72 Acute Parenchymal Disease in Pediatric Patients
 Diseases of the Airways
Diseases of the Airways
Status Asthmaticus
Although unusual anatomic conditions of the lower airways can occur in pediatric patients (Table 72-1), status asthmaticus and bronchiolitis are probably the most common causes of lower-airway disease encountered in the pediatric ICU. Asthma is common in the industrialized world, and the overall mortality rate attributable to asthma in the United States is estimated at 2.6 deaths per million children per year.1 Recurrent hospitalizations, previous ICU admissions, and the need for mechanical ventilatory support have been identified as risk factors for death from asthma.2 Status asthmaticus is characterized by acute, severe airway obstruction due to bronchoconstriction that is refractory to initial management with supplemental oxygen, inhaled bronchodilators, and corticosteroids. The pathophysiology of this condition begins with a precipitant that triggers contraction of hyperresponsive bronchial smooth muscle, mucus secretion, and mucosal edema, all of which lead to the obstruction of large and small airways (Figure 72-1). Hyperinflation from airflow limitation and premature closure of lower airways in expiration leads to increased end-expiratory lung volume3 and an increased respiratory workload, which ultimately set the stage for alveolar hypoventilation and hypoxemia. An abrupt and profound acidosis can develop when respiratory compensation for accumulated inorganic acids ceases to occur.3 On physical examination, the child with status asthmaticus can appear anxious or lethargic, will often demonstrate accessory muscle use, and depending on the quality of air entry, can demonstrate either cough with profound inspiratory and/or expiratory wheezing and prolongation of audible expiration, or a silent chest. An exaggerated pulsus paradoxus can often be demonstrated, a finding that reflects the profoundly negative intrapleural pressures generated by these patients during spontaneous respiration.
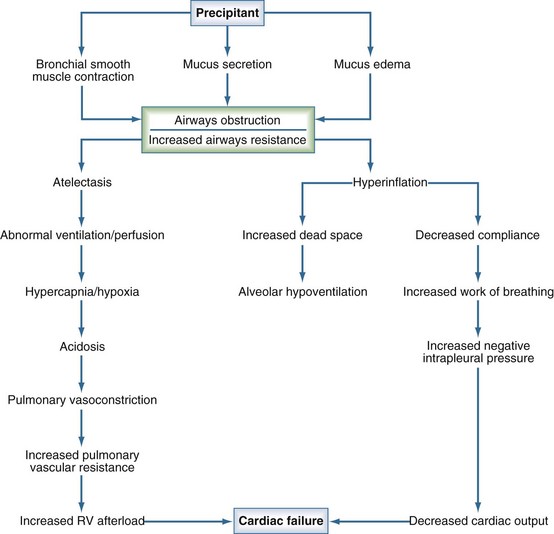
Figure 72-1 Pathophysiology of status asthmaticus.
(Modified from Helfaer M, Nichols D, Rogers M. Lower airway disease: bronchiolitis and asthma. In: Rogers M, editor. Textbook of Pediatric Intensive Care. 3rd ed. Baltimore: Williams and Wilkins; 1996, p. 141.)
Therapy
Supportive therapy for status asthmaticus begins with maintaining the airway, monitoring the quality of respirations, and ensuring euvolemia. Standard medical therapies for these patients include bronchodilators and corticosteroids, and several adjunct therapies have been investigated as possible rescue agents in difficult cases (Table 72-2). Short-acting β-agonist agents, which mediate airway smooth muscle relaxation via local β2 receptors,3 are the most commonly used bronchodilators for status asthmaticus. Among these agents, albuterol is the most widely used. Unlike epinephrine and isoproterenol, albuterol is relatively β2 selective,3 and it is most commonly administered by nebulization. It is typically given at a dose of 0.15 mg/kg (up to 2.5 mg/dose) on a frequent intermittent basis, but only a small fraction of the nebulized dose may actually be delivered to the lung, particularly in critically ill infants and children who are intubated with small tracheal tubes.4–6 Several studies have demonstrated that small doses of nebulized β-agonist given in rapid sequential fashion produce sustained improvements in forced expiratory volume more often than when larger doses are given less frequently,7,8 and there is also evidence to suggest that continuous nebulization of the drug may actually lead to more rapid and sustained clinical improvement.9
TABLE 72-2 Selected Pharmacotherapies for Status Asthmaticus
| Nebulized Therapies | Albuterol (0.5%), 0.15 mg/kg/dose (0.03 mL/kg/dose) inhaled q 1-6 h as needed (PRN) Continuous inhalation 0.5 mg/kg/h |
| Ipratropium, 0.25-0.5 mg inhaled q 4-6 h | |
| Racemic epinephrine (2.25%), 0.25-0.5 mL inhaled q 1 h PRN | |
| Subcutaneous (SQ) Therapies | Epinephrine (1 : 1000), 0.01 mg/kg/dose (0.01 mL/kg/dose) SQ (max 0.5 mL/dose) |
| Intravenous (IV) Therapies | Terbutaline, 10 µg/kg IV × 1, followed by 0.4-6.0 µg/kg/min IV infusion |
| Magnesium sulfate, 25-50 mg/kg IV over 20 minutes (max 2 g/dose) | |
| Methylprednisolone, 1 mg/kg/dose IV q 6 h |
In recent years, a preparation of the therapeutically active isomer of albuterol (levalbuterol) has become available. Levalbuterol appears to be effective when administered to children with stable asthma.10 There are no controlled trials presently available to evaluate its use in children with acute exacerbations of the disease. Inhaled anticholinergic agents such as ipratropium also have a role in the management of severe bronchospasm in children with asthma. Addition of inhaled ipratropium to inhaled β-agonists has been associated with favorable changes in pulmonary function, especially in children with severe asthma.11,12 For patients who do not respond to inhaled bronchodilators, it is possible to administer β-agonist therapy intravenously (IV). In some countries, the IV preparation of albuterol is available, which allows for an alternative administration route for this β2-selective agent. In the United States where IV albuterol is not available, terbutaline, which has some β2 selectivity, is a reasonable alternative. Although terbutaline has not been associated with clinically significant cardiac toxicity in most pediatric patients,3,13 many clinicians advise monitoring the electrocardiogram (ECG) and serum troponin level during its administration.
For as long as the inflammatory basis for asthma has been recognized, corticosteroids have had an important role in the management of status asthmaticus. The use of corticosteroids has been demonstrated to significantly improve airways obstruction in patients with severe acute asthma.14 The parenteral route is the method of choice for administering these agents to the critically ill child, and it is important to understand that fatal anaphylaxis to these drugs has been reported.15,16 Methylprednisolone is one of the most commonly used agents for acute severe asthma. Because of its half-life, steady-state levels can be achieved relatively quickly, and although dosing regimens vary, it is probably most appropriate to dose the drug every 6 hours. There does not seem to be any advantage to administering massive doses of glucocorticoids in status asthmaticus.17 If methylprednisolone is not available, equipotent doses of another glucocorticoid may be used.
Magnesium has been investigated for use in status asthmaticus because of its potential to augment the effects of bronchodilators by causing relaxation of airway smooth muscle. A recent randomized controlled trial in adults demonstrated that 2 g IV magnesium sulfate improves pulmonary function when administered as an adjunct to nebulized β-agonists and IV corticosteroids in patients with especially low forced expiratory volume in the first second of expiration (FEV1) (<20% of predicted).18 Although magnesium is occasionally added to standard therapy in pediatric status asthmaticus, the evidence supporting its use in this population is limited.19
Enthusiasm for the use of methylxanthines (theophylline, aminophylline) in pediatric asthma has fluctuated over time. These drugs act primarily as phosphodiesterase inhibitors, but the mechanism of their effects in asthma is not well understood. A recent randomized controlled trial investigated the effects of aminophylline in 163 children with status asthmaticus. Aminophylline was administered to these children as an adjunct to nebulized β-agonists, nebulized anticholinergics, and parenteral corticosteroids.20 The results of this trial suggested that aminophylline improved pulmonary function and may have averted intubation in a portion of those patients who received it.20 Although aminophylline may have a role in the treatment of severe status asthmaticus that is not responding to standard therapies, the potential for its widespread use is limited by its narrow therapeutic index.3
Bronchiolitis
Bronchiolitis is a clinical term implying an invasion of the large and small airway respiratory epithelium by inflammatory cells in the setting of acute respiratory illness. The primary cause of bronchiolitis is respiratory syncytial virus (RSV), which is responsible for 45% to 75% of cases, although parainfluenza viruses, rhinoviruses, adenoviruses, influenza viruses, enteroviruses, and Mycoplasma pneumoniae can produce the syndrome as well. RSV dependably produces yearly epidemics occurring during the winter and spring months. Infection with RSV is nearly universal among infants and children by 2 years of age. Although hospitalization rates vary seasonally and regionally, a recent study cited an average hospitalization rate between 3 per 1000 among children younger than 5 years of age, and 17 per 1000 among children younger than 6 months.21 Among all hospitalized children, the percentage requiring intensive care has been reported as 7% to 9% among patients without comorbidity and as high as 20% to 37% in those with preceding cardiac disease, chronic lung disease, prematurity, immunocompromise, and age younger than 6 weeks.22 Patients with these coexisting conditions are also at increased risk of mortality from RSV23 and have been identified as candidates to receive monthly prophylaxis with an RSV antigen–specific monoclonal antibody (Palivizumab [MedImmune Inc., Gaithersburg, Maryland]) during RSV season. However, recent epidemiologic data indicate that most RSV-infected children have no significant comorbidities, suggesting that prevention strategies targeting only medically complex patients may have minimal impact on the overall disease burden.21
RSV transmission can occur either by direct contact with contagious secretions or by exposure to aerosolized particles from the respiratory mucosa.24 The incubation period varies from 2 to 8 days,24 symptoms tend to escalate over 3 to 5 days, and convalescence can be prolonged up to several weeks in the most vulnerable small infants. On histologic examination, reappearance of ciliated respiratory epithelium commonly takes more than 2 weeks.24 Viral shedding from the respiratory tract typically occurs over 3 to 8 days but may also continue for up to 4 to 6 weeks in small infants. Symptoms typically begin with signs of upper respiratory illness, including fever, coryza, and possibly otitis media. Small infants commonly present with lethargy and central apnea25 early in the course of illness. Cough and tachypnea soon develop as the illness progresses to the lower airways, usually 1 to 3 days following incubation.24 Wheezing produced by flow limitation in peripheral airways is a nearly universal finding and may be due in large part to intermittent obstruction of large and small airways with necrotic epithelial debris, edema, and mucus24 rather than to the bronchospasm more commonly seen in asthma. Radiographic findings are often nonspecific but commonly include hyperinflation, peribronchial thickening, subsegmental consolidation, and multiple areas of atelectasis or infiltration involving most frequently the right middle and right upper lobes. A large prospective study of RSV-infected hospitalized children found that secondary bacterial infection occurred in only 1.2% of the study cohort, establishing that risk of bacterial disease is low in RSV bronchiolitis, despite potentially suggestive radiographic findings and the widespread empirical use of broad-spectrum antimicrobial agents in these patients.26
Therapy
Treatment of the infant or child with bronchiolitis is primarily supportive. Many years of clinical experience with empirical use of symptomatic medical therapies have failed to determine a clear role for any of these agents in the management of this disease. Data on the use of medical therapies in critically ill children with bronchiolitis is especially scant. Aerosolized ribavirin, a synthetic guanosine analog with broad-spectrum antiviral activity, is currently the only specific therapy approved for hospitalized infants with RSV bronchiolitis.24 In general, it has been shown to improve oxygenation and clinical status scores and reduce inflammatory mediators associated with ongoing wheezing in patients with RSV.24 A meta-analysis of three studies on the use of ribavirin in ventilated patients showed a small but significant decrease in ventilator days associated with the use of this agent.27 Nonetheless, prospects for widespread administration of this agent or even additional large-scale trials to further evaluate its role are limited by the technical challenges, cost, and occupational hazards associated with its use.28–30
Widespread use of bronchodilators and corticosteroids for the management of bronchiolitis is common despite the absence of evidence for improved clinical outcomes in critically ill children.27 There are presently no randomized controlled trials that have evaluated the efficacy of bronchodilators in critically ill children with bronchiolitis.31 Moreover, a recent large randomized controlled trial,32 as well as a systematic review,33 have failed to establish that any bronchodilator produces a significant improvement in relevant outcome measures in less severely ill hospitalized children with bronchiolitis. A few small studies have associated some short-term physiologic benefit with the use of corticosteroids and immune globulin in critically ill infants and children with bronchiolitis, but the efficacy of these therapies in altering outcomes in this population remains unproven.27 Following from the observation that critically ill children with severe bronchiolitis demonstrate surfactant deficiency and dysfunction, a great deal of interest surrounds the use of exogenous surfactant to modify the course of bronchiolitis in intubated patients. A number of small underpowered trials have been conducted on this topic,34–36 but the available data are not sufficient to provide a reliable estimate of surfactant’s effects in this setting.37 Moreover, the interpretation of this literature is complicated by the fact that the choice of surfactant preparation, the dosing regimen, and the mechanical ventilation strategy vary across studies, and each of these could have an important effect on outcome.37 An ongoing multicenter randomized controlled trial evaluating the impact of the synthetic surfactant, lucinactant (Discovery Laboratories, Warrington, Pennsylvania), on duration of mechanical ventilation among children younger than 2 years of age with acute hypoxemic respiratory failure38 may provide additional insight into surfactant’s therapeutic role in critically ill patients with bronchiolitis. Because future prospects for providing lasting immunity to RSV remain doubtful,24 there is an ongoing need for large multicenter studies to identify therapies which may benefit critically ill children with this disease.
Mechanical Ventilation
The need for mechanical ventilation in the patient with lower airways disease commonly arises from failure of ventilation and resulting hypercapnia. Hypoxemia and recurrent apnea, which are common in young infants with bronchiolitis, also frequently precipitate the institution of ventilatory support. Assuming adequate airway protection, oxygenation, and respiratory drive, it is probably best to avoid intubation in the patient with lower airways disease unless the overall clinical status of the child warrants the risk of augmenting airway hyperreactivity through airway instrumentation.39 To this end, there are several adjunct therapies that may obviate the need for intubation when added to aggressively applied conventional therapies. An inspired mixture of helium and oxygen (heliox) has been used to alleviate airflow limitation in pediatric patients. Owing to its low density and reduced Reynolds number, helium is able to convert turbulent gas flow to laminar flow in airways, and its clinical effect is generally immediate. Because it is an inert gas, it can potentially lower airway resistance without toxicity. When given as 60% to 80% of the total inspired gas mixture, helium can produce more efficient delivery of oxygen as well as nebulized drugs.40
The use of heliox in patients with lower-airway disease has generally produced inconsistent results. A small randomized controlled trial in spontaneously breathing children with status asthmaticus demonstrated that administration of heliox improves respiratory mechanics by lowering the pulsus paradoxus, increasing peak flow, and decreasing the dyspnea index, which may decrease the need for mechanical ventilation.41 In another small series, a 60 : 40 heliox mixture administered to 7 intubated patients resulted in a 15% to 50% reduction in peak inspiratory pressure and a 30% to 60% reduction in PaCO2.42 A recent literature review on the use of heliox in patients of all ages with acute asthma concluded that it may be useful in the short-term management of these patients, but any clinical advantage attributable to its use seems to diminish over time.43 There is little evidence available on the use of heliox in critically ill patients with bronchiolitis. This issue was prospectively investigated in a nonrandomized study of 38 nonintubated infants with RSV bronchiolitis admitted to an ICU.44 The investigators were able to demonstrate favorable changes in respiratory status through the first 4 hours of heliox administration and a significant decrease in ICU length of stay among infants who received heliox therapy.44 In a small randomized crossover study of RSV-positive, nonintubated patients, clinical indicators of respiratory status improved during heliox administration, particularly among children with more severe disease.45 However, many of the patients required another form of respiratory support, and the study was not designed to evaluate longer-term outcomes such as ICU length of stay.45
The application of noninvasive forms of mechanical support such as continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BiPAP) using either a nasal interface or full face mask has potential advantage in the patient with adequate respiratory drive. Careful titration of applied CPAP (or positive end-expiratory pressure [PEEP]) noninvasively may prevent premature airway closure during expiration and decrease gas trapping (see later discussion). The patient who develops high levels of intrinsic PEEP due to hyperinflation manifests an increased work of breathing and, ultimately, respiratory muscle fatigue, which may precipitate dramatic and rapid clinical deterioration. Noninvasive respiratory support may allow unloading of the muscles of respiration without adding to airway reactivity and has been used with success in managing asthma as well as bronchiolitis.46–48
In the patient with respiratory failure for whom noninvasive mechanical support is not feasible, intubation and mechanical ventilation is warranted. As tracheal intubation is performed in the patient with airways disease, the clinician should be watchful for complications arising from the transition to positive-pressure ventilation. In the spontaneously breathing child with severe airway obstruction, profoundly negative intrathoracic pressures develop in order to generate lung inflation. These conditions produce maximal venous return as right atrial pressure remains subatmospheric.49 The transition to positive-pressure ventilation in this setting increases juxtacardiac pressures and right ventricular afterload, resulting in decreased venous return, decreased left ventricular compliance, and decreased left ventricular end diastolic volume,49 with risk of hypotension and cardiac arrest.3
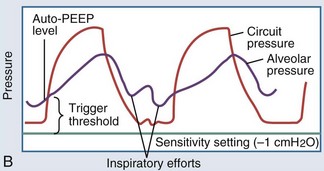
In intubated patients with status asthmaticus or bronchiolitis, low elastic recoil and increased airway resistance due to bronchoconstriction, airway edema, and mucus plugging contribute to regional gas trapping and dynamic hyperinflation (Figure 72-2, A). Gas trapping can also be exacerbated by the patient’s forced expiratory efforts, during which increased abdominal pressure is transmitted to the pleural space, potentiating premature airway closure and the development of excess or intrinsic PEEP (“auto-PEEP”). The magnitude of the auto-PEEP reflects the degree of dynamic hyperinflation in patients with severe asthma.50 Dynamic hyperinflation and auto-PEEP have an adaptive purpose in increasing the elastic recoil pressure of the lung to a level that would eventually allow complete evacuation of inhaled volume.50 However, this increase in lung volume takes place at the expense of an unfavorable change in pulmonary compliance. Other potential consequences of dynamic hyperinflation and auto-PEEP include air leak, hemodynamic compromise from sustained elevations in pulmonary vascular resistance, and increased inspiratory workload from the patient’s attempts to drop the ventilator circuit pressure below the total PEEP level (applied or set PEEP plus auto-PEEP) to trigger a breath (see Figure 72-2, B). The development of gas trapping and auto-PEEP can be inferred if the flow-versus-time waveform on the ventilator console shows initiation of inspiratory flow before the expiratory flow from the preceding breath reaches zero. Alternatively, the ventilator can quantify auto-PEEP by allowing the alveolar pressure to equilibrate with pressure at the airway opening during an end-expiratory hold maneuver. The accuracy and reliability of each of these techniques rest on the premise that all lung units communicate with the airway opening, which may not be true if bronchial obstruction is severe.51
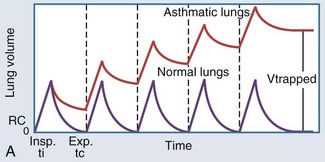
Figure 72-2 A, Dynamic hyperinflation.
Expiratory flow limitation in the asthmatic lung (upper tracing) causes incomplete evacuation of lung volume at end exhalation. Repetitive cycles of gas trapping lead to excess pressure accumulation at end exhalation (“auto-PEEP”), with a progressive shift toward ventilation on the less compliant (upper and outer) portion of the pressure-volume curve (see also Figure 35-3).
(Adapted from Stather DR, Stewart TE. Clinical review: mechanical ventilation in severe asthma. Crit Care 2005;9:581-7.)
In summary, initial ventilator settings in patients with lower-airway disease should be guided by observation, auscultation, careful ventilator waveform analysis, and attention to inspiratory plateau pressure. Ultimately, the choice of ventilator mode is not as important as a thorough understanding of how any mode might be strategically manipulated to alleviate the pathophysiology of gas trapping and auto-PEEP. It is generally preferable to allow the patient to breathe in a spontaneous ventilator mode, using a strategy of permissive hypercapnia. In spontaneously breathing mechanically ventilated patients, applied PEEP can be titrated cautiously upward as needed to improve respiratory mechanics to a level not exceeding 80% of the auto-PEEP, or until the plateau pressure begins to exceed a tolerable limit, which is usually around 30 cm H2O.51,52 If controlled ventilation is necessary, it is preferable to apply the lowest minute ventilation that provides adequate gas exchange.53 The use of neuromuscular blocking agents should be limited to the shortest feasible course because of their potentially detrimental effect on the relationship between ventilation and perfusion, and because of the risk of myopathy when these agents are administered together with corticosteroids.54 High-frequency oscillatory ventilation (see later discussion) has been used to rescue a limited number of pediatric patients with asthma and bronchiolitis who demonstrate respiratory failure refractory to management with conventional ventilation.55 One recent report recommends the use of high distending pressures to decrease airway resistance, as well as low frequencies, longer expiratory times, and muscle relaxation to minimize gas trapping.56
Sedation is an important component of managing intubated patients with lower-airway disease. Besides alleviating distress and promoting synchrony with the ventilator, sedative agents can be helpful adjuncts in limiting carbon dioxide production and reducing mechanical ventilatory requirements.51 Ketamine, a dissociative anesthetic with sympathomimetic and bronchodilatory properties, is often used for sedation in the intubated asthmatic child.57 Because of its favorable effects on airway reactivity, the inhalational anesthetic, isoflurane, may be a useful adjunct to managing severe status asthmaticus in the intubated child who is difficult to sedate or unresponsive to other therapies. The mechanism underlying its bronchodilatory properties is not well understood.58 Although isoflurane has a better safety profile than halothane when used for this purpose, periodic monitoring of renal function may be advisable in the child who requires prolonged therapy with this agent.58
 Diseases of the Alveoli
Diseases of the Alveoli
Viral Pneumonia
Defined as acute respiratory symptoms accompanied by parenchymal infiltrates on chest x-ray, pneumonia is a common syndrome in children and is most commonly caused by viral or bacterial pathogens.59 Important viral pathogens responsible for pneumonia in infants and children include RSV, influenza, parainfluenza, and adenovirus. As previously discussed, each of these is agents is also capable of producing the clinical syndrome of bronchiolitis in infants and children. The precise infectious etiology for pediatric viral pneumonias may be suggested by the physical examination, the age of the patient, and seasonal incidence patterns. Confirmatory testing through microbiologic analysis is generally sought to facilitate therapeutic decision making and cohorting of similarly affected patients. RSV is the most common viral cause of lower respiratory infection in infancy60 and primarily infects the small airways. Influenza is another very important cause of pediatric pneumonia. Infection rates in healthy children are estimated at 10% to 40% each year, and approximately 1% of these children require hospitalization.60 The course of up to 25% of infected children is complicated by lower respiratory tract disease.60 Neonates and children up to 5 years of age, especially those with underlying lung disease, congenital heart disease, immunocompromise, and other chronic conditions, seem to be at special risk for influenza pneumonia.60 Neonates are at risk for especially severe influenza syndromes which may also include apnea and sepsis.60 Infants and children older than 6 months of age, especially those in high-risk categories, are candidates for annual vaccination against influenza.61 Antiviral therapy for A and B strains of influenza are now available and can be considered for patients of appropriate age who are at high risk of complicated or severe disease.60 When administered within 48 hours of disease onset, amantadine, which is approved for use in children older than 1 year of age, may decrease the severity of influenza A disease, but data in young patients are limited.60 Oseltamivir, a neuraminidase inhibitor active against both A and B strains of influenza, has been demonstrated to decrease symptom duration when administered early in disease. When originally licensed for pediatric administration, oseltamivir was not approved for use in infants younger than 1 year.62 However, increased experience using oseltamivir in smaller infants during the 2009 H1N1 influenza pandemic produced some consensus on appropriate dosing guidelines in this age group.63 Unlike RSV, influenza is commonly associated with secondary bacterial pneumonia that is typically caused by Streptococcus pneumoniae or Staphylococcus aureus, making it especially important to consider appropriate empirical antimicrobial therapy when clinically appropriate.64,65 Parainfluenza viruses are also responsible for causing pneumonia in children, and seasonal epidemics commonly occur in autumn.60 Primary infection tends to occur in young children 2 to 6 years of age, and recurrent infection is generally less severe, except perhaps in the immunocompromised host.60 Finally, adenoviruses have been reported to cause up to 20% of pneumonias in children younger than 5 years of age, and the mortality rate attributable to the disease in this population has been reported as high as 20%.66 In neonates, adenovirus can produce an especially severe syndrome of disseminated disease and sepsis, which can present in the first 10 days of life.66 The incubation period is generally 2 to 14 days,60 and the virus can produce a profound and destructive lower-respiratory process. Necrotizing bronchitis, purulent exudative alveolitis, and hyaline membrane formation have been identified on autopsy specimens of affected patients.66 Survivors of severe adenoviral infections commonly demonstrate chronic sequelae such as recurrent wheezing and bronchiolitis obliterans.66
Bacterial Pneumonia
Most commonly, bacterial presence is established in the lower respiratory tract as a result of oropharyngeal overgrowth of environmentally acquired pathogens and subsequent introduction of these secretions into the lower airways. Children with aspiration syndromes, immunodeficiencies, and malformations of the respiratory tract are at increased risk of bacterial lower respiratory infection.67 Bacterial pathogens remain an important cause of potentially lethal pediatric pneumonias in the developing world, and they are the most important cause of severe pneumonia in Europe and North America, especially when complicated by parenchymal necrosis and/or parapneumonic effusion.59 It is challenging to establish a causal role for specific bacteria when these agents are normally found in the upper airway secretions, the specimen most commonly sampled for microbiologic diagnosis in children. The best data regarding the etiology of community acquired pneumonia come from lung-puncture studies revealing that S. pneumoniae, Hemophilus influenzae, and S. aureus are among the most important causes.59 Since the introduction of a conjugate vaccine against H. influenzae type B (Hib) in 1988, the incidence of invasive disease in infants and young children attributable to this organism has declined by 99%.60 Other serotypes of the organism, including nonencapsulated strains, may also cause pneumonia in children.60
A comprehensive review of necrotizing pneumonia cases occurring in predominantly immunocompetent children admitted to Children’s Hospital Boston between 1990 and 2005 indicates that parenchymal necrosis appears to be an increasingly common complication of pediatric bacterial pneumonia.68 In this series, S. pneumoniae was the predominant inciting organism, accounting for 22% of cases. Since 2002, many more organisms, including methicillin-sensitive S. aureus, methicillin-resistant S. aureus, Fusobacterium species, Pseudomonas species, and other Streptococcus species, have emerged as important causes of necrotizing pneumonia as well. Despite the short-term morbidity in these children, conservative management (consisting mainly of antibiotics and chest drainage) appeared sufficient to produce resolution of clinical symptoms within 2 months of hospital discharge, and marked improvement of imaging findings within 6 months.
Recent studies on the epidemiology of pediatric pneumonia complicated by parapneumonic effusion indicate that the incidence of empyema appears to have risen during the 1990s.69–71 During that period, S. pneumoniae was isolated most commonly from patients with empyema, followed by Streptococcus pyogenes and S. aureus.70,71 As in the case of necrotizing pneumonia, temporal trends in the epidemiology of pediatric empyema in the United States show a shift in causative organisms after the year 2000, when the heptavalent pneumococcal conjugate vaccine (PCV) was licensed for widespread use. A large case series reported from Texas Children’s Hospital indicates that since 2000, S. aureus has overtaken S. pneumoniae as the most common bacterial pathogen isolated from children with empyema, and the majority of S. aureus isolates in this cohort were methicillin resistant.69 In addition, nonvaccine serotypes (particularly serotypes 1, 3, and 19A) predominate among causes of pneumococcal empyema in the post-PCV era.70,72 The overall impact of widespread vaccination with PCV on the incidence of pediatric empyema across the United States is less clear. In Utah, where pneumococcal serotype 1 has always been prevalent, the incidence of pediatric empyema is still rising, while data from Texas Children’s Hospital show a decrease in the incidence of empyema since the vaccine became available.69,70
In neonates and young infants up to about 3 months of age, group B Streptococcus (GBS), Listeria monocytogenes, and gram-negative enteric organisms are the major causes of pneumonia and sepsis.60,67 Widespread maternal intrapartum antibiotic prophylaxis has influenced the incidence of perinatal GBS infection as well as its antimicrobial resistance patterns.73 The incidence of GBS sepsis has declined among very low-birth-weight infants in the era of ampicillin prophylaxis, while the incidence of Escherichia coli sepsis (largely resistant to ampicillin) has increased in the same time period.73 Perinatally acquired Chlamydia trachomatis is another important cause of lower respiratory tract infection in infants up to 12 weeks of age.67 Although uncommon, periodic epidemics of infection with Bordetella pertussis occur among incompletely immunized infants and children.67 Apnea and intermittent cyanosis progressing to respiratory failure and shock can develop in young infants infected with B. pertussis, and clinicians should have a relatively low threshold for admitting these patients to the ICU.
Therapy
In the clinical setting, one is often faced with having to select empirical antimicrobial therapy before arriving at a definitive viral or bacterial diagnosis. The presence of a focal alveolar process on chest radiographs, especially if accompanied by significant parapneumonic effusion, evidence of parenchymal necrosis, and/or abnormal peripheral blood counts and C-reactive protein, all add considerably to the predictive value for the presence of bacterial disease.59 Before demonstrating evidence of localized infection, neonates and young infants may demonstrate nonspecific but potentially ominous signs of lethargy, hypothermia, and apnea. Infants younger than 3 months of age should be treated with both ampicillin and gentamicin, and consideration should be given to adding a third-generation cephalosporin in severe cases.59 Investigation and empirical coverage for infection with B. pertussis should also be considered in infants with severe respiratory disease that features profound peripheral lymphocytosis, paroxysmal cough, and/or apnea.
For the critically ill child with community-acquired bacterial pneumonia, reasonable coverage may be assured with a third-generation cephalosporin,59,67 although some centers advocate the use of clindamycin as a second empirical agent. A macrolide antibiotic can be added in cases where infection with atypical agents such as Mycoplasma pneumoniae and Chlamydia pneumoniae is possible, particularly in patients with sickle cell disease.59,74 Although emerging resistance to penicillins in S. pneumoniae is widely recognized, high doses of cephalosporins are still appropriate in the majority of penicillin-nonsusceptible strains, so long as concurrent meningitis is not suspected, but the addition of vancomycin may be warranted in some cases.59,75 If infection with S. aureus is possible, an antistaphylococcal penicillin such as oxacillin should be added unless local resistance patterns warrant the use of vancomycin.59 In patients at risk for aspiration pneumonia and in immunocompromised children, special consideration should be given to administration of two antibiotics effective against gram-negative organisms (such as Pseudomonas) and to optimizing coverage for anaerobic organisms.
Management of pleural effusion is another important consideration in the care of the patient with bacterial pneumonia. Although drainage of parapneumonic effusions is indicated under certain circumstances, satisfactory recovery may occur in many cases without intervention.76 Recently an evidence-based clinical practice guideline was developed for the medical and surgical treatment of parapneumonic effusions in adults.77 The panel issued management suggestions according to the underlying risk of poor clinical outcome, based on effusion size and loculation as well as chemical and microbiologic analysis of the pleural fluid.77 Pleural fluid drainage was recommended for large effusions occupying more than 50% of the hemithorax, whether or not loculation or pleural thickening is present. Drainage was also recommended for purulent effusions, those with positive culture or Gram stain, or those with pH less than 7.20 as measured by a blood gas analyzer.77 In situations where drainage is indicated, more complex or invasive options such as thoracoscopic or “open” procedures are likely to be necessary for sufficient control of the effusion.77 It must be emphasized that the consensus panel’s recommendations are based primarily on case series, historical controls, and expert opinion.77
The literature on parapneumonic effusion in children also does not presently provide robust evidence on which to base clinical intervention. The effect of image-guided needle aspiration versus percutaneous pigtail catheter drainage was examined in a 5-year retrospective study of pediatric parapneumonic effusions.78 When comparing outcomes in the two groups, the authors found no difference in length of stay but did report a significant decrease in the need for second intervention in patients who received a chest drain.78 Other independent predictors for second intervention in their study population included loculation of pleural fluid and pH less than 7.2. A combination of low glucose and low pH in the pleural fluid specimen was especially predictive of the need for reintervention.78 The decision to perform thoracostomy drainage in pediatric patients with parapneumonic effusion may depend on the clinical context in which it occurs. In cases where significant pleural fluid organization has taken place, some favor the administration of intrapleural thrombolytics to facilitate evacuation of fluid through the chest drain.79 Studies assessing the efficacy of this practice have produced conflicting results. In one uncontrolled case series, 54 of 58 children (93%) with pneumonia complicated by empyema who received intrapleural tissue plasminogen activator (tPA) did not require additional surgical drainage.80 However a randomized controlled trial that enrolled 454 adults with empyema showed no outcome benefit attributable to the administration of intrapleural thrombolytics, compared to chest drainage and routine supportive care alone.81 In recent years, video assisted thoracoscopic surgery (VATS) has gained popularity as a way to facilitate chest drainage through inspection of the pleural space, disruption of adhesions, and placement of chest drains in strategic locations.79 To date, at least two prospective pediatric trials have failed to identify an outcome advantage attributable to VATS when compared to thrombolytic-enhanced chest drainage and routine supportive therapy for empyema.82,83
Acute Lung Injury and Acute Respiratory Distress Syndrome
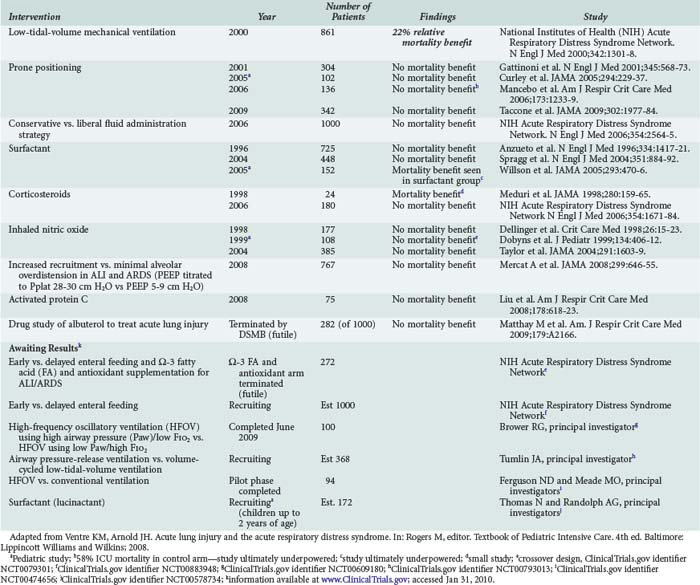
What was once known as adult respiratory distress syndrome is now called acute respiratory distress syndrome (ARDS) in an effort to acknowledge its prevalence in the pediatric population. A syndrome of lung injury featuring permeability edema leading to hypoxic respiratory failure had been described in adults for many years, but consensus criteria for the diagnosis of the syndrome did not enter the scientific literature until 1994.84 Once clear diagnostic criteria were established for ARDS and acute lung injury (ALI), the less severe form of the disease, large-scale randomized controlled trials began and have added considerably to our understanding of the epidemiology and outcomes of both conditions (Table 72-3). Both ALI and ARDS may arise as a consequence of primary pulmonary disease or as a feature of systemic pathophysiology that is nonpulmonary in origin. Using contemporary diagnostic criteria, ARDS is estimated to account for 1% to 4% of all PICU admissions, or approximately 10% of all children requiring mechanical ventilatory support.85,86 Pneumonia, which was responsible for 35% of cases in a recent epidemiologic study, appears to have overtaken sepsis as the most common cause of pediatric ARDS.85 Reported mortality rates for pediatric ARDS have fluctuated over time, depending on the criteria used to identify cases, the presence of important comorbidities such as immunocompromise and nonpulmonary organ failures among patients in the cohort, and the quality and consistency of supportive care provided in the ICU. Recent reported mortality rates for pediatric ARDS range from 8% in a prone-positioning trial87 in which the investigators protocolized nearly every conceivable aspect of supportive therapy, to 22% in a recent large prospective cohort study, a figure more comparable to the mortality rates reported from contemporary adult ARDS trials.52,85 The last decade has seen the completion of many multicenter trials designed to investigate the effects of various adjuvant therapies in pediatric and adult ALI and ARDS (Table 72-4). So far, tidal volume reduction during mechanical ventilation stands as the only intervention proven to offer a significant mortality benefit to patients with ALI and ARDS.
TABLE 72-3 American-European Consensus Criteria for Acute Lung Injury and Acute Respiratory Distress Syndrome
| Acute Lung Injury | Acute Respiratory Distress Syndrome |
|---|---|
| Acute onset | Acute onset |
| Bilateral pulmonary infiltrates on chest radiography | Bilateral pulmonary infiltrates on chest radiography |
| PAOP ≤ 18 mm Hg or no clinical evidence LA hypertension | PAOP ≤ 18 mm Hg or no clinical evidence LA hypertension |
| PaO2/FIO2 ratio ≤300 | PaO2/FIO2 ratio ≤200 |
Adapted from Bernard GR, Artigas A, Brigham KL et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818-24.
Mechanical Ventilation
Mechanical ventilatory support of the patient with ALI and ARDS is often necessary to provide adequate oxygenation. In relatively stable patients, noninvasive ventilation may be effective when instituted early in the disease process. This method has been used successfully in the management of acute hypoxic respiratory failure in a heterogenous population of adult patients88 and in a more selected population of immunocompromised adult patients.89 Each of these randomized controlled trials showed that early use of noninvasive ventilation decreased the need for intubation and reduced the risk of death in the ICU and in the hospital. Data on the use of noninvasive positive-pressure ventilation (NIPPV) in pediatric patients are limited, but several case series report success with the application of this technique in children with alveolar disease.90,91 In one study, noninvasive BiPAP was used to support pediatric patients with pneumonia, acute chest syndrome and sickle-cell disease, underlying chronic hypoventilation syndromes, and postoperative hypoventilation with atelectasis.90 The authors reported favorable changes in respiratory rate, heart rate, and oxygenation among all patients receiving noninvasive support, and 91% of respiratory failure episodes in their study were reversed without the need for intubation.90
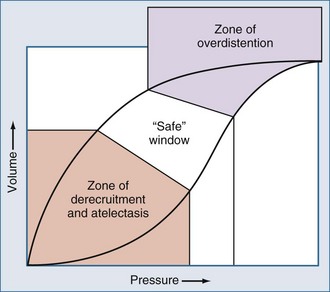
When noninvasive techniques are not appropriate or have failed, tracheal intubation is warranted. It has been well established in a number of animal and human studies that the mechanical ventilation strategy can have a profound influence on the course of disease and overall clinical outcome.52,92–95 Chief among these is the landmark multicenter study conducted by the ARDS Network (ARDSnet) investigators, which established that ALI and ARDS patients randomized to receive tidal volumes of 6 cc/kg ideal body weight had a mortality reduction of 22% relative to those who received ventilation using “traditional” tidal volumes of 12 cc/kg ideal body weight.52 Remarkably, this trial also demonstrated a greater reduction in plasma levels of the proinflammatory cytokine, interleukin 6 (IL-6), among those patients randomized to receive lower tidal volumes, suggesting that reducing the magnitude of phasic stretch during mechanical ventilation can actually attenuate the systemic inflammatory response. Over the last decade, much attention has been given to the provision of “lung-protective” mechanical ventilation in patients with acute lung injury and ARDS. Lung-protective ventilation involves (1) preservation of end-expiratory lung volume by judicious use of PEEP to minimize atelectrauma; (2) minimization of cyclic stretch; and (3) avoidance of parenchymal overdistension at end-inspiration by limiting tidal volume and transpulmonary pressure.52,92–95
When oxygenation failure is refractory to conventional ventilation, high-frequency oscillatory ventilation (HFOV) is an alternative modality that is well established in the pediatric population. During HFOV, lung recruitment is maintained by application of a relatively high mean airway pressure with superimposed pressure oscillations at a frequency of 3 to 15 Hz.95 Because maximal recruitment is maintained throughout the respiratory cycle, and ventilation is achieved using very small phasic changes in pressure and volume, this technique allows the lung to be ventilated above the critical opening pressure of injured lung units while avoiding end-inspiratory overdistension of more compliant lung units (Figure 72-3).96–98 This “open-lung” strategy of mechanical ventilation can capitalize on pulmonary hysteresis to achieve satisfactory gas exchange at lower alveolar pressures (see Figure 72-3). In 1994, a prospective multicenter randomized clinical study compared HFOV and conventional mechanical ventilation in pediatric patients with diffuse alveolar disease or air leak syndromes.99 Patients in the HFOV arm showed rapid and sustained improvements in oxygenation without suffering adverse effects on ventilation.99 Ultimately these patients showed a decreased incidence of ventilator-associated lung injury, as evidenced by a decreased need for supplemental oxygen at 30 days, and demonstrated improved outcomes compared to their cohorts in the conventional arm, particularly when HFOV was instituted within 72 hours of intubation.99 The oxygenation index (OI), defined as (MAP × FIO2 × 100)/PaO2, used often in the pediatric literature to quantify oxygenation failure, was shown to discriminate between survivors and nonsurvivors in the first 72 hours of therapy.99 Furthermore, the time at which changes in the OI were found to occur seemed to influence the likelihood of survival: an OI ≥42 at 24 hours predicted mortality with an odds ratio of 20.8, a sensitivity of 62%, and a specificity of 93%.99 In the time since this study was published, other investigators have helped establish that the OI seems to be a time-sensitive predictor of survival in patients with hypoxic respiratory failure, and OI trends can be used to facilitate decisions about the need for extracorporeal support in patients with acute hypoxic respiratory failure.100
 Diseases of the Interstitium
Diseases of the Interstitium
The interstitial lung diseases (ILD) in children are a diverse group of rare conditions that involve alteration of the alveolar wall, infiltration and fibrosis of the pulmonary interstitium, and loss of functional alveolar-capillary units.101 The major clinical findings include abnormal gas exchange, tachypnea, and crackles, as well as the potential for both restrictive and obstructive pulmonary physiology.102 There are numerous potential etiologies, ranging from primary congenital abnormities of the alveolar-capillary unit which present in early infancy, to acquired syndromes of chronic interstitial disease referable to infection, recurrent aspiration, or symptomatic cardiovascular disease (Table 72-5).101 In children, as in adults, the morbidity and mortality of these diseases are high,103,104 but the frequency distribution of specific etiologies may be very different in the two populations. The prevalence of specific ILD subtypes in the pediatric population has shifted in recent years, following publication of an international consensus statement on ILD classification.105 In the past, usual interstitial pneumonitis (UIP) and respiratory bronchiolitis had occasionally been described in children, but recent revisions to the classification and essential diagnostic criteria for each ILD subtype now cast doubt on whether either of these conditions actually exist in the pediatric population.105 On the other hand, several varieties of ILD are uniquely found in infancy, such as disorders of lung growth and development, neuroendocrine cell hyperplasia, follicular bronchitis/bronchiolitis, cellular interstitial pneumonitis, idiopathic pulmonary hemorrhage of infancy, and chronic pneumonitis of infancy due to congenital abnormalities of surfactant dysfunction.102 Overall, infectious etiologies may be relatively common in the pediatric population, accounting for perhaps 20% of pediatric ILD in some series.101,103 Given the wide variety of potential etiologies in ILD, a systematic approach to the diagnostic workup has been suggested.103 While history and physical exam have a role in the initial evaluation of a child with suspected ILD, noninvasive tests such as serologies, cultures, chest radiographs, high-resolution chest computed tomography (CT) scans, pulmonary function testing, barium swallow, pH studies, and echocardiograms will more often allow the clinician to arrive at a specific diagnosis.102,103 In those children in whom an etiology still cannot be determined, more invasive studies such as bronchoalveolar lavage, cardiac catheterization, and lung biopsy should be considered.103 Results of biopsy specimens may be particularly important to guide decision making in critically ill children who are not responding to therapy.
TABLE 72-5 Consensus Classification of Interstitial Lung Diseases
| Histologic Patterns | Clinical/Radiologic/Pathologic Diagnosis |
|---|---|
| Usual interstitial pneumonia1 | Idiopathic pulmonary fibrosis/cryptogenic fibrosis alveolitis |
| Nonspecific interstitial pneumonia | Nonspecific interstitial pneumonia |
| Organizing pneumonia | Cryptogenic organizing pneumonia2 |
| Diffuse alveolar damage | Acute interstitial pneumonia |
| Respiratory bronchiolitis1 | Respiratory bronchiolitis interstitial lung disease |
| Desquamative interstitial pneumonia | Desquamative interstitial pneumonia |
| Lymphoid interstitial pneumonia | Lymphoid interstitial pneumonia |
| Other Forms of Interstitial Lung Disease | |
| Primary pulmonary disorders | Alveolar hemorrhage syndromes |
| Aspiration syndromes | |
| Radiation or drug-induced lung disease | |
| Hypersensitivity pneumonitis | |
| Infectious or postinfectious chronic lung disease | |
| Pulmonary alveolar proteinosis | |
| Pulmonary infiltrates with eosinophilia | |
| Pulmonary lymphatic disorders | |
| Pulmonary microlithiasis | |
| Pulmonary vascular disorders | |
| Systemic disorders with pulmonary involvement | Connective tissue disease |
| Histiocytosis | |
| Lipid storage disease | |
| Neurocutaneous syndromes | |
| Malignancies | |
| Sarcoidosis | |
| Inborn errors of metabolism | |
1Not described in children; 2Previously known as bronchiolitis obliterans organizing pneumonia.
Adapted from Fan LL, Deterding RR, Langston C. Pediatric interstitial lung disease revisited. Pediatr Pulmonol 2004;38:369–78.
Therapy
As many of the etiologies for pediatric ILD may begin with an inflammatory response to lung injury, treatment of children with this condition commonly involves the use of antiinflammatory agents such as corticosteroids. A favorable response to corticosteroids among children with ILD may be evident in only 40% of cases,106 and this variability may reflect the diverse potential causes of the disease. In cases where concerns about long-term administration of corticosteroids arise, steroid-sparing antiinflammatory agents such as azathioprine, cyclophosphamide, methotrexate, cyclosporine, and IV gammaglobulin have been used.102 There is also a great deal of experience with the use of hydroxychloroquine in the management of pediatric ILD, although its use has been associated with the development of hepatic toxicity and retinopathy in children.106 Ultimately, identifying and controlling underlying causes and contributing issues are very important when this is possible.
 Complex Parenchymal Diseases
Complex Parenchymal Diseases
Bronchopulmonary Dysplasia
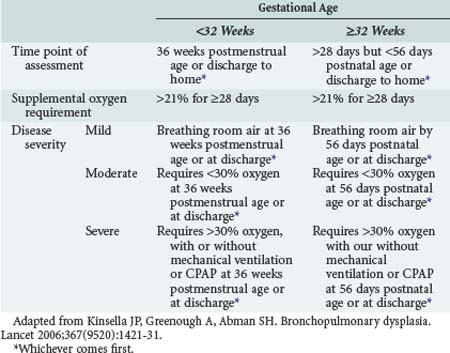
Bronchopulmonary dysplasia (BPD) is a term used to describe histopathologic changes in the lungs of neonates exposed to mechanical ventilation who go on to demonstrate radiologic abnormalities and supplemental oxygen dependence at 36 weeks postmenstrual age.107 Heterogeneous alveolar consolidation, squamous metaplasia of airway epithelium, hyperplasia of mucus glands, peribronchial fibrosis, airway smooth muscle hypertrophy, and vascular lesions of pulmonary hypertension once typified BPD-related histopathologic changes when the disease was first described by Northway and colleagues in 1967.108,109 The past 2 decades have witnessed a shift in the histopathologic features of BPD away from cystic, metaplastic, and fibroproliferative changes toward a more uniform distribution of lung aeration across fewer, larger, and more simplified alveoli.110,111 This progression likely documents the effects from more than 20 years of widespread intratracheal surfactant administration to preterm infants, as well as a trend toward the use of lung-protective ventilatory strategies and other improvements in the supportive care of these patients. Ten years ago, a consensus conference convened by the National Institutes of Health refined the diagnostic criteria for BPD in order to acknowledge its evolution into a disease with mild, moderate, or severe manifestations, depending on the intensity of respiratory support an infant requires at the point of assessment (Table 72-6).112 The revised criteria better represent the array of clinical manifestations seen in contemporary BPD and should facilitate the execution of clinical trials to identify subpopulations of infants who are likely to benefit from specific therapies.
In the current era, BPD is most likely to develop in premature infants who are born at a gestational age when alveolar development is not yet complete, and whose birthweight is less than 1000 to 1200 g.108,113 Clinically, the BPD syndrome is associated with airway hyperreactivity and intermittent airway obstruction, leading to increased work of breathing, recurrent wheezing, chronic abnormalities of gas exchange, and potentially significant pulmonary hypertension.108 Focal airway collapse consistent with tracheomalacia and/or bronchomalacia has also been documented in these infants,114 but their pathogenesis in this context is unknown. The spectrum of pathology observed in BPD patients is believed to derive from an inflammatory response to lung injury; numerous investigations have identified mediators of inflammation in the bronchoalveolar lavage (BAL) fluid of infants with chronic lung disease.115 Our present understanding of the pathogenesis of chronic lung injury in the neonate mirrors what has been learned from laboratory and clinical investigations of this process in older children and adults, but it is also important to recognize that in preterm infants, perinatally or postnatally acquired inflammatory lung injury takes place against a background of disrupted alveolar development. This is a key distinction between BPD and ARDS or ALI that develops in mature infants and older children, and likely accounts for the persistence of pulmonary morbidity in the BPD population into early adolescence.111 In any event, preterm neonates with respiratory failure may be especially susceptible to ventilator-associated lung injury because surfactant deficiency, high chest wall compliance, and a dynamic functional residual capacity (FRC) that is near closing capacity in this age group may potentiate cycles of derecruitment and reinflation that have been shown to promote the development of lung injury in humans and animal models, including surfactant-deficient preterm animals.92–94,116,117 Mechanical ventilatory techniques targeted to promote alveolar recruitment and maintain lung volume have in fact decreased the incidence of ventilator-associated lung injury in neonates. Numerous large prospective, randomized, controlled trials have found a lower incidence of chronic lung disease among high-risk infants supported with HFOV compared to cohorts who are supported with conventional phasic ventilation, with no apparent increase in the development of intracranial hemorrhage or other significant morbidities.118–120
Pulmonary edema from cardiogenic and noncardiogenic causes, infectious issues, and exposure to high concentrations of supplemental oxygen are other factors important in the pathogenesis of BPD. Premature infants may be at special risk from exposure to high concentrations of supplemental oxygen because they are deficient in the antiproteases and antioxidant enzymes that have a role in modulating the injurious effects from the proliferation of reactive oxygen species.107
Therapy
In the past 5 years, methylxanthines have emerged as having a potentially important role in the prevention of BPD. A large multicenter, randomized, controlled trial found that 36% of 963 very low-birth-weight infants who received caffeine in the first 10 days of life remained dependent on supplemental oxygen at 36 weeks postmenstrual age, compared to 47% in the placebo group (P < 0.001).121 Positive-pressure respiratory support was also discontinued 1 week earlier in the intervention group (P > 0.001). For those infants in whom BPD cannot be prevented, medications that may be useful in producing short-term improvements in their pulmonary mechanics include bronchodilators, corticosteroids, and diuretics (Table 72-7).107,108,122 Aerosolized β-agonists may be useful in the management of smooth muscle–mediated bronchospasm in the infant with chronic lung disease, but the consequent decrease in airway smooth muscle tone may aggravate airway collapse in the infant with tracheomalacia or bronchomalacia.114 Diuretics may be especially helpful in the management of these infants because many demonstrate a tendency to accumulate fluid in the pulmonary interstitium on the basis of alterations in pulmonary vascular resistance, plasma oncotic pressure, capillary permeability, and impaired lymphatic drainage.115 Judicious use of diuretics can also facilitate the delivery of adequate nutrition to the infant with chronic lung disease.115 Inhaled nitric oxide (iNO) has also been studied for its potential role in treating refractory hypoxemia in infants with chronic lung disease. Case series have documented improvements in oxygenation with the use of iNO, including in infants with intercurrent infection, with a sustained response reported in some cases.123,124
TABLE 72-7 Pharmacotherapies Commonly Used in the Management of Infants with Bronchopulmonary Dysplasia
| Inhaled Therapies | Albuterol (0.5%) 0.15 mg/kg/dose inhaled q 1-6 h PRN Continuous nebulization 0.5 mg/kg/h |
| Ipratropium 0.25-0.5 mg/dose inhaled q 4-6 h | |
| Fluticasone 44 mcg BID (maintenance therapy) (Max 440 µg/d) |
|
| Diuretic Therapies | Furosemide 1-2 mg/kg/dose IV/po q 6 h |
| Chlorothiazide 10-20 mg/kg/d IV divided q 12 h <6 months: 20-40 mg/kg/d PO divided q 12 h ≥6 months: 20 mg/kg/d PO divided q 12 h |
|
| Spironolactone 1.5-3.3 mg/kg/d PO divided q 6-24 h | |
| GI Therapies | Metoclopramide 0.1-0.2 mg/kg/dose IV/PO q 6 h (Max 10 mg/dose) |
| Ranitidine 1 mg/kg/dose IV q 8 h 2-3 mg/kg/dose PO q 12 h |
|
| Other Therapies | Caffeine citrate 20 mg/kg IV × 1 (load) followed by 5 mg/kg/d |
Lower respiratory tract infection is one of the most common reasons for hospital readmission in the first year of life for infants with BPD, and accounts for a significant fraction of these pulmonary exacerbations.113 Other potential causes for BPD exacerbations include aspiration syndromes, worsening pulmonary hypertension, and the evolution of clinically important systemic-to-pulmonary collateral vessels.108 Therefore, the diagnostic approach to the infant with BPD who demonstrates unexplained deterioration may include dynamic airway studies as well as echocardiography and in certain cases, cardiac catheterization.108 Treatment of these episodes is supportive and often includes empirical antibiotic coverage for potential infectious causes.
Congenital Diaphragmatic Hernia
Management of the infant with congenital diaphragmatic hernia (CDH) is one of the greatest clinical challenges the intensive care clinician encounters. The Bochdalek hernia is the most common form and occurs when abdominal contents herniate into the thoracic cavity through a posterolateral diaphragmatic defect, usually at around the 10th week of gestation. This phase of gestation concurrently includes the branching of bronchi and pulmonary arteries, and this crucial process may be interrupted by the growing mass of herniated viscera.125 On the other hand, the discovery that administering the teratogen nitrofen to mid-gestation rats results in diaphragmatic defects in the developing fetus as well as a spectrum of anomalies in other organ systems similar to what is seen in humans with CDH suggests that the pathogenesis of this syndrome may originate from fetal exposure to an agent that causes generalized maldevelopment from that point forward.126–129 The complex pathology associated with congenital diaphragmatic hernia in humans includes a hypoplastic and abnormally muscularized pulmonary arterial tree.125 Other congenital anomalies are associated with CDH in up to 39% of cases. Congenital cardiac disease is the most commonly associated feature and most frequently involves some degree of cardiac hypoplasia, although a wide variety of structural cardiac anomalies may be associated with CDH.130 Genitourinary, gastrointestinal, neurologic, and skeletal defects are also commonly described.125 Adjunct medical therapies have not managed to improve the discouraging survival statistics of these infants, whose mortality rate is traditionally reported in the range of 50%. Nonetheless, there are experienced centers that have reported more encouraging results in recent years by adopting strategic forms of mechanical support of these patients that incorporate much of what has been learned about modulating the pulmonary and hemodynamic consequences of mechanical ventilation.
Therapy
In infants with CDH, as in those with BPD, intensive care management is directed at managing their lower airways disease, alveolar disease, and abnormal pulmonary vascular reactivity. Initial medical stabilization of the infant with CDH includes endotracheal intubation and nasogastric decompression. It is preferable to obtain preductal (i.e., right radial) arterial access when possible. Information from preductal blood gases should guide clinical intervention, because it reflects the status of the cerebral circulation. Initially, echocardiography is suggested to rule out structural cardiac disease, and it may be repeated as necessary throughout the clinical course to determine evidence of ongoing right-to-left shunting as well as estimates of right ventricular pressure and function in response to therapy.125 Inhaled nitric oxide has been used in infants with CDH with varying results, and a role for the drug in reducing the need for extracorporeal membrane oxygenation (ECMO) or in improving survival among these patients was not established by a large, randomized controlled trial on the use of iNO in neonates with pulmonary hypertension.131 In general, evidence supporting the use of iNO in the management of infants with CDH is limited to small case series and individual case studies.132–134 In CDH, as in BPD, deficient alveolar development may explain the limited potential benefit from iNO.124 A limited number of reports have addressed the possibility of targeting an array of potential mechanisms behind pulmonary hypertension in CDH, including interference with calcium-mediated platelet activation and vasoconstriction (prostaglandin analogues), inhibition of endothelin-mediated vasoconstriction (bosentan), and inhibition of phosphodiesterase metabolism (sildenafil, milrinone), but none have been able to establish a clear outcome benefit for any of these agents in infants with this disease.135 At least one source has raised concern about the potential for hepatotoxicity when bosentan is used in infants.136
Recommendations for the optimal timing of surgical repair in infants with CDH have evolved over time. It was once considered appropriate to refer these infants for immediate repair. Growing experience with the mechanical support of CDH patients, along with the observation that pulmonary vascular resistance and reactivity as well as pulmonary compliance could become more favorable within days after birth, have since created a trend toward delaying surgical repair until a satisfactory level of physiologic stability can be achieved.125,137
Mechanical Ventilation
Given what is presently known about ventilator-associated lung injury, it is logical to apply lung-protective ventilation strategies to infants with chronic lung disease as well as to infants with CDH. Although the technique has not been traditionally applied to neonates, permissive hypercapnia is in fact well tolerated by most infants with these conditions.138–140 Because of the heterogeneity of airspace involvement in BPD and CDH, regional hyperinflation can easily occur. Therefore it makes sense to maintain end-expiratory lung volume with a careful titration of PEEP, and limit tidal volume to 4 to 6 cc/kg in order to ventilate at the area of maximal compliance on the pressure-volume curve.141 While managing these patients, monitoring tidal volume at the endotracheal tube is important because compressible volume losses in the ventilator circuit can be significant. Judicious use of sedation and the use of spontaneous ventilation (such as flow-triggered pressure support) may improve matching of ventilation to perfusion and may allow optimal patient-ventilator synchrony.
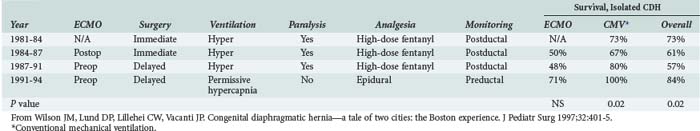
A review of all infants with CDH managed at Children’s Hospital Boston revealed a significant increase in survival from 44% to 69% during the period in which permissive hypercapnia was used to manage these infants, with even higher survival rates noted in infants without coexisting heart disease (Table 72-8).142 Of note, neither the introduction of ECMO nor delaying surgical repair was associated with significant increases in survival in this single-center historical experience.142 Other case series have also reported favorable results using kinder and gentler ventilatory strategies rather than more aggressive techniques that attempt to control pulmonary vascular resistance.137,143,144 These observations suggest that ventilator-associated lung injury greatly contributes to excess mortality in infants with CDH,137,142 and it is possible that a survival benefit attributable to ECMO may emerge as lung-sparing mechanical ventilation is more widely applied.142 At least one single-center experience suggests that epidural analgesia in the postoperative period facilitates spontaneous ventilation and may further improve pulmonary outcomes in these infants.142
TABLE 72-8 Therapeutic History and Outcomes for Congenital Diaphragmatic Hernia, Children’s Hospital, Boston
Over the past decade, experience with the use of HFOV in infants with CDH has grown. For those clinicians who opt to use HFOV in this population, it is essential to understand that infants with CDH do not have inherently recruitable lungs, and attempts to improve gas exchange by applying high levels of mean airway pressure can actually increase the dead-space fraction and may result in both lung injury and potentially dangerous elevations in pulmonary vascular resistance.145 Therefore, centers experienced in the use of HFOV in infants with CDH generally recommend trying to limit the mean airway pressure to 16 cm H2O or less.145 The Hospital for Sick Children in Toronto has developed an HFOV protocol for infants with CDH that emphasizes maintaining a preductal SaO2 above 85%, tolerating hypercarbia with a compensated pH, and initiation of HFOV when the peak inspiratory pressure on conventional ventilation exceeds 25 cm H2O. This group has reported a significant improvement in the survival of CDH infants since implementing this set of guidelines in 1995.145
 Weaning the Pediatric Patient from Mechanical Ventilation
Weaning the Pediatric Patient from Mechanical Ventilation
Although it is clear that it is best to discontinue mechanical ventilatory support as soon as feasible, a great deal of controversy surrounds ventilator mode selection, the pace of weaning, and timing of separation from mechanical support in children. In the largest pediatric study presently available in the literature, the use of specific weaning modes and ventilator weaning protocols was evaluated against standard care (no defined protocol) for mechanically ventilated infants and children.146 Patients with alveolar disease as well as lower-airway disease were included, but those older than 2 years of age with status asthmaticus and those with congenital diaphragmatic hernia were excluded. In this study, 182 intubated spontaneously breathing children who met standardized bedside criteria for extubation readiness were randomized to the protocolized application of pressure-support ventilation (PSV), volume-support ventilation (VSV), or no protocol.146 There were no significant differences among the three treatment groups in extubation failure rates, and most children were weaned from the ventilator in 2 days or less.146 In children who were successfully extubated, the median duration of ventilator weaning did not significantly differ according to mode of ventilation.146
2000 Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301-1308.
Flori HR, Glidden DV, Rutherford GW, et al. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171:995-1001.
Courtney SE, Durand DJ, Asselin JM, Hudak ML, Aschner JL, Shoemaker CT. High-frequency oscillatory ventilation versus conventional mechanical ventilation for very-low-birth-weight infants. N Engl J Med. 2002;347:643-652.
1997 Inhaled nitric oxide and hypoxic respiratory failure in infants with congenital diaphragmatic hernia. The Neonatal Inhaled Nitric Oxide Study Group (NINOS). Pediatrics. 1997;99:838-845.
Randolph AG, Wypij D, Venkataraman ST, et al. Effect of mechanical ventilator weaning protocols on respiratory outcomes in infants and children: a randomized controlled trial. JAMA. 2002;288:2561-2568.
1 Akinbami L. The state of childhood asthma, United States, 1980-2005: advance data from vital and health statistics; no 381. Hyattsville, Maryland: National Center for Health Statistics; 2006.
2 Turner MO, Noertjojo K, Vedal S, et al. Risk factors for near-fatal asthma. A case-control study in hospitalized patients with asthma. Am J Respir Crit Care Med. 1998;157(6 Pt 1):1804-1809.
3 Werner HA. Status asthmaticus in children: a review. Chest. 2001;119(6):1913-1929.
4 Bisgaard H. Delivery of inhaled medication to children. J Asthma. 1997;34(6):443-467.
5 MacIntyre NR, Silver RM, Miller CW, et al. Aerosol delivery in intubated, mechanically ventilated patients. Crit Care Med. 1985;13(2):81-84.
6 Ahrens RC, Ries RA, Popendorf W, et al. The delivery of therapeutic aerosols through endotracheal tubes. Pediatr Pulmonol. 1986;2(1):19-26.
7 Robertson CF, Smith F, Beck R, et al. Response to frequent low doses of nebulized salbutamol in acute asthma. J Pediatr. 1985;106(4):672-674.
8 Heimer D, Shim C, Williams MHJr. The effect of sequential inhalations of metaproterenol aerosol in asthma. J Allergy Clin Immunol. 1980;66(1):75-77.
9 Papo MC, Frank J, Thompson AE. A prospective, randomized study of continuous versus intermittent nebulized albuterol for severe status asthmaticus in children. Crit Care Med. 1993;21(10):1479-1486.
10 Milgrom H, Skoner DP, Bensch G, et al. Low-dose levalbuterol in children with asthma: safety and efficacy in comparison with placebo and racemic albuterol. J Allergy Clin Immunol. 2001;108(6):938-945.
11 Rebuck AS, Chapman KR, Abboud R, et al. Nebulized anticholinergic and sympathomimetic treatment of asthma and chronic obstructive airways disease in the emergency room. Am J Med. 1987;82(1):59-64.
12 Schuh S, Johnson DW, Callahan S, et al. Efficacy of frequent nebulized ipratropium bromide added to frequent high-dose albuterol therapy in severe childhood asthma. J Pediatr. 1995;126(4):639-645.
13 Chiang VW, Burns JP, Rifai N, et al. Cardiac toxicity of intravenous terbutaline for the treatment of severe asthma in children: a prospective assessment. J Pediatr. 2000;137(1):73-77.
14 Fanta CH, Rossing TH, McFadden ERJr. Glucocorticoids in acute asthma. A critical controlled trial. Am J Med. 1983;74(5):845-851.
15 Schonwald S. Methylprednisolone anaphylaxis. Am J Emerg Med. 1999;17(6):583-585.
16 Kamm GL, Hagmeyer KO. Allergic-type reactions to corticosteroids. Ann Pharmacother. 1999;33(4):451-460.
17 Manser R, Reid D, Abramson M. Corticosteroids for acute severe asthma in hospitalised patients. Cochrane Database Syst Rev 2003;1:CD001740.
18 Silverman RA, Osborn H, Runge J, et al. IV magnesium sulfate in the treatment of acute severe asthma: a multicenter randomized controlled trial. Chest. 2002;122(2):489-497.
19 Ciarallo L, Sauer AH, Shannon MW. Intravenous magnesium therapy for moderate to severe pediatric asthma: results of a randomized, placebo-controlled trial. J Pediatr. 1996;129(6):809-814.
20 Yung M, South M. Randomised controlled trial of aminophylline for severe acute asthma. Arch Dis Child. 1998;79(5):405-410.
21 Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(6):588-598.
22 Wang EE, Law BJ, Stephens D. Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) prospective study of risk factors and outcomes in patients hospitalized with respiratory syncytial viral lower respiratory tract infection. J Pediatr. 1995;126(2):212-219.
23 Stretton M, Ajizian SJ, Mitchell I, et al. Intensive care course and outcome of patients infected with respiratory syncytial virus. Pediatr Pulmonol. 1992;13(3):143-150.
24 Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344(25):1917-1928.
25 Anas N, Boettrich C, Hall CB, et al. The association of apnea and respiratory syncytial virus infection in infants. J Pediatr. 1982;101(1):65-68.
26 Hall CB, Powell KR, Schnabel KC, et al. Risk of secondary bacterial infection in infants hospitalized with respiratory syncytial viral infection. J Pediatr. 1988;113(2):266-271.
27 Davison C, Ventre KM, Luchetti M, et al. Efficacy of interventions for bronchiolitis in critically ill infants: a systematic review and meta-analysis. Pediatr Crit Care Med. 2004;5(5):482-489.
28 Wald ER, Dashefsky B. Ribavirin. Red Book Committee recommendations questioned. Pediatrics. 1994;93(4):672-673.
29 Turner RB. Red Book recommendations on ribavirin challenged. Pediatrics. 1994;93(5):873. author reply 873-874
30 Decker JA, Seitz TA, Shults RA, et al. Occupational exposures to aerosolized pharmaceuticals and control strategies. Scand J Work Environ Health. 1992;18(Suppl. 2):100-102.
31 Davison C, Luchetti M, Randolph A. Systematic review of treatments for critically ill infants with bronchiolitis (Abstract). Crit Care Med. 29(A144), 2001.
32 Wainwright C, Altamirano L, Cheney M, et al. A multicenter, randomized, double-blind, controlled trial of nebulized epinephrine in infants with acute bronchiolitis. N Engl J Med. 2003;349(1):27-35.
33 Kellner JD, Ohlsson A, Gadomski AM, et al. Bronchodilators for bronchiolitis. Cochrane Database Syst Rev 2000;2:CD001266.
34 Luchetti M, Ferrero F, Gallini C, et al. Multicenter, randomized, controlled study of porcine surfactant in severe respiratory syncytial virus-induced respiratory failure. Pediatr Crit Care Med. 2002;3(3):261-268.
35 Tibby SM, Hatherill M, Wright SM, et al. Exogenous surfactant supplementation in infants with respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1251-1256.
36 Luchetti M, Casiraghi G, Valsecchi R, et al. Porcine-derived surfactant treatment of severe bronchiolitis. Acta Anaesthesiol Scand. 1998;42(7):805-810.
37 Ventre K, Haroon M, Davison C. Surfactant therapy for bronchiolitis in critically ill infants. Cochrane Database Syst Rev 2006;3:CD005150.
38 Lucinactant for treatment of acute hypoxemic respiratory failure in children up to two years old [cited January 7, 2010. Available from. www.clinicaltrials.gov.
39 Roberts JS, Bratton SL, Brogan TV. Acute severe asthma: differences in therapies and outcomes among pediatric intensive care units.[comment]. Crit Care Med. 2002;30(3):581-585.
40 Anderson M, Svartengren M, Bylin G, et al. Deposition in asthmatics of particles inhaled in air or in helium-oxygen. Am Rev Respir Dis. 1993;147(3):524-528.
41 Kudukis TM, Manthous CA, Schmidt GA, et al. Inhaled helium-oxygen revisited: effect of inhaled helium-oxygen during the treatment of status asthmaticus in children. J Pediatr. 1997;130(2):217-224.
42 Gluck EH, Onorato DJ, Castriotta R. Helium-oxygen mixtures in intubated patients with status asthmaticus and respiratory acidosis. Chest. 1990;98(3):693-698.
43 Ho AM, Lee A, Karmakar MK, et al. Heliox vs air-oxygen mixtures for the treatment of patients with acute asthma: a systematic overview. Chest. 2003;123(3):882-890.
44 Martinon-Torres F, Rodriguez-Nunez A, Martinon-Sanchez JM. Heliox therapy in infants with acute bronchiolitis. Pediatrics. 2002;109(1):68-73.
45 Hollman G, Shen G, Zeng L, et al. Helium-oxygen improves Clinical Asthma Scores in children with acute bronchiolitis. Crit Care Med. 1998;26(10):1731-1736.
46 Padman R, Lawless ST, Kettrick RG. Noninvasive ventilation via bilevel positive airway pressure support in pediatric practice. Crit Care Med. 1998;26:169-173.
47 Beasley JM, Jones SE. Continuous positive airway pressure in bronchiolitis. Br Med J (Clin Res Ed). 1981;283(6305):1506-1508.
48 Soong WJ, Hwang B, Tang RB. Continuous positive airway pressure by nasal prongs in bronchiolitis. Pediatr Pulmonol. 1993;16(3):163-166.
49 Miro A, Pinsky M. Cardiopulmonary interactions. In: Fuhrman B, Zimmerman J, editors. Pediatric Critical Care. 2nd ed. St. Louis: Mosby; 1998:250-260.
50 Koh Y. Ventilatory management of patients with severe asthma. Int Anesthesiol Clin. 2001;39(1):63-73.
51 Stather DR, Stewart TE. Clinical review: mechanical ventilation in severe asthma. Crit Care. 2005;9(6):581-587.
52 Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301-1308.
53 Darioli R, Perret C. Mechanical controlled hypoventilation in status asthmaticus. Am Rev Respir Dis. 1984;129(3):385-387.
54 Leatherman JW, Fluegel WL, David WS, et al. Muscle weakness in mechanically ventilated patients with severe asthma. Am J Respir Crit Care Med. 1996;153(5):1686-1690.
55 Priebe GP, Arnold JH. High-frequency oscillatory ventilation in pediatric patients. Respir Care Clin North Am. 2001;7(4):633-645.
56 Duval EL, van Vught AJ. Status asthmaticus treated by high-frequency oscillatory ventilation. Pediatr Pulmonol. 2000;30(4):350-353.
57 Youssef-Ahmed MZ, Silver P, Nimkoff L, et al. Continuous infusion of ketamine in mechanically ventilated children with refractory bronchospasm. Intensive Care Med. 1996;22(9):972-976.
58 Tobias JD, Garrett JS. Therapeutic options for severe, refractory status asthmaticus: inhalational anaesthetic agents, extracorporeal membrane oxygenation and helium/oxygen ventilation. Paediatr Anaesth. 1997;7(1):47-57.
59 McIntosh K. Community-acquired pneumonia in children. N Engl J Med. 2002;346(6):429-437.
60 Bednash G. The decreasing supply of registered nurses: inevitable future or call to action? JAMA. 2000;283(22):2985-2987.
61 Reduction of the influenza burden in children. Pediatrics. 2002;110(6):1246-1252.
62 Whitley RJ, Hayden FG, Reisinger KS, et al. Oral oseltamivir treatment of influenza in children. Pediatr Infect Dis J. 2001;20(2):127-133.
63 Smith J, Ariano R, Toovey S. The use of antiviral agents for the management of severe influenza. Crit Care Med. 38(Suppl), 2010. (in press)
64 O’Brien KL, Walters MI, Sellman J, et al. Severe pneumococcal pneumonia in previously healthy children: the role of preceding influenza infection. Clin Infect Dis. 2000;30(5):784-789.
65 Siberry GK. Complications of influenza infection in children. Pediatr Ann. 2000;29(11):683-690.
66 Singh-Naz N, Rodriguez W. Adenoviral infections in children. Adv Pediatr Infect Dis. 1996;11:365-388.
67 Lichenstein R, Suggs A, Campbell J. Pediatric pneumonia. Emerg Med Clin North Am. 2003;21(2):437-448.
68 Sawicki GS, Lu FL, Valim C, et al. Necrotising pneumonia is an increasingly detected complication of pneumonia in children. Eur Respir J. 2008;31(6):1285-1291.
69 Schultz KD, Fan LL, Pinsky J, et al. The changing face of pleural empyemas in children: epidemiology and management. Pediatrics. 2004;113(6):1735-1740.
70 Byington CL, Korgenski K, Daly J, et al. Impact of the pneumococcal conjugate vaccine on pneumococcal parapneumonic empyema. Pediatr Infect Dis J. 2006;25(3):250-254.
71 Byington CL, Spencer LY, Johnson TA, et al. An epidemiological investigation of a sustained high rate of pediatric parapneumonic empyema: risk factors and microbiological associations. Clin Infect Dis. 2002;34(4):434-440.
72 Tan TQ, Mason EOJr, Wald ER, et al. Clinical characteristics of children with complicated pneumonia caused by Streptococcus pneumoniae. Pediatrics. 2002;110(1 Pt 1):1-6.
73 Stoll BJ, Hansen N, Fanaroff AA, et al. Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N Engl J Med. 2002;347(4):240-247.
74 Dean D, Neumayr L, Kelly DM, et al. Chlamydia pneumoniae and acute chest syndrome in patients with sickle cell disease. J Pediatr Hematol Oncol. 2003;25(1):46-55.
75 Friedland IR. Comparison of the response to antimicrobial therapy of penicillin-resistant and penicillin-susceptible pneumococcal disease. Pediatr Infect Dis J. 1995;14(10):885-890.
76 Chonmaitree T, Powell KR. Parapneumonic pleural effusion and empyema in children. Review of a 19-year experience, 1962-1980. Clin Pediatr. 1983;22(6):414-419.
77 Colice GL, Curtis A, Deslauriers J, et al. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. [erratum appears in Chest 2001 Jan;119(1):319]. Chest. 2000;118(4):1158-1171.
78 Mitri RK, Brown SD, Zurakowski D, et al. Outcomes of primary image-guided drainage of parapneumonic effusions in children. Pediatrics. 2002;110(3):e37.
79 Ampofo K, Byington C. Management of parapneumonic empyema. Pediatr Infect Dis J. 2007;26(5):445-446.
80 Hawkins JA, Scaife ES, Hillman ND, et al. Current treatment of pediatric empyema. Semin Thorac Cardiovasc Surg. 2004;16(3):196-200.
81 Maskell NA, Davies CW, Nunn AJ, et al. U.K. controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med. 2005;352(9):865-874.
82 St Peter SD, Tsao K, Spilde TL, et al. Thoracoscopic decortication vs tube thoracostomy with fibrinolysis for empyema in children: a prospective, randomized trial. J Pediatr Surg. 2009;44(1):106-111. discussion 111
83 Sonnappa S, Cohen G, Owens CM, et al. Comparison of urokinase and video-assisted thoracoscopic surgery for treatment of childhood empyema. Am J Respir Crit Care Med. 2006;174(2):221-227.
84 Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818-824.
85 Flori HR, Glidden DV, Rutherford GW, et al. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171(9):995-1001.
86 Randolph AG, Meert KL, O’Neil ME, et al. The feasibility of conducting clinical trials in infants and children with acute respiratory failure. Am J Respir Crit Care Med. 2003;167(10):1334-1340.
87 Curley MA, Hibberd PL, Fineman LD, et al. Effect of prone positioning on clinical outcomes in children with acute lung injury: a randomized controlled trial. JAMA. 2005;294(2):229-237.
88 Ferrer M, Esquinas A, Leon M, et al. Noninvasive ventilation in severe hypoxemic respiratory failure: a randomized clinical trial. Am J Respir Crit Care Med. 2003;168(12):1438-1444.
89 Hilbert G, Gruson D, Vargas F, et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure.[comment]. N Engl J Med. 2001;344(7):481-487.
90 Padman R, Lawless ST, Kettrick RG. Noninvasive ventilation via bilevel positive airway pressure support in pediatric practice. Crit Care Med. 1998;26(1):169-173.
91 Piastra M, Antonelli M, Chiaretti A, et al. Treatment of acute respiratory failure by helmet-delivered non-invasive pressure support ventilation in children with acute leukemia: a pilot study. Intensive Care Med. 2004;30(3):472-476.
92 Ranieri VM, Suter PM, Tortorella C, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1999;282(1):54-61.
93 Slutsky AS, Tremblay LN. Multiple system organ failure. Is mechanical ventilation a contributing factor? Am J Respir Crit Care Med. 1998;157(6 Pt 1):1721-1725.
94 Hernandez LA, Peevy KJ, Moise AA, et al. Chest wall restriction limits high airway pressure-induced lung injury in young rabbits. J Appl Physiol. 1989;66(5):2364-2368.
95 Doctor A, Arnold JH. Mechanical support of acute lung injury: options for strategic ventilation. New Horizons. 1999;7(3):359-373.
96 Froese AB. High-frequency oscillatory ventilation for adult respiratory distress syndrome: let’s get it right this time!. Crit Care Med. 1997;25(6):906-908.
97 Arnold JH. High-frequency ventilation in the pediatric intensive care unit. Pediatr Crit Care Med. 2000;1(2):93-99.
98 Ventre KM, Arnold JH. High frequency oscillatory ventilation in acute respiratory failure. Paediatr Respir Rev. 2004;5(4):323-332.
99 Arnold JH, Hanson JH, Toro-Figuero LO, et al. Prospective, randomized comparison of high-frequency oscillatory ventilation and conventional mechanical ventilation in pediatric respiratory failure. Crit Care Med. 1994;22(10):1530-1539.
100 Trachsel D, McCrindle BW, Nakagawa S, et al. Oxygenation index predicts outcome in children with acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2005;172(2):206-211.
101 Langston C, Fan LL. The spectrum of interstitial lung disease in childhood. Pediatr Pulmonol. 2001;23(Suppl):70-71.
102 Fan LL, Deterding RR, Langston C. Pediatric interstitial lung disease revisited. Pediatr Pulmonol. 2004;38(5):369-378.
103 Fan LL, Kozinetz CA, Deterding RR, et al. Evaluation of a diagnostic approach to pediatric interstitial lung disease. Pediatrics. 1998;101(1 Pt 1):82-85.
104 Fan LL, Mullen AL, Brugman SM, et al. Clinical spectrum of chronic interstitial lung disease in children. J Pediatr. 1992;121(6):867-872.
105 American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165(2):277-304.
106 Fan LL, Langston C. Chronic interstitial lung disease in children. Pediatr Pulmonol. 1993;16(3):184-196.
107 Barrington KJ, Finer NN. Treatment of bronchopulmonary dysplasia. A review. Clin Perinatol. 1998;25(1):177-202.
108 Abman SH, Groothius JR. Pathophysiology and treatment of bronchopulmonary dysplasia. Current issues. Pediatr Clin North Am. 1994;41(2):277-315.
109 Northway WHJr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. 1967;276(7):357-368.
110 Bhandari A, Bhandari V. Pitfalls, problems, and progress in bronchopulmonary dysplasia. Pediatrics. 2009;123(6):1562-1573.
111 Kinsella JP, Greenough A, Abman SH. Bronchopulmonary dysplasia. Lancet. 2006;367(9520):1421-1431.
112 Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723-1729.
113 Eber E, Zach MS. Long-term sequelae of bronchopulmonary dysplasia (chronic lung disease of infancy). Thorax. 2001;56(4):317-323.
114 McCubbin M, Frey EE, Wagener JS, et al. Large airway collapse in bronchopulmonary dysplasia. J Pediatr. 1989;114(2):304-307.
115 Bancalari E. Neonatal Chronic lung disease. In: Fanaroff A, Martin R, editors. Neonatal-Perinatal Medicine: Diseases of the Fetus and Infant. St. Louis: Mosby; 2002:1057-1070.
116 Wada K, Jobe AH, Ikegami M. Tidal volume effects on surfactant treatment responses with the initiation of ventilation in preterm lambs. J Appl Physiol. 1997;83(4):1054-1061.
117 Taskar V, John J, Evander E, et al. Surfactant dysfunction makes lungs vulnerable to repetitive collapse and reexpansion. Am J Respir Crit Care Med. 1997;155(1):313-320.
118 Clark RH, Gerstmann DR, Null DMJr, et al. Prospective randomized comparison of high-frequency oscillatory and conventional ventilation in respiratory distress syndrome. Pediatrics. 1992;89(1):5-12.
119 Gerstmann DR, Minton SD, Stoddard RA, et al. The Provo multicenter early high-frequency oscillatory ventilation trial: improved pulmonary and clinical outcome in respiratory distress syndrome. Pediatrics. 1996;98(6 Pt 1):1044-1057.
120 Courtney SE, Durand DJ, Asselin JM, et al. High-frequency oscillatory ventilation versus conventional mechanical ventilation for very-low-birth-weight infants. N Engl J Med. 2002;347(9):643-652.
121 Schmidt B, Roberts RS, Davis P, et al. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354(20):2112-2121.
122 Cabal LA, Larrazabal C, Ramanathan R, et al. Effects of metaproterenol on pulmonary mechanics, oxygenation, and ventilation in infants with chronic lung disease. J Pediatr. 1987;110(1):116-119.
123 Clark PL, Ekekezie II, Kaftan HA, et al. Safety and efficacy of nitric oxide in chronic lung disease. Arch Dis Child Fetal Neonatal Ed. 2002;86(1):F41-F45.
124 Mariani G, Barefield ES, Carlo WA. The role of nitric oxide in the treatment of neonatal pulmonary hypertension. Curr Opin Pediatr. 1996;8(2):118-125.
125 Greenholz SK. Congenital diaphragmatic hernia: an overview. Semin Pediatr Surg. 1996;5(4):216-223.
126 Greer JJ, Allan DW, Babiuk RP, et al. Recent advances in understanding the pathogenesis of nitrofen-induced congenital diaphragmatic hernia. Pediatr Pulmonol. 2000;29(5):394-399.
127 Migliazza L, Xia H, Alvarez JI, et al. Heart hypoplasia in experimental congenital diaphragmatic hernia. J Pediatr Surg. 1999;34(5):706-710. discussion 710-1
128 Migliazza L, Otten C, Xia H, et al. Cardiovascular malformations in congenital diaphragmatic hernia: human and experimental studies. J Pediatr Surg. 1999;34(9):1352-1358.
129 Migliazza L, Xia H, Diez-Pardo JA, et al. Skeletal malformations associated with congenital diaphragmatic hernia: experimental and human studies. J Pediatr Surg. 1999;34(11):1624-1629.
130 Fauza DO, Wilson JM. Congenital diaphragmatic hernia and associated anomalies: their incidence, identification, and impact on prognosis. J Pediatr Surg. 1994;29(8):1113-1117.
131 Inhaled nitric oxide and hypoxic respiratory failure in infants with congenital diaphragmatic hernia. The Neonatal Inhaled Nitric Oxide Study Group (NINOS). Pediatrics. 1997;99(6):838-845.
132 Henneberg SW, Jepsen S, Andersen PK, et al. Inhalation of nitric oxide as a treatment of pulmonary hypertension in congenital diaphragmatic hernia. J Pediatr Surg. 1995;30(6):853-855.
133 Russell IA, Zwass MS, Fineman JR, et al. The effects of inhaled nitric oxide on postoperative pulmonary hypertension in infants and children undergoing surgical repair of congenital heart disease. Anesth Analg. 1998;87(1):46-51.
134 Leveque C, Hamza J, Berg AE, et al. Successful repair of a severe left congenital diaphragmatic hernia during continuous inhalation of nitric oxide. Anesthesiology. 1994;80(5):1171-1175.
135 van den Hout L, Sluiter I, Gischler S, et al. Can we improve outcome of congenital diaphragmatic hernia? Pediatr Surg Int. 2009;25(9):733-743.
136 Kinsella JP, Ivy DD, Abman SH. Pulmonary vasodilator therapy in congenital diaphragmatic hernia: acute, late, and chronic pulmonary hypertension. Semin Perinatol. 2005;29(2):123-128.
137 Azarow K, Messineo A, Pearl R, et al. Congenital diaphragmatic hernia—a tale of two cities: the Toronto experience. J Pediatr Surg. 1997;32(3):395-400.
138 Wung JT, James LS, Kilchevsky E, et al. Management of infants with severe respiratory failure and persistence of the fetal circulation, without hyperventilation. Pediatrics. 1985;76(4):488-494.
139 Varughese M, Patole S, Shama A, et al. Permissive hypercapnia in neonates: the case of the good, the bad, and the ugly. Pediatr Pulmonol. 2002;33(1):56-64.
140 Mariani G, Cifuentes J, Carlo WA. Randomized trial of permissive hypercapnia in preterm infants. Pediatrics. 1999;104(5 Pt 1):1082-1088.
141 Carlo WA, Martin R, Fanaroff A. Assisted ventilation and complications of respiratory distress. In: Fanaroff A, Martin RJ, editors. Neonatal-Perinatal Medicine: Diseases of the Fetus and Infant. St. Louis: Mosby; 2002:1011-1025.
142 Wilson JM, Lund DP, Lillehei CW, et al. Congenital diaphragmatic hernia–a tale of two cities: the Boston experience. J Pediatr Surg. 1997;32(3):401-405.
143 Wung JT, Sahni R, Moffitt ST, et al. Congenital diaphragmatic hernia: survival treated with very delayed surgery, spontaneous respiration, and no chest tube. J Pediatr Surg. 1995;30(3):406-409.
144 Dworetz AR, Moya FR, Sabo B, et al. Survival of infants with persistent pulmonary hypertension without extracorporeal membrane oxygenation. Pediatrics. 1989;84(1):1-6.
145 Bohn D. Congenital diaphragmatic hernia. Am J Respir Crit Care Med. 2002;166(7):911-915.
146 Randolph AG, Wypij D, Venkataraman ST, et al. Effect of mechanical ventilator weaning protocols on respiratory outcomes in infants and children: a randomized controlled trial. JAMA. 2002;288(20):2561-2568.