160 Acute Pulmonary Complications in Pregnancy
 Pulmonary Physiology in Pregnancy
Pulmonary Physiology in Pregnancy
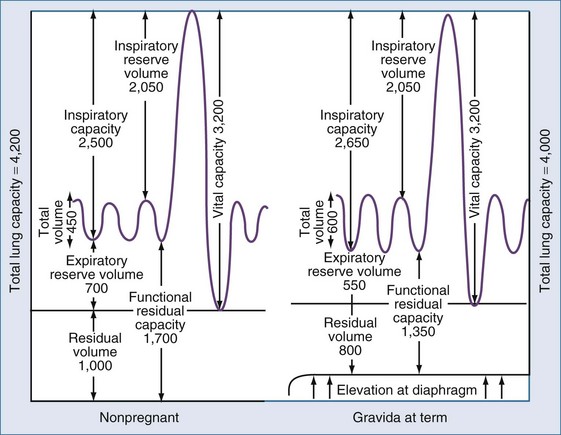
A number of physiologic changes affect respiration during pregnancy. Normal pregnancy is associated with a 20% increase in oxygen consumption and a 15% increase in metabolic rate. During the first trimester, minute ventilation is increased while respiratory rate remains the same. Although one might assume that lung volume during pregnancy would decrease owing to the rise in the maternal diaphragm, tidal volume (VT) is actually increased by 40% over baseline values. The increase in VT is thought to be due to the increase in circulating progesterone that affects the respiratory center.1 Arterial blood gas measurements reflect a respiratory alkalosis that is compensated by a metabolic acidosis that results in a relatively normal pH. PaCO2 usually ranges from 28 to 32 mm Hg. Functional residual capacity (FRC), residual volume, and total lung volume are decreased near term. Because of this decrease, respiratory distress occurs more rapidly in the gravid than in the nongravid state. The function of the large airways as measured by forced expiratory volume at 1 second (FEV1), and peak expiratory flow rate (PEFR) is essentially unchanged throughout pregnancy.2
Figure 160-1 illustrates the graphic relationship of pulmonary changes.
 Asthma
Asthma
Epidemiology
Asthma is one of the most common pulmonary problems in pregnant women; recent studies report that approximately 8% are affected.3 The disease is characterized by hyperactive airways leading to episodic bronchoconstriction. The role of inflammatory mediators in the pathogenesis of asthma has become apparent in recent years, leading to earlier use of inflammatory medications in the treatment of exacerbations.
Effects of Asthma on Pregnancy
Asthma may be triggered by environmental allergens, medications, especially aspirin or nonsteroidal antiinflammatory drugs (NSAIDs), or stress.4 Most exacerbations are marked by cough, wheezing, and dyspnea. Rapid therapeutic intervention at the time of an exacerbation is imperative to prevent impaired maternal and fetal oxygenation, because uncontrolled asthma can increase maternal morbidity. In several studies, even after controlling for confounding variables, adverse pregnancy outcomes are more pronounced in patients with asthma. These include low birth weight, preeclampsia, preterm birth, and stillbirth.5,6
Whereas historical data have shown an increase in perinatal death and low birth weight,7 Fitzsimmons and colleagues observed low birth weight in only those patients treated for status asthmaticus.8 In addition, Schatz and colleagues noted that intrauterine growth restriction was directly related to lung function as measured by FEV1.9
Effect of Pregnancy on Asthma
Numerous studies have observed that the course of asthma may be affected by pregnancy. Gluck et al. found that on average, asthma improved in 36% of women during pregnancy, remained unchanged in 41%, and worsened in 23%.10 Schatz et al., in an analysis of 366 pregnancies in which patient status was followed by objective criteria, found that asthma improved in 28%, remained unchanged in 33%, and worsened in 35%. Fifty-nine percent of the patients had similar asthma control in successive pregnancies.11
Fetal sex may influence asthma in pregnancy. In one study, mothers who gave birth to boys were more likely to report improved asthma symptoms.12 Dodds and colleagues also found that the use of medications to treat asthma was less common in mothers of boys.13 While a number of hypotheses have been proposed, including alterations in progesterone and the role of leukotrienes, changes in not one of these mediators can explain the varied course of the pregnant asthmatic.14
Management
The National Asthma Education and Prevention Program (NAEP) issued specific guidelines regarding asthma treatment. In 1993, the Working Group on Asthma and Pregnancy established criteria for diagnosis and treatment in the gravid population (Figure 160-2).15
Pharmacologic therapy is the mainstay of asthma treatment. Most drugs used in the treatment of asthma are thought to be safe in pregnancy. Inhaled β-agonists are the most frequently used in asthma treatment. A prospective study of inhaled β-agonists in 259 pregnancies showed no change in the rate of congenital malformation, perinatal mortality, low birth weight, or complications of pregnancy.16 There is little role for the use of oral β-agonists, which may have more adverse systemic symptoms and are no more effective than inhaled drugs.
Inhaled corticosteroid therapy remains the mainstay of antiinflammatory treatment of asthma. Corticosteroids have also been advocated as first-line therapy in patients with mild asthma.17 Studies have demonstrated that with asthma, those taking an inhaled corticosteroid were four times less likely than their nontreated counterparts to suffer an exacerbation.18 Another randomized study noted that there was a 55% reduction in readmission rates for acute asthma in patients using inhaled beclomethasone.19 Inhaled corticosteroids can increase the effectiveness of β-adrenergic agents by inducing the formation of new β receptors. Because beclomethasone is the most studied of the inhaled corticosteroids in pregnancy, it is recommended as first-line therapy.15 However, if patients are well controlled on other corticosteroid preparations, it is suggested they be continued on their current medication, because all inhaled corticosteroids are labeled by the U.S. Food and Drug Administration (FDA) as pregnancy class C. Other antiinflammatory medications used in the treatment of asthma (e.g., cromolyn sodium and nedocromil sodium) appear to be less effective than inhaled corticosteroids in reducing asthma symptoms.
Intravenous corticosteroids have no increased benefits over oral corticosteroids in the treatment of acute exacerbations.20 Methylprednisolone, hydrocortisone, and prednisone are safe for use in pregnancy, unlike betamethasone or dexamethasone, because very little active drug crosses the placenta.
Leukotriene pathway moderators have been shown to improve pulmonary function, as measured by FEV1.21 Zafirlukast and montelukast are rated FDA category B; however, there is little experience with these drugs in pregnancy, and their role is undetermined.
The treatment of asthma requires patient education to provide optimization in the preconceptional period and during the pregnancy to provide optimum outcome. Box 160-1 offers a suggested schematic for the treatment of asthma in pregnancy.
Box 160-1
Treatment of Asthma in Pregnancy
Moderate Asthma
Status Asthmaticus
Status asthmaticus is a rare complication in pregnancy. Diagnosis is established by a PaO2 of less than 70 mm Hg, a PaCO2 of greater than or equal to 35 mm Hg, or a measured expiratory flow of less than 25% of expected. Because of impending respiratory failure, these patients should be managed in a critical care unit. Aggressive treatment of status asthmaticus is mandatory to protect the mother and fetus. Maternal mortality may be as high as 7% and fetal mortality as high as 11% despite adequate treatment. Epinephrine is not contraindicated in pregnancy during a respiratory emergency. Criteria for intubation in the gravida with status asthmaticus include (1) inability to maintain PaO2 of greater than 60 mm Hg despite supplemental oxygen; (2) inability to maintain a PCO2 of less than 40 mm Hg; (3) evidence of maternal exhaustion, with worsening acidosis (pH < 7.2) despite intensive bronchodilator therapy; and (4) altered maternal consciousness.15
When traditional treatment proves to be ineffective, a number of therapies have been reported beneficial. The use of a helium-oxygen mixture that has been reported to be effective in nonpregnant studies has been used safely in pregnancy.22
 Pulmonary Edema
Pulmonary Edema
Etiology
Other causes of acute pulmonary edema in pregnancy include amniotic fluid embolism, aspiration, and the need for massive transfusion after hemorrhage.23
 Acute Respiratory Distress Syndrome
Acute Respiratory Distress Syndrome
Etiology
The causes of acute respiratory distress syndrome (ARDS)24–27 in pregnancy include preeclampsia, sepsis, aspiration, pyelonephritis, intrauterine infections, acute fatty liver of pregnancy, and amniotic fluid embolism.28 In a review of 83 cases of ARDS associated with pregnancy, it was noted that among the causes of ARDS, 35 cases were attributed to uniquely obstetric conditions.29 In addition, it was noted that varicella pneumonia and pyelonephritis were associated with ARDS. These conditions rarely trigger ARDS in immunocompetent adults. De Vaciana et al. pointed out that development of lung injury in pregnancy correlates with known physiologic changes including increased blood volume, decreased colloid osmotic pressure, and an unchanged critical lung closing volume despite a diminished FRC.30
Management
Management of ARDS includes diagnosis, maternal stabilization, fetal monitoring, investigation and treatment of underlying causes, and in many cases, evaluation for delivery.29
Contemporary thinking regarding the treatment of ARDS has found that a lung-protective ventilator strategy is the first therapy that has been found to improve outcomes in ARDS. It has been noted in numerous studies that decreasing the VT from the standard of 12 mL/kg to 6 mL/kg or less and peak inspiratory pressures to less than 30 cm H2O from 50 cm H2O have resulted in decreased morbidity and mortality in patients with ARDS.31 There has been much discussion in the literature concerning permissive hypercapnia and its use in preventing lung injury. However, there have been no controlled studies in pregnancy, and it is the opinion of the author that increasing PaCO2 in the pregnant patient should be undertaken with caution.
A number of other methods have been discussed in the treatment of ARDS, including inhaled nitric oxide, prostacyclin, surfactant, and inverse ratio ventilation. Currently these modalities cannot be recommended, because they have not been shown to decrease morbidity and mortality. Other trials considering prone ventilation and corticosteroids in late ARDS appear promising but have not been proven in large prospective randomized trials.32–34
Fetal surveillance during ARDS may be more difficult because drugs used to sedate the mother can affect fetal heart rate and variability. Sedatives, anxiolytics, hypnotics, and nondepolarizing agents are not contraindicated in pregnancy. In addition, preterm contractions and labor may present a problem due to maternal hypoxemia. The clinician is cautioned against starting tocolytic therapy before achieving adequate maternal oxygenation. If tocolysis in needed, β-agonists such as terbutaline should be avoided because of the risk of increased pulmonary capillary permeability and increased demands on cardiac load. Magnesium sulfate is not strictly contraindicated but also may increase pulmonary capillary permeability. The use of NSAIDs may be the best choice for tocolysis because they have been proven to improve ARDS in animal models.29 Consultation with a maternal-fetal specialist is recommended to assist the intensivist in caring for these complex patients.
The timing of delivery of the patient with ARDS is a question that must be addressed by the clinician. Some authors advocate delivery after maternal stabilization, citing the possible “therapeutic effect” of delivery. Whitty and colleagues failed to demonstrate any significant benefit to delivery.33 It is this author’s opinion that delivery should be considered on a case-by-case basis, carefully weighing the risk/benefit ratio to the mother and fetus.
Box 160-3 represents a reasonable management scheme for the patient with ARDS.
Box 160-3
Management of the Patient with ARDS
 Embolism
Embolism
Clinical signs of a pulmonary embolism include unexplained tachycardia, dyspnea, diaphoresis, and a nonproductive cough. The workup for a suspected pulmonary embolism should include normal laboratory studies (arterial blood gases) and an electrocardiogram in conjunction with radiographic testing.35 Pregnancy should not prevent obtaining appropriate radiographic studies. In patients with a high clinical index of suspicion for thromboembolic phenomena, definitive diagnosis is imperative (Box 160-4). Ventilation-perfusion scans are recommended as the first diagnostic test. Spiral computed tomography has replaced ventilation-perfusion scanning in many centers as an initial test. Pulmonary angiography is still the gold standard for offering definitive diagnosis. All of the aforementioned tests use less than the 5 rads of radiation exposure that has been associated with fetal teratogenesis. The use of an abdominal shield further decreases fetal exposure.
Box 160-4
Treatment of Pulmonary Embolism in Pregnancy
 Pneumonia
Pneumonia
Concern over the H1N1 virus has reinforced the seriousness of influenza infection in pregnant patients. Historical data show that during an influenza pandemic, mortality rates among pregnant women are unusually high. Neuzil et al. noted that even during a normal season, compared to their postpartum counterparts, pregnant women were more likely to be hospitalized.36 The risk of hospitalization was highest in the third trimester, with women nearly 5 times more likely to be hospitalized than the postpartum control group. Influenza-related morbidity occurs in 10.5 of 10,000 pregnant women, compared to a rate of 1.91 of 10,000 in nonpregnant controls. Influenza pneumonia mortality in pregnancy has been noted to range from 12.5% to 42.1%.38
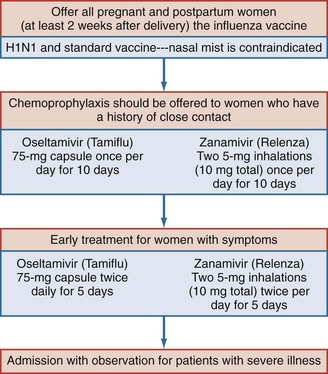
Contemporary management of influenza infection in pregnancy includes the use of antiviral medications for preventing and treating the disease. Amantadine and rimantadine have been shown to be effective in shortening the course and duration of disease in influenza A and influenza B. Recently, oseltamivir (Tamiflu) and Zanamivir (Relenza) has been recommended for prevention of influenza infection. Current Centers for Disease Control and Prevention (CDC) guidelines recommend that treatment be initiated for pregnant women (including patients until 2 weeks postpartum) with documented exposure to influenza virus and those patients who present with symptoms in the first 48 hours of illness, regardless of gestational age. Medication should be started at the first sign of symptoms; awaiting confirmation of the diagnosis and delaying therapy could result in rapid progression of disease. In the 2009 flu season, 6% of deaths were in pregnant women, even though only 1% of the population is pregnant at any given time. Data suggest that the use of antiviral medications can significantly reduce perinatal morbidity and mortality. Since 1995, the CDC has recommended that all pregnant women receive influenza immunizations. There has been some discussion regarding the use of thimerosal, which is used in the standard influenza vaccine; most authorities feel that the thimerosal-free vaccine when available is preferable. It is the opinion of the author that all pregnant patients who present with respiratory symptoms after exposure to viral illness should be hospitalized for observation.37–39 Changes in maternal respiratory physiology during pregnancy can make progression from mild respiratory distress to respiratory distress rapid and unpredictable36 (see Figure 160-1).
Key Points
Cole DE, Taylor TL, McCullough DM, Shoff CT, Derdak S. Acute respiratory distress syndrome in pregnancy. Crit Care Med. 2005;33:S269-S278.
Jenkins TM, Troiano NH, Graves CR, et al. Mechanical ventilation in an obstetric population: characteristics and delivery rates. Am J Obstet Gynecol. 2003;188:549-552.
Schatz M, Dombrowski MP. Asthma in pregnancy. N Engl J Med. 2009;360:182-189.
An excellent review article that evaluates asthma, pregnancy, and considerations for treatment.
Tomlinson MW, Caruthers TJ, Whitty JE, Gonik B. Does delivery improve maternal condition in the respiratory-compromised gravida? Obstet Gynecol. 1998;91:108-111.
Robertson L, Greer I. Thromboembolism in pregnancy. Curr Opin Obstet Gynecol. 2005;17:113-116.
Provides a current summary regarding the treatment of venous thromboembolism during pregnancy.
Graves CR. Pneumonia in pregnancy. Clin Obstet Gynecol. 2010;53:329-336.
1 England S, Fahri L. Fluctuations in alveolar CO2 and base excess during the menstrual cycle. Respir Physiol. 1976;26:157-161.
2 Stenius-Aarniala B, Piirila P, Teramo K. Asthma and pregnancy: A prospective study of 198 pregnancies. Thorax. 1987;43:12-18.
3 Kwon HL, Triche EW, Belanger K, Bracken B. The epidemiology of asthma during pregnancy: prevalence, diagnosis and symptoms. Immunol Allergy Clin North Am. 2006;26:29-62.
4 Schmidt GA, Hall JB. Pulmonary disease. In: Barron WM, Lindheimer MD, editors. Medical Disorders of Pregnancy. St. Louis: Mosby; 2000:197.
5 Enriquez R, Griffin MR, Carroll KN, et al. Effect of maternal asthma and asthma control on pregnancy and perinatal outcome. J Allergy Clin Immunology. 2007;120:625-630.
6 Wen SW, Demissie K, Lui S. Adverse outcomes in pregnancies of asthmatic women: results from a Canadian population. Ann Epidemiol. 2001;11:7-12.
7 Jana N, Vasishtak SC, Khunnu B. Effect of bronchial asthma on the course of pregnancy, labour and perinatal outcome. J Obstet Gynecol. 1995;21:227-232.
8 Fitzsimmons R, Greenberger PA, Patterson R. Outcome of pregnancy in women requiring corticosteroids for severe asthma. J Allergy Clin Immunol. 1986;78:349-353.
9 Schatz M, Dombrowski MP, Wise R, et al. Spirometry is related to perinatal outcome in pregnant women with asthma. Am J Obstet Gynecol. 2006;194:120-126.
10 Gluck JC, Gluck PA. The effects of pregnancy on asthma: The effect of pregnancy on the course of asthma. Immunol Allergy Clin North Am. 2006;26:63-80.
11 Schatz M, Harden K, Forsythe A, et al. The course of asthma during pregnancy, postpartum and with successive pregnancies: A prospective analysis. J Allergy Clin Immunol. 1988;81:509-517.
12 Beecroft N, Cochrane GM, Milburn HJ. The effect of fetal sex on asthma during pregnancy: Blind prospective study. BMJ. 1998;317:856-857.
13 Dodds L, Armson BA, Alexander S. Use of asthma drugs is less among women pregnant with boys rather than girls. BMJ. 1999;318:1011.
14 Tan KS, McFarkane LC, Lipworth BJ. Modulation of airway reactivity and diurnal peak flow variability in asthmatics receiving oral contraceptive pills. Am J Respir Crit Care Med. 1997;25:461-466.
15 National Asthma Education Program. Management of Asthma during Pregnancy. Report of the Working Group on Asthma and Pregnancy. Bethesda, MD: National Institutes of Health; 1993. NIH publication No. 93-3279
16 Schatz M, Zeigler RS, Harden KM, et al. The safety of inhaled [beta] agonist bronchodilators during pregnancy. J Allergy Clin Immunol. 1997;100:301-306.
17 Schatz M, Dombrowski MP, Wise R, et al. The relationship of asthma medications use to perinatal outcomes. J Allergy Clin Immunol. 2004;113:1040-1045.
18 Jaeschke R, O’Bryne PM, Mejza F, et al. The safety of long acting beta agonists among patients using inhaled corticosteroids: systemic review and metaanalysis. Am J Respir Crit Care Med. 2008;178:1009-1016.
19 Nelson HS, Carr W, Nathan R, Portnoy JM. Update of the safety of long acting beta agonists in combination with inhaled corticosteroids for the treatment of asthma. Ann Allergy Asthma Immunol. 2009;102:11-15.
20 Perlow JH, Montgomery D, Morgan MA, et al. Severity of asthma and perinatal outcome. Am J Obstet Gynecol. 1992;167:963-967.
21 Bakhierva LN, Jones JL, Schatz M, et al. Safety of leukotriene receptor antagonist in pregnancy. J Allergy Clin Immunol. 2007;119:618-625.
22 George R, Berkenbosch JW, Fraser RF, Tobias JO. Mechanical ventilation during pregnancy using helium oxygen mixture in a patient with respiratory failure due to status asthmaticus. J Perinatol. 2001;21:395-398.
23 Dorschera DR, Hall JB, Schmidt GA. Critical illness. In: Barron WM, Lindheimer MD, editors. Medical Disorders of Pregnancy. St. Louis: Mosby; 2000:252.
24 Ashbaugh DG, Bigelow DB, Petty TL, et al. Acute respiratory distress in adults. Lancet. 1967;2:319-323.
25 Wyncoll DLA, Evans TW. Acute respiratory distress syndrome. Lancet. 1999;354:497-501.
26 Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818-824.
27 Tomashehefski JF. Pulmonary pathways of adult respiratory distress syndrome. Clin Chest Med. 1990;11:593-619.
28 Mabie WC, Barton JR, Sibai BM. Adult respiratory distress in pregnancy. Am J Obstet Gynecol. 1992;167:950-957.
29 Cole DE, Taylor TL, McCullough DM, Shoff CT, Derdak S. Acute respiratory distress syndrome in pregnancy. Crit Care Med. 2005 Oct;33(10 Suppl):S269-S278.
30 DaVaciana M, Towers CV, Major CA, et al. Pregnancy injury associated with appendicitis in pregnancy: Who is at risk? Am J Obstet Gynecol. 1994;171:1008-1013.
31 Fan E, Needham DM, Stewart TE. Ventilatory management of acute lung injury and acute respiratory distress syndrome. JAMA. 2005 Dec 14;294(22):2889-2896.
32 McIntyre RC, Pulido EJ, Bensard DD, et al. Thirty years of clinical trial in acute respiratory distress syndrome. Crit Care Med. 2000;28:3314-3331.
33 Tomlinson MW, Caruthers TJ, Whitty JE, Gonik B. Does delivery improve maternal condition in the respiratory-compromised gravida? Obstet Gynecol. 1998;91:108-111.
34 Jenkins TM, Troiano NH, Graves CR, et al. Mechanical ventilation in obstetric population: Characteristic and delivery rates. Am J Obstet Gynecol. 2003;188:549-552.
35 Robertson L, Greer I. Thromboembolism in pregnancy. Curr Opin Obstet Gynecol. 2005 Apr;17(2):113-116.
36 Neuzil KM, Reed GW, Mitchel EF, Simonsen L, Griffen MR. Impact of influenza on acute pulmonary hospitalizations in pregnant women. Am J Epidemiol. 1998;148:1094-1102.
37 Graves CR. Acute pulmonary complications during pregnancy. Clin Obstet Gynecol. 2002;45:369-376.
38 Centers for Disease Control and Prevention. H1N1 updated interim recommendations for obstetric health providers. www.cdc.gov, 2009. Available at
39 Graves CR. Pneumonia in pregnancy. Clin Obstet Gynecol. 2010;53:329-336.