Acute pancreatitis
General description
Acute pancreatitis is a common cause for emergency hospital admission, with approximately 40 cases per year for each 100 000 population in Scotland,1 Norway2 and Sweden.3 There has been a steady increase in the incidence and a slight reduction in case mortality, although not population mortality, over the past 45 years.4 In approximately 80% of patients, acute pancreatitis is a rapidly-resolving condition requiring little more than analgesia and a short period of intravenous fluid resuscitation, with the remainder developing a multisystem illness characterised by a systemic inflammatory response with a variable degree of organ dysfunction.
Pathophysiology
Experimental models have shed some light on the mechanism by which pancreatic duct obstruction induces acute pancreatitis. Changes in the pattern of enzyme secretion within pancreatic acinar cells, coupled with intracellular zymogen activation, are considered the important early events in the development of acute pancreatitis. Disruption of the paracellular barrier allows release of pancreatic enzymes into the paracellular space. Inflammatory cells and inflammatory mediators may further exacerbate the acinar cell injury.4 Research has shown that many of these early events can be triggered by an increase in intracellular calcium.5
The mechanism of alcohol-induced acute pancreatitis is less clear, but alcohol has been shown to increase the sensitivity of acinar cells to cholecystokinin hyperstimulation, resulting in enhanced intracellular protease activation.6 Alcohol also influences acinar cell calcium homeostasis, but several alternative theories have been proposed.
Natural history
Acute pancreatitis varies from a mild, self-limiting attack to a severe life-threatening illness and, for this reason, patients are often classified as having either mild or severe acute pancreatitis (see Box 13.1). This rather simplistic description ignores the wide variety of clinical behaviour that can be observed in these patients but helps to focus attention on the subgroup of patients who develop complications. Currently, the internationally accepted classification of acute pancreatitis and its complications is set out in the paper arising from the Atlanta Conference.7 Improved understanding of treatment concepts and the dynamic nature of the pathophysiology has rendered a number of the concepts outlined in the Atlanta Conference outdated and a revision has recently been published.8
Within this framework different patterns of disease have emerged. Multicentre trials in acute pancreatitis have enabled prospective study of severe acute pancreatitis and several important points have emerged. Firstly, the majority of patients who develop severe acute pancreatitis have evidence of early systemic organ dysfunction.9 It is exceptional for a patient to have no evidence of organ failure in the first week of illness and to subsequently develop a significant late local complication. Secondly, most patients who develop organ failure have evidence of this at the time of admission or very shortly thereafter.10 Thirdly, while the tendency is for early organ dysfunction to recover without further problems, worsening organ failure is associated with a high mortality.9,11,12
The majority of patients with severe early organ dysfunction will have pancreatic necrosis on computed tomography (CT) scan. A significant proportion (30–40%) of patients with pancreatic necrosis will develop secondary pancreatic infection, usually in the second to third week after admission,13 which may be associated with a deterioration in organ failure. Patients who have infected pancreatic necrosis complicated by multiple organ failure represent a formidable management challenge.
Diagnosis
In the majority of patients the diagnosis of acute pancreatitis is relatively easy, characterised by a clinical presentation of sudden severe epigastric pain radiating through to the back. Vomiting within the first 24 hours is very frequently severe and contributes to dehydration. Other signs and symptoms such as tachycardia, tachypnoea and circulatory collapse are dependent on the severity of the attack. A raised serum amylase (at least three times the upper limit of normal) supports the diagnosis in >95% of cases. Serum amylase estimation may be inaccurate in association with hyperlipidaemia, where a raised urinary amylase can be diagnostic. Serum lipase may be marginally more accurate but is not commonly available in routine clinical practice. CT can confirm the diagnosis where doubt exists, or in patients with delayed presentation, and it should therefore be very uncommon for the diagnosis to be made at laparotomy (Fig. 13.1).
Aetiology
Genetic defects
Genetic familial defects of the cationic trypsinogen gene14 (N29I, RII7H) and the cystic fibrosis gene (CTFR) may be associated with recurrent pancreatitis, but severe acute inflammatory changes are uncommon.
Iatrogenic causes
Drug-induced acute pancreatitis may occur following ingestion of a number of drugs;15 those most commonly implicated are valproic acid, azathioprine, L-asparaginase and corticosteroids. However, unless gallstone disease has been excluded with confidence it is unwise to ascribe acute pancreatitis to a particular drug. Repeat exposure to the same drug again causing acute pancreatitis is the strongest evidence of a direct association.
Inflammatory
Autoimmune pancreatitis is a rare condition, considered part of the IgG4-related autoimmune disease spectrum.16 This presents as abdominal pain associated with homogeneous gland enlargement with a well-defined edge on CT, an increased IgG4/IgG ratio and a periductal lymphoplasmocytic infiltrate on biopsy. This may also be associated with abnormalities in the extrahepatic biliary tree resembling sclerosis cholangitis and a response to steroids is diagnostic. Focal autoimmune pancreatitis may prove difficult to differentiate from carcinoma. There are established associations with other autoimmune diseases (polyarteritis nodosa, systemic lupus erythematosus, vasculitis) and inflammatory bowel disease (Crohn’s and ulcerative colitis), and many are now considered part of the autoimmune spectrum, although only a small proportion appear to have an association with IgG4 serum or tissue abnormalities.
Physiological
Sphincter manometric abnormalities
Type 1 pancreatic sphincter dysfunction17 may be associated with hyperamylasaemia and abnormalities on sphincter manometry, as part of a global gut dysmotility spectrum. Managament of sphincter spasm may only partly resolve the patient’s symptoms. Conventional treatment involves endoscopic sphincterotomy, but the risk of post-ERCP pancreatitis in these patients is high (30%).
Assessment of severity
The dynamic nature of organ dysfunction in patients presenting with acute pancreatitis has been well described,9 and for over 30 years authors have explored ways of ‘predicting’ those patients with more severe disease. Overall mortality, whether early or late, is also associated with the development and persistence of organ failure.18 This was indirectly shown, if not recognised, 25 years previously with the development of the predictive multifactorial scoring systems – Ranson,19 Glasgow20 and APACHE II21 – which, rather than predicting the subsequent development of organ failure, more accurately identified established multisystem organ dysfunction. Their principal use is to remind the inexperienced of the multisystem nature of the disease process, or as a method of stratifying patients within a study protocol. Of the multiple factor scoring systems, APACHE II provides the best prediction of mortality but the mainstay of assessment remains repeated, careful clinical observation.22
Single biochemical measures
C-reactive protein (CRP)
The most widely studied single predictive marker is serum CRP. The major advantage of CRP is its routine availability in clinical practice. Patients with clinically severe pancreatitis usually have a CRP > 200 mg/L, with a practical cut-off being 150 mg/L, but its serum peak is not reached for 36–48 h. The positive predictive value of CRP is similar to that of APACHE II22 but its major use is in monitoring the clinical course during the acute and recovery phase.
Other single predictive markers
Peak levels of interleukin-6 (IL-6) occur within 24 h and this has aroused interest in its use as a predictor of outcome. IL-6 is a pro-inflammatory cytokine induced by stimuli such as tumour necrosis factor (TNF) and interleukin-1 (IL-1). Other single predictive markers that have been studied include trypsinogen activation peptide (TAP), leucocyte polymorph neutrophil (PMN) elastase, TNF and serum procalcitonin. Procalcitonin is the precursor of the hormone calcitonin and is raised in the presence of an inflammatory response, particularly where this is bacterial in origin. Serum procalcitonin is a promising marker of both severe acute pancreatitis and of infected pancreatic necrosis in the later phase.22,23
Intra-abdominal hypertension (IAH)
IAH is recognised as a contributing factor to organ dysfunction in the context of a variety of acute abdominal processes. Most of the literature to date focuses on trauma patients, but there is increasing interest in its role in patients with severe acute pancreatitis (SAP). There are data to suggest that raised intra-abdominal pressure (IAP) may be associated with disease severity,24 organ failure and mortality in SAP.25 There are, however, no data to suggest improved outcome following surgical decompression for raised IAP in acute pancreatitis, and indeed this may be harmful. At present the monitoring of IAP cannot be recommended outside of a clinical trial.
Imaging
All patients with acute pancreatitis should have an ultrasonic assessment of the biliary tree within 24 h of admission.26 In those with gallstones, the majority will have mild disease, and this will facilitate definitive treatment of cholelithiasis prior to discharge. In the emergency situation, ultrasonography can be difficult due to a number of factors, including the presence of intraluminal bowel gas or lack of patient cooperation. Therefore, in patients with a negative initial US, and no other obvious aetiological factor, the US should be repeated prior to discharge before excluding gallstones as a potential aetiological factor.
Role of CT
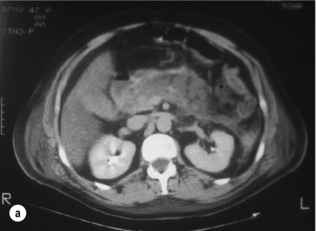
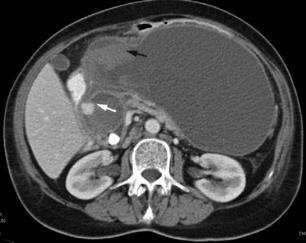
The main role of CT in the early phase of acute pancreatitis is to clarify the diagnosis in cases where there is diagnostic uncertainty. In patients with severe acute pancreatitis, particularly when complicated by MODS, early CT helps to exclude other pathology such as intestinal perforation, gut ischaemia or dissecting aortic aneurysm. Dynamic contrast-enhanced CT can also be used to assess disease severity (Fig. 13.2) and predict the potential for complications.27 Although not widely used for this purpose clinically in the UK, it is routinely used in many other countries, and can be useful for stratification of patients in clinical trials. The CT Severity Index (CTSI)27 combines a score for the radiological pancreatic and peripancreatic abnormalities with a weighting for the extent of necrosis. More recently there are reports of perfusion CT, which may be a useful modality for detecting early, subclinical ischaemic changes in the pancreas that then lead to pancreatic necrosis.
Role of magnetic resonance (MR)/magnetic resonance cholangiopancreatography (MRCP)
Magnetic resonance imaging (MRI) offers a realistic alternative to contrast-enhanced CT28 in the assessment of patients with acute pancreatitis. The avoidance of cumulative radiation exposure, potentially nephrotoxic iodinated contrast media, and the excellent contrast sensitivity and spatial resolution would make it an attractive alternative. Axial T1- and T2-weighted scans produce images analogous to those of CT. Gadolinium contrast enhancement can infer viability and improve anatomical definition. Heavily T2-weighted image acquisition, using a single breath hold and long repetition (TR) and echo (TE) times, results in little signal being produced by solid tissue, and a high signal from static fluid in the biliary and pancreatic ducts, enabling images anatomically comparable to those of ERCP to be produced.
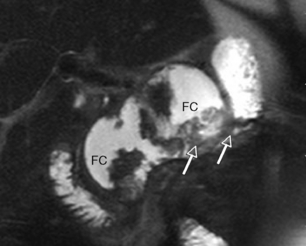
Whilst technically feasible in most centres, the MR environment is unsuitable for patients requiring significant circulatory or respiratory support, and at present few centres have the capability to perform MR-guided intervention. Contrast-enhanced CT therefore remains the imaging modality of choice for assessment and intervention in severe acute pancreatitis. However, MR has a role in the follow-up of acute inflammatory collections, where it is superior to CT in determining the extent of solid material within a collection (Fig. 13.3), and in excluding choledocholithiasis in selected patients.
Management
Management of acute pancreatitis in the UK is still based on two key guideline documents, now 726 and 1029 years old, respectively. The initial management of patients presenting with acute pancreatitis should be directed at the early identification and management of organ failure, most frequently renal and respiratory dysfunction.
At present there are no established end-points of resuscitation to confirm that tissue perfusion and oxygen delivery have been restored adequately in patients with acute pancreatitis. Aggressive fluid resuscitation is often required, and monitoring the response to this relies upon traditional markers, in particular urine output, blood pressure and pulse oximetry. Physiological markers of resuscitation, for example acid–base balance from an arterial blood gas, may be helpful in detecting clinically occult hypoperfusion. Patients who do not respond to initial resuscitation, or who have evidence of organ dysfunction, should be transferred to a critical care environment for more invasive and intensive monitoring with central venous and arterial catheters. Respiratory failure should be treated with humidified oxygen, and this will be guided by continuous pulse oximetry and arterial blood gas analysis. Deteriorating respiratory function is a sign of disease progression. As many as half of all deaths from acute pancreatitis occur in less than 7 days, and the majority of these occur within 72 h of admission.1 There is evidence that patients managed in specialist institutions have a reduced risk of early death and this may be an indication that management of early MODS could be improved.
Specific medical management
Prevention of infection
In patients who survive the early, systemic complications of acute pancreatitis, secondary infection of pancreatic necrosis is the most important late complication. Infection occurs in 30–40% of patients with a minimum 30% pancreatic necrosis13 and is responsible for the majority of late deaths from acute pancreatitis. Secondary infection manifests as escalating sepsis or a deterioration in organ failure scores, usually in the second (36%) and third (71%) weeks of the illness.30
The role of prophylactic antibiotics to prevent secondary infection has been widely studied. The most recent Cochrane review31 found no evidence of a significant reduction in mortality with antibiotic prophylaxis and no difference in the incidence of infected pancreatic necrosis. Even non-pancreatic infections showed no significant difference with antibiotic therapy. It was noted, however, that all of the studies were underpowered and a definitive answer to this question will require better quality clinical trials. The most recent meta-analysis of 14 trials including 841 patients also found no evidence of benefit with antibiotic prophylaxis.32
Nutritional support
Nutritional delivery in the patient with acute pancreatitis: The key study in this regard was the randomised study of Kalfarentzos et al. in 1996, who randomised 38 patients with severe acute pancreatitis to total parenteral nutrition (TPN) or nasojejunal feeding.33 The most recent Cochrane review, including eight randomised trials, demonstrated a reduction in mortality, systemic complications and surgical intervention in patients given enteral nutrition.34
Most experience to date has been with enteral feeding distal to the duodenojejunal flexure. More recently, four randomised studies have shown nasogastric feeding to be a practical alternative to jejunal feeding.35 All studies in this area are underpowered and it is therefore difficult to recommend this feeding route in routine clinical practice.
Disease modulation through content or mode of delivery: There has been interest in the role of the intestine in the pathophysiology of multiple organ failure in critical illness, with loss of gut barrier function potentially leading to endotoxaemia and the systemic inflammatory response syndrome (SIRS). In a small study from Leeds,36 the authors reported a reduction in the inflammatory response and organ failure in those receiving enteral support, but unfortunately there were only 13 patients with severe disease, limiting the validity of the conclusions. There have been several trials comparing so-called ‘immunonutrition’ with standard enteral feeding in critically ill patients, but so far no evidence of benefit has been demonstrated in acute pancreatitis.37 Similarly, there has been interest in the role of ‘probiotics’, but a randomised trial from the Netherlands found an increase in fatal complications in the probiotic group, with an unexpectedly high incidence of intestinal necrosis.38
Other medical therapies
Inhibition of pancreatic secretion: Pharmacological attempts to suppress pancreatic function have included intravenous glucagon, somatostatin and, more recently, the somatostatin analogue octreotide. On the basis of the available literature there is no justification for the use of octreotide or any other pharmacological inhibitor of pancreatic secretion in acute pancreatitis.
Inhibition of pancreatic enzymes: Many studies have evaluated the concept of supporting the endogenous antiprotease defence mechanisms. Randomised trials of intravenous aprotinin (Trasylol), gabexatemesilate, intraperitoneal aprotinin, and both low- and high-dose fresh frozen plasma (FFP) have shown no therapeutic benefit.
Inhibition of the inflammatory response: Following initial promising results with the platelet-activating factor antagonist, lexipafant, a multicentre, randomised, placebo-controlled study of anticytokine therapy recruited 1518 patients. This study recruited only those patients with symptoms of less than 48 h duration and was restricted to those with predicted severe attacks. Not only was there no difference in mortality between groups, the incidence of local complications, length of ICU stay, hospital stay and change in organ failure scores were all similar in the three study groups.
Role of ERCP: There have now been three published randomised trials addressing this issue, and four smaller studies. Contradicting an earlier Cochrane review,39 the most recent meta-analysis40 has shown early ERCP in patients with either predicted mild or severe acute biliary pancreatitis without acute cholangitis did not lead to a significant reduction in the risk of overall complications and mortality. There is no role for urgent ERCP in patients with mild disease. All patients with jaundice who exhibit signs of sepsis should undergo urgent ERCP and sphincterotomy as cholangitis may coexist with acute pancreatitis and hyperamylasaemia, but there appears to be little role for early ERCP outside this scenario.
Definitive management issues
Prevention of recurrent acute pancreatitis
The timing of cholecystectomy will obviously depend on the clinical situation. In patients recovering from mild acute biliary pancreatitis, definitive management of the gallstones to prevent a further attack should ideally be achieved during the same admission, and certainly no later than 4 weeks following discharge from hospital.26 This will normally involve a cholecystectomy (laparoscopic or open) with intraoperative cholangiography, or alternatively duct imaging by MRCP (or EUS) followed by cholecystectomy. Elderly patients or those with significant medical comorbidity may be managed by an endoscopic sphincterotomy, although this may not be as effective as definitive surgery in preventing further attacks.
In severe acute pancreatitis, interval cholecystectomy should be performed when the inflammatory process has subsided and the procedure is potentially easier.29
Investigation of non-gallstone-associated pancreatitis
Following resolution of an attack of acute pancreatitis, an assessment of potential aetiological factors is an important aspect of care, and a diagnosis of idiopathic pancreatitis should be made in less than 20% of patients.26 Evaluation of the initial acute attack should include an adequate history (alcohol/drugs/familial), biochemical tests (liver function tests/lipids (hypertriglyceridaemia)/calcium) and biliary ultrasound. If these investigations are normal, axial imaging (CT or MR/MRCP) may be appropriate to exclude a mechanical cause. Patients in whom no cause is identified following these investigations should be considered for EUS, as a cause will be identified in the majority of cases.41 A cholecystectomy or biliary sphincterotomy is justified in patients with recurrent idiopathic pancreatitis in whom microlithiasis or biliary sludge is identified. With the increasing use of EUS in these patients, it is increasingly recognised that many of these patients will have changes consistent with early chronic pancreatitis,41 rather than biliary microlithiasis as suggested by earlier studies. The prevalence of microlithiasis appears to be higher in regions where gallstone disease is the predominant aetiology.
Peripancreatic fluid collections
Management of a pseudocyst
Percutaneous drainage: Results of percutaneous drainage suggest wide variation in success (40–96%)42 and the introduction of infection is a risk that must be considered. In practical terms, the risk of pancreatic fistula limits this approach and there is evidence that it can make subsequent surgical intervention more hazardous if this becomes necessary.43 In our practice we restrict the use of percutaneous drainage to occasional patients with infected, fluid-predominant collections, particularly where there is a degree of systemic organ dysfunction. Increasingly, however, endoscopic or laparoscopic drainage is employed.
Endoscopic drainage: The technique of endoscopic cystgastrostomy as first described by Baron et al.,44 initially by blind puncture of a cyst bulging into the stomach wall using a side-viewing endoscope, has been subsequently refined using endoscopic ultrasound guidance. EUS guidance enables drainage of cysts where no intraluminal bulge is present and also helps avoid intervening vessels. Further procedures may be required to facilitate drainage, particularly where cysts are large or where there is much necrotic debris, but we have not found it necessary to pursue active endoscopic necrosectomy in this group of patients. Where disruption of the pancreatic duct has occurred, transpapillary duct stenting can aid resolution. Baron and colleagues have recently updated their experience in 104 patients with walled-off pancreatic necrosis with successful resolution in 95 patients (91%). The mean time to intervention was 63 days and mean duration of treatment was 4.1 months. The complication rate, mainly haemorrhage and perforation, was 14%.45
Surgical drainage of an acute post-inflammatory collection: Surgery may be considered as a primary mode of intervention in selected patients, particularly for patients with large, necrosis-predominant collections without infection or systemic organ dysfunction. This can be readily achieved by a laparoscopic transgastric procedure, or a direct cyst-enterostomy, and allows simultaneous laparoscopic cholecystectomy where appropriate. In those patients in whom endoscopic drainage fails to achieve complete resolution, or a cyst recurs, simple surgical drainage is rarely an option as this often results from separation of the head/body and tail of the pancreas due to prior necrosis of the central pancreas – termed a ‘disconnected tail’. These patients often require a challenging distal pancreatico-splenectomy.
Management of necrosis
1. True pancreatic necrosis – minimal separation of devitalised tissue with a high solid/fluid ratio.
2. Transitional pancreatic necrosis with partial but incomplete separation.
3. Organised pancreatic necrosis (OPN) – good separation of devitalised tissue within a fluid-filled cavity and formation of a fibrous wall lined with granulation tissue.
4. Pseudocyst – almost complete resolution of any solid component and a well-formed fibrous wall lined with granulation tissue.
The necrotic process associated with pancreatitis tends to involve both the pancreatic parenchyma and surrounding adipose tissue. Indeed, significant quantities of necrotic peripancreatic tissue can be present with an essentially viable gland. Complications relate to the extent of the necrotic process, and in particular the extent of parenchymal necrosis. Early aggressive debridement in the absence of infection has been advocated. However, mortality in this series was 25% overall, and the only randomised study of early versus late (> 12 days) necrosectomy was discontinued as a result of the mortality rate in the early treatment group (56% vs. 27%).46 The general principle is now to withhold surgery in the early phase of disease, operating for complications ideally once the acute inflammatory insult has subsided.
Management of sterile necrosis
The development of retroperitoneal necrosis secondary to acute pancreatitis is not in itself an indication for intervention. Pancreatic necrosis, where sterile, can usually be adequately treated by conservative means.47 There remains debate regarding the role of debridement in patients failing to respond to conservative treatment. Early debridement does not improve outcome; however, some specialists advocate debridement in patients with continuing organ dysfunction after several weeks, but this may also be detrimental.48 The majority of sterile post-inflammatory fluid collections progress into organised pancreatic necrosis, which can be managed with low morbidity and mortality, and our policy is to delay intervention where possible. The management of these patients is discussed in the section dealing with fluid collections associated with necrosis.
Management of infected necrosis (early phase, 2–6 weeks)
Infected pancreatic necrosis has previously been described as the most feared surgical complication of acute pancreatitis. This led to the development of protocol-driven management in the 1990s, aimed at the early identification of secondary infection within necrosis. The presence of a persistent SIRS response made clinical differentiation between SIRS and sepsis difficult and this led to CT- or US-guided fine-needle aspiration (FNA) of the pancreatic or peripancreatic collections as a diagnostic test. FNA was considered the cornerstone of management as it was thought infected necrosis mandated radical surgical intervention.49,50
Methods of necrosectomy: The traditional approach to infected necrosis was open laparotomy/debridement. These approaches are falling from favour with increasing evidence that minimally invasive intervention may reduce morbidity/mortality;51–53 however, they remain the method of choice in some countries.
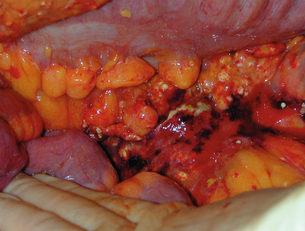
Open laparotomy/debridement: The technique of pancreatic debridement involves a wide exposure of the abdomen, usually through a bilateral subcostal/rooftop incision. Both colonic flexures are mobilised to expose the retroperitoneum and the lesser sac entered via the gastrocolic omentum, or occasionally the transverse mesocolon. Pus is aspirated from the abscess cavity, leaving the solid component behind, which is then removed by ‘blunt finger’ dissection (Fig. 13.4). Tissue that will not come away by finger teasing should be left in situ to demarcate and subsequent removal at a later procedure. The procedure may also include a cholecystectomy, operative cholangiogram and feeding jejunostomy.
Methods for postoperative management of the debridement cavity after laparotomy are as follows:
• With drainage/‘closed packing’. Simple drainage, often with multiple retroperitoneal tube drains, was the conventional approach to the postoperative management of the debrided pancreatic and peripancreatic bed. Whilst mortality was less than with resective procedures, multiple second-look laparotomies were often required for residual sepsis. The initial results of Warshaw and colleagues reported respectable mortality figures of 24% using this technique. Their technique has been modified using multiple soft Penrose drains containing cotton gauze to pack the cavity following completion of the necrosectomy.54 These are subsequently removed at intervals, allowing the cavity to collapse around the drains. Their reported mortality rate using this technique is the lowest in the literature (6.2%), although the series included patients with sterile necrosis (11%) and pancreatic abscess (39%), and only 14% had both sepsis syndrome and a positive culture requiring early intervention, which is indicative of the difficulties in interpretation of the available literature.
• With open packing. Bradley and colleagues from Atlanta have been the principal proponents of the open laparostomy technique.55 In this, at the conclusion of the debridement, the lesser sac is packed with lubricated cotton gauze and the abdomen left open, allowing planned re-explorations every few days until granulation tissue forms. Enteric fistula and secondary haemorrhage are not uncommon, and the technique is rarely performed as a first option. Surgical packing and planned re-operation is, however, sometimes required to control blood loss from the retroperitoneum following the development of an intraoperative coagulopathy, a lavage system being created, following correction of the coagulopathy, at the time of subsequent pack removal.
• With closed lavage. Postoperative closed lavage as described by Beger et al.56 is the most popular method for postoperative sepsis control following open debridement, the aim of the lavage being the continuous removal of devitalised necrotic material and bacteria. Several (four to six) large-diameter tube drains are inserted in the lesser sac and throughout the abdomen, and the abdomen closed. Continuous lavage is then commenced, our own preference being for CAPD dialysis fluid (Dianil 7, Baxter Healthcare, potassium free, Iso-osmolar) warmed through a blood warmer and delivered at 500 mL/h. The lavage is continued, for around 3–4 weeks on average, until the return fluid is clear and the patient has no residual signs of systemic sepsis. This technique has been adopted with minor variations by centres on both sides of the Atlantic.
Minimally invasive approaches to infected necrosis: Minimally invasive surgery has been shown consistently to be associated with a lesser activation of the inflammatory response than equivalent open surgery, and there is experimental evidence suggesting that local sepsis and the inflammatory response may be lessened by a minimally invasive rather than an open technique. The widespread belief that formal necrosectomy is required has recently been challenged and there is evidence that patients can resolve following simple percutaneous drainage or following limited necrosectomy. The PANTER trial and several prospective cohort series51–53 have suggested that by minimising the massive inflammatory ‘hit’ of open pancreatic necrosectomy, a minimally invasive approach to the management of infected pancreatic necrosis may lessen the risk of post-procedural organ failure, respiratory and wound morbidity in these patients. However, there is as yet no evidence that one minimal approach is superior to another.
• Percutaneous drainage. Freeny et al.,57 combining aggressive CT-guided percutaneous drainage with continuous post-drainage lavage, showed that nearly half the pancreatic abscesses may resolve. However, more than half of these patients required subsequent surgical intervention for residual sepsis. Drain occlusion is common due to necrotic debris and repeated drains may be necessary. Simple drainage, even with small-diameter drains, may be associated with complete resolution and within the PANTER trial ‘step-up’ arm, 35% of patients were successfully managed by small-bore (4-mm) percutaneous drainage alone.
• Minimally invasive surgery. Simple percutaneous or endoscopic drainage alone may result in complete resolution; however, they also have a useful role providing initial sepsis control, associated with an improvement in organ dysfunction. Careful drain management is required to recognise drain blockage early and to prevent recurrent sepsis. As a result of the difficulties in maintaining drain patency, we have developed a technique58 to allow minimally invasive drainage, and in addition removal of the necrotic component. This involves the intraoperative dilatation of a previously placed CT-guided percutaneous drain tract and subsequent necrosectomy using a urological rigid-rod lens system (Fig. 13.5). Complete resolution of sepsis and necrosis can occur without recourse to further surgery, with a reduced need for postoperative organ support compared to the open procedures. In other centres as well as within our own patient cohort, this technique has significantly reduced mortality.52 The Dutch Pancreatitis study group have popularised a video-assisted retroperitonael debridement technique (VARD variation of the Fagniez technique) through a small 5-cm incision in the left flank. Their management approach has evolved from being initially performed on all patients with infected necrosis, to now being used as a ‘step-up’ approach should initial percutaneous drainage fail to control sepsis. The Dutch group have completed a randomised trial51 comparing this minimally invasive two-stage ‘step-up’ approach with open necrosectomy and have shown a reduction in early organ failure (respiratory) and late morbidity, but the study was underpowered to address mortality.
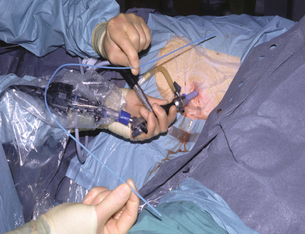
Figure 13.5 Percutaneous necrosectomy showing the rod lens scope and a guiding catheter to ensure accurate drain placement.
• Endoscopic necrosectomy. The principle of tract dilatation and minimally invasive necrosectomy has also been used with the endoscopic approach. Seifert et al.59 have reported the dilatation of an endocyst-gastrotomy tract, allowing insertion of the endoscope into the retroperitoneum and subsequent piecemeal debridement. More recently the use of multiple transgastric cyst gastrostomy puncture sites has been reported, allowing nasocystic lavage and stent-assisted drainage through alternative drainage sites with good sepsis control.60
Management of pancreatic abscess
A pancreatic abscess by definition is an infected, fluid-predominant acute collection (pseudocyst) with little or no necrosis, and is therefore suitable for minimally invasive drainage. The endoscopic technique of Baron described above has been used in this situation with reasonably good effect, but there is a significant risk of haemorrhage from blind puncture of the abscess wall. The EUS-guided endoscopic technique appears to be associated with a lower morbidity and has a reported resolution of sepsis of nearly 90%.61 Laparoscopic cyst gastrostomy may be an effective alternative.
Specific late complications
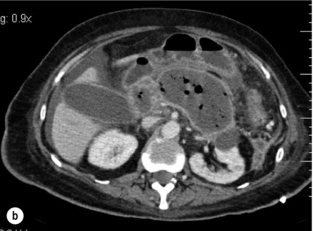
Life-threatening haemorrhage may rarely occur acutely in pancreatic necrosis within the first week following presentation and requires mesenteric embolisation or surgical exploration. Haemorrhage is, however, a relatively common problem following prior necrosectomy as a postoperative reactionary haemorrhage due to a combination of having a large raw surface, partly controlled sepsis and exposed major vessels, leading to reactionary haemorrhage (Fig. 13.6). Bleeding may be arterial or venous. Urgent surgical intervention and ligation of proximal visceral vessels may be suggested; however, the combination of haemorrhage and subsequent laparotomy frequently precipitates escalating organ failure and death. Angiography and embolisation, with endovascular metal coils, is therefore the treatment of choice.
References
1. McKay, C.J., Evans, S., Sinclair, M., et al, High early mortality rate from acute pancreatitis in Scotland, 1984–1995. Br J Surg. 1999;86(10):1302–1305. 10540138
2. Appelros, S., Borgstrom, A., Incidence, aetiology and mortality rate of acute pancreatitis over 10 years in a defined urban population in Sweden. Br J Surg. 1999;86(4):465–470. 10215815
3. Halvorsen, F.A., Ritland, S., Acute pancreatitis in Buskerud County, Norway. Incidence and etiology. Scand J Gastroenterol. 1996;31(4):411–414. 8726312
4. Yadav, D., Lowenfels, A.B., Trends in the epidemiology of the first attack of acute pancreatitis: a systematic review. Pancreas. 2006;33(4):323–330. 17079934
5. Criddle, D.N., McLaughlin, E., Murphy, J.A., et al, The pancreas misled: signals to pancreatitis. Pancreatology. 2007;7(5–6):436–446. 17898533
6. Katz, M., Carangelo, R., Miller, L.J., et al, Effect of ethanol on cholecystokinin-stimulated zymogen conversion in pancreatic acinar cells. Am J Physiol. 1996;270(1, Pt 1):G171–G175. 8772515
7. Bradley, E.L., 3rd., A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11 through 13, 1992. Arch Surg. 1993;128(5):586–590. 8489394
8. Banks, P.A., Bollen, T.L., Dervenis, C., et al, Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–111. 23100216
9. Buter, A., Imrie, C.W., Carter, C.R., et al, Dynamic nature of early organ dysfunction determines outcome in acute pancreatitis. Br J Surg. 2002;89(3):298–302. 11872053
10. McKay, C.J., Curran, F., Sharples, C., et al, Prospective placebo-controlled randomized trial of lexipafant in predicted severe acute pancreatitis. Br J Surg. 1997;84(9):1239–1243. 9313702
11. Isenmann, R., Rau, B., Beger, H.G., Early severe acute pancreatitis: characteristics of a new subgroup. Pancreas. 2001;22(3):274–278. 11291929
12. Johnson, C.D., Abu-Hilal, M., Persistent organ failure during the first week as a marker of fatal outcome in acute pancreatitis. Gut. 2004;53(9):1340–1344. 15306596
13. Beger, H.G., Bittner, R., Block, S., et al, Bacterial contamination of pancreatic necrosis. A prospective clinical study. Gastroenterology. 1986;91(2):433–438. 3522342
14. Whitcomb, D.C., Ulrich, C.D., 2nd., Hereditary pancreatitis: new insights, new directions. Bailliere’s Best Pract Res Clin Gastroenterol. 1999;13(2):253–263. 11030605
15. Underwood, T.W., Frye, C.B., Drug-induced pancreatitis. Clin Pharm. 1993;12(6):440–448. 8403815
16. Chari, S.T., Current concepts in the treatment of autoimmune pancreatitis. JOP. 2007;8(1):1–3. 17228127
17. Hogan, W.J., Geenen, J.E., Dodds, W.J., Dysmotility disturbances of the biliary tract: classification, diagnosis, and treatment. Semin Liver Dis. 1987;7(4):302–310. 3324349
18. McKay, C.J., Buter, A., Natural history of organ failure in acute pancreatitis. Pancreatology. 2003;3(2):111–114. 12748419
19. Ranson, J.H., Rifkind, K.M., Roses, D.F., et al, Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet. 1974;139(1):69–81. 4834279
20. Blamey, S.L., Imrie, C.W., O’Neill, J., et al, Prognostic factors in acute pancreatitis. Gut. 1984;25(12):1340–1346. 6510766
21. Wilson, C., Heath, D.I., Imrie, C.W., Prediction of outcome in acute pancreatitis: a comparative study of APACHE II, clinical assessment and multiple factor scoring systems. Br J Surg. 1990;77(11):1260–1264. 2253005
22. Gravante, G., Garcea, G., Ong, S.L., et al, Prediction of mortality in acute pancreatitis: a systematic review of the published evidence. Pancreatology. 2009;9(5):601–614. 19657215
23. Mofidi, R., Suttie, S.A., Patil, P.V., et al, The value of procalcitonin at predicting the severity of acute pancreatitis and development of infected pancreatic necrosis: systematic review. Surgery. 2009;146(1):72–81. 19541012
24. Al-Bahrani, A.Z., Abid, G.H., Holt, A., et al, Clinical relevance of intra-abdominal hypertension in patients with severe acute pancreatitis. Pancreas. 2008;36(1):39–43. 18192879
25. Zhang, W.F., Ni, Y.L., Cai, L., et al, Intra-abdominal pressure monitoring in predicting outcome of patients with severe acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2007;6(4):420–423. 17690042
26. , UK guidelines for the management of acute pancreatitis. Gut. 2005;54(Suppl. 3):iii1–iii9. 15831893
27. Balthazar, E.J., Robinson, D.L., Megibow, A.J., et al, Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990;174(2):331–336. 2296641
28. Viremouneix, L., Monneuse, O., Gautier, G., et al, Prospective evaluation of nonenhanced MR imaging in acute pancreatitis. J Magn Reson Imaging. 2007;26(2):331–338. 17654731
29. Uhl, W., Warshaw, A., Imrie, C., et al, IAP guidelines for the surgical management of acute pancreatitis. Pancreatology. 2002;2(6):565–573. 12435871
30. Beger, H.G., Rau, B., Mayer, J., et al, Natural course of acute pancreatitis. World J Surg. 1997;21(2):130–135. 8995067
31. Villatoro, E., Mulla, M., Larvin, M., Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev. 2010;(5) CD002941. 20464721
32. Wittau, M., Mayer, B., Scheele, J., et al, Systematic review and meta-analysis of antibiotic prophylaxis in severe acute pancreatitis. Scand J Gastroenterol. 2011;46(3):261–270. 21067283 Most recent review of the role of antibiotics in acute pancreatitis.
33. Kalfarentzos, F., Kehagias, J., Mead, N., et al, Enteral nutrition is superior to parenteral nutrition in severe acute pancreatitis: results of a randomized prospective trial. Br J Surg. 1997;84(12):1665–1669. 9448611 This initial randomised trial has since been supported by seven others.
34. Al-Omran, M., Albalawi, Z.H., Tashkandi, M.F., et al, Enteral versus parenteral nutrition for acute pancreatitis. Cochrane Database Syst Rev. 2010;(1) CD002837. 20091534
35. Petrov, M.S., Correia, M.I., Windsor, J.A., Nasogastric tube feeding in predicted severe acute pancreatitis. A systematic review of the literature to determine safety and tolerance. JOP. 2008;9(4):440–448. 18648135
36. Windsor, A.C., Kanwar, S., Li, A.G., et al, Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis. Gut. 1998;42(3):431–435. 9577354
37. Petrov, M.S., Loveday, B.P., Pylypchuk, R.D., et al, Systematic review and meta-analysis of enteral nutrition formulations in acute pancreatitis. Br J Surg. 2009;96(11):1243–1252. 19847860
38. Besselink, M.G., van Santvoort, H.C., Buskens, E., et al, Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371(9613):651–659. 18279948
39. Ayub, K., Imada, R., Slavin, J., Endoscopic retrograde cholangiopancreatography in gallstone-associated acute pancreatitis. Cochrane Database Syst Rev. 2004;(4) CD003630. 15495060
40. Petrov, M.S., van Santvoort, H.C., Besselink, M.G., et al, Early endoscopic retrograde cholangiopancreatography versus conservative management in acute biliary pancreatitis without cholangitis: a meta-analysis of randomized trials. Ann Surg. 2008;247(2):250–257. 18216529 The most recent review of the role of ERCP in acute pancreatitis.
41. Yusoff, I.F., Raymond, G., Sahai, A.V., A prospective comparison of the yield of EUS in primary vs. recurrent idiopathic acute pancreatitis. Gastrointest Endosc. 2004;60(5):673–678. 15557941
42. Bhattacharya, D., Ammori, B.J., Minimally invasive approaches to the management of pancreatic pseudocysts: review of the literature. Surg Laparosc Endosc Percutan Tech. 2003;13(3):141–148. 12819495
43. Nealon, W.H., Walser, E., Surgical management of complications associated with percutaneous and/or endoscopic management of pseudocyst of the pancreas. Ann Surg. 2005;241(6):948–960. 15912044
44. Baron, T.H., Thaggard, W.G., Morgan, D.E., et al, Endoscopic therapy for organized pancreatic necrosis. Gastroenterology. 1996;111(3):755–764. 8780582
45. Gardner, T.B., Coelho-Prabhu, N., Gordon, S.R., et al, Direct endoscopic necrosectomy for the treatment of walled-off pancreatic necrosis: results from a multicenter U.S. series. Gastrointest Endosc. 2011;73(4):718–726. 21237454
46. Mier, J., Leon, E.L., Castillo, A., et al, Early versus late necrosectomy in severe necrotizing pancreatitis. Am J Surg. 1997;173(2):71–75. 9074366 The only randomised trial of early surgery in acute pancreatitis.
47. Buchler, M.W., Gloor, B., Muller, C.A., et al, Acute necrotizing pancreatitis: treatment strategy according to the status of infection. Ann Surg. 2000;232(5):619–626. 11066131
48. Uomo, G., Visconti, M., Manes, G., et al, Nonsurgical treatment of acute necrotizing pancreatitis. Pancreas. 1996;12(2):142–148. 8720660
49. Rau, B., Pralle, U., Mayer, J.M., et al, Role of ultrasonographically guided fine-needle aspiration cytology in the diagnosis of infected pancreatic necrosis. Br J Surg. 1998;85(2):179–184. 9501810
50. Gerzof, S.G., Banks, P.A., Robbins, A.H., et al, Early diagnosis of pancreatic infection by computed tomography-guided aspiration. Gastroenterology. 1987;93(6):1315–1320. 3678750
51. van Santvoort, H.C., Besselink, M.G., Bakker, O.J., et al, A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362(16):1491–1502. 20410514
52. Raraty, M.G., Halloran, C.M., Dodd, S., et al, Minimal access retroperitoneal pancreatic necrosectomy: improvement in morbidity and mortality with a less invasive approach. Ann Surg. 2010;251(5):787–793. 20395850
53. van Santvoort, H.C., Bakker, O.J., Bollen, T.L., et al, A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology. 2011;141(4):1254–1263. 21741922
54. Fernandez-del Castillo, C., Rattner, D.W., Makary, M.A., et al, Debridement and closed packing for the treatment of necrotizing pancreatitis. Ann Surg. 1998;228(5):676–684. 9833806
55. Bradley, E.L., 3rd., Management of infected pancreatic necrosis by open drainage. Ann Surg. 1987;206(4):542–550. 3662663
56. Beger, H.G., Buchler, M., Bittner, R., et al, Necrosectomy and postoperative local lavage in necrotizing pancreatitis. Br J Surg. 1988;75(3):207–212. 3349326
57. Freeny, P.C., Hauptmann, E., Althaus, S.J., et al, Percutaneous CT-guided catheter drainage of infected acute necrotizing pancreatitis: techniques and results. Am J Roentgenol. 1998;170(4):969–975. 9530046
58. Carter, C.R., McKay, C.J., Imrie, C.W., Percutaneous necrosectomy and sinus tract endoscopy in the management of infected pancreatic necrosis: an initial experience. Ann Surg. 2000;232(2):175–180. 10903593
59. Seifert, H., Faust, D., Schmitt, T., et al, Transmural drainage of cystic peripancreatic lesions with a new large-channel echo endoscope. Endoscopy. 2001;33(12):1022–1026. 11740644
60. Varadarajulu, S., Phadnis, M.A., Christein, J.D., et al, Multiple transluminal gateway technique for EUS-guided drainage of symptomatic walled-off pancreatic necrosis. Gastrointest Endosc. 2011;74(1):74–80. 21612778
61. Giovannini, M., Pesenti, C., Rolland, A.L., et al, Endoscopic ultrasound-guided drainage of pancreatic pseudocysts or pancreatic abscesses using a therapeutic echo endoscope. Endoscopy. 2001;33(6):473–477. 11437038