are associated with pancreatic sufficiency. EMBO J. 1995;14:876-883.
191 Noone PG, Zhou Z, Silverman LM, et al. Cystic fibrosis gene mutations and pancreatitis risk: Relation to epithelial ion transport and trypsin inhibitor gene mutations. Gastroenterology. 2001;121:1310-1319.
192 Gomez Lira M, Patuzzo C, Castellani C, et al. CFTR and cationic trypsinogen mutations in idiopathic pancreatitis and neonatal hypertrypsinemia. Pancreatology. 2001;1:538-542.
193 Yoshida K, Toki F, Takeuchi T, et al. Chronic pancreatitis caused by an autoimmune abnormality: Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995;40:1461-1568.
194 Khalid A, Whitcomb DC. The importance of autoimmune pancreatitis. Gastroenterology. 2001;121:1518-1520.
195 Ohana M, Okazaki K, Hajiro K, et al. Multiple pancreatic masses associated with autoimmunity. Am J Gastroenterol. 1998;93:99-102.
196 Ito T, Nakano I, Koyanagi S, et al. Autoimmune pancreatitis as a new clinical entity: Three cases of autoimmune pancreatitis with effective steroid therapy. Dig Dis Sci. 1997;42:1458-1468.
197 Hammano H, Kawa S, Horiuchi A, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732-738.
198 Okazaki K, Uchida K, Ohana M, et al. Autoimmune-related pancreatitis is associated with autoantibodies and a Th1/Th2-type cellular immune response. Gastroenterology. 2000;118:573-581.
199 Raina A, Yadav D, Krasinskas AM, et al. Evaluation and management of autoimmune pancreatitis: Experience at a large US center. Am J Gastroenterol. 2009;104:2295-2306.
200 Irie H, Honda H, Baba S, et al. Autoimmune pancreatitis: CT and MR characteristics. AJR Am J Roentgenol. 1998;170:1321-1327.
201 Furukawa N, Muranaka T, Yasumori K, et al. Autoimmune pancreatitis: Radiologic findings in three histologically proven cases. J Comput Assist Tomogr. 1998;22:880-883.
202 Farrell JJ, Garber J, Sahani D, et al. EUS findings in patients with autoimmune pancreatitis. Gastrointest Endosc. 2004;60:927-936.
203 Levy MJ, Reddy RP, Wiersema MJ, et al. EUS-guided trucut biopsy in establishing autoimmune pancreatitis as the cause of obstructive jaundice. Gastrointest Endosc. 2005;61:467-472.
204 Mizuno N, Bhatia V, Hosoda W, et al. Histological diagnosis of autoimmune pancreatitis using EUS-guided trucut biopsy: A comparison study with EUS-FNA. J Gastroenterol. 2009;44:742-750.
205 Kamisawa T, Tu Y, Nakajima H, et al. Usefulness of biopsying the major duodenal papilla to diagnose autoimmune pancreatitis: A prospective study using IgG4-immunostaining. World J Gastroenterol. 2006;12:2031-2033.
206 Chari ST, Smyrk TC, Levy MJ, et al. Diagnosis of autoimmune pancreatitis: The Mayo Clinic experience. Clin Gastroenterol Hepatol. 2006;4:1010-1016.
207 Choi EK, Kim MH, Kim JC, et al. The Japanese diagnostic criteria for autoimmune chronic pancreatitis: Is it completely satisfactory? Pancreas. 2006;33:13-19.
208 Kim KP, Kim MH, Kim JC, et al. Diagnostic criteria for autoimmune chronic pancreatitis revisited. World J Gastroenterol. 2006;12:2487-2496.
209 Chen RY, Adams DB. IgG4 levels in non-Japanese patients with autoimmune sclerosing pancreatitis. N Engl J Med. 2002;346:1919.
210 Erkelens GW, Vleggaar FP, Lesterhuis W, et al. Sclerosing pancreato-cholangitis responsive to steroid therapy. Lancet. 1999;345:43-44.
211 Tanaka S, Kobayashi T, Nakanishi K, et al. Corticosteroid-responsive diabetes mellitus associated with autoimmune pancreatitis. Lancet. 2000;356:910-911.
212 Pitchumoni CS, Thomas E, Balthazar E, et al. Chronic calcific pancreatitis in association with celiac disease. Am J Gastroenterol. 1977;68:358-361.
213 Regan PT, DiMagno EP. Exocrine pancreatic insufficiency in celiac sprue: A cause of treatment failure. Gastroenterology. 1980;78:484-487.
214 Fernanez LB, DePaula A, Prizont R, et al. Exocrine pancreas insufficiency secondary to gluten enteropathy. Am J Gastroenterol. 1970;53:564-569.
215 Tandon BN, George PK, Sama SK, et al. Exocrine pancreatic function in protein-calorie malnutrition disease of adults. Am J Clin Nutr. 1969;22:1476-1482.
216 Pitchumoni CS. Pancreas in primary malnutrition disorders. Am J Clin Nutr. 1973;26:374-379.
217 Rhodes RA, Tai HH, Chey WY. Impairment of secretin release in celiac sprue. Am J Dig Dis. 1978;23:833-839.
218 Maton PN, Selden AC, Fitzpatrick ML, et al. Defective gallbladder emptying and cholecystokinin release in celiac disease: Reversal by gluten-free diet. Gastroenterology. 1985;88:391-396.
219 Dimagno EP, Go WL, Summerskill WH. Impaired cholecystokinin-pancreozymin secretion, intraluminal dilution, and maldigestion of fat in sprue. Gastroenterology. 1972;63:25-32.
220 Patel RS, Johlin FCJr, Murray JA. Celiac disease and recurrent pancreatitis. Gastrointest Endosc. 1999;50:823-827.
221 Delhaye M, Engelholm L, Cremer M. Pancreas divisum: Congenital anatomic variant or anomaly? Contribution of endoscopic retrograde dorsal pancreatography. Gastroenterology. 1985;89:951-958.
222 Burtin P, Person B, Charneau J, et al. Pancreas divisum and pancreatitis: A coincidental association? Endoscopy. 1991;23:55-58.
223 Soehendra N, Kempeneers I, Nam VC, et al. Endoscopic dilatation and papillotomy of the accessory papilla and internal drainage in pancreas divisum. Endoscopy. 1986;18:129-132.
224 Liquory C, Lefebvre JF, Canard JM, et al. Le pancreas divisum: Etude clinique et therapeutique chez I’homme apropos de 87 cas. Gastroenterol Clin Biol. 1986;10:820-825.
225 Lans JI, Geenen JE, Johanson JF, et al. Endoscopic therapy in patients with pancreas divisum and acute pancreatitis: A prospective, randomized, controlled clinical trial. Gastrointest Endosc. 1992;38:430-434.
226 Boerma D, Huibregtse K, Gulik TM, et al. Long-term outcome of endoscopic stent placement for chronic pancreatitis associated with pancreas divisum. Endoscopy. 2000;32:452-456.
227 Ertan A. Long-term results after endoscopic pancreatic stent placement without pancreatic papillotomy in acute recurrent pancreatitis due to pancreas divisum. Gastrointest Endosc. 2000;52:9-14.
228 Heyries L, Barthet M, Delvasto C, et al. Long-term results of endoscopic management of pancreas divisum with recurrent acute pancreatitis. Gastrointest Endosc. 2002;55:376-381.