43 Acute Pain
THE PRACTICE OF PAIN MANAGEMENT in children continues to advance. Since the early 1980s, clinicians have come to recognize that neonates and infants experience pain and process those learning experiences. Research has demonstrated the adverse long-term consequences of unrelieved pain, including harmful neuroendocrine responses, disrupted eating and sleep cycles, and increased pain perception during subsequent painful experiences.1–3 Disparities in pain treatment led organizations, such as the Agency for Healthcare Research and Quality (AHRQ) and the American Pain Society (APS), to provide guidelines and the Joint Commission (formerly the Joint Commission on Accreditation of Healthcare Organizations [JCAHO]) to issue mandates that further enhanced the practice of pediatric pain management.4–6 The availability of reliable and valid pain assessment tools for children and governmental incentives encouraged the inclusion of children in analgesic drug trials. Sufficient research data regarding children’s pain became available, making it possible to develop pediatric evidence-based pain management guidelines. Many children’s hospitals now have dedicated specialized multidisciplinary pain teams that manage acute and chronic pain. The increasing use of regional analgesia techniques led to the development of the Pediatric Regional Anesthesia Network (PRAN), a registry of practice patterns and complications of regional anesthetics in children. An enormous expansion of the breadth of techniques for acute pain management in children, the establishment of pediatric pain services, and the investigation and introduction of innovative modalities of therapy all attest to the importance accorded to this aspect of perioperative care.
Developmental Neurobiology of Pain
Investigators have examined indices suggestive of cortical activation, including near-infrared spectroscopy7 and electroencephalography,8 in response to noxious events. Using near-infrared spectroscopy, a unilateral heelstick (performed for clinical purposes) produces signal changes suggestive of contralateral cortical activation.7–9
Despite these lines of evidence, the nature of pain in neonates, viewed as conscious suffering, remains unknown. Other investigators have looked for long-term consequences of painful events (with or without treatment) in humans and in animal models. Despite attempts by these investigators to correct for confounding factors, in our view, the interpretation of these studies, especially in humans, should be quite cautious. Neonates who undergo painful procedures are commonly those who are more medically ill. It appears difficult to distinguish consequences of pain per se from the consequences of other factors, such as prematurity, critical illness (including episodes of hypoxia or ischemia), deprivation of tactile and social contact, and nutritional deprivation. Many clinicians and investigators have adopted the view that, in the absence of better information about either the nature of suffering experienced by neonates or the potential adverse consequences of pain in terms of long-term development, caregivers should err on the side of providing, rather than withholding, analgesia. Although this is a compelling perspective, it is important to highlight three concerns: (1) in general, available studies have had difficulty showing effects of routine administration of analgesia (e.g., morphine infusions) on immediate behavioral indices of distress in neonates undergoing intensive care; (2) repeated or prolonged administration of anesthetics and sedatives in animal models have been shown to have deleterious effects on brain development, (the human implications of these animal studies remain unclear at this time [see also Chapters 6 and 23]);10–16 and (3) as will be detailed later, younger organisms develop tolerance to opioids and benzodiazepines more rapidly than older organisms, so that the management of tolerance and withdrawal has now become a nearly universal consequence of prolonged administration of these medications to critically ill neonates, infants, and children.
Pain Assessment
The International Association for the Study of Pain (IASP) has defined pain as an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage. The IASP and others have acknowledged that the inability to communicate verbally, as in the preverbal, nonverbal, or the cognitively impaired, does not preclude the possibility that an individual is experiencing pain and is in need of appropriate pain management.17,18 Physicians and nurses have been very creative in developing tools to evaluate pain in children of all ages; most of these tools are discussed below. Table 43-1 summarizes various pain assessment tools in terms of appropriate age, target population, ease of use, and practicality.
TABLE 43-1 Appropriate Pain Assessment Measures by Age-Group: Self Report, Observational/Behavior, and for the Cognitively Impaired
| Self-Report Tools | Appropriate Age-Groups | Comments |
|---|---|---|
| Faces Pain Scale | 3-18 years | Simple and quick to use; extensively validated in healthy schoolchildren with postoperative and cancer pain |
| Oucher | 3-18 years | Photographic for ≥3-year-olds, numeric 0-10 scale for ≥6-year-olds; less clinical utility and feasibility compared to other faces scales |
| Manchester Pain Scale | 3-18 years | Panda bear faces eliminate gender and ethnic bias; tested in emergency department setting |
| Computer Face Scale | 4-18 years | Offers option for continuous rather than categorical format; good construct validity; preferred by children over the Wong Baker Faces Scale; further testing needed |
| Sydney Animated Facial Expression Scale (SAFE) | 4-18 years | Animated version of Faces Pain Scale; rated by children as easiest to use; no psychometric advantage compared to other scales |
| Visual Analog Scale (VAS) | 6-18 years | Simple and quick to use; requires the concepts of order, magnitude, and seriation (the ability to place or visualize in series); widely used across settings; preferred to other self-report tools by children ≥8 years old and adolescents |
| Numeric Rating Scale (NRS) | 7-18 years | Simplest and most commonly used in clinical as well as research settings |
| Observational/Behavioral Measures | ||
| Comfort Scale | 0-18 years | Developed for use in intensive care settings; useful in mechanically ventilated children and in the postoperative setting |
| Face, Legs, Activity, Cry, Consolability (FLACC) | 2 months to 7 years | Excellent pragmatic and psychometric qualities; widely adopted in clinical and research settings; has been translated into several languages other than English |
| Children’s Hospital of Eastern Ontario Pain Scale (CHEOPS) | 1-7 years | Good psychometric properties; lengthy with inconsistent scoring among categories; cumbersome; extensively used both in clinical and research settings |
| Cognitively Impaired Children | ||
| Revised FLACC | All ages | Allows for scoring individualized pain behaviors; good psychometric properties; highest clinical utility compared to the Non-Communicating Children’s Pain Checklist−Postoperative Version (NCCPC-PV) and Nurses’ Assessment of Pain Intensity (NAPI) |
| Non-Communicating Children’s Pain Checklist (NCCPC) | All ages | Requires 5-minute observation period; comprehensive but cumbersome; used in clinical and research setting |
| University of Wisconsin Pain Scale | All ages | Inconsistent scoring style compared to other clinical scoring systems; scoring style may permit flexibility but limits precision |
| The Pain Indicator for Communicatively Impaired Children | All ages | Useful for pain assessment in cognitively impaired children in the home setting. |
Self-Report Measures
Because pain is a subjective experience, self-report measures, in which a patient is asked to quantify the severity of the pain between 0 (no pain) and 10 (maximum pain), are considered to most accurately reflect acute pain. Because many children lack the cognitive skills to use such scales, pain assessment measures that include developmentally appropriate self-report tools, behavioral-observational tools, and physiologic-biologic measures have been developed. Given the multidimensional nature of the individual pain experience, and the complexity and inherent biases associated with self-report, use of unidimensional numeric scales alone to reflect pain is overly simplistic.19–22 Therefore, regardless of the measure used, it must be emphasized that a complete pain assessment is more than just a number attempting to quantify the severity of pain. Estimating the impact of pain on the suffering and the quality of the individual’s life, targeting appropriate therapeutic measures, and evaluating the effectiveness of such measures are additional key components of a global and ongoing pain assessment and treatment strategy.
Faces Pain Scales
Faces pain scales comprise a series of line diagrams of faces with expressions of increasing distress.23–28 Some versions have a smiling face whereas others have a neutral face to represent the “no pain” end of the scale (Fig. 43-1). Unlike the numeric scales, the faces scales do not require the concept of magnitude or seriation and can therefore be used by preschool aged children. The Wong Baker Faces Pain Scale has been extensively studied and its reliability and validity confirmed in children 3 to 18 years of age. Strong correlations have been reported between the Wong Baker Scale scores and other faces scales, the Visual Analog Scale (VAS), as well as nurses’ ratings based on behavior.29–32 Recent data suggest that versions with the smiling face at the no-pain end of the spectrum, such as the Wong Baker Scale, may overestimate pain because children without pain, but with distress from other sources, may be reluctant to choose the smiling face.28 The Wong-Baker scale was preferred by children to the numeric rating scale, the graphic rating scale, and the Color Analog Scale.23,25,30,33 Overall, the Faces Pain Scale–Revised is the faces scale with the largest support for its validity.34
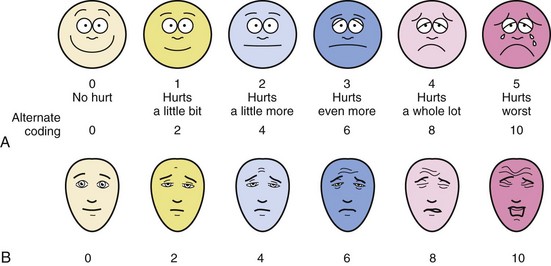
FIGURE 43-1 A, The Wong-Baker Faces Pain Scale. B, The Bieri Faces Pain Scale.
(B modified from Bieri D, Reeve RA, Champion GD, et al. The Faces Pain Scale for the self-assessment of the severity of pain experienced by children: development, initial validation, and preliminary investigation for ratio scale properties. Pain 1990;41:139-50.)
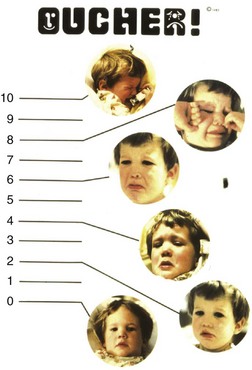
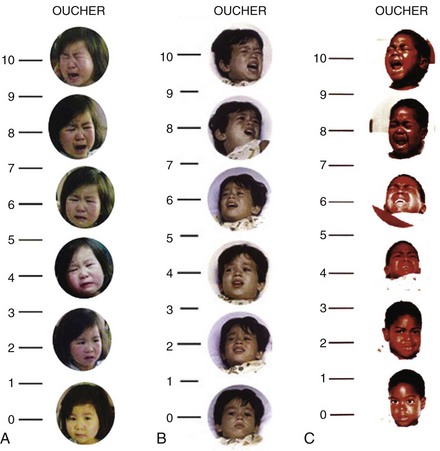
Oucher
The Oucher combines a photographic faces scale with a 0 to 10 vertical numeric scale. Different versions of the Oucher incorporate photographs of Caucasian, African American, Asian, and Hispanic children to minimize biases related to ethnicity (Fig. 43-2 and E-Fig. 43-1, A to C).35,36 Strong correlations have been demonstrated between Oucher scores and those obtained using the Pieces of Hurt tool, faces pain scales, and VAS.37–39 The Oucher also demonstrates responsivity, that is, the ability to detect change in pain intensity before and after surgery and after administration of an analgesic.37 The numerical rating component of the Oucher requires that the child be able to count to 10 and has been used successfully in children older than 6 years.
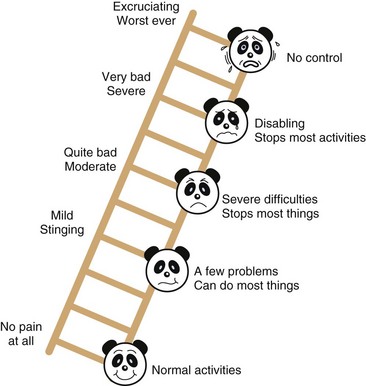
The Manchester Pain Scale
The Manchester Pain Scale (Fig. 43-3), which was designed to overcome the gender and ethnic biases of the Oucher, is composed of a pain ruler on which panda facial images are superimposed.40 It includes verbal descriptors of the extent of pain and how pain possibly interferes with normal functions. A study of children presenting to an emergency department found a very good correlation between scores assigned using the Manchester Scale and the Oucher.40
Novel Self-Report Tools
These self-report tools use a categorical format and the static faces do not allow for “fine tuning” of the ratings before a final assessment regarding the severity of pain is reached.41 In recent years, there has been interest in developing computer-based self-report assessment tools that use a continuous rather than categorical format.42
The Computer Face Scale allows the child to adjust the shape of the mouth of a cartoon face from smiling to frowning and simultaneously to adjust the eyes from completely open to completely closed![]() .41,43 The suggested benefits of this scale include increased sensitivity (given the ability to select from a wide range of faces) and computerized storage of the results, with ready access and data display. Preliminary work with this scale has demonstrated its construct validity and it was preferred by children over the Wong Baker Faces Scale.41
.41,43 The suggested benefits of this scale include increased sensitivity (given the ability to select from a wide range of faces) and computerized storage of the results, with ready access and data display. Preliminary work with this scale has demonstrated its construct validity and it was preferred by children over the Wong Baker Faces Scale.41
The Sydney Animated Facial Expression Scale (SAFE) is an animated version of the Faces Pain Scale44 and comprises of a series of 101 faces (Video 43-1![]() ). To administer this scale, the child pushes the left or right arrow key on a computer causing the expression of the single face to change until it corresponds with the child’s pain intensity (www.usask.ca/childpain/research/safe). At this point, a keystroke records a score between 0 and 100. The SAFE scale was rated to be easiest to use by children aged 4 to 16 years compared with other scales, including the Faces Pain Scale, the Color Analog Scale, and Pieces of Hurt,45 although it offered no psychometric advantage over the other scales. At this time, further research with this tool is needed before its role can be clearly defined.
). To administer this scale, the child pushes the left or right arrow key on a computer causing the expression of the single face to change until it corresponds with the child’s pain intensity (www.usask.ca/childpain/research/safe). At this point, a keystroke records a score between 0 and 100. The SAFE scale was rated to be easiest to use by children aged 4 to 16 years compared with other scales, including the Faces Pain Scale, the Color Analog Scale, and Pieces of Hurt,45 although it offered no psychometric advantage over the other scales. At this time, further research with this tool is needed before its role can be clearly defined.
Numeric Scales
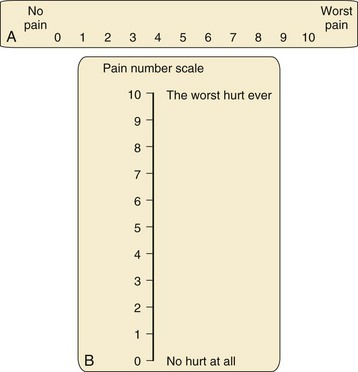
Visual Analog Scale
Several versions of the VAS are available, including horizontal and vertical lines, word anchors representing extremes of pain, and lines with divisions and numeric values (Fig. 43-4). When using the vertical versions of this scale, the severity of the pain increases as one ascends the ladder. Although moderate to strong correlations have been reported between the VAS, faces pain scales, and the Oucher,37,46 the effect of user age on VAS ratings are conflicting.
Numeric Rating Scale
The Numeric Rating Scale (NRS) is the simplest and most commonly used numeric scale in which the child rates the pain from 0 (no pain) to 10 (worst pain). Its validity has been established with good correlations between NRS and Faces Pain Scale-Revised scores in children 7 to 17 years of age and NRS and VAS scores in children 9 to 17 years of age.47 An important caveat when using numeric scales is to be sure of the denominator that the child is using. For example a pain score of 9 on a 0 to 100 scale would reflect mild pain and may not require treatment whereas a score of 9 on a 0 to 10 scale would reflect severe pain that warrants aggressive treatment.
Selection Criteria
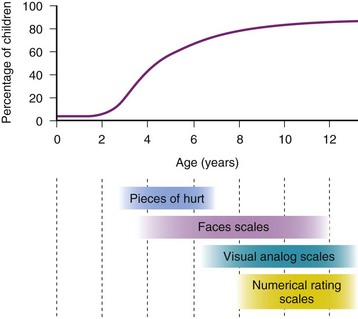
Selection of a self-report tool for a child requires careful consideration of the age and cognitive and developmental level. Figure 43-5 depicts the percentages of children of different ages who are able to self-report their pain and the tools most appropriate for various age ranges. Children who are unable to use a self-report tool may be able to report their pain intensity using simple words, such as “small,” “medium,” and “big.” However, self-reports of pain are subject to the modulating influences of a number of factors, including the child’s previous pain experience and response to treatment, psychosocial factors, and parental preferences and influences. In many cases, therefore, it may be necessary to complement self-reported pain scores with behavioral observations, particularly in preschool-aged children. Regardless of the tool selected, assessment of postoperative pain is greatly facilitated by the introduction of the concept of rating pain and of the tool itself during the preoperative preparation of the child.
Observational-Behavioral Measures
Despite several age-appropriate methods for self-reporting, assessing pain in children who are unable or unwilling to self-report depends on observations of their behaviors. Five behaviors that have been shown to be reliable, specific, and sensitive when predicting analgesic requirements are facial expression, vocalization or cry, leg posture, body posture, and motor restlessness.48 Variations in these behaviors have been used in several observational pain tools. Table 43-2 describes the content validity of some of the observational tools that are commonly used in clinical practice. Behavior checklists provide a list of pain behaviors that are marked as present or absent and the extent of pain is estimated on the basis of the number of behaviors present at the time of the assessment.49,50 Behavior rating scales also incorporate a rating of the intensity or frequency and duration of each behavior.51 Global rating scales provide a rating of the observer’s global impression of the child’s pain.
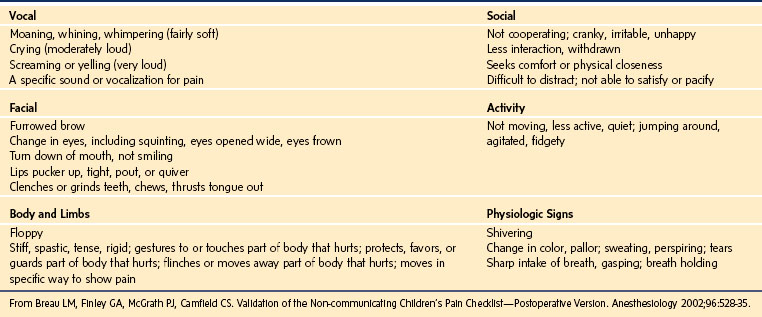
TABLE 43-2 Content Validity of Behavioral Pain Tools: Categories of Behavior in Pain Assessment Tools
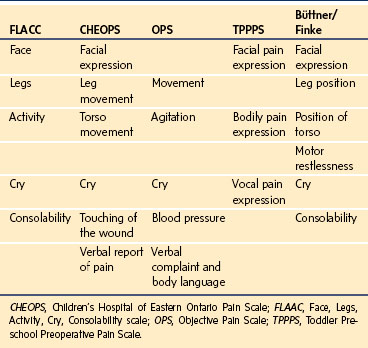
Children’s Hospital of Eastern Ontario Pain Scale
The Children’s Hospital of Eastern Ontario Pain Scale (CHEOPS), one of the earliest behavioral rating scales (Table 43-3),52 incorporates six categories of behavior scored individually from 0 to 2 or 1 to 3 and then sums them to provide a pain score ranging from 4 to 13. Scores of 6 or less indicate no pain. Its validity and reliability for brief painful events and for postoperative pain has been well established, with good to excellent correlations with faces pain scales and the VAS.46,53 However, the time required to complete the evaluation and inconsistent scoring among categories of the CHEOPS makes it cumbersome and impractical to use in a busy clinical setting.
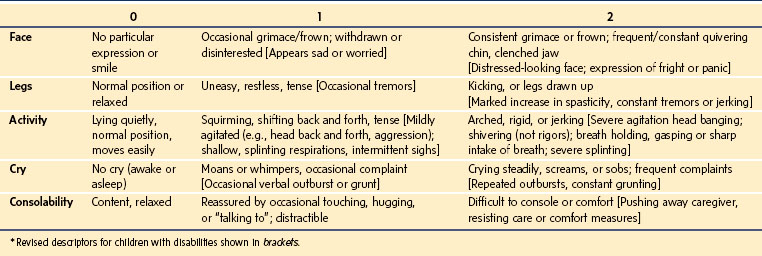
Face, Legs, Activity, Cry, Consolability Scale
The Face, Legs, Activity, Cry, Consolability (FLACC) scale was developed in an effort to improve on the pragmatic qualities of the existing behavioral pain tools by providing a simple framework for quantifying pain behaviors in children.51 This tool includes five categories of behaviors previously found to reliably correlate with pain in young children, including: facial expression, leg movement, activity, cry, and consolability (Table 43-4).48 The acronym FLACC facilitates recall of these categories, each of which is scored from 0 to 2 to provide a total pain score ranging from 0 to 10. The FLACC tool has been extensively tested and determined to have good inter-rater reliability and excellent validity based on changes in pain scores from before to after analgesic administration and excellent correlation with the Objective Pain Scale (OPS), the CHEOPS, the Toddler Preschool Preoperative Pain Scale (TPPPS), and good correlation with self-reported pain scores using faces pain scales.51,53–55 The FLACC scale has been translated into several languages, including Chinese, Swedish, French, Italian, Portuguese, Norwegian, and Thai.
Comfort Scale
The Comfort scale (Table 43-5), developed for use in an intensive care setting, consists of six behavioral and two physiologic measures, each of which has five response categories, thereby allowing detection of subtle changes in the child’s distress.56 Initial evaluation of the Comfort scale found acceptable inter-rater reliability and good correlations with VAS scores in 37 mechanically ventilated infants.56 Another study evaluated the reliability and validity of the Comfort scale as a postoperative pain instrument in children after thoracic or abdominal surgery.57 This study found good to excellent inter-rater agreement for all categories except respiratory response, for which there was moderate agreement. Additionally, strong correlations between Comfort and VAS pain scores support the use of the Comfort scale as a postoperative pain measurement instrument in children.
After a systematic review of observational pain measures, the FLACC and the CHEOPS52 scales were recommended for assessment of pain associated with medical procedures, the FLACC for postoperative pain, and the Comfort Scale for pain in children in critical care.19 Despite the extensive science supporting the use of behavioral tools, it may be difficult to separate behaviors caused by pain from those caused by other sources of distress in some children.58 Accurate pain assessment in children, therefore, requires careful consideration of the context of the behaviors. Input from the parents or caregivers may be valuable as proxy measures, although some parents may lose objectivity in such a situation. Similarly, a regular caregiver may best assess older children with significant developmental delay. When in doubt regarding the source of distress, a trial of analgesics is appropriate and may be both diagnostic and therapeutic.
Limitations of PAIN Assessment
It remains unclear whether integration of routine pain assessment into clinical practice significantly improves patient outcomes. A critical review of the studies that addressed this question determined that in 2 of 6 studies, children experienced a reduction in pain intensity when a standardized pain assessment tool was used, in 2 studies there was no change in pain intensity, and in 2 studies pain intensity decreased when pain assessment was combined with pain management interventions.59 Studies that examined sustainability of the benefits over time reported conflicting results, and most studies were identified to have major methodologic problems.60,61 Additional investigation is required to determine whether routine pain assessment has any effect on pain outcomes.
Despite the large body of evidence supporting the psychometric properties of numerous structured pain assessment tools described previously and elsewhere, there remains considerable variability in the interpretation of the clinical relevance of pain scores.22 Attempts have been made to define what range of pain scores is associated with a perceived need for medicine or what magnitude of change in pain score is associated with a perception of better or worse pain.62–64 A survey of 6- to 16-year-old hospitalized children found that a median pain score of 3 on a 0-to-6 Faces Pain Scale was associated with the child’s perceived need for medicine.62 Others have reported that a 10-mm change in a 0-to-100-mm VAS score was the minimum difference whereby children in the emergency department perceived their pain to be slightly better or slightly worse.63 In the postoperative period, children with a median pain score of 6 on a 0-to-10 NRS scale perceived the need for an analgesic whereas those with a score of 3 felt there was “no need” for treatment.64 In addition, children felt “a little better” or “worse” if the NRS scale changed by at least 1. Despite these findings, there was large variability and overlap in scores associated with these outcomes.
It has been suggested that the widespread adoption of a pain score as the fifth vital sign may contribute to the overprescribing of analgesics and sedatives.65 A review of trauma center site surveys reported a fivefold increase in deaths from excessive pain medicines during two time periods (1994 through 1998 and 2000 through 2004). Evaluations of the effectiveness of pain treatment algorithms based on numerical pain scores have yielded conflicting results. One study reported increased prescription for opioid and nonopioid analgesics, an increased administration of nonopioids, and reduced pain scores in children who received postoperative pain treatment based on a pain score–based algorithm.66 Children whose pain management was algorithm-based experienced more nausea, but no other adverse effects. In contrast, hospitalized adults whose pain management was based on a numerical pain treatment algorithm, experienced a twofold increase in episodes of oversedation and a 49% increase in opioid-related adverse drug events.67 This latter study highlights the potential for harm when numeric pain scores alone are used guide decisions regarding pain treatment. A comprehensive approach to pain assessment that includes consideration of the child’s self-reporting (when available), combined with behavioral observation and the overall clinical context, is required to direct treatment decisions.68
Special Considerations for the Cognitively Impaired Child
Children who are cognitively impaired experience pain more frequently than cognitively intact children because of a number of inherent conditions, such as spasticity, muscle spasms, the need for assistive devices for positioning and mobility, and the need for invasive surgical procedures. Indeed, as many as 60% of children with cerebral palsy undergo orthopedic surgery by 8 years of age, and many of them require repeated procedures.69 Yet both children and adults who are cognitively impaired receive fewer analgesics than those who are cognitively intact with similar painful conditions.70,71 Barriers to effective pain management in the cognitively impaired include the complexity of pain assessment in those who cannot verbalize their pain, outdated beliefs that these children have altered or blunted pain perception, limited evidence for the safety and efficacy of analgesic regimens, and an exaggerated concern regarding opioid adverse effects, particularly respiratory depression. Difficulties with pain assessment have led to the virtual exclusion of these children from clinical drug trials, leading to deficits in our knowledge of how to effectively manage their pain. A survey of clinicians who treat children who are cognitively impaired identified inadequate pain assessment tools and inadequate training and knowledge of providers as significant barriers to effective pain management, despite respondents beliefs that children who are cognitively impaired perceive pain to a similar extent as cognitively intact children.72
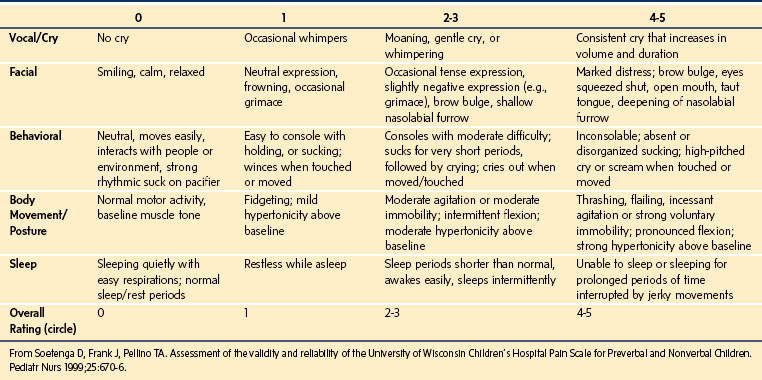
The University of Wisconsin Pain Scale for Preverbal and Nonverbal Children
This scale is composed of five behavior categories with four descriptors for each (E-Table 43-1).73 The overall rating using this tool is not a sum of scores of individual behaviors but a score assigned on a 0- to 5-scale based on the clinician’s judgment relative to assessment of individual categories. The scoring style of this tool does allow for flexibility, but limits its precision. This scale has been tested in 59 preverbal children and 15 children who were nonverbal because of cognitive impairment. Although these investigators reported good validity and reliability in their overall sample, the reliability and validity of this tool for the subset of children with cognitive impairment was not reported.
The Non-Communicating Children’s Pain Checklist—Postoperative Version
This tool comprises a checklist of 27 pain behaviors across six categories.74 Each of these behaviors (E-Table 43-2) is scored on a 0- to 3-point scale based on the frequency of observation of that behavior over a 10-minute observation period. The scores of all items are summed to provide a total pain score. This tool has been evaluated in 25 children who were cognitively impaired,74 with good inter-rater reliability in four of the six behavior categories and good correlations between the Non-Communicating Children’s Pain Checklist—Postoperative Version (NCCPC-PV) scores and VAS scores. Although this checklist provides a comprehensive pain assessment method for children with cognitive impairment undergoing surgery, it may be cumbersome for frequent and repeated pain assessments in the clinical setting.
E-TABLE 43-2 Noncommunicating Children’s Pain Checklist—Postoperative Version
| Vocal | Social |
From Breau LM, Finley GA, McGrath PJ, Camfield CS. Validation of the Non-communicating Children’s Pain Checklist—Postoperative Version. Anesthesiology 2002;96:528-35.
The Pain Indicator for Communicatively Impaired Children
One group of investigators interviewed parents and/or caregivers of 30 communicatively impaired children regarding cues they used to identify pain in their child.75 Six core pain cues were reported by 90% of the caregivers as signs of definite or severe pain in their child (E-Table 43-3). Each of these cues is scored on a 4-point Likert scale (not at all, a little, often, all the time), based on the frequency of occurrence of the behavior over the observation period. Caregivers of children with severe cognitive impairment, who evaluated this scale at home over a 7-day period, reported no significant relationship between crying and the presence of pain. Yet, they found that a “screwed up or distressed looking face” had the strongest relationship with the presence of pain. In fact, they found that facial expression alone correctly identified 71% of children in pain and 93% of those not in pain, with an overall correct classification rate of 87%. This tool provides a simple method of assessing pain in children with cognitive impairment in the home setting. Further testing of this tool is required in the hospital setting, and using shorter observation periods, to determine its feasibility of use by clinicians.
E-TABLE 43-3 Pain Indicator for Communicatively Impaired Children (PICIC)
From Stallard P, Williams L, Velleman R, et al. The development and evaluation of the pain indicator for communicatively impaired children (PICIC). Pain 2002;98:145-9.
Face, Legs, Activity, Cry, Consolability Observational Tool
Initial evaluation of the FLACC tool in children with cognitive impairment found a good correlation between scores assigned independently by different observers and by parent global ratings of pain.76 Although measures of exact agreement between observers were acceptable for the face, cry, and consolability categories, measure of agreement for the legs and activity categories were less acceptable, likely because of coexisting motor impairments such as spasticity. The FLACC tool was therefore revised to incorporate additional descriptors of behaviors most consistently associated with pain in children with cognitive impairment (Table 43-6).77 Inter-rater reliability for the total FLACC scores, as well as for each of the categories, improved when the evaluation included the revised FLACC (r-FLACC) in 52 cognitively impaired children. Also, good correlation between FLACC, parent, and child scores supported its criterion validity. FLACC scores were noted to decrease after an opioid was administered, supporting the construct validity of the tool. The pragmatic attributes of the r-FLACC were compared with those of the Nurses’ Assessment of Pain Intensity (NAPI) and the NCCPC-PV.78 Clinicians using these tools to score pain rated the complexity as less and the relative advantage and overall clinical utility of the FLACC and the NAPI to be greater compared with the NCCPC-PV, suggesting that these tools may be more readily adopted into clinical practice.
Strategies for Pain Management
Pain is a complex phenomenon that occurs because of the transmission of nociceptive stimuli from the peripheral nervous system through the spinal cord to the cerebral cortex. Pain perception is further influenced by emotions, behavior, and previous pain experiences via multiple synapses in the limbic system, frontal cortex, and thalamus. Given the complexity of the pain mechanism, effective treatment of pain requires the use of multimodal therapies that target multiple sites along the pain pathways, as illustrated in Figure 43-6. Analgesics with additive or synergistic activity and different adverse effect profiles should be selected so that adequate analgesia can be provided with fewer adverse consequences. Thus pain can be treated at the peripheral level using local anesthetics, peripheral nerve blockade, nonsteroidal antiinflammatory drugs (NSAIDs), antihistamines, or opioids. At the spinal cord level, pain can be treated with local anesthetics, neuraxial opioids, α2-adrenoceptor agonists, and N-methyl-d-aspartate (NMDA) receptor antagonists. Finally, at the cortical level systemic opioids, α2-agonists, and voltage-gated calcium channel α2δ proteins (targets for anticonvulsants) can be used.79 Most cases of moderate to severe pain are best treated with a combination of analgesic techniques.
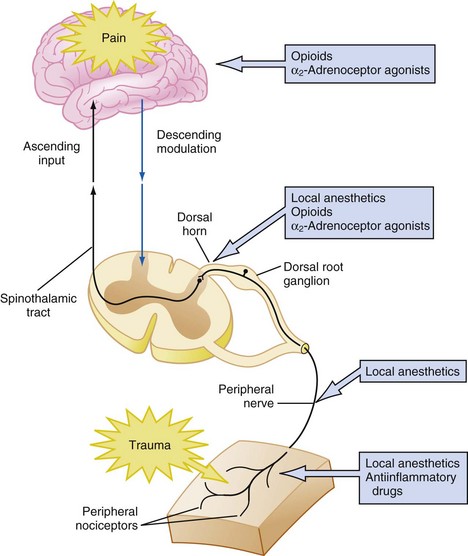
FIGURE 43-6 Schematic diagram of the pain pathways and multimodal measures to provide pain relief.
(From http://old.cvm.msu.edu/courses/VM545/Evans/Pain%20Management%20PDA.htm.)
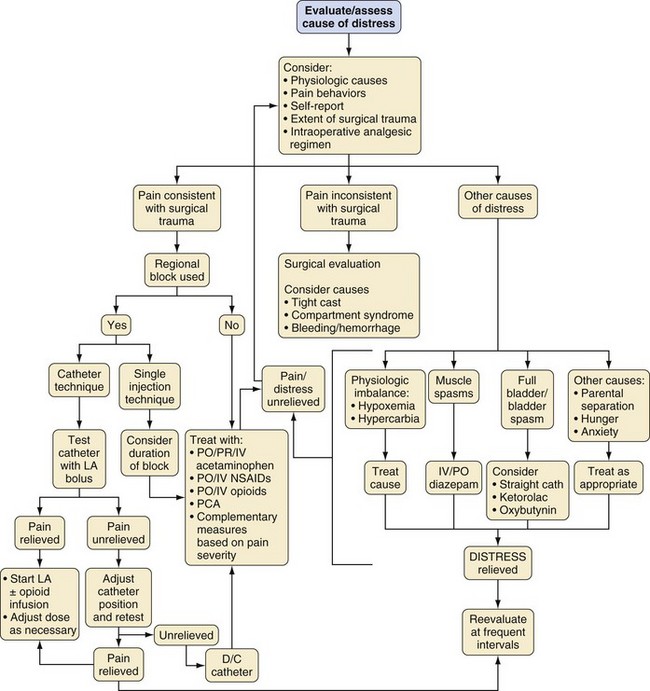
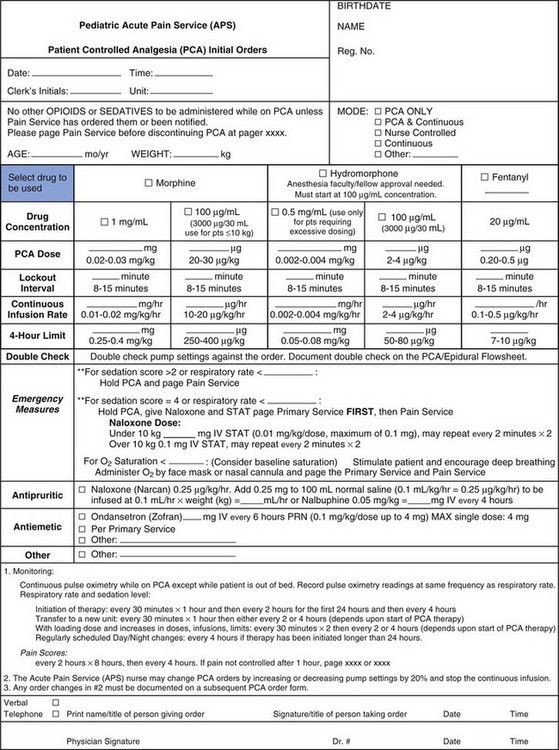
The strategy for postoperative pain management is an integral part of the preanesthetic plan, so that informed consent for procedures, such as placement of peripheral or regional blocks, can be obtained (see Chapters 41 and 42). Additionally, appropriate teaching for techniques, such as patient-controlled analgesia (PCA), should begin in the preoperative period. An honest discussion with the child that, although some discomfort is inevitable, every effort will be made to minimize pain after surgery, decreases the anxiety related to the perioperative experience. This, together with the use of nonpharmacologic techniques, may even reduce the need for opioids and other analgesics. Selection of an analgesic regimen requires careful consideration of a number of factors, including scope and requirements of the surgical procedure, age and cognitive abilities of the child, the child’s previous pain experience and response to treatment, underlying medical conditions that might alter the response to pain medications, and child and family preferences. The goal should be for the child to emerge from anesthesia in reasonable comfort, because it is generally easier to maintain analgesia in a pain-free child than to achieve analgesia in a child with severe pain. Figure 43-7 presents a flowchart describing strategies for assessment and management of acute postoperative pain in a child.
Surgical Considerations
The scope and requirements of the surgical procedure, as well as specific postoperative issues, should be discussed with the surgical team before choosing an analgesic regimen, particularly if a regional technique is planned. For example, the site of placement of an epidural catheter and choice of epidural solution will differ in a child with a vertical midline incision from a child with a transverse suprapubic incision. With certain procedures, an epidural catheter may intrude into the surgical field or access to the catheter site in the postoperative period may be obscured by a cast or dressing. In such cases, the catheter may be tunneled subcutaneously away from the surgical field. Alternatively, one or more epidural catheters may be placed under direct vision by the surgeon at the end of the procedure (e.g., spinal fusion or selective dorsal rhizotomy).80–83 Postoperative pain is managed by the pain service, using infusion of local anesthetic and/or opioid solutions through the catheter.80 Painful muscle spasms after certain procedures are often well managed with continuous epidural analgesia.80,84,85 Refractory spasms of the bladder, which can be quite problematic after some surgeries (e.g., ureteral reimplantation), can also be effectively treated with NSAIDs (e.g., ketorolac) or anticholinergics.86 Intravesical bupivacaine has also been used to manage bladder spasm.87,88 Muscle spasms after orthopedic surgery may be prevented by dense levels of regional blockade, but may also require supplementation with oral or parenteral benzodiazepines if epidural analgesia alone is ineffective. Epidural blockade may favorably alter diaphragmatic mechanics after thoracotomy and upper abdominal surgery. This effect is likely a result of the motor blockade of the intercostal muscles and alteration in the resting length of the diaphragm, and not solely a result of reversal of diaphragmatic inhibition.89–92 However, it remains uncertain whether analgesia alone, achieved by systemic opioids or central neuraxial blockade, is of value in diminishing postoperative diaphragmatic inhibition or significantly improving postoperative pulmonary function.93,94 Effective analgesia, however, does improve child compliance with measures such as deep breathing and early mobilization, thereby reducing the incidence of postoperative complications.95
Child-Related Considerations
Age and Cognitive Abilities
Analgesic techniques, such as infiltration of the wound with local anesthetics, peripheral nerve blocks, or regional blockade that minimize the use of opioids and central respiratory depressants, may be ideal for preterm or very young infants with impaired central respiratory drive.96,97 Acetaminophen can be a useful adjunct, because when used within its recommended dose range it has a large therapeutic window with few untoward effects. Although the judicious use of opioids is not contraindicated, preterm or term infants younger than 1 month of age who receive these medications require careful observation and monitoring to detect respiratory depression.98 The use of local anesthetics in infants also requires more careful attention to dose, to avoid accumulation and toxicity.
Although analgesia for the preterm infant was often neglected in the past, we now understand that these infants have reduced thresholds to painful or noxious stimuli when compared with older children.99 Most of the neural pathways that conduct nociception from the periphery through the central nervous system (CNS) are present and functional at 24 weeks gestational age, although the central connections, particularly in the thalamocortical pathways that are involved in the integration and perception of conscious pain, are not as well developed.100–102 Controversy remains as to the meaning and implications of this neural immaturity. Opioid receptors and responses are present in the spinal cord at the time of birth, although spinal glial inflammatory mechanisms are immature. Because these mechanisms are central to the cyclooxygenase (COX-1 and COX-2) responses, this may imply that there is limited or no analgesic response to NSAIDs and COX inhibitors in preterm infants or neonates, whereas opioid responses are active. GABAergic pathways, which play an important role in the effects of analgesics and anesthetics, can be either excitatory or inhibitory, depending on the stage of development.103 The neuroplasticity that is characteristic of these infants may be a double-edged sword. Animal models and some clinical evidence suggest that repeated noxious stimuli may result in heightened sensitivity to nociceptive input and adverse behavioral sequelae.2,10,104–107 On the other hand, nerve injury in infant animals may result in less pain than it does in older animals.106,107 In humans, the neural injury to the brachial plexus after shoulder dystocia during delivery rarely results in chronic pain.108 It may be that there are both vulnerable periods and periods of greater resiliency during development, so that the consequences of pain in our youngest children may not be easily predictable.
Older infants and toddlers who are expected to experience moderate to severe pain may be adequately treated with oral opioids when oral intake resumes. Alternatively, low-dose continuous opioid infusions, nurse-controlled analgesia,109 or regional blockade may be required in those undergoing extensive surgery. Nonpharmacologic techniques, such as child life therapy and the presence of a comforting parent, can do much to supplement analgesic therapy.
Preschool and school-aged children have greater fears and better understanding of the postoperative experience than do their younger counterparts. Most cognitively intact children 7 years of age or more are able to understand the concept of patient-controlled analgesia (PCA), which may be helpful in giving a sense of control back to the child during a period in which all other aspects of control are removed.110 Such issues of control and dependency assume even greater importance in adolescents; allowing them to participate in decision-making will contribute to the success of any analgesic technique.110 Regional techniques are excellent for providing analgesia in all age groups and are associated with a reduced incidence of adverse effects compared with systemic opioids (e.g., nausea, vomiting, excessive sedation, dysphoria and respiratory depression). Children with significant developmental delay require special consideration of their physical disability, as well as cognitive abilities, although in most cases the pharmacologic actions of the drugs are not altered.
Previous Pain Experience
A detailed history regarding the child’s previous pain experience, analgesic history, response to treatment, and adverse effects from previous analgesic regimens should be carefully considered when selecting a pain management technique. An opioid-naive child undergoing surgery for the first time requires smaller doses of opioids for a smaller duration compared with a child with chronic pain who has developed opioid tolerance as a result of long-term or repeated opioid use. Analgesic selection should also be modified based on the effectiveness of analgesics for that particular child in the past. For example, a child with a history of not responding to codeine may be deficient in the cytochrome P-450 2D6 isoenzyme (see also Chapter 6). These children cannot metabolize codeine (methylmorphine) to morphine (its active moiety) and experience reduced analgesia after codeine. Ineffective conversion of codeine to morphine may be present in up to 7% to 10% of Caucasian children, whereas the incidence of fast metabolizers is 5% in North America. These incidences differ with ethnicity, with a significantly greater incidence of polymorphisms in North African descendants.111–115 On the other hand, another polymorphism, present in about 0.5% of children, results in rapid demethylation of codeine to morphine, producing exaggerated sedation and respiratory depression when codeine is administered.116,117 Overall, we strongly discourage routine use of codeine as a first-line opioid for children. Oxycodone, hydrocodone, hydromorphone, or morphine are superior alternatives to codeine. Alternatively, if pain is of moderate or smaller intensity, another class of analgesics (e.g., NSAIDs) can be substituted.
Pharmacologic Treatment of Pain
Nonopioid Analgesics
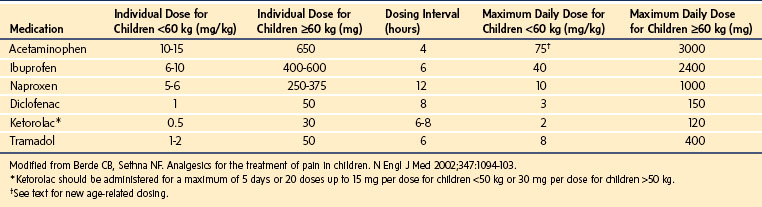
Nonopioid analgesics may be used as sole agents for the treatment for mild pain and as important adjuncts for the multimodal treatment of moderate to severe pain. Although most nonopioid analgesics produce dose-dependent responses, they are limited by a ceiling effect in the analgesia achieved, that is, larger doses of the medication provide no additional analgesia. Hence, more severe pain is resistant to therapy from these medications alone.118 Therefore they are frequently prescribed in combination with opioids to reduce both the opioid requirements and the adverse effects (Table 43-7).
Acetaminophen
Acetaminophen is the most common antipyretic and nonopioid analgesic used in children. It exerts its analgesic effects by blocking central and peripheral prostaglandin synthesis, reducing substance P–induced hyperalgesia, and modulating the production of hyperalgesic nitric oxide in the spinal cord.119–121 In addition, it has been suggested that acetaminophen produces analgesia via activation of descending serotonergic pathways.122–124 However, it is likely that its primary site of action may be inhibition of prostaglandin H2 synthetase at the peroxidase site.123 Effective analgesic and antipyretic effects have been described with plasma concentrations of 5 to 20 µg/mL125–129; a target effect-site concentration of 10 µg/mL reduces pain after tonsillectomy by 3.6/10 pain units.130 The total daily dose of acetaminophen via any route is age- and weight-based but should not exceed 75 mg/kg for children; term and preterm infants require further downward dosing adjustment (60 mg/kg and 45 mg/kg, respectively).
The recommended dose for oral administration is 10 to 15 mg/kg every 4 hours. Acetaminophen has a wide margin of safety when administered in the recommended therapeutic dose range. However, hepatotoxicity has been reported with doses only slightly above the recommended 10- to 15-mg/kg/dose orally for a total of five doses or 75 mg/kg/day, suggesting that acetaminophen may have a narrow therapeutic index in some children.131,132 Because of these reports and on the advice of a U.S. Food and Drug Administration panel, the manufacturers have reduced the maximum single dose of oral acetaminophen in adults to 650 mg and the maximum daily dose to 3 grams. Acetaminophen is available in a wide variety of formulations, alone or in combination with decongestants, for oral use in a variety of cold remedies, and with opioids for the treatment of moderate to severe pain. There are currently more than 600 over-the-counter acetaminophen-containing products, increasing the risk of an overdose because children may take more than one formulation that contains the drug. Frequent review of medications and parental education is needed to minimize the risk of overdose. In the past, pediatric liquid formulations of acetaminophen as in infant drops were commonly supplied in larger concentrations than that in elixirs, resulting in dosing errors. The current recommendation is to standardize liquid formulations to a single concentration of 32 mg/mL. Acetaminophen can be given orally before surgery; both gastric fluid volume and pH are unchanged after acetaminophen was administered orally 90 minutes before induction of anesthesia.133
Slow and unpredictable absorption of acetaminophen after rectal administration results in variable blood concentrations, with peak concentrations reached between 60 and 180 minutes after administration.128,134,135 There is a dose-response relationship for rectal acetaminophen. The morphine-sparing effects of 40 mg/kg and 60 mg/kg of rectal acetaminophen were greater than those of 20 mg/kg and placebo in children undergoing ambulatory surgery.136 In children undergoing orthopedic surgery, a loading dose of 40 mg/kg rectal acetaminophen followed by 20 mg/kg every 6 hours yielded serum concentrations of 10 to 20 µg/mL, with no evidence of accumulation over a 24-hour period.134 This dosing scheme is now the one most commonly recommended when the rectal route is employed.
After IV administration of acetaminophen, analgesic onset occurs in 15 minutes and that of antipyresis in 30 minutes.137,138 IV paracetamol rapidly penetrates the blood-brain barrier in children, yielding detectable concentrations in the cerebrospinal fluid (CSF) within 5 minutes of administration, and peak CSF concentrations within 57 minutes after injection (compared with 2 to 3 hours after rectal or oral administration), thus explaining the fast onset of its analgesic and antipyretic effects.139 A large multicenter trial reported that 1 gram of IV paracetamol and 2 grams of IV propacetamol (equivalent to 1 gram acetaminophen) provided superior analgesia with a reduced need for morphine compared with placebo in adults after lower extremity joint replacement.140 The propacetamol group experienced a greater incidence of local skin reactions and pain on injection compared with the IV paracetamol group. A controlled randomized trial reported that both rectal acetaminophen, 40 mg/kg, and IV acetaminophen, 15 mg/kg, administered after induction of anesthesia in children undergoing adenotonsillectomy, provided good analgesia for the first 6 hours after surgery.141 However, children who received acetaminophen rectally had a greater duration of analgesia and did not require rescue analgesia as early as those in the IV group.141 This is attributable to the slow absorption of rectal acetaminophen causing sustained effective concentrations. A prospective randomized trial comparing rectal acetaminophen to IV propacetamol in infants after craniofacial surgery reported that the IV formulation provided superior analgesia,142 in part because of reduced bioavailability of acetaminophen by the rectal route.
Another controlled randomized study compared the analgesic efficacy and side effects of fentanyl-placebo versus fentanyl-acetaminophen administered via PCA in 6- to 24-month-old children undergoing ureteroneocystostomy.143 Children in the acetaminophen group required significantly less fentanyl and demonstrated a reduced incidence of vomiting and excessive sedation compared with those in the placebo group. Lastly, a large retrospective study reported no differences in alanine transaminase and γ-glutamyl transferase concentrations, and a progressive decrease in aspartate aminotransferase levels, in term and preterm neonates before, during and after IV acetaminophen injection.144
Nonsteroidal Antiinflammatory Drugs
NSAIDs provide excellent analgesia for mild to moderate pain resulting from surgery, injury, and disease. Their principle mechanism of action is via inhibition of the enzyme prostaglandin H2 synthetase at the COX site, causing a reduction in the production of prostaglandins at the site of tissue injury, and attenuation of the inflammatory cascade. In addition to their peripheral effects, the NSAIDs have also been shown to exert a direct spinal action by blocking the hyperalgesic response induced by activation of spinal glutamate and substance P receptors.145 Decreased production of leukotrienes, activation of serotonin pathways, and inhibition of excitatory amino acids, NMDA-mediated hyperalgesia, and central inhibition of prostaglandin biosynthesis have been proposed as additional mechanisms of action.146,147 The COX-1 enzyme is present in the brain, gastrointestinal tract, kidneys, and platelets and is expressed constitutively. It preserves gastric mucosal integrity and function, platelet aggregation, and renal perfusion. COX-2 expression is induced by inflammation or tissue injury. Selective COX-2 inhibitors reduce inflammation but have less effect on gastric mucosal function and have fewer effects on platelet aggregation, thereby resulting in fewer adverse effects. Their deleterious effects on renal perfusion, however, are no different than the nonselective COX drugs, because COX-2 is constitutively expressed in renal tissues and may be involved in prostaglandin-dependent renal homeostatic processes.148 The risks of renal toxicity increase in the presence of hypovolemia, cardiac failure, preexisting renal dysfunction, or with the concurrent use of other nephrotoxic drugs. Reports of thrombotic cardiovascular and CNS events after both long-term and short-term use in adults led to withdrawal of two of the COX-2 inhibitors, rofecoxib and valdecoxib from the market.149,150 Similar data are unavailable to date in children; consequently, the risk of these agents causing thrombotic complications in children remains unknown. Most pediatric studies have evaluated the use of nonselective COX medications. In adult studies, COX-2 inhibitors have generally, but not always, produced analgesia roughly equivalent to that of traditional NSAIDs. Ibuprofen, one of the oldest orally administered NSAIDs, has been used extensively for treatment of fever and pain related to surgery, trauma, arthritis, menstrual cramps, and sickle cell disease. A large, controlled, randomized, double-blind study reported a greater decrease in VAS pain scores with ibuprofen than with acetaminophen or codeine in children presenting to the emergency department with acute pain after musculoskeletal trauma.151 Additionally, more children who received ibuprofen had VAS scores less than 30 on a 0-to-100-mm VAS scale than in the other two groups. The recommended dose of ibuprofen is 6 to 10 mg/kg every 6 hours. Like acetaminophen, ibuprofen is available in a variety of formulations and concentrations, placing children at risk for an overdose. For pediatric use, ibuprofen is available as:
 Concentrated drops containing 50 mg ibuprofen in 1.25 mL
Concentrated drops containing 50 mg ibuprofen in 1.25 mL
 Oral suspension containing 100 mg of ibuprofen in 5 mL
Oral suspension containing 100 mg of ibuprofen in 5 mL
 Junior-strength chewable tablets or caplets containing 100 mg of ibuprofen in each
Junior-strength chewable tablets or caplets containing 100 mg of ibuprofen in each
Diclofenac provides effective analgesia after minor surgical procedures in children. It is available only as an oral tablet in the United States, but it is available as a suppository and in the injectable form in several countries. The pediatric dose of diclofenac is 1 mg/kg every 8 hours orally, 0.5 mg/kg rectally, and 0.3 mg/kg IV.152 The oral and rectal doses reflect bioavailabilities of 0.36, 0.35, and 0.6 for suspension, dispersible tablets, and suppository, respectively. When diclofenac was administered rectally, the relative bioavailability was greater and the peak concentration was reached earlier than after enteric coated tablets administered orally.153 Children who received diclofenac experienced comparable analgesia to those who received caudal bupivacaine or IV ketorolac for inguinal hernia repair.154–156 In children undergoing tonsillectomy and/or adenoidectomy, diclofenac yielded superior analgesia with less supplemental opioid dosing, less nausea and vomiting, and earlier resumption of oral intake compared with acetaminophen.157,158 Although there are occasional reports of increased bleeding and restlessness in the recovery room in children who received diclofenac compared with those who had received papaveretum during tonsillectomy,159 a Cochrane review established that NSAIDs did not cause any increase in bleeding that required a return to the operating room (OR) for children. There was significantly less nausea and vomiting with NSAIDs compared with alternative analgesics, suggesting their benefits outweigh their negative aspects.160
Ketorolac, indomethacin and ibuprofen are the only injectable NSAIDs available in the United States. Indomethacin is the only NSAID used for closure of patent ductus arteriosus in preterm neonates. The IV formulation of ibuprofen is only labeled for adults in the United States. Clinical trials are currently under way in children. Ketoprofen and diclofenac are other injectable NSAIDs that are available outside the United States. A large multicenter study compared the risks of serious adverse events from IV ketorolac, ketoprofen, and diclofenac in more than 11,000 adults undergoing major surgery.161 The results indicated that 1.4% of adults experienced a serious adverse outcome, including surgical site bleeding (1%), death (0.17%), severe allergic reactions (0.12%), renal failure (0.09%), and gastrointestinal bleeding (0.04%), with no differences in outcomes among the groups; similar large-scale studies are not available for children.
Ketorolac has been shown to provide postoperative analgesia similar to opioids, in children of all ages.162–165 Its benefits include lack of opioid adverse effects (respiratory depression, sedation, nausea, and pruritus) making it an attractive choice for the treatment of postoperative pain. However, in common with all NSAIDs, it carries risks of platelet dysfunction, gastrointestinal bleeding, and renal dysfunction. Ketorolac (1 mg/kg) given to 18 preterm and term neonates undergoing painful procedures in the OR or the neonatal intensive care unit,163 revealed reduced pain scores (Neonatal Infant Pain Scale) with no incidents of systemic or local bleeding and no hematologic, hepatic, or renal complications (note that this dose is twice the usually recommended dose of 0.5 mg/kg). Similarly, no adverse effects on surgical drain output, renal or hepatic function tests, or oxygen saturation after major surgery were noted in 37 infants and toddlers between 6 and 18 months of age.166 Children in that study received continuous morphine infusions postoperatively, confounding the evaluation of the analgesic efficacy of ketorolac. Finally, ketorolac has been used to supplement opioid analgesia, with no increase in renal or bleeding complications in infants and children after open heart surgery.167–169 Nevertheless, because ketorolac can reduce renal blood flow, many recommend that its course be limited to 48 to 72 hours, and that renal function be checked if a course of administration greater than 72 hours is required. In single dose studies, the pharmacokinetics (PK) of ketorolac in infants less than 12 months of age appear to be homogeneous, although there was a trend toward reduced clearance in the infants less than 6 months of age.170
In an effort to avoid the respiratory depressant effects of opioids after airway surgery, several studies investigated the safety and benefits of ketorolac in children undergoing tonsillectomy.171–175 These early reports of ketorolac adversely skewed analyses of the adverse effects of NSAIDs after tonsillectomy. All but one of these studies171 found a two- to fivefold increase in bleeding complications, including measured blood loss, ease of achieving hemostasis, and bleeding episodes in the postanesthesia care unit (PACU), necessitating reexploration and hospital admission in some cases. Two of these studies were terminated prematurely, when preliminary data showed an unacceptably greater risk of bleeding in children who had received ketorolac.172,175 In one of these two studies, ketorolac was given at the end of surgery after hemostasis had been achieved.172 The benefits of ketorolac, including adequacy of analgesia, resumption of oral intake, and reduction in nausea, vomiting, and sedation were modest. This issue is further confounded by conflicting results yielded by two meta-analyses that evaluated the related literature. In one of these, the use of aspirin, but not of NSAIDs (diclofenac or ibuprofen), significantly increased the risk of post-tonsillectomy hemorrhage compared with either acetaminophen with codeine or tramadol for postoperative analgesia.176 The other reported no increase in intraoperative blood loss, postoperative bleeding, or admission because of bleeding, but did show a statistically significant increase in the rate of reoperation for bleeding in children who received an NSAID in the postoperative period compared to those who did not.177 Taken together, these data suggest that the use of NSAIDs during or after tonsillectomy is best avoided, and alternative analgesics, such as acetaminophen and tramadol, be considered to reduce opioid requirements. A large multicenter study in adults found that the risk of gastrointestinal and operative site bleeding associated with ketorolac was larger and clinically important when ketorolac was used in larger doses, in older subjects, and for more than 5 days.178
Another contentious issue regarding NSAIDs relates to their effects on bone healing and their use in children undergoing spinal fusion. Prostaglandins play an integral role in bone metabolism and significantly influence bone resorption and formation; however, their effects on bone formation predominate. NSAIDs inhibit the formation of prostaglandins, thereby raising the concern that they could promote nonunion after spinal fusion. Studies in rabbits and some studies in adults have reported a greater incidence of nonunion or pseudarthrosis, particularly with the use of large doses of ketorolac.179,180 However, no differences in curve progression, hardware failure, pseudarthrosis, or need for reoperation have been found in children and adolescents who received ketorolac in the immediate postoperative period compared with those who did not.181–183 Of note, the majority of the pediatric data are from otherwise healthy children with idiopathic scoliosis, making it problematic to extrapolate these data to children with comorbidities or those with neuromuscular scoliosis. There is no unique advantage of the IV route with NSAIDs. There is also no evidence that IV ketorolac is a more potent analgesic than comparable (i.e., equipotent) doses of a number of other NSAIDs, administered by oral or rectal routes.184
A recent meta-analysis of the use of NSAIDs for postoperative pain included 27 studies and compared 567 children who received NSAIDs to 418 children who did not.185 This study found that coadministration of NSAIDs and opioids during the perioperative period decreased opioid requirement in the PACU and the first 24 hours after surgery, decreased pain intensity in the PACU, and postoperative nausea and vomiting (PONV) during the first 24 hours postoperatively. Additionally, coadministration of acetaminophen with NSAIDS and opioids reduced pain intensity for the first 24 hours postoperatively. Other investigators demonstrated up to a 30% opioid-sparing effect in children who receive acetaminophen and diclofenac in addition to PCA. Therefore, in the absence of contraindications, it has been recommended that NSAIDs be used as part of a multimodal regimen to manage postoperative pain and to decrease opioid consumption in children.185
Tramadol
Tramadol is a synthetic analogue of codeine that exerts its analgesic properties by two complementary mechanisms. One of its metabolites has a weak affinity for the µ opioid receptor with no affinity for the δ or the κ receptors. In addition to its mild opioid effects, it also inhibits serotonin and norepinephrine uptake. Its main advantages over opioids include reduced incidences of respiratory depression, sedation, nausea, and vomiting. Additionally, because it does not inhibit prostaglandin synthesis, it does not cause the adverse effects commonly reported with NSAIDs, including peptic ulceration and renal and platelet dysfunction. Adverse effects associated with its use include nausea and vomiting (9% to 10% of cases), pruritus (7%), and rash (4%).186 It is known to cause dizziness and its use has been associated with seizures. Tramadol is available only in tablet form alone or in combination with acetaminophen in the United States. However, it is available in a liquid formulation (and as oral drops for infants), as a suppository, and as an injectable solution in other countries, allowing for greater flexibility of dosing. Therefore, it has been used to provide analgesia by a number of routes, including oral, rectal, IV (including PCA devices), into the caudal epidural space, and by local infiltration.
Tramadol is used for postoperative pain treatment in children undergoing ambulatory surgery and has also been used when transitioning from IV opioids to oral analgesics. Two doses of tramadol (1 mg/kg and 2 mg/kg orally) were compared in children who were being transitioned from morphine PCA. Children who received 2 mg/kg required fewer supplemental analgesics with no difference in adverse effects compared with those who had received 1 mg/kg.186 Tramadol, 2 mg/kg IV, produced similar analgesia and sedation, with fewer episodes of oxygen desaturation compared with morphine 0.1 mg/kg IV, in children with obstructive sleep apnea undergoing adenotonsillectomy.187 Tramadol has also been found to produce a similar analgesic effect as that of ilioinguinal and iliohypogastric nerve blocks in children undergoing herniorraphy.188 The tramadol group, however, experienced a greater incidence of nausea and vomiting. Tramadol PCA has also been found to provide adequate analgesia with less sedation, earlier awakening, and earlier extubation in children undergoing atrial or ventricular septal defect repair compared with those who received morphine via PCA.189
Tramadol has also been effective when administered via the neuraxial space. Caudal tramadol (2 mg/kg) produced reliable postoperative analgesia comparable to that produced by caudal morphine (30 µg/kg) in children undergoing inguinal hernia repair.190 No additional pain medications were required in the first 24 hours in more than 90% of children in each group. Rigorous drug-specific neurotoxicity studies, however, are lacking. Another study compared the analgesic efficacy of 2 mg/kg tramadol administered IV or by peritonsillar infiltration in children undergoing adenotonsillectomy.191 Both groups experienced excellent analgesia in the first hour. However, the local infiltration group experienced more prolonged analgesia and required fewer rescue doses of acetaminophen compared with the IV group. Overall, tramadol appears to be an analgesic of medium potency with a low incidence of adverse effects that may be used alone for mild to moderate pain and for its opioid-sparing effect in children with severe pain.
Ketamine
There has been increasing interest in the use of ketamine, an NMDA-receptor antagonist, in the treatment of both chronic and acute pain. Its professed benefits include an opioid-sparing effect, avoidance of opioid tolerance, prevention of central sensitization and wind-up, mitigation of opioid-induced hyperalgesia, and provision of synergistic analgesia in multimodal regimens by virtue of its own antinociceptive properties. Case series in children with intractable pain resulting from advanced stages of cancer have reported reduction in opioid requirement, decreased opioid adverse effects, improvement in pain control and function, and increased ability to interact with their families.192–194
Studies evaluating the use of ketamine alone or in combination with opioids for acute postoperative pain in children have yielded equivocal results. In one study, children undergoing tonsillectomy who received IV ketamine 0.5 mg/kg after induction or at the end of surgery experienced reduced pain scores and required fewer rescue analgesics compared with those who received placebo.195 All children in this study received a standardized analgesic regimen, including rectal diclofenac before the start of surgery and oral acetaminophen at scheduled intervals postoperatively. Another study reported reduced pain scores and reduced requirement for rescue analgesics in children who received ketamine as a bolus dose and by infusion that began before the start of a tonsillectomy, compared with those who received a single bolus dose of ketamine at the end of surgery.196 Intramuscular (IM) ketamine 0.5 mg/kg has also produced equivalent analgesia in terms of similar pain scores and need for rescue analgesics compared with IM morphine 0.1 mg/kg as sole analgesics for tonsillectomy.197 Other studies have found no such benefits when ketamine was compared with placebo for tonsillectomy, urologic, and orthopedic surgery.198–201
A meta-analysis of 35 randomized controlled trials compared 567 children who received ketamine as an adjuvant analgesic by a variety of routes for a variety of surgical procedures with 418 who did not receive ketamine.202 This study found that, although the use of ketamine was associated with reduced pain intensity in the PACU and a reduced need for nonopioids, it did not demonstrate an opioid-sparing effect. A systematic review of 37 studies included 4 studies in children, two of which demonstrated beneficial effects of ketamine administered as an adjuvant analgesic and two found no benefits.203 The investigators could draw no conclusions regarding the use of ketamine as an adjuvant analgesic. The use of ketamine in all the above studies was associated with only a few mild and self-limiting adverse effects. Further investigation is needed to evaluate the benefits of low-dose ketamine for acute postoperative pain before its routine use can be recommended.
Opioid Analgesics
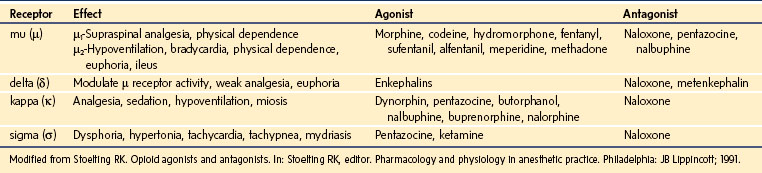
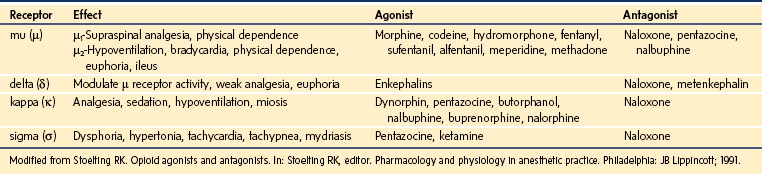
Opioids are indicated for moderate to severe pain after surgery or trauma, for acute painful crisis in children with sickle cell disease, as well as for chronic painful conditions such as cancer. Opioids mimic the effects of endogenous ligands known as endorphins, exerting their effects by binding to specific opioid receptors located at presynaptic and postsynaptic sites in the brain, spinal cord, and peripheral nerve cells. Opioid receptors in the CNS are classified as µ, κ, δ, and σ.204–206 Activation of these receptors causes neuronal inhibition by decreasing the release of excitatory neurotransmitters from presynaptic terminals. The µ receptors are further subdivided into µ1 receptors, responsible for supraspinal analgesia and physical dependence, and µ2 receptors, responsible for respiratory depression, bradycardia, physical dependence, and gastrointestinal dysmotility.207 Activation of the κ receptors causes analgesia without significant respiratory depression, whereas activation of the σ receptors causes dysphoria, tachycardia, tachypnea, hypertonia, and mydriasis. The δ receptors modulate the activity of the µ receptors.
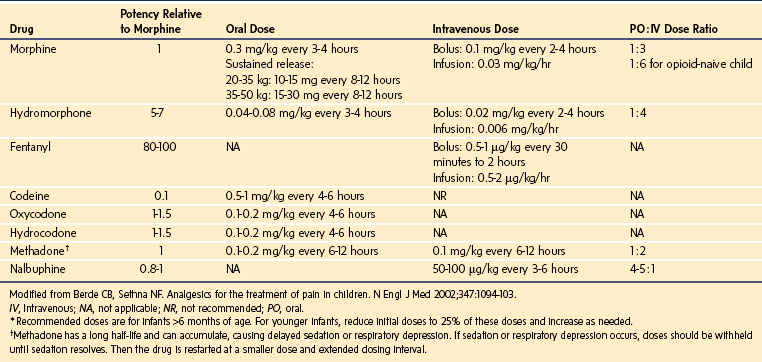
Drugs that exert their effects on opioid receptors are classified as agonists, antagonists, partial agonists, and mixed agonist-antagonists. Agonists are neurotransmitters that bind to a receptor and exert their pharmacologic effects. Antagonists, on the other hand, bind to the receptor but do not initiate any effects; yet by occupying the receptor they block the effects of agonists. Partial agonists have reduced intrinsic activity and produce less than a maximal response. They act as antagonists as well because they block the agonists from access to the receptor. The mixed agonist-antagonist drugs act as agonists at certain opioid receptors and antagonists at others. E-Table 43-4 depicts the various opioid receptors, their effects, as well as the drugs that exert activity on each of them. The opioids that are used most commonly in the management of pain are µ-receptor agonists, including morphine, hydromorphone, the fentanyls, methadone, hydrocodone, and oxycodone. Of these, morphine is the opioid that is most commonly used as first-line therapy for moderate to severe pain in children and, consequently, is the agent with which clinicians have the greatest experience. Table 43-8 lists the relative potencies and suggested initial doses of the opioids in common clinical use. Developmental pharmacology, PK, and side effects of opioids are discussed in depth in Chapter 6.
Delivery Techniques
Oral Administration
Although codeine has been widely used as an oral opioid analgesic, our strong preference is to avoid prescribing it in almost all situations, for several reasons. First, in recommended doses, it is a weak analgesic. Second, because it is a prodrug that requires conversion via demethylation to morphine (as detailed in Chapter 6), there is marked developmental and pharmacogenetic variation in this conversion that may result in ineffective conversion and thus reduced analgesia in some cases, or an overdose in others. Third, when the dosing is escalated, the frequencies of adverse effects, such as nausea, vomiting, constipation, and dysphoria increase. It is important to note that with active metabolites of codeine excreted in breast milk, there has been a report of an opioid overdose in a neonate who was breastfed by a mother who was an extensive metabolizer.208
Methadone is a synthetic opioid with a very prolonged elimination half-life (mean of 19 hours) in children between 1 and 18 years of age, and a large bioavailability (approximately 80%) after oral administration. Oral or IV methadone has been considered a good alternative to the use of continuous opioid infusions because repeated dosing at intervals of every 4 to 8 hours can achieve relatively stable plasma drug concentrations.209 Although it is used most frequently to facilitate weaning of opioid-tolerant children, it has also been recommended for postoperative analgesia and for transitioning children from parenteral to oral opioid therapy.210–212 Methadone is especially useful for children with cancer, burns, or other serious illnesses who require a long-acting oral opioid, because it is available in an elixir formulation. Unlike some sustained-release formulations of other opioids, oral methadone is also relatively inexpensive. Note that crushing tablets of most sustained-release formulations of other opioids renders them into immediate-release, relatively short-acting medications. Methadone should be thought of as virtually a combination analgesic. It is supplied as a racemic mixture. The l-isomer acts as a µ opioid, whereas the d-isomer acts as an antagonist at the NMDA subclass of excitatory amino acid receptors. Action at NMDA receptors makes methadone uniquely effective in the treatment of neuropathic pain. This NMDA-blocking action, and a differential activation of receptor-mediated endocytosis versus protein kinase activation,213,214 may lead to a relatively slower rate of development of tolerance for methadone compared with some other opioids. Despite these advantages of methadone, it requires careful titration and repeated reassessment to avoid delayed oversedation. This challenge in methadone dosing is due, in part, to its slow and widely variable clearance, as well as to its effects on NMDA antagonism, generating incomplete cross tolerance on conversion to methadone from other opioids. In opioid-naive subjects, a single dose of IV morphine is roughly equipotent to a single dose of methadone. Although morphine has active metabolites, the slower clearance of methadone compared with morphine translates in opioid-naive subjects, into daily IV methadone requirements that are roughly one-third those of morphine. However, in the setting of marked opioid tolerance, such as in the case of children with advanced cancer or in the setting of intensive care, the equipotent daily dose of IV methadone may be as small as one-tenth the preceding daily dose of IV morphine.129,209,215–217 A convenient web-based calculation tool (www.globalrph.com/narcoticonv.htm) has synthesized the information from these and other studies to aid in opioid conversions in both opioid-naive and opioid-tolerant subjects. In our practice, this calculation tool appears quite useful, although it must be noted that it has not received independent assessment for use in children. Smartphone applications for multiple platforms are also available.
Intravenous Administration
Intermittent IV injections with opioids of short or moderate duration administered on an as-needed basis (pro re nata, or PRN) do not achieve stable blood concentrations and predispose to periods of excessive sedation alternating with periods of inadequate analgesia. Yet this technique remains the most common method of treating postoperative pain in many centers. A partial solution to this problem is to prescribe the opioid at closer intervals (such as 2 hourly) and then use a “reverse-PRN” schedule, in which the medication is offered at the prescribed interval but the child can choose to take it or refuse it. Children should be assessed frequently, with the goal of administering the next dose before moderate to severe pain recurs. The use of a long-acting opioid, such as methadone, has been recommended to provide more prolonged and even periods of analgesia than could be achieved with shorter-acting opioids, approaching the efficacy of continuous infusions.218 However, careful titration of dosing and frequent assessment of the child are required because of methadone’s slow and variable clearance. Alternatively, administration of shorter-acting opioids via continuous infusion or a PCA device should be considered.
Continuous IV opioid infusions are an excellent means of providing analgesia to children with moderate to severe pain who are unable to use PCA, such as infants, young children, and those who are cognitively impaired or physically disabled.219 Once a therapeutic blood concentration of the opioid is achieved by administering an initial loading dose, an infusion rate can be selected to maintain that concentration without excessive fluctuations. Additionally, rescue doses of IV opioids may be required for breakthrough pain. Opioids, however, cause a dose-dependent respiratory depression by shifting the CO2 response curve, reducing its slope, and decreasing the hypoxic ventilatory response. Residual and synergistic effects of sedatives and hypnotics in the early postoperative period further increase the risk of opioid-induced respiratory depression. This is particularly true in preterm and term infants because of age-related differences in elimination and clearance of opioids and other sedating medications (see also Chapter 6). This is of particular concern with the use of continuous opioid infusions because inappropriate dosing or prolonged elimination may lead to drug accumulation, placing children at risk for side effects. In a recent prospective audit of 10,726 opioid infusions in the UK and Ireland, the overall risk of permanent harm was found to be 1 in 10,000 cases, and serious events without permanent harm 1 in 383, with half of the serious events being respiratory depression.220 Therefore, the rate of the infusion should be carefully selected, based on the child’s age, comorbidities, and clinical condition. Additionally, children who receive opioid infusions should be monitored and assessed frequently for depth of sedation and respiratory rate. The onset of sedation is an important clinical index of incipient respiratory depression and should alert the nursing staff and physicians to decrease the infusion rate and observe the child more closely. Use of continuous pulse oximetry is widely recommended during continuous opioid infusions, especially in opioid-naive children and other children at increased risk for respiratory depression. Another method of IV opioid delivery is via PCA, which is discussed below. With any infusion technique, scrupulous attention must be paid to protocols for checking pump settings to avoid errors. Pump programming errors, none of which caused serious harm, but which had the potential to do so, occurred in 17 instances in the UK audit, all from a single center, highlighting the critical importance of system safeguards to prevent patient harm.220
Intramuscular and Subcutaneous Routes
Intermittent IM and subcutaneous injections of opioids are obsolete because they are frightening and unpleasant for children and are often perceived as worse than the pain for which they are administered.221 Additionally, they have the PK disadvantage of unpredictable and erratic uptake if regional blood flow is impaired, and they produce pronounced wide swings in blood concentrations. The goal of maintaining an even level of analgesia is thus nearly impossible to achieve with these routes of administration. An important exception is the use of indwelling subcutaneous catheters for continuous infusions and PCA as in palliative care.
Selection of Opioids for Parenteral Use
Morphine is the opioid most commonly used for postoperative analgesia and has been extensively studied in all pediatric age groups. After major abdominal, thoracic, and orthopedic surgery, children who received continuous morphine infusions had reduced pain scores compared with those who received intermittent IM or IV injections.222–224 However, other investigators were only able to demonstrate reduced pain scores with continuous morphine infusions compared with intermittent IV injections of morphine in children between 1 and 3 years of age, and not in infants in the first year of life.225,226 Similarly, evidence of the beneficial effects of opioid analgesia in ameliorating the postoperative response to surgical stress are conflicting. A significant reduction in serum β-endorphin concentrations has been reported in neonates after the initiation of a continuous infusion of morphine in the postoperative period.227 In neonates whose lungs were mechanically ventilated, both epinephrine and norepinephrine concentrations decreased significantly after the initiation of morphine or fentanyl infusions.228 However, β-endorphin concentrations decreased only in the children who received fentanyl. When the effects of continuous infusions of morphine were compared with those of intermittent IV injections of morphine on the stress response in children between 1 and 3 years of age, reduced glucose concentrations in the continuous infusion group suggested only a modest ablation of the stress response in this age group.225
Several studies have described the PK of morphine administered as a continuous infusion and evaluated the pharmacodynamic effects of morphine on respiratory indices in neonates, infants, and children after various surgical procedures. In children 14 months to 17 years of age who underwent cardiac surgery,229 morphine infusions were adjusted between 10 and 50 µg/kg/hr to minimize discomfort and avoid excessive sedation. Supplemental boluses of 100 µg/kg morphine were administered for breakthrough pain. Steady-state morphine concentrations were achieved in 4 hours. Those children who could self-report their pain reported good analgesia with morphine concentrations in excess of 12 ng/mL. Morphine infusions of 10 to 30 µg/kg/hr yielded mean serum concentrations between 10 and 22 ng/mL with less than 2% experiencing evidence of respiratory depression (Paco2 greater than 50 mm Hg). Furthermore, children who received morphine infusions of 10 to 30 µg/kg/hr breathed spontaneously after extubation of the trachea, and those who were weaned from assisted to spontaneous ventilation maintained a normal Paco2. On the other hand, 60% (3 of 5 children) who received a greater infusion rate of morphine, 40 to 50 µg/kg/hr, experienced hypercarbia (Paco2 48 to 66 mm Hg). A subsequent study by the same investigators evaluated the severity of respiratory depression in infants and children aged 2 days to 18 months treated with morphine. Of those whose morphine concentrations exceeded 20 ng/mL, approximately 70% experienced respiratory depression (Paco2 greater than 55 mm Hg and/or a depressed slope of the CO2 response curve) compared with 15% to 28% of those whose concentrations were less than 20 ng/mL.230 The investigators suggested a steady-state morphine concentration of 20 ng/mL as a threshold concentration for respiratory depression in this age group.
Previous studies determined that the clearance of morphine is impaired in preterm infants and that clearance increases with postconception age.231 Additionally, morphine clearance is impaired in full-term infants up to 1 to 2 months of age, at which time it is comparable with that in older children and adults.98,232 Preterm and full-term neonates, therefore, have a narrower therapeutic window for morphine analgesia compared with older children. Indeed, these groups have reduced morphine requirements postoperatively, requiring fewer rescue doses of morphine when receiving continuous infusions or intermittent bolus doses.233 Therefore, opioids should be carefully titrated in infants in a monitored environment with significantly reduced continuous infusion rates. Based on PK modeling and morphine clearance predictions, a target morphine concentration of 10 ng/mL can be achieved with morphine infusions ranging from 5 µg/kg/hr in term neonates to 16 µg/kg/hr in 1- to 3-year-old children (see Chapter 6).234
Pharmacodynamic differences between infants and children have been postulated as the mechanism responsible for the greater sensitivity of infants (compared with older children) to the respiratory depressant effects of opioids. However, this may not be the case. Although rodent data suggest that the brain concentrations of opioids in neonates are greater than those in older children at similar serum concentrations,235 these findings may not be applicable to humans. Neonatal rats have a relatively immature brain and a far more permeable blood-brain barrier than that in human infants. Consequently, the rodent may not be an appropriate model to depict the human condition.236 It appears that the “increased sensitivity” is related, at least in part, to PK variables, perhaps in some measure as a result of a neonate’s decreased conjugating ability.
Regardless of the mechanism, respiratory depression remains the most feared adverse effect of opioids administered by any route. Neonates and infants younger than 6 months of age are at greater risk for opioid-induced respiratory depression because the ventilatory responses to airway obstruction, hypoxemia, and hypercapnia are immature at birth and mature over the first several months of life in preterm as well as full-term infants (see also Figs. 4-8 and 4-9). Indeed, there was a 4.5% incidence of failure to wean from the ventilator and a 13.5% incidence of apnea (30 seconds or more that required intervention) or severe respiratory depression in spontaneously breathing neonates who received opioids for postoperative pain.237 Another report of a 3-year surveillance period for adverse drug reactions described 15 children aged 2 days to 17 years who experienced opioid-induced respiratory depression.238 Respiratory depression in the latter study was defined as apnea, hypoxemia, cyanosis, a marked decrease in respiratory rate, or a need for naloxone. Although this study was unable to define the incidence of respiratory depression because the denominator was unknown, it did identify several predisposing factors, including age younger than 1 year (7 of 15 children), drug errors (including prescription and administration errors; 6 of 15 children), concurrent medical problems (diminished respiratory reserve, hepatic, and/or renal impairment), and concurrent sedative drugs. The prospective UK audit found 14 cases of respiratory depression (out of 10,726 total infusions, or 0.13%), 10 with nurse-controlled anesthesia (NCA), 2 with continuous infusions, and 2 with PCA.220 Potentially contributing risk factors in half of the cases included very young age and neurodevelopmental, respiratory, or cardiac disease. In contrast to the above studies, no case of respiratory depression was reported in 110 children older than 3 months of age who received opioid infusions postoperatively.239 Interpretation of this literature is confounded by different monitoring techniques and different definitions of respiratory depression. For instance, in the latter study, a 4.5% incidence of clinically significant hypoxemia was reported but was not included in their definition of respiratory depression. Additionally, children in that study were monitored with hourly documentation of respiratory rate, but oxygen saturation was not monitored after discharge from PACU, thereby reducing their ability to detect the more subtle episodes of respiratory depression. In summary, the results of these studies suggest that children who receive opioids require careful monitoring for respiratory depression, with appropriate age-based reduction of dosage, particularly for neonates and infants younger than 6 months of age.
The most common adverse effect of opioid therapy is nausea and vomiting. One study reported nausea and vomiting in 34 of 80 children (42.5%) who received postoperative morphine infusions. These were well managed with antiemetic therapy in all but 2 children who required discontinuation of the opioids.239 In the same study, the incidence of pruritus and urinary retention were both 13% and that of dysphoria was 7%. Seizures have been reported in two neonates who had received bolus doses of morphine followed by infusions of 32 and 40 µg/kg/hr and whose serum morphine concentrations were 61 and 90 ng/mL, respectively.240 Irregular jerking movements, as well as one case of a generalized seizure, have been reported in children 1 to 15 years of age receiving postoperative morphine infusions.222 Metoclopramide, 0.10 to 0.15 mg/kg (100 to 150 µg/kg) given IV, is an effective antiemetic but may also cause sedation and dystonia. The serotonin-receptor antagonist antiemetics, such as ondansetron and dolasetron, have the advantage of virtually eliminating the risk of dystonic or oculogyric reactions that occur with phenothiazines, butyrophenones, and metoclopramide. However, headaches occur in a small number of those who receive serotonin-receptor antagonists. A “microdose” naloxone infusion (0.25 to 1.0 µg/kg/hr) reverses the incidence of both nausea and pruritus after opioids without affecting the analgesia or opioid consumption.241,242 A more recent dose-escalation study demonstrated that doses of 1 to 1.65 µg/kg/hr resulted in greater efficacy in reducing side effects, particularly pruritus, without degrading analgesia.242 It is likely that these results may be generalized to other routes of opioid administration.
Opioid-induced bowel dysfunction reported in more than 90% of patients on opioid therapy, occurs by blocking propulsive peristalsis, inhibiting secretion and increasing reabsorption of intestinal fluids, and decreasing the activity of excitatory and inhibitory neurons in the myenteric plexus. Bowel dysfunction manifests as abdominal distension and bloating, delayed gastric emptying, and constipation. Aggressive prophylactic measures, including osmotic, lubricant, or stimulant laxatives, should be prescribed early in the course of treatment. A newer selective gastrointestinal peripheral µ-opioid receptor antagonist, methylnaltrexone, was approved for use in adults in 2008. Although adult studies have shown promising results with the use of this agent,243,244 its pediatric use has been described in only one case report.245 A neonate who experienced a severe ileus during fentanyl administration after major abdominal surgery was noted to have resolution of the signs within 15 minutes of IV methylnaltrexone (0.15 mg/kg). She received 5 daily doses of methylnaltrexone without reversal of analgesia or occurrence of withdrawal.245
Fentanyl may be a useful substitute for morphine in children with hemodynamic instability, in whom a decrease in peripheral vascular tone is undesirable, and in whom histamine release caused by morphine is not well-tolerated. Additionally, its rapid onset of analgesia makes it ideal for children with severe escalating pain who require urgent pain relief. Fentanyl is metabolized by the liver into an inactive metabolite, norfentanyl, which is excreted via the kidneys. It is 80 to 100 times more potent than morphine. Although its elimination half-life is significantly less than that for morphine, its context-sensitive half-life during chronic infusion increases exponentially as a result of growing tissue storage (see Chapter 6). Like morphine, the elimination half-life of fentanyl in neonates is nearly twice that in adults, predisposing them to a greater risk for accumulation compared with older infants.246,247 As with morphine, a reduction in hepatic blood flow in very young infants further decreases fentanyl conjugation. For a given bolus of fentanyl, plasma concentrations in infants between 3 months and 1 year of age are less than those in older children and adults.248 This finding is consistent with the almost twofold greater clearance of fentanyl in children compared with neonates. In children 18 days to 14 years of age who were mechanically ventilated, the clearance of fentanyl was age related yet quite variable, with the slowest clearance occurring in infants younger than 6 months of age and the most rapid in those between 6 months and 6 years of age.249 The clearance of fentanyl is slow in preterm infants, with the clearance correlating with the postnatal age.250
Fentanyl is known to cause all of the adverse effects reported with opioids, including pruritus, nausea, vomiting, constipation, and sedation. Respiratory depression and chest wall and glottic rigidity, however, are its most feared adverse effects. One study compared the incidence of respiratory depression in full-term and former preterm infants and young infants receiving 2 µg/kg bolus doses of fentanyl every 2 hours or a continuous infusion of 1 µg/kg/hr after abdominal or thoracic surgery.251 Randomization was terminated prematurely because of a sixfold greater incidence of apnea that required intervention in the bolus dose group compared with the continuous infusion group (89% vs. 14%). The continuous infusion arm was continued for another 20 children, resulting in a 25% incidence of apnea in this group. In contrast, the incidence of respiratory depression (based on transcutaneous Paco2 measurements and the incidence of apnea) for a given plasma fentanyl concentration in infants 1 to 12 months of age and children 1 to 5 years of age was less than that in adults undergoing hernia repair or other peripheral surgery.252 Differences in surgical procedures and the inclusion of preterm infants in the former study may account for the significant difference in the incidence of apnea found in these two studies.
Although chest wall rigidity usually occurs after the rapid bolus administration of high-dose fentanyl, it has also been reported in an infant after a low-dose continuous infusion of fentanyl. Chest wall rigidity was reported in 9% of preterm and full-term neonates who received an average of 4.9 µg/kg over a 2 to 3 minute period for a procedure or for perioperative analgesia.253 In every case, naloxone reversed the chest wall rigidity. Additionally, a case of chest wall rigidity has been reported in a preterm neonate after high-dose fentanyl was administered to the parturient before a cesarean section.254 Although administration of naloxone has been used successfully to treat cases of chest wall rigidity, severe cases associated with rapid oxygen desaturation may require the use of neuromuscular blocking drugs and mechanical ventilation.
The use of continuous fentanyl infusions in infants and children has been associated with a rapid development of tolerance, as indicated by a steady increase in infusion rate to maintain the desired effect255,256 and a large incidence of opioid withdrawal syndrome after termination of the infusion.256,257 The incidence of opioid withdrawal is directly related to the total dose administered and the duration of infusion.256,257 Neonatal abstinence syndrome has been reported in 21 of 37 neonates (57%) after continuous fentanyl infusions during extracorporeal membrane oxygenation.256 Both a total fentanyl dose in excess of 1.6 mg/kg and extracorporeal membrane oxygenation that lasted more than 5 days were predictors of opioid withdrawal. A similar incidence has been reported in 23 children 1 week to 22 months of age who received continuous fentanyl infusions during mechanical ventilation.257 This study also found that a total dose of 1.5 mg/kg of fentanyl over 5 days was associated with a greater than 50% incidence of withdrawal symptoms. Furthermore, a total dose of 2.5 mg/kg as a continuous infusion over 9 days was 100% predictive of the occurrence of withdrawal. Finally, movement disorder and irritability have been reported after withdrawal of fentanyl infusion in five infants who were mechanically ventilated.258 None of the infants who developed the movement disorder had received another opioid after withdrawal of fentanyl, whereas five of eight controls who did not develop withdrawal during the same period had received a substitute opioid. These data suggest that opioid withdrawal occurs earlier and with greater frequency after fentanyl infusions compared with other opioids. Therefore it seems prudent to use fentanyl infusions for pain relief during periods of hemodynamic instability, such as in the early postoperative period, and to transition to another opioid, such as morphine, as soon as the child is stabilized. Children who require fentanyl infusions for 5 days or more should undergo a slow taper (e.g., 10% decrease every 12 hours) or be transitioned to another parenteral or oral opioid regimen.
Hydromorphone has a spectrum of action similar to that of morphine. Adult opioid equipotency data suggest that it is 3.5 to 7 times as potent as morphine.259–261 The only pediatric study that was performed in children with mucositis pain after bone marrow transplant reported that a 7 : 1 conversion ratio of morphine to hydromorphone, underestimated hydromorphone requirements by 27%.262 These data suggest that a 5 : 1 conversion ratio may be more appropriate, particularly in children with chronic pain. Despite its widespread use, there are very few studies that evaluated the use of hydromorphone in children. A Cochrane review of studies related to the use of hydromorphone for acute and chronic pain in adults and children found little difference between the analgesic efficacy and adverse effect profile of morphine and hydromorphone.263 However, several of the studies in this review included small numbers of patients, some were of low quality and only four of them included children. It remains common practice to prescribe a trial of hydromorphone in children who experience unacceptable side effects with morphine.
Meperidine is an opioid that has been used clinically for many years.264,265 Its potency is approximately one-tenth that of morphine. Accumulation of its active metabolite, normeperidine (which has CNS stimulant properties), after repeated doses of meperidine, places children at risk for seizures.266 Therefore, its use has been restricted to the treatment of postoperative shivering267,268 or rigors after amphotericin. A single dose of dexmedetomidine (0.5 µg/kg) has been used successfully for the treatment of postoperative shivering and may replace meperidine for this indication.269 Although its short-term use continues by some clinicians for procedural sedation and analgesia, it is preferable to use other analgesics for this purpose. Meperidine is not recommended for PCA or as a continuous infusion in children.
Patient-Controlled Analgesia
PCA was first studied in adults in 1965. The initial interest with this technique was as a research tool for the study of pain. By the early 1970s, it was identified as an excellent strategy for treating pain in the clinical setting, with studies demonstrating that pain relief was achieved by PCA with relatively smaller doses of opioids and with greater patient satisfaction than with conventional methods.270 However, it was not until the late 1980s that PCA was studied in children.271 Since that time, it has become the preferred method for opioid delivery in children older than 5 to 6 years of age for acute pain, as well as chronic pain associated with cancer or sickle cell disease.262,271–274 The primary benefit of PCA is that it allows children to titrate the analgesic to the extent of their pain. The goal is for the child to self-regulate a blood opioid concentration within the therapeutic range. Most children strike a balance between adequate pain relief on the one hand and adverse effects of the drug on the other. This approach, which grants the child some degree of autonomy, is the rationale given for the fact that pain is an entirely subjective and individual experience and that opioid metabolism and pain perception are quite variable among individuals. It also reduces the apprehension that older children and adolescents have about pain relief because they can control it and they can tailor the opioid delivery to the extent of pain they have at a given time, for example, before physical therapy, removal of tubes or drains, dressing changes, or getting out of bed. Additionally, the use of PCA avoids delays in administration of analgesics associated with standard “as needed” orders of IV opioids and allows smaller doses of opioids to be delivered more frequently without increasing nursing workload. Therefore, PCA is thought to provide more consistent pain relief with less total opioid dosing, resulting in fewer side effects, such as sedation, nausea, and vomiting. Purportedly, children using PCA report better analgesia and reduced pain scores compared with children who have to rely on the nursing staff to administer analgesics when they are in pain. These and other benefits of PCA have been extensively touted in the medical literature,274–276 as well as in the lay press.277 Recently, however, risks associated with PCA use have also been highlighted and are discussed later.278–280 Recognition of these risks has led to recommendations for careful dosing and monitoring of all children who are receiving opioids, particularly those receiving continuous infusions and those with specific risk factors.281
Child training is a necessary part of PCA, because successful use of PCA requires that both the child and family understand how it works.282 The instructions should be clear that the pump should be activated whenever the child feels pain, that children cannot give themselves “too much medication” because of the computer lockout interval, that the child should not wait for severe pain to activate the pump, and that a dose can also be given in anticipation of painful stimuli, such as ambulation or chest physiotherapy. Most importantly, PCA does not mean parent-controlled analgesia, and parents should never activate the pump unless specifically authorized to do so by the primary care or pain service physician (see Nurse/Caregiver-Controlled Analgesia, later).281
Choice of Drug and Drug Dosages
Morphine remains the most common opioid administered via PCA, with hydromorphone and fentanyl being second-line drugs usually reserved for children who are intolerant to morphine. Suggested initial dosages for opioids via PCA for opioid-naive children are presented in Table 43-9. Children with opioid tolerance will require adjustments to these settings, taking into account the previous opioid history and the opioid doses that the child was receiving before the acute painful stimulus. Indeed, one study reported that children with sickle cell disease self-administered more than double the dose of morphine via PCA, required more nonopioid adjuvant analgesics, reported greater pain scores, and stayed in the hospital for twice the duration compared with non–sickle cell disease–affected children after laparoscopic cholecystectomy.283
Fentanyl PCA has been used with success as a first-line and a secondary drug in children with cancer pain, as well as acute postoperative pain.276,284 Most of the adverse effects, including nausea and pruritus, were mild and easily managed. However, some reported an overall incidence of apnea and hypoxemia of 3.5% in 212 children receiving PCA, of whom 144 had received fentanyl.276 Finally, children who received tramadol PCA after heart surgery were extubated earlier and had less sedation, comparable pain scores, and a similar incidence of emesis as those who received morphine PCA.189 The IV formulation of tramadol is not yet available in the United States, but some studies from Europe and China support its use in the postoperative period.189,285 The benefits that hydromorphone PCA offers over morphine PCA in the chronic and acute pain settings require further investigation.
Pump Settings
Most PCA pumps have five settings to adjust:
 A loading dose of opioid ranging from 0.025 to 0.1 mg/kg morphine divided into incremental doses is usually given to establish adequate analgesia before therapy is turned over to the child, because self-administered doses with this technique are generally small. A sufficient interval between incremental doses must be allowed, so that the morphine achieves its peak effect before the next dose, thereby avoiding an overdose. If PCA is started in the PACU, opioid doses administered during surgery must be considered before prescribing a loading dose. Additionally, it may be desirable to administer the loading dose via the PCA pump so that it is included in the initial 4-hour or hourly limit of the PCA. That is because children who receive IV-PRN doses of opioids in the PACU, followed by initiation of PCA, may be at risk for oversedation and respiratory depression resulting from opioid stacking. Children who have received opioids toward the end of surgery, those who awaken in comfort, or those who receive nerve blocks may not need a loading dose and may start to use the demand doses as needed on awakening.
A loading dose of opioid ranging from 0.025 to 0.1 mg/kg morphine divided into incremental doses is usually given to establish adequate analgesia before therapy is turned over to the child, because self-administered doses with this technique are generally small. A sufficient interval between incremental doses must be allowed, so that the morphine achieves its peak effect before the next dose, thereby avoiding an overdose. If PCA is started in the PACU, opioid doses administered during surgery must be considered before prescribing a loading dose. Additionally, it may be desirable to administer the loading dose via the PCA pump so that it is included in the initial 4-hour or hourly limit of the PCA. That is because children who receive IV-PRN doses of opioids in the PACU, followed by initiation of PCA, may be at risk for oversedation and respiratory depression resulting from opioid stacking. Children who have received opioids toward the end of surgery, those who awaken in comfort, or those who receive nerve blocks may not need a loading dose and may start to use the demand doses as needed on awakening.
 A patient bolus dose, that is, the dose that will be administered with each child’s activation of the pump, must be prescribed. These small boluses are usually in the range of 0.01 to 0.02 mg/kg of morphine in opioid-naive subjects.
A patient bolus dose, that is, the dose that will be administered with each child’s activation of the pump, must be prescribed. These small boluses are usually in the range of 0.01 to 0.02 mg/kg of morphine in opioid-naive subjects.
 A lockout interval of usually 5 to 15 minutes prevents a child from activating the pump until the full effect from the previous bolus is achieved, and it should correspond to the time from IV injection to the peak effect of the drug.
A lockout interval of usually 5 to 15 minutes prevents a child from activating the pump until the full effect from the previous bolus is achieved, and it should correspond to the time from IV injection to the peak effect of the drug.
 A continuous basal infusion ranging from 0.00 to 0.02 mg/kg/hr of morphine (or more, in opioid-tolerant subjects) may be used in selective cases (see later).
A continuous basal infusion ranging from 0.00 to 0.02 mg/kg/hr of morphine (or more, in opioid-tolerant subjects) may be used in selective cases (see later).
 A maximum hourly dose or a 4-hour limit may be chosen to limit the cumulative amount of drug a child can administer. Once this limit is reached, the child cannot activate the pump until the 4-hour limit has passed. Four-hour limits allow for increased flexibility in dosing over greater periods of time and pain intensity. Typically, the maximum hourly dose ranges from 0.05 to 0.1 mg/kg and 4-hour limits from 0.25 to 0.4 mg/kg of morphine in opioid-naive subjects. This amount may be chosen based on the average hourly use of morphine during the past 24 hours or, in children started on PCA immediately after surgery, at the reduced range of the dosage scale. Figure 43-8 presents sample PCA orders, including choice of drugs, dosing, and suggested monitoring.
A maximum hourly dose or a 4-hour limit may be chosen to limit the cumulative amount of drug a child can administer. Once this limit is reached, the child cannot activate the pump until the 4-hour limit has passed. Four-hour limits allow for increased flexibility in dosing over greater periods of time and pain intensity. Typically, the maximum hourly dose ranges from 0.05 to 0.1 mg/kg and 4-hour limits from 0.25 to 0.4 mg/kg of morphine in opioid-naive subjects. This amount may be chosen based on the average hourly use of morphine during the past 24 hours or, in children started on PCA immediately after surgery, at the reduced range of the dosage scale. Figure 43-8 presents sample PCA orders, including choice of drugs, dosing, and suggested monitoring.
Continuous Basal Infusions (CBI)
The use of a CBI of the opioid to supplement child-administered doses remains a subject of controversy. The rationale for the use of CBI is to maintain near-therapeutic plasma opioid concentrations, particularly during periods of sleep when there may be no self-administered doses, as illustrated in Figure 43-9, A. On the other hand, as depicted in Figure 43-9, B, a child who receives only PCA bolus dosing with no CBI is likely to awaken with unrelieved pain that may require multiple doses to again achieve adequate pain relief. Decreased nocturnal awakenings secondary to pain, improved restfulness or sleep patterns, reduced total opioid consumption, fewer adverse effects, and improved analgesic effectiveness have all been proposed as potential reasons in favor of using CBI. However, the use of CBI commits the child to receiving a fixed dose of opioid regardless of the level of sedation, and has the theoretical potential for overriding one of the inherent safety features of PCA, that is, an excessively sedated or somnolent child is unlikely to push the button and therefore receives no additional opioid but, with a fixed infusion, drug may accumulate (Fig. 43-9, C ), with the potential for hypoventilation.286 Furthermore, it has also been argued that programming errors with CBI can lead to more serious adverse events because the opioid medication is delivered regardless of the child’s level of sedation.285,287
Some adult studies have suggested that the use of CBI has limited benefit in terms of efficacy and is associated with a greater incidence of opioid adverse effects, including respiratory depression.288–290 Studies in children, however, have yielded conflicting results.273,274,291–295 Children 7 to 19 years of age who received PCA with CBI after orthopedic surgery reported significantly reduced pain scores compared with those who received PCA boluses alone or IM morphine.274 There were no differences in morphine consumption or in opioid adverse effects among the three groups, with no incidents of respiratory depression. Notably, child satisfaction was greatest in the PCA with CBI group. Similar pain scores with improved sleeping patterns have also been reported with the use of PCA with CBI, compared with those who received PCA alone, on the first two postoperative nights in children after abdominal surgery. No incidents of respiratory depression or excessive sedation were reported in either group.273 Children who received CBI with PCA or NCA in one study reported slightly reduced pain scores without differences in morphine use or adverse effects after spine fusion surgery compared with those who received PCA or NCA alone.295 In contrast, others have reported greater morphine use and a greater incidence of hypoxemia with similar pain scores in children who received PCA plus CBI after surgery compared with those who received PCA alone.293,294 A subsequent study found that children who received PCA with a CBI of morphine (4 µg/kg/hr) experienced fewer adverse effects and less hypoxemia than children who received PCA with 20 µg/kg/hr CBI or those who received PCA bolus doses alone, noting similar pain scores in all three groups.292 Both of the PCA with CBI groups reported better sleep at night than did the PCA alone group. Differences in opioid doses, age groups, and surgical procedures may account, in part, for these apparent conflicting observations in the pediatric studies. Based on these studies and our own experience, we hold the view that the use of CBI may be beneficial in some children, although it requires careful selection of dose, based on the surgical severity and the child’s comorbid conditions and vigilant monitoring to ensure safety. CBI should be used as a routine for most children with pain resulting from cancer, for most children with mucositis resulting from cancer treatment or bone marrow transplantation, and for a significant percentage of children with sickle cell vasoocclusive episodes. At our institutions, the standard practice is to use CBI, unless limited by somnolence or hypoventilation, for the first night for a majority of children undergoing selected major painful surgeries, such as scoliosis surgery, pelvic osteotomies, and thoracotomies.
Nurse/Caregiver-Controlled Analgesia
Activation of the PCA pump by the bedside nurse, a parent, or a caregiver (such as a grandparent) has been used with success in children who are unable to push the button because of young age or because of physical or cognitive impairments.275,276,295–297 In much of the literature, and in some policy statements by the Joint Commission and other organizations, there is, in our view, too little distinction between activation of PCA pumps by nurses and activation by nonclinician surrogates. The term caregiver is used variably in this literature; here it is used to mean nonclinician surrogates. In pediatrics, this means primarily parents and other family members. A small study of 12 children who received NCA after spine fusion surgery reported adequate analgesia, parent and nurse satisfaction, and no complications.295 Children who received NCA received smaller total morphine doses compared with those who were able to use the PCA device themselves, likely because of the tendency for nurses to underestimate their patients’ pain. A larger observational study of 212 children who received parent- or nurse-controlled analgesia with morphine, fentanyl, or hydromorphone reported effective analgesia (pain scores 3/10 or 2/5 or below) in more than 80% of children.276 Pruritus occurred in 8% and vomiting in 15% of the children on the first day of treatment. Nine children (4.2%) required naloxone for the following: apnea (N = 4), hypoxemia (N = 1), excessive sedation (N = 3), or to facilitate extubation (N = 1). Six of these children had significant comorbid conditions, and 5 received additional sedatives. These investigators emphasized the importance of close monitoring to minimize risk and permit early intervention when using nurse/caregiver-controlled analgesia (NCA/CCA). Another study found that the incidence of overall adverse events in opioid-naive children who received NCA was similar to that in children who received PCA after surgery (22% and 24%, respectively).297 However, children who were able to self-administer PCA required only minor interventions (stimulation, reduction in opioid dosage, or supplemental oxygen), whereas those who received NCA were more likely to require more aggressive interventions, such as opioid reversal, airway management, or escalation of care. This study found that cognitive impairment and opioid dose on the first postoperative day were independent predictors of adverse events. Although the mean time to the occurrence of adverse events was 16 to 27 hours, some events occurred during the third postoperative day, suggesting that monitoring, including continuous pulse oximetry, should be continued as long as the PCA is used. In a study of children with cancer, five respiratory and/or neurologic serious adverse effects were reported, with 1 child requiring naloxone during the 576 days of treatment with NCA/CCA.296 In that study, pulse oximetry was used only at the discretion of the provider, perhaps reducing the investigator’s ability to recognize hypoxemia in some children. Furthermore, the reduced incidence of adverse events may be explained by the fact that most children in that study were not opioid naive and may have developed some degree of opioid tolerance. A study of 10,000 pediatric patients receiving NCA further confirms the safety and tolerability of this delivery method.109
For safety reasons, however, it is important to distinguish between authorized and unauthorized use of the PCA button by an individual other than the child. Several reports describe serious adverse events, including excessive sedation, severe respiratory depression, respiratory arrest, and death, attributed to unauthorized activation of the PCA device by parents, spouses, other family members, and health care providers.297–301 The practice of PCA by proxy has therefore come under scrutiny and its safety questioned by the Joint Commission and the Institute for Safe Medication Practices (ISMP).302–304 In 2004, the Joint Commission issued a sentinel event alert based on PCA errors reported to the U.S. Pharmacopeia. Of 460 errors that resulted in death or some level of harm to the patient, 15 resulted from PCA by proxy, including 12 attributed to family members, 2 to a nurse, and 1 to a pharmacist.302 In interpreting this report and some other reports, it should be emphasized that many do not cite denominator data (total numbers of patients receiving PCA vs. NCA/CCA), so that it is difficult to assign numbers for relative or absolute risks of these techniques. Recognition of these risks has led the Joint Commission and ISMP to strongly recommend that specific policies and procedures be developed and implemented related to the use of PCA by individuals other than the child. Such policies must address the following issues:
 Appropriate selection of children. Many pediatric centers restrict parent-controlled analgesia to children with chronic pain, those who require palliative care, and in special circumstances for children who undergo repeated and extensive surgery. In pediatric centers worldwide, NCA is increasingly being used, with appropriate observation, for both opioid-naive and opioid-tolerant children.
Appropriate selection of children. Many pediatric centers restrict parent-controlled analgesia to children with chronic pain, those who require palliative care, and in special circumstances for children who undergo repeated and extensive surgery. In pediatric centers worldwide, NCA is increasingly being used, with appropriate observation, for both opioid-naive and opioid-tolerant children.
 The specific process of identifying suitable caregivers who will be allowed to activate the PCA button.
The specific process of identifying suitable caregivers who will be allowed to activate the PCA button.
 Communication among clinicians, including the primary service physician, the pain service team, and the primary bedside nurse, regarding the suitability of CCA.
Communication among clinicians, including the primary service physician, the pain service team, and the primary bedside nurse, regarding the suitability of CCA.
 An educational plan for health care providers involved in the decision-making.
An educational plan for health care providers involved in the decision-making.
 Education of caregivers, including pain assessment, recognition of opioid adverse effects, and scenarios when not to activate the PCA button (e.g., when the child is asleep or somnolent). The caregiver should be encouraged to call the nursing staff when in doubt.
Education of caregivers, including pain assessment, recognition of opioid adverse effects, and scenarios when not to activate the PCA button (e.g., when the child is asleep or somnolent). The caregiver should be encouraged to call the nursing staff when in doubt.
 Monitoring protocols, including assessment of sedation depth, respiratory status, and pain assessment at regular intervals. It must be emphasized that the primary responsibility for monitoring the safety and effectiveness of analgesia remains with the nursing staff. Electronic monitoring, including pulse oximetry, is widely used in this setting.
Monitoring protocols, including assessment of sedation depth, respiratory status, and pain assessment at regular intervals. It must be emphasized that the primary responsibility for monitoring the safety and effectiveness of analgesia remains with the nursing staff. Electronic monitoring, including pulse oximetry, is widely used in this setting.
Risks and Adverse Events with PCA
Despite its numerous benefits, the use of PCA has been associated with a wide range of adverse effects, adverse events, and unfavorable outcomes in adults.278,279,299,305–308 Although some adverse effects from PCA therapy may be attributed to the opioid drugs themselves or to patient comorbidities, a significant number of harmful effects occur as a result of human error, with incorrect prescribing, dispensing, administration, or equipment failure. E-Table 43-5 describes the causes of PCA medication errors as identified by the ISMP.303–305309 Increasing awareness of preventable adverse events from PCA has led to improved pump technology directed at minimizing the likelihood of programming errors, including the development of smart PCA pumps that use bar-coded syringes and an integral bar-code reader to prevent incorrect programming of drug concentration. Potential PCA pump errors have been reduced with these “smart pumps.”310 Children are at greater risk for medication-related adverse events resulting from calculation errors in drug doses (because all doses are based on body weight or body surface area) and to developmental differences in PK. However, data for PCA-related adverse events in children are limited.280,311–313 The reported incidence of respiratory depression in children receiving PCA ranges from 0% to 25%.297,311,312,314,315 Risk factors for respiratory depression identified by these studies include cumulative opioid dose, use of basal infusions, concomitant administration of sedatives, and comorbid conditions, including renal failure and cognitive impairment. Recognition of the risks from PCA316,317 has led organizations, such as the ISMP, to emphasize the importance of monitoring children who use PCA and of detailed child and staff education regarding its use.318
| Improper child selection |
PCA, Patient-controlled analgesia.
Monitoring the Child Using PCA
Despite the recommendation from the ISMP that practitioners should identify children at risk for opioid-related respiratory depression and define the appropriate level of monitoring, there remains no consensus regarding the risk-to-benefit ratio and effectiveness of any specific forms of monitoring for children receiving PCA, NCA, or CCA. The Anesthesia Patient Safety Foundation (APSF) recommended the use of continuous respiratory monitoring (minimally pulse oximetry and a continuous measure of respiratory rate) for children receiving PCA, neuraxial, or serial doses of parenteral opioids.319 Additionally, the APSF recommended that reliable alerting methods, such as audible alarms, central stations, or pagers, be implemented to ensure timely and appropriate clinician response to deteriorating respiratory status of those receiving opioid therapy. Recently a nurse notification system was implemented at one of the authors’ institutions, where pulse oximetry alarms generated automated nurse call-light notification after 15 seconds, a page to the bedside nurse and charge nurse after 1 minute, and an emergency group page after 3 minutes of sustained oxygen desaturation.297 The impact of implementing such technology on the incidence of PCA-related adverse events requires investigation. It must be emphasized that pulse oximetry can detect hypoventilation only if the child is breathing room air. Oximetry is not a measure of ventilation but rather of oxygenation. The use of supplemental oxygen interferes with the ability of pulse oximetry to detect respiratory depression by delaying the onset of desaturation.320,321
Side stream sampling of end-tidal CO2 via a nasal cannula or noninvasive capnography detects respiratory depression earlier and more frequently than does pulse oximetry or periodic checks of respiratory rate in adults who are receiving opioids via PCA, or for procedural sedation/analgesia.322,323 Although similar data for children receiving PCA therapy are not available, studies in children undergoing procedural sedation in the emergency department and intensive care unit have reported that capnography detected respiratory events that would have been unrecognized by pulse oximetry, periodic respiratory rate monitoring, and/or clinical examination.324–327 Additionally, these studies identified instances of respiratory depression that were recognized by abnormal capnography before oxygen desaturation was detected. Thus capnography may provide the earliest warning of impending respiratory compromise and may alert clinicians to carefully evaluate the children under their care and adjust opioid doses accordingly. Conversely, in real-world practice, capnography cannulas can be difficult to maintain in proper position in children on a busy postoperative ward, readings can be influenced by mouth breathing, and this technology also has the potential for false-positive and false-negative conclusions. Newer technology incorporates a fully integrated PCA system with modules for continuous monitoring of oxygen saturation and end-tidal CO2.328 Some of these pumps also have a feature that shuts off PCA delivery if preset threshold parameters for oxygen saturation and end-tidal CO2 are reached. Studies evaluating the benefits of such technology in reducing PCA-related adverse events in children are needed. Although electronic monitoring has an important role in patient safety, all available methods are imperfect. Moreover, they generate frequent false alarms that annoy children and families, disturb the restorative sleep of both children and parents, and contribute to desensitization of nurses’ vigilance. Despite these limitations of electronic monitoring, it has been reported that the use of computerized physician order entry and involvement of dedicated pediatric pain teams improved compliance with routine monitoring and increased the likelihood of early identification of adverse events.329
Regional Blockade and Analgesia
The use of local anesthetics, both with and without the addition of central neuraxial opioids and other adjuncts, offers many advantages in the postoperative setting. Blockade with long-acting local anesthetics, or continuous peripheral nerve blockade with infusion of local anesthetics via catheters and elastomeric infusion pumps can provide postoperative analgesia for outpatient surgery so that a child can be discharged home in comfort. Reducing or eliminating the need for systemic analgesics diminishes the potential for adverse effects associated with their use (see Chapters 41 and 42). Regional blockade affords the ability to provide excellent analgesia to children who might otherwise not tolerate larger doses of opioids. This group includes some neonates, especially preterm and former preterm infants who are at risk for apnea; children with problems of central ventilatory control, respiratory disease, precarious airways; or those who risk obstruction with sedation (e.g., children with obstructive sleep apnea).
There are few absolute contraindications to regional blockade. Anatomic anomalies, such as myelodysplasia, sacral dysgenesis, and other abnormalities, either disrupting the epidural space or making access to it impossible, may prevent the performance of a caudal or epidural block. A report of epidural analgesia in children with myelodysplasia, however, suggests that catheters may be used safely in these children when placed at a level above the anatomic neural abnormality.330 In cases involving these types of anatomic anomalies, we encourage consultation with experts in pediatric regional anesthesia, prior review of imaging studies, and consideration of fluoroscopic guidance. A needle and block should never be placed through infected tissue or in close proximity to it. Children with burn injuries may be candidates for continuous regional techniques, provided the burned area is distant from the catheter insertion site (see Chapter 34). We do not believe that the benefits of regional analgesia outweigh the potential risks inherent in inserting catheters through burned tissue or close to it.
Sepsis presents a similar problem. In general, it is not advisable to place caudal or epidural catheters in children who are septic, for fear of seeding the epidural space during a period of bacteremia. Peripheral nerve, plexus, or intrapleural catheters may pose less of a problem in this regard, but there are no data to provide guidance regarding this issue. Coagulopathy and thrombocytopenia are relative contraindications to regional anesthesia, with mild abnormalities in hemostasis not necessarily precluding a regional block. In unusual cases, and with proper consideration of risk-benefit issues, fresh frozen plasma or platelets can be infused at the time of a regional procedure to provide temporary correction of coagulopathy. The considerations regarding regional anesthesia, coagulopathy, and anticoagulation are complex and have been reviewed extensively for adults by consensus groups from the American and European Societies of Regional Anesthesia, and suggested guidelines regarding regional blockade in the anticoagulated patient can be found in Table 41-7.331 In the absence of additional pediatric data, we recommend that clinicians review these adult publications as provisional guides for pediatric regional anesthesia as well. When placing a catheter for continuous blockade, consideration of the state of coagulation must include the time of catheter withdrawal as well as placement. If a child is to receive postoperative anticoagulation, for example, a continuous block should not be considered unless anticoagulation therapy can be stopped for 2 hours before the catheter is removed.
There is no consensus on the timing of a regional block (at the beginning or end of the surgical procedure). Placing a single-injection caudal block before incision confers a similar duration of postoperative analgesia, after a brief surgical procedure of 1 hour or less, as placing it at the end of surgery. For example, the times from recovery until the first request for analgesics after caudal blocks placed before incision or after surgery for inguinal herniorrhaphy were similar.332 For more prolonged procedures, the block may be renewed with a second caudal injection before emergence or a catheter placed and redosed at appropriate intervals (usually approximately 1.5 hours). A volume of half of the original dose is usually sufficient if less than 2 hours have elapsed. A reduced concentration of local anesthetic is usually effective for postoperative analgesia. Adjunctive additives, such as clonidine, have also been shown in some studies to prolong the action of “single shot” central neuraxis and some peripheral blocks (see later discussion) permitting a single-injection block placed before the incision to augment both intraoperative and postoperative analgesia for longer operations. In cases of major surgery on the extremities and shoulders, there is a growing trend toward placement of indwelling plexus or peripheral nerve catheters for local anesthetic infusions for several days, both for adults and children, as detailed subsequently in the section on catheter techniques.333,334
Evidence suggests that placing a block at the beginning of surgery offers several potential advantages.335 Although preemptive or preventive analgesia is a reproducible phenomenon in laboratory studies, the results in humans have been conflicting. For example, initial studies in adults demonstrated a dramatic decrease in the incidence of phantom limb pain when an epidural block was administered before an amputation, although subsequent studies did not consistently reaffirm the initial observations.336–338 Similarly, children who receive intraoperative neural blockade may experience less postoperative pain than those managed with general anesthesia alone, with the duration of analgesia in some cases lasting beyond the pharmacologic action of the block. On the other hand, a blinded study of caudal anesthesia administered either before or after inguinal surgery failed to show a difference in postoperative analgesia.339
It is theorized that interruption of nociceptive impulses at the spinal cord level attenuates imprinting of painful stimuli on the sensory cortex or forestalls the development of spinal cord hyperexcitability and “wind up,” thereby reducing the neural input and persistent postoperative pain.338,340–343 It has also become increasingly evident however, that if preemptive or preventative analgesia is to have a beneficial effect, other conditions must be met: the block must be of sufficient duration in relation to the nociceptive stimulus, it must extend into the postoperative period, and it must be effective at preventing central transmission of the nociceptive signals.335 This third requirement suggests that a multimodal analgesic approach may offer the greatest benefit. The presence of poorly controlled preoperative pain may sensitize the CNS, rendering pain difficult to control via intraoperative or postoperative interventions.344 Additionally, epidural opioids have been shown to reduce the inflammatory response after surgery in adults, as indicated by interleukin-2 concentrations, suggesting that attenuating the stress response to surgery may improve postoperative analgesia.345
Further evidence suggests that local anesthetic infiltration of the incision site, especially when performed in conjunction with a regional anesthetic technique, may be an effective means of providing prolonged analgesia after surgery.346,347 This simple and effective approach can be used before or at the end of virtually any surgical procedure. A major limitation of wound infiltration with currently available local anesthetics is that the duration of analgesia is usually only 4 to 6 hours. Because postoperative pain commonly persists for several days, it would be more useful to administer local anesthetics for 2 to 4 days. To achieve this, the surgeon must place a multi-orifice catheter in the tissue planes of the wound during closure, through which local anesthetics can be infused.334,348–352 Several commercially available kits using these “soaker hoses” have been shown to be effective. A disposable elastomeric pump, filled with local anesthetic that infuses local anesthetic continuously into the surgical tissues, can be placed at the end of surgery and the child sent home with it infusing for several days. Although this is an effective supplemental strategy to achieve postoperative analgesia, practitioners should be aware of complications that have been reported in adults.353 A note of caution: the concentration of local anesthetics, such as bupivacaine, and the infusion rate must be carefully prepared to avoid a local anesthetic overdose if such an approach is planned, particularly after chest surgery and in neonates and small infants. A novel method of providing prolonged analgesia that is currently under investigation involves injection of a suspension of biodegradable polymer microspheres or lipospheres that contain bupivacaine. Dexamethasone can further prolong the duration of this method of block. After injection, these microspheres release bupivacaine in a controlled manner to provide blockade of peripheral nerves for periods of 2 to 6 days, depending on dose, formulation, and site of injection.354–357 An alternative experimental approach to providing prolonged analgesia is the use of modified neurotoxins. Site 1 sodium channel blockers, such as tetrodotoxin and neosaxitoxin, have very strong affinity for sodium channels in vitro. Tetrodotoxin and neosaxitoxin are nonneurotoxic, and do not significantly block sodium channels in the myocardium.358
In animals, the combination of these toxins with bupivacaine, epinephrine, or clonidine markedly prolongs the nerve block and reduces systemic toxicity. Neosaxitoxin shows no cardiotoxicity in animals,359 and has shown promise in a recent Phase 2 clinical trial for abdominal surgery in adults.360
Peripheral nerve and plexus blocks tend to provide blocks of greater duration than do central neuraxial blocks, the former lasting 8 to 12 hours, and on occasion exceeding 24 hours. Depending on the nature of the surgery, this may permit the child to transition to nonopioid analgesics at the time the block wears off, thereby eliminating or reducing the use of opioids and their potential untoward effects. Children undergoing outpatient surgery may be discharged after a single-injection regional block, but follow-up the next day with the family is necessary to ensure that the block has receded and no complications have developed. This is especially the case after peripheral nerve blocks. Parents must further be cautioned that there may be some degree of motor blockade present, and that the blocked limb must be protected from injury. If a lower extremity is blocked, assistance with ambulation is mandatory. Techniques for regional anesthesia and analgesia are discussed in detail in Chapters 41 and 42.
Choice of Local Anesthetics, Additives, and Dosing
Dilute long-acting local anesthetics, such as bupivacaine 0.125% to 0.25% or ropivacaine 0.1% to 0.2%, are the most commonly used local anesthetics for regional blockade. In Europe and Canada, levobupivacaine is available and has the advantage of smaller risk of toxicity than bupivacaine (see Chapter 41). Ropivacaine and levobupivacaine have the dual advantages when compared with bupivacaine of a relatively prolonged duration of action and decreased motor blockade. Epinephrine (1 : 200,000) is often added to bupivacaine to decrease systemic absorption and increase duration of action but should be omitted when a digital or penile block is performed, because of the risk of inducing ischemia by direct vasoconstriction. (Note that the avoidance of epinephrine for digital blocks is based on limited and historical evidence, and current proponents of the practice have not found adverse complications to occur).361–363
Initial studies concluded that the optimal concentration of bupivacaine that provides maximum sensory blockade without motor blockade for caudal analgesia was 0.125%,364 although subsequent studies demonstrated that the optimal concentration was actually 0.175% (7 mL of 0.25% bupivacaine combined with 3 mL of saline).365 More concentrated solutions of bupivacaine (0.2% to 0.25%) may be used for blocks that do not significantly affect motor function, such as for an ilioinguinal-iliohypogastric nerve block after herniorrhaphy. Bupivacaine 0.5% offers no advantage in most settings, and limits the volume that can be administered because of the risk of toxicity. The maximum allowable dose of bupivacaine is 2.5 mg/kg. Furthermore, the use of solutions with many different concentrations may increase the risk of calculation errors and potential overdoses. For this reason, it is prudent to restrict the choice of the concentrations of local anesthetics to a manageable number. Our clinical impression is that thoracic epidural analgesia after Nuss bar placement may be enhanced with a denser blockade. For these blocks, motor block is of less significance because most lung expansion is produced by the diaphragm.92 For thoracic epidural blocks, we use ropivacaine 0.2% with an opioid and clonidine.
Ropivacaine, an l-enantiomer amide local anesthetic, is widely used in children, particularly in neonates and infants.366–369 In both pediatric and adult studies, the duration of analgesia after ropivacaine is similar to that after bupivacaine. Although data in adults suggest that there is a more selective sensory than motor blockade, this has not been demonstrated in studies of immature animals or children.366–368,370–376 Ropivacaine, is less cardiotoxic than bupivacaine. In terms of developing terminal apnea, infant rats tolerate 1.5 times the dose of ropivacaine as compared with bupivacaine. The doses for the onset of respiratory distress and seizures are similarly increased with ropivacaine.368 These differences are more pronounced in infant rats than in adult rats. Animal data suggest that the toxic thresholds for both CNS and cardiovascular toxicity are increased 20% to 30% with this agent in both adults and infants, although seizures can still occur with ropivacaine if the dose exceeds the toxic threshold. The authors recommend using a levorotatory enantiomer (levo-enantiomer), such as ropivacaine, in preference to bupivacaine in infants younger than 6 months of age, because of the potential increased margin of safety.369,377 Another local anesthetic with a similar decreased toxicity is levobupivacaine (the levorotatory isomer of bupivacaine). This drug possesses properties similar to bupivacaine with a moderate reduction in the toxicity risks inherent with bupivacaine.378–380 Levobupivacaine has become difficult to obtain in the United States, although it is commonly used in place of bupivacaine elsewhere.381
Opioids have been injected in the epidural and intrathecal space for analgesia in children, both with and without local anesthetics. Central neuraxial opioids have been used for more than two decades to produce effective analgesia in children. However, opioids administered into the central neuraxis have the potential to cause delayed respiratory depression, and are generally avoided in the outpatient setting to ensure the safety of children after discharge.382 Their use is described in detail later.
Adjuvant drugs have been added to the local anesthetic administered for caudal blockade to prolong the duration of the sensory block, an obviously desirable attribute for a single-injection block. However, their use is not without controversy. Many drugs have been injected into the epidural space with only limited laboratory evidence for safety and lack of neurotoxicity, and some caution is necessary.383 Clonidine, 0.5 to 2 µg/kg, has been shown to lack evidence of neurotoxicity. It increases the duration of analgesia after bupivacaine in caudal blocks by approximately 3 hours, with insignificant hemodynamic effects, mild sedation, and no delay in recovery times.384–390 Despite these reported beneficial effects of clonidine, one double-blind investigation found no difference between IV and caudally administered clonidine in a dose of 2 µg/kg as an adjunct to caudal blocks using bupivacaine, and another found no difference in duration or quality of analgesia when compared with caudal bupivacaine alone.391,392 Conversely, when clonidine was used in conjunction with levobupivacaine, children who received clonidine in the caudal space demonstrated a significant delay in the need for rescue analgesia and reduced pain scores compared with those who received IV clonidine, suggesting that the prolonged analgesia occurs at a spinal cord site of action.389,390,393 Caudal clonidine produces less nausea, itching, ileus, and urinary retention than opioid additives, although it may increase postoperative somnolence or respiratory depression at doses in excess of 1 µg/kg, particularly in the neonate.392,394–396 The clearance of clonidine in infants is approximately one-third that of older children.397 The preponderance of evidence supports the use of clonidine to prolong the analgesic effect of central neuraxial blocks, but we caution against the use of more than 1 µg/kg for outpatient procedures, especially in younger infants, because of an increased risk of respiratory depression.
Preservative-free ketamine has also been used for caudal analgesia, both alone and in combination with bupivacaine. Doses of 0.5 mg/kg appear to provide adequate analgesia, without untoward behavioral effects, such as those reported with IV or oral administration of ketamine.398 When combined with bupivacaine, the duration of analgesia approached 24 hours.203,399,400 In a comparison with IV ketamine, caudal (S)-(+)-ketamine significantly prolonged the duration of analgesia, despite similar plasma concentrations.400 One clinical study suggested a specific spinal site of action. Only preservative-free ketamine should be used because preservatives have been associated with neurotoxicity.401,402 In the United States, preservative-free ketamine is currently unavailable.386,403
Choice of Block and Techniques
Single-Injection Techniques
There are many circumstances in which the simplicity and duration of action of a single-injection block is desirable. Both neuraxial blocks and peripheral plexus and nerve blocks can be effective as single-injection techniques. The caudal block remains the most commonly used technique in pediatric regional anesthesia. It provides analgesia of the lower extremity and lower abdomen, and is easily and quickly performed in most infants and children (see Chapter 41). When an operation is performed on both legs it is often preferable to bilateral lower extremity blocks.
The use of ultrasound makes the identification of plexuses and peripheral nerves much easier in an anesthetized child. Peripheral nerve stimulation using Teflon-coated needles remains an effective technique, but is becoming less frequently used as familiarity with ultrasound and its benefits increases. It is plausible, but unproven, that ultrasound reduces the risk of injury to nerves and adjacent structures (see Chapters 41 and 42).404 To precisely position the needle in the target area and avoid piercing adjacent structures, one must develop skill in coordinating its trajectory with the ultrasound image so that the needle tip does not pass out of the plane of view. Although it remains unknown whether ultrasound and more precise needle placement will reduce nerve injury, there is evidence that it reduces the volume of local anesthetic needed to produce an effective block.405,406 Ultrasound visualization of the local anesthetic surrounding a nerve or plexus provides reliable confirmation that a block will be successful. Nerve blocks of the lower extremity can, in some circumstances, be used instead of caudal blockade (see Chapters 41 and 42). They provide a field of analgesia limited to the operative site, have a prolonged duration of analgesia (usually at least twice that of caudal block), and eliminate some of the potential undesirable effects of central neuraxis blockade, such as urinary retention, motor blockade (wobbly legs), and, occasionally, numbness. The fascia iliaca block, which produces analgesia in the distribution of the femoral, obturator, and lateral femoral cutaneous nerves, has been described in children as an excellent alternative for postoperative analgesia of a lower extremity.407 Blockade of the femoral nerve, the sciatic nerve, and the nerves of the ankle is easily performed in children by using similar techniques to those in adults (see Chapters 41 and 42). Regional blockade of the upper extremity may involve an axillary block, interscalene, supraclavicular, or infraclavicular block, or less commonly, blockade of the individual nerves at the level of the arm or wrist. Intercostal nerve blocks may be used after thoracic or upper abdominal surgery. This block may be considered for procedures of limited scope, such as open-lung biopsy or thoracostomy for drainage, but the duration of blockade is limited to several hours. In our view, for most thoracic procedures, the duration of single-injection percutaneous intercostal blockade is generally too short to warrant the risk of pneumothorax. For open-chest procedures, intercostal blocks by the surgeon pose a low risk and may be indicated, although again, their effectiveness is limited by their short duration. Epinephrine is commonly included in the local anesthetic for these blocks to limit the rate of uptake. More extensive surgery anticipated to cause postoperative pain of longer duration may be best managed with a catheter (continuous infusion) technique.
Ilioinguinal-iliohypogastric nerve block provides excellent postoperative analgesia for inguinal herniorrhaphy, a common outpatient procedure in children (see Chapters 41 and 42). It appears similar in efficacy to a caudal block, with a duration of analgesia of at least 4 hours when bupivacaine with epinephrine is used. For orchiopexy, ilioinguinal nerve block has been found to be as effective as caudal blockade to the T10 level in a randomized and blinded investigation.408 In our experience, however, postoperative analgesia for procedures that involve considerable manipulation and traction on the spermatic cord and testis may be better managed with caudal blockade. Penile block is effective for both circumcision and distal, simple hypospadias repair. More extensive procedures on the penis, especially repair of penile-scrotal hypospadias, require a caudal, rather than a penile block, to produce effective analgesia.409 The use of single-injection neuraxial opioids is an additional technique that can provide longer-lasting analgesia, but must be used in inpatients only, because of the need for respiratory monitoring. Intrathecal morphine has been administered to infants and children for several decades and provides long-acting analgesia (up to 24 hours) after a single injection.410,411 It is absolutely essential that only preservative-free preparations of the drug be used because preservative-containing solutions may result in injury to the central neuraxis. Doses ranging from 5 to 10 µg/kg are usually chosen. Because of the hydrophilicity of this agent, respiratory monitoring is mandated. Respiratory depression, as well as pruritus, nausea, and urinary retention, are reported after intrathecal morphine. We most commonly employ this modality for analgesia after posterior spinal fusion. The drug is often administered by the surgeon under direct vision of the dura, but if administered at the beginning of the case, it has been reported to reduce intraoperative blood loss.412,413 This route of administration has also been employed before cardiac surgery.414 The effective intrathecal dose of opioid is roughly one-fifth to one-tenth that of the epidural dose, and the duration of action, especially with morphine, is significantly prolonged. Therefore, observation in a monitored setting must be continued for 24 hours or until no further evidence of respiratory compromise exists (without the use of naloxone). A novel extended-release preparation of morphine uses a liposomal foam suspension of the drug (DepoDur, EKR Therapeutics, Bedminster, N.J.) that is injected in the lumbar epidural space (note that it should not be used intrathecally) and provides 48 hours of analgesia, with no increase in untoward effects compared with conventional neuraxial morphine.415–418 To date there are no pediatric data, but clinical trials in children are currently in progress.
Catheter Techniques
A catheter placed in the epidural space or adjacent to a nerve or plexus can be used to provide continuous uninterrupted analgesia for prolonged periods after surgery. These catheters are commonly used for about 3 days but may be used for more prolonged periods in selected situations. With proper care, catheters tunneled under the skin may be left in situ for more than 7 days.419,420 Catheters can also be placed in intrapleural or extrapleural and/or retropleural locations to provide analgesia after thoracic surgery.421 In our experience, intrapleural analgesia reduces but does not eliminate the need for systemic opioids, especially if thoracostomy drains are present. We rarely use this technique because toxic concentrations of local anesthetic and seizures have been reported.422 Continuous extrapleural, intercostal, and paravertebral catheters can be placed either through the operative field or percutaneously. In adult studies, and in a smaller series of pediatric studies, these techniques appear to provide excellent analgesia at rest, but with movement they provide only partial opioid sparing. Some clinicians regard these techniques as providing many of the advantages of thoracic epidural analgesia, with the potential for reducing the risks and adverse effects of thoracic epidural analgesia.
Continuous regional blockade is remarkably effective and safe, although as with any technique, monitoring for untoward effects is necessary to prevent complications. New technology for the delivery of drugs to peripheral nerves and plexuses have made it possible for children to receive the benefits of continuous neural blockade after discharge from the hospital or day surgery unit.333 Catheters may deliver local anesthetics and other medications via controlled infusion devices that use a pressurized elastomer-bulb reservoir that controls the infusion rate with a flow limiter (ON-Q pump, I-Flow LLC, Lake Forest, Calif.; Infusor, Baxter, Deerfield, Ill.; Accufusor, Moog Medical Devices, Salt Lake City; Easypump, B Braun Melsungen AG, Melsungen, Germany; and others) (E-Fig. 43-2). These devices can be used at home after discharge, for infusion of medication into a tissue plane to provide a continuous field blockade or a continuous peripheral nerve block. Infusion of local anesthetics in the subcutaneous tissues at the incision site or into the surgical plane using these devices provides prolonged analgesia in both adults and children. Several types of these infusion systems are available, including ones that have options for fixed, variable, or continuous infusion rates with a bolus option. The latter two must be used with caution in smaller children in whom local anesthetic toxicity from excessive dosage may be a risk; we generally use the fixed-rate devices. When continuous peripheral nerve or plexus blocks are used for outpatients, a carefully designed system must be in place to follow these children, to avoid complications and achieve early detection of potentially adverse events. Daily follow-up, either by visiting nurses or by phone, is mandatory. We are aware of a small number of anecdotal cases of possible excessive doses of local anesthetic from a continuous infusion pump used at home that resulted in adverse effects, although no catastrophic events have been reported.
Caudal and Epidural Catheters.
With experience and proper equipment, the lumbar route is feasible at any age, but specific expertise is required for infants and toddlers. Practitioners who have less experience with lumbar epidural catheterization in children should consider placing the catheter via the caudal route for children less than 6 years of age. In infants and children up to about 6 years of age, catheters may be advanced freely from the caudal canal cephalad to lower thoracic levels with excellent success.423 This is possible in part because young children have a less developed vascular plexus and more compact and globular fat than do older children and adults.423,424 However, other authors describe less reliability with caudal-to-thoracic advancement of catheters in children larger than 10 kg.425 This has been attributed to the more mature composition of the contents of the epidural space and the lumbar lordosis that occurs with walking. With age, the epidural fat appears to lose the spongy gelatinous character noted in infants and the spaces between the fat globules become less distinct.423,424 Catheters made of nylon or polyamide may be less likely to kink beneath the skin and seem to thread more easily than those made of Teflon or other materials. The catheter should never be advanced if resistance is felt. Similar difficulties in advancing catheters that resulted in catheters looping backwards, or kinking, or puncturing the dura have been reported in neonates weighing less than 3.5 kg.426 In one series of 20 preterm infants, epidurography revealed misplaced catheters in 3 infants or 15%. New data suggest that misplacement of threaded catheters in all ages may be more common than is generally recognized.427 In this series of 724 epidurograms, unexpected misplacement was detected in 11 or 1.5%, including 3 intravascular catheters, despite negative test doses, 2 intrathecal without cerebrospinal fluid aspiration, 4 that were intraperitoneal, and 1 each in the rectum and psoas compartment. These authors recommended epidurography in all cases, although this is not the current standard of practice. If specific dermatomal placement of a catheter is sought, one should consider obtaining an imaging study to confirm the dermatomal level of the tip of the catheter, and the aforementioned data suggest that imaging might be advisable whenever a catheter is advanced to a level substantially more rostral than its insertion level.
As an alternative to epidurography, a nerve-stimulating catheter may be used, which allows real-time monitoring of the location of the tip of the catheter as it is advanced cephalad (with the stylet in place, using one system available in Canada), or at least confirmation of tip location on removal of the stylet (using a convenient modification of equipment readily available in the United States).428,429 The technique requires the use of saline loss-of-resistance and avoiding air bubbles in the epidural space or in the catheter-connector-injection system, because air impedes electrical conduction. In addition, neuromuscular blockade must be avoided because it abolishes the motor response. Used in conjunction with a nerve stimulator set to very low milliamperage (approximately 6 mA), the muscles supplied by a nerve root will twitch as the catheter approaches the segments that supply that dermatome, thereby confirming the catheter tip’s location. Specifically, twitches in the feet and ankles occur with catheter tips around L5 to S1, hip flexion occurs with catheter tips around T12 to S1, abdominal muscle twitches without hip flexion imply thoracic positioning above T12, intercostal muscle twitches imply midthoracic tip positioning. Finger twitches would imply advancement to around T1. This technique can also be used to detect catheter malpositioning. Bilateral twitching at a current less than 0.6 mA generally implies subarachnoid positioning. Unilateral twitches in a narrow motor distribution at a current less than 1 mA may suggest advancement out a root foramen. Unilateral twitches at a current less than 1 mA in a very broad motor distribution may indicate subdural positioning. Absence of twitches as the current is increased to about 15 mA (in the absence of air bubbles or neuromuscular blockade) generally indicates that the epidural catheter is not in the epidural space. Our experience is that the use of one of these confirmatory techniques can help avoid problems with failed or incomplete blocks in the postoperative period.
Choice of drugs for epidural infusions depends on a number of factors, including site of surgery, site of the epidural catheter tip, and child risk factors. Local anesthetics, lipophilic opioids, and, to some extent, clonidine all have more restricted cephalad distribution of action during infusions, compared with hydrophilic opioids, such as hydromorphone or morphine. Consequently, optimal positioning of the catheter tip during insertion can improve the analgesic action postoperatively. Experience in adults suggests that when local anesthetics are used, analgesia is optimized when the tip of the epidural catheter is positioned at or slightly above the dermatomal levels involved in the surgery. This effect may be more pronounced when children are moving than when they are at rest. Positioning at levels slightly above the dermatomal levels involved in surgery is more relevant for surgery in lumbosacral dermatomes compared with upper thoracic dermatomes because of the greater distance between root level and dorsal horn level in these two circumstances.430
Placement of catheters at thoracic levels requires consideration of risk-benefit trade-offs. Therefore, if a catheter tip is at the lumbar or caudal level and the surgery is in thoracic and upper abdominal dermatomes, continuous infusions containing local anesthetics alone or with lipophilic opioids, such as fentanyl or sufentanil, are likely to be ineffective. Increasing the infusion volume is limited by maximum allowable systemic local anesthetic concentrations. In this circumstance, a reasonable alternative is to administer hydrophilic opioids (e.g., morphine or hydromorphone) through lumbar or caudal catheters. The hydrophilic properties of these opioids permit a greater rostral spread, such that a wider range of dermatomes can be covered after caudal or lumbar catheter placement, with only small increases in dose for surgical sites remote from the catheter tip. This same characteristic, unfortunately, also appears to increase the risk for adverse effects, including respiratory depression, as a result of rostral spread of morphine to the central respiratory center in the brainstem. Hydromorphone may offer some advantages because its rostral spread is intermediate compared with fentanyl and morphine and it causes slightly less pruritus compared with morphine.431,432 Lumbar administration of hydromorphone spreads sufficiently to provide thoracic analgesia.433 It should be noted that the potency of hydromorphone relative to morphine in the epidural space, is less than when it is administered systemically (2-3 : 1 epidural vs. 5 : 1 systemic).434 Such a technique, however, precludes the optimal use of local anesthetics, and the considerable benefit that may accrue from a multimodal approach to regional analgesia.
A thoracic epidural catheter can be placed in an awake, sedated, or anesthetized child. Some clinicians are hesitant to perform thoracic epidural puncture in anesthetized children because of the inability to use child reports (e.g., paresthesia or lancinating pain) as an indicator of improper catheter or needle placement. However, there are few, if any, data to suggest that the risk of inserting a thoracic epidural catheter in an anesthetized child by an experienced clinician is actually greater than attempting such placement in an awake child who may not be able to remain still, nor is it clear that a well-sedated child can accurately report sensations of paresthesia or differentiate the discomfort of needle passage from more ominous pain.435 The prospective data from the British National Epidural Audit do not support the contention that there is increased risk with this practice, and those data are corroborated by the PRAN study, which found no neural injury regardless of patient state during epidural placement.436–438 We believe that data and experience support the safety of placement of thoracic epidural catheters in anesthetized children by anesthesiologists trained and experienced in this procedure, although the tolerances for error are smaller than in adults and older children.439–441
Successful management of children with an epidural catheter requires carefully coordinated monitoring protocols, nursing management, and medical management of drug selection and dosing. As with PCA or continuous-infusion opioids, the orders should be standardized and written in consultation with the nursing staff, so that misinterpretations are less likely to occur (Fig. 43-10). Because the single most sensitive monitor of children receiving epidural opioids is the nurse, rather than a mechanical or electronic device, education of the nursing staff is of paramount importance to ensure safety. Nursing staff can also assess the adequacy of analgesia and thereby help to titrate the drug dose. Catheter insertion sites should be inspected at least once daily by the pain service, both for the integrity of the dressing and for any evidence of erythema or skin infection (Fig. 43-11). When continuous infusions are used, the tubing connecting the infusion pump to the catheter should not have any injection ports and it should be clearly labeled as an epidural catheter to preclude unintended epidural administration of drugs intended for IV use. We use color-coded tubing and a color-coded drug cassette for the continuous administration of regional agents. Although the same pumps are used for IV PCA administration, it is immediately obvious what route the drug is intended for simply by recognizing the tubing and cassette color.
FIGURE 43-10 Sample epidural orders.
(Modified from the University of Michigan Hospitals & Health Centers.)
A variation of traditional epidural analgesia and PCA is the technique of patient-controlled epidural analgesia (PCEA).442 With this variation of traditional PCA, children are generally maintained with an infusion of epidural analgesics (frequently opioid alone or an opioid–local anesthetic mixture) and have the capability of self-administering supplemental doses when needed. When using this technique, the background infusion is used to provide the majority of the analgesia and the child can add to this when needed. It must be emphasized that the time needed for a bolus dose to effect a change with epidural administration is more prolonged than it is with IV agents. Therefore, with PCEA, lockout intervals are greater (often 15 to 30 minutes) than with PCA.443,444 The considerations one would employ for choosing this technique include the same child-monitoring factors for IV PCA and epidural analgesia, and maximum local anesthetic doses must be carefully calculated to cover the contingency of the greatest possible activation of the PCEA demand doses. “Smart pump” technology has been demonstrated to improve safety of this technique.445
Selection of Drugs and Doses.
Local anesthetics, opioids, and adjuvant agents were discussed previously; this section addresses the specifics of drug choices for continuous infusion through indwelling catheters. Many drugs and combinations have been administered via continuous infusion to the epidural space to provide postoperative analgesia.390 The most common choices involve mixtures of local anesthetics and opioids, such as bupivacaine and fentanyl, although, increasingly, clonidine is being added (Table 43-10). Continuous infusions for peripheral nerve and plexus blocks are generally limited to local anesthetics, as evidence for the efficacy of other agents in these blocks is equivocal at best.
The choice of drug is based on several factors: the age and size of the child, the operation, and the underlying medical conditions that may decrease the margin of safety of one of the agents. The volume of solution required to fill the epidural space on a milliliter-per-kilogram basis appears to decrease with age; therefore, older children and adolescents may require less volume than infants and young children, based on weight. In children over 1 year of age, continuous infusions of ropivacaine up to 0.4 mg/kg/hr for up to 72 hours produced stable blood concentrations of unbound ropivacaine without evidence of accumulation or toxicity.446,447 In very young or preterm infants, however, the risk of accumulation of the amino-amide local anesthetics, and thus the potential for toxicity, is particularly problematic.448,449 Neonates are at increased risk for potential local anesthetic toxicity because of decreased protein binding (resulting in increased unbound drug) and possibly immature drug metabolism. The manifestations of local anesthetic toxicity in infants and neonates may be more difficult to recognize than in adults. Hence, we recommend a conservative maximum infusion dose for bupivacaine of 0.2 mg/kg/hr for no more than 48 hours in neonates.450–452 Because safe infusion rates of bupivacaine in the neonate frequently provide insufficient analgesia when wide dermatomal coverage is needed, the amino-ester local anesthetic chloroprocaine may be used in an epidural infusion instead.453 Although the amino-amide local anesthetics are slowly cleared in neonates and young infants, the amino-ester chloroprocaine is cleared extremely rapidly, even in preterm infants, via ester hydrolysis, with an elimination half-life of several minutes.453 This permits large doses and infusion rates with a reduced risk for systemic toxicity. Previous concerns regarding neurotoxicity after chloroprocaine involved a succession of formulations with preservatives, including metabisulfite, methylparaben, and ethylenediaminetetraacetic acid, although the current formulation is preservative-free. An epidural solution of 1% to 1.5% chloroprocaine can be infused at rates of 0.2 to 0.8 mL/kg/hr. If chloroprocaine is not available, the levorotatory enantiomers ropivacaine or levobupivacaine may be preferable to bupivacaine in this age group, because of their better toxicity profile.
When a block is not previously established in the OR, it is useful to dose catheters with local anesthetic (without opioids) at a volume of 0.05 mL/kg per spinal segment or between 0.5 and 1 mL/kg of local anesthetic, not to exceed 5 mg/kg of lidocaine, or 2.5 mg/kg of bupivacaine or ropivacaine. Some clinicians administer a loading dose of opioid as well, such as 1 µg/kg of fentanyl or 1 to 3 µg/kg of hydromorphone. Bupivacaine infusion rates should not exceed 0.4 mg/kg/hr (e.g., 0.4 mL/kg/hr for 0.1% bupivacaine) for children, because toxicity may result.454 Similarly, starting epidural fentanyl infusion rates should not exceed 1 µg/kg/hr. The upper limits of the epidural fentanyl dose should be determined by clinical effect. If epidural local anesthetic infusions are begun without either opioids or clonidine (e.g., 0.0625% to 0.125% bupivacaine) and inadequate analgesia occurs at infusion rates of 0.3 to 0.4 mL/kg/hr (a maximum dosage of 0.4 mg/kg/hr), further increases in local anesthetic infusion rate or concentration should be avoided. Instead, correct placement of the catheter should be confirmed (e.g., with an epidurogram or chloroprocaine/lidocaine test dose) if dermatomal levels cannot be unequivocally determined. If the catheter is properly located, an epidural opioid or clonidine can be added to the local anesthetic infusion. It is imperative to confirm that an epidural catheter is properly functioning immediately after an infant or child arrives in the PACU, or if there is any question of its proper location later. If an amino-amide local anesthetic has been given during or after surgery as an initial bolus followed by a continuous infusion, then use of a repeat bolus dose of these amino amides may result in a “stair casing” of plasma concentrations, with a risk for systemic toxicity. Although administration of epidural or systemic opioids may provide analgesia, they may not clarify the site of the epidural catheter. For this reason, confirmation of the catheter location is imperative whenever the clinical picture is not clear. Several means of accomplishing this are described later.
Local anesthetic and opioid combinations (e.g., 0.1% bupivacaine or ropivacaine with fentanyl 2 to 3 µg/mL or hydromorphone 3 to 5 µg/mL at infusion rates of 0.2 to 0.4 mL/kg/hr), via lumbar epidural catheters or caudal catheters advanced to a lumbar position, provide adequate analgesia in the majority of children undergoing lower abdominal or lower extremity surgery. Fentanyl alone (0.3 µg/kg/hr) has been shown to provide 90% effective postoperative analgesia after a levobupivacaine block was established during surgery.455 Epidural opioids should be used with great caution, or at considerably reduced doses, in children at risk for apnea or hypoventilation (e.g., former preterm infants, children with chronic respiratory failure or disorders of central control of ventilation, or those with obstructive sleep apnea). In very young infants, epidural infusions of both local anesthetics and opioids and other adjuvants can be used safely, but require increased surveillance and reduced initial infusion rates. This is because (1) drug clearance may be reduced; (2) protein binding, which is decreased, may increase the free serum drug concentrations; and (3) titration to clinical end points (e.g., pain scoring) is less precise. A double-blind randomized study demonstrated that the combination of 0.1% bupivacaine with 1 µg/mL of fentanyl provided superior analgesia compared with local anesthetic alone, with no increase in adverse effects, when infused through thoracic catheters in infants under 6 months of age after thoracotomy.456 In contrast, another study found no incremental benefits in children after abdominal surgery from the addition of fentanyl to epidural catheters with greater local anesthetic concentrations (e.g., 0.125% bupivacaine at 0.3 mL/kg/hr).455,457 The use of fentanyl as an adjunct may increase PONV.457 In some centers, application of epidural infusions, especially with opioids, for children younger than 3 to 6 months of age is restricted to intensive care areas. Acetaminophen or NSAIDs can also be administered if adjunctive analgesia is needed.
If the catheter tip is positioned at the thoracic dermatomes, bupivacaine-fentanyl85,95,458–461 or ropivacaine-fentanyl solutions may be used for thoracic and upper abdominal surgery.462 Infusion rates may need to be decreased for thoracic catheters compared with lumbar catheters because the capacitance or volume of the epidural space appears to be less than in lumbar regions. The authors have noted that some older children and adolescents require greater than expected infusion rates to achieve adequate dermatome levels for analgesia. Using hydromorphone in place of fentanyl may be of benefit in these instances, as its greater hydrophilicity results in wider spread. If thoracic placement is not possible, other agents may be administered through lumbar or caudal catheters. For example, epidural bolus doses of morphine 0.03 mg/kg (30 µg/kg) may be administered every 6 to 12 hours as needed.463 Because the time to onset of analgesia is between 30 and 90 minutes, the doses must be administered at the earliest sign of return of pain. Alternatively, an epidural morphine infusion may be administered at a rate of 0.005 mg/kg/hr (5 µg/kg/hr; doses usually range from 4 to 8 µg/kg/hr) after a small initial bolus. We now rarely use epidural morphine and prefer a continuous epidural infusion of hydromorphone when greater spread is desired, as its adverse effect profile appears to be preferable. Studies in adults suggest an epidural potency ratio of between 2 and 3 : 1, compared with 5 : 1 for systemic morphine.433 For thoracic or upper abdominal epidural infusions, hydromorphone is commonly administered at infusion rates of 0.001 to 0.003 mg/kg/hr (1 to 3 µg/kg/hr). When mixed with a local anesthetic for lumbar epidural infusion, concentrations of 3 to 5 µg/mL of hydromorphone yield a solution that results in an appropriate infusion rate of both drugs.
Hydrophilic opioids, especially when administered by infusion, require prolonged careful observation (see later discussion). If somnolence, shallow breathing, or hypopnea occurs, the infusion must be stopped, not simply decreased, until these effects subside, and appropriate therapy instituted (Table 43-11). Rather than administering a large dose of naloxone, it is preferable to titrate it at 1 µg/kg every 1 to 2 minutes until the respiratory drive improves, assuming that the airway can be adequately supported. In this manner, analgesia can be preserved. The duration of action of naloxone is considerably less than the respiratory depressant effect of the neuraxial opioids, thus continued respiratory monitoring is required and usually an infusion of naloxone (0.25-1 µg/kg/hr) until resolution of the respiratory depression. Common epidural infusions and alternative modalities for pain management of sample cases are provided in Appendix 43-1.
TABLE 43-11 Treatment of Untoward Effects of Epidural Opioids
| Adverse Effect | Treatment | Infusion Rate |
|---|---|---|
| Pruritus | Naloxone, 0.5-1 µg/kg IV or infusion at 0.25-1 µg/kg/hr | No change, or decrease opioid concentration, or decrease by 10%-20% (if dermatome level permits) |
| Nalbuphine, 0.025 mg/kg IV every 6 hours | ||
| Nausea and vomiting | NPO for 24 hours | Decrease opioid concentration or decrease by 10%-20% (if dermatome level permits) |
| Naloxone, 0.5 µg/kg IV or infusion at 0.25-1 µg/kg/hr | ||
| Ondansetron 0.1 mg/kg (maximum 4 mg) IV every 6 hours | ||
| Metoclopramide, 0.1-0.15 mg/kg IV every 6-8 hours | ||
| Urinary retention | Bladder catheterization (one time; some institutions keep urinary catheters in these children routinely) | Decrease opioid concentration |
| Naloxone, 0.5 µg/kg IV or infusion at 0.25-1 µg/kg/hr | ||
| Indwelling urinary catheter | ||
| Respiratory depression;* child unarousable, hypoxemic, hypercarbic, or apneic | Oxygen by mask; assisted ventilation if needed | Discontinue infusion; consider possibility of intrathecal catheter migration (check by aspirating) |
| Naloxone, 5-10 µg/kg IV | ||
| Transfer child to monitored setting until episode is fully resolved. | ||
| Consider naloxone infusion (0.25-1 µg/kg/hr) |
IV, Intravenous; NPO, nothing by mouth.
*Stop infusion until the child is alert, if using morphine or hydromorphone.
Adjuvant agents, particularly clonidine, can be administered continuously via epidural catheter, most commonly in combination with or in place of local anesthetics and opioids. The primary analgesic effect appears to be via an α-adrenergic mechanism. Although epidural clonidine has been found to be less potent in terms of analgesic efficacy compared with epidural opioids, such as fentanyl, it has occasionally been substituted for neuraxial opioids because it is associated with a smaller incidence of untoward effects, such as respiratory depression, nausea, and pruritus.464–467 This also suggests that the combination of all three agents, each at smaller concentrations than required individually, might be beneficial at reducing the incidence of adverse effects while optimizing analgesia, but carefully controlled studies have yet to be performed.
Risks and Untoward Effects
Adverse effects associated with regional analgesia can be grouped according to those caused by the technique (e.g., the catheter) and those related to the medications (e.g., local anesthetics, opioids, other adjuvants). Three recent large prospective studies have confirmed the safety of regional anesthetics in children, detecting very small complication rates.436–438468
Local anesthetics in the dilute concentrations used for postoperative analgesia have a very small incidence of untoward effects. Motor blockade occurs less frequently with dilute concentrations, but can be observed even with small concentrations of bupivacaine, such as 0.1%. As discussed earlier, children with neuromuscular disorders causing motor weakness should be observed carefully for exacerbation of their motor weakness. Motor weakness and blockade can result in several problems. Inability or difficulty in ambulation may impede recovery. The inability of a child to move a limb can result in skin breakdown or peripheral nerve compression injuries, especially if meticulous attention to frequent repositioning does not occur. We are aware of a case of complex regional pain syndrome in the distribution of the peroneal nerve that arose in this manner. Motor blockade should be avoided, if at all possible, and frequent examinations may quantify motor function using the Bromage score, if possible (Table 43-12). At our institutions, motor function is assessed every 8 hours along with the other vital signs pertinent to regional blockade (see Fig. 43-10). If motor blockade occurs, the clinician should (1) reduce the concentration of local anesthetic, (2) pay strict attention to padding and to frequent repositioning of the extremity involved, and (3) consider stopping the infusion until motor function returns. Rapid onset of more profound motor block during epidural infusion should raise immediate concern about erosion of the catheter into the subarachnoid space. Motor blockade may occur more frequently with peripheral nerve or plexus analgesia than with epidural blocks, and is subject to the same precautions. When children are discharged home with a peripheral nerve catheter, it is particularly important to teach the parents how to deal with motor blockade.
TABLE 43-12 Bromage Score* for Assessment of Motor Function of the Legs
| Full motor function: can flex hip, knee, and ankle | 0 |
| Can’t flex hip (unable to perform straight-leg lift) | 1 |
| Can’t flex hip or knee | 2 |
| Can’t flex hip, knee, or ankle | 3 |
*Note that alternative scoring schemes for the Bromage scale exist that range from 1-4 (sometimes denoted I-IV) that directly correspond to the 0-3 scale depicted here.
The PK of bupivacaine have been studied in infants and children after both single-injection doses469 and continuous infusions. Data suggest that in children younger than 6 months of age, reduced protein binding increases the free fraction of circulating bupivacaine, which increases the risk of bupivacaine toxicity.470 Neonates and infants with impaired hepatic blood flow may be at particular risk for bupivacaine accumulation. Seizures and cardiac arrest have been reported when large doses of bupivacaine have been administered, usually after excessive infusion rates or unintended intravascular injection. Limited information is available regarding extended administration over days, although low concentration solutions (and therefore low total doses) reduce the risk for toxicity. l-Enantiomers of amino amides (ropivacaine and levobupivacaine) have toxic thresholds that are greater than that of bupivacaine by as much as 30%, and such drugs should be considered in smaller children and for blocks that require greater infusion rates and for greater durations. A particular “hidden” risk occurs when a block is functioning less than optimally and bolus doses are administered in an attempt to broaden the dermatome level. Even though the infusion rate is kept within the recommended range, the bolus doses can raise the blood concentration above the toxic threshold. Whenever a local anesthetic is administered by continuous infusion, nursing and medical staff must be aware of the signs and symptoms of local-anesthetic toxicity, be vigilant in monitoring for the early detection of those signs, and be familiar with the use of lipid rescue protocols to treat toxicity if it occurs. The use of lipid emulsion for treating local anesthetic toxicity is discussed in detail in Chapter 41. Tachyphylaxis during prolonged administration of local anesthetics is a theoretic consideration, although no systematic studies have addressed this phenomenon in children. In animal models, tachyphylaxis is accelerated by hyperalgesia and prevented by agents that prevent hyperalgesia at spinal sites. One rationale for coadministration of opioids or clonidine in epidural infusions may be to reduce the incidence or severity of tachyphylaxis. As discussed in Chapter 41, hemodynamic stability is maintained in children younger than 6 years of age during spinal or epidural blockade, even with extensive sympathetic blockade. As children approach school age, however, rapid position changes may produce hemodynamic responses after extensive sympathetic blockade, and this must be considered in children who receive continuous epidural blockade. Orthostatic changes may be exacerbated when epidural clonidine is infused, and extra caution should be taken.
Neuraxial opioids confer several potential adverse effects, including respiratory depression, nausea, urinary retention, and pruritus. Indeed, despite their proven analgesic efficacy, not all authorities are proponents of their use because of these effects, preferring other adjuvants instead.471 The most effective means of treating or preventing the adverse effects is the use of opioid antagonists or mixed agonist-antagonists. This approach directly targets the etiology of the signs rather than simply treating their manifestations. Low-dose naloxone infusions, which have been shown to be effective in children receiving parenteral opioids, are also effective for treating the adverse effects of neuraxial opioids, although pediatric data are lacking.472–476 We have used similar rates to those shown effective in the PCA studies, beginning at 0.25 µg/kg/hr.241 This infusion rate can be titrated upwards as needed to 1 µg/kg/hr without adversely affecting analgesic efficacy.242
The most worrisome and dangerous complication is respiratory depression. The lipophilic agents (fentanyl and sufentanil) have the widest therapeutic indices. This results from greater receptor binding in the substantia gelatinosa of the spinal cord adjacent to the area of drug administration, thus limiting the rostral spread of the drug. Despite the fact that the hydrophilic opioids, hydromorphone and morphine, pose a greater risk for respiratory depression, the incidence remains low. Nevertheless, reports in the pediatric literature of respiratory depression after epidural administration of morphine demonstrate that vigilance in monitoring is mandatory.382,477,478 The incidence of complications with caudal morphine is reduced if a dose of 0.033 mg/kg (33 µg/kg) or less is used in children older than 1 year of age.463 The adequacy of analgesia at this dose was virtually unchanged from 0.1 mg/kg (100 µg/kg), with the exception of a prolongation of action with the greater dose. The hallmark of impending overdose of central neuraxial opioids is increasing sedation and decreasing DEPTH AND RATE of respirations. It is important to recognize that the depth of respiration must be assessed, not just the rate, because children frequently develop decreased tidal volume before the respiratory rate decreases, leading to alveolar hypoventilation and the potential for hypercarbia and hypoxemia.479
Pruritus is a common adverse effect associated with epidural or intrathecal opioid use, occurring in as many as 30% to 70% of children. Antihistamines are less effective antipruritics in this situation, because the primary mechanism is a central opioid, not a histamine, effect. Thus opioid antagonists, used in small doses, are most effective. Again, low-dose infusions of naloxone can be employed with good results.242,472,473,475,476 Some practitioners have found low-dose nalbuphine to be effective to antagonize pruritus (25 to 50 µg/kg every 6 hours, PRN).473,474 Although we have found this treatment to be effective, others have found it to be no more effective than placebo in reducing pruritus in children.480
Nausea and vomiting can also occur in association with opioids (both systemic and neuraxial) and may be more common with morphine than with fentanyl. Children who are fasted during the first 24 hours after surgery do not vomit excessively, even when given caudal morphine.481 As with all other opioid adverse effects, nausea and vomiting respond to the previously mentioned doses of naloxone or mixed agonist-antagonists, such as nalbuphine. Some antiemetics, such as antihistamines and butyrophenones (e.g., droperidol), may cause sedation and should be used with caution. Serotonin receptor antagonists, such as ondansetron (0.1 to 0.15 mg/kg, maximum 4 mg) or dolasetron (0.35 mg/kg, maximum 12.5 mg), may be effective and not cause sedation.482 Metoclopramide in doses of 0.1 to 0.15 mg/kg (100 to 150 µg/kg) IV every 6 to 8 hours may provide adequate relief, with less sedation than other drugs of its class. It is sometimes prudent to decrease the infusion rate of the epidural (if the block level permits) or the opioid concentration in the infusate when untoward effects require treatment. If additional analgesia is required, acetaminophen, oral NSAIDs, or ketorolac may be administered. Untoward effects of epidural opioids and their treatment are summarized in Table 43-11.
Urinary retention is a relatively common complication of neuraxial opioids. Studies of single-injection caudal blocks have found that this complication does not occur when epidural local anesthetics are used, only when neuraxial opioids are added.372 Neuraxial opioids depress detrusor contractility in a dose-dependent manner, and this effect may actually outlast the analgesic effect of the drug by hours.483,484 Different approaches have been used for this problem, including indwelling urinary catheterization and the use of opioid antagonists.485
Concerns have been raised that regional analgesia could mask or delay the detection of compartment syndrome of an extremity because of the intensity or quality of analgesia produced by neural blockade.486 Despite the theoretical worries, this has not been demonstrated in numerous studies, and indeed, the opposite has been observed, that the onset of pain in a patient with previously adequate analgesia from an epidural or nerve block is an early warning sign that may herald the onset of compartment syndrome.487,488 The concentration of local anesthetics used for postoperative analgesia appears to be inadequate to mask the intensity of pain caused by compartment syndrome. In addition, epidural blockade is not effective in controlling the discomfort from intense pressure (as demonstrated by the parturient’s ability to perceive pressure during labor contractions despite being pain-free). The loss of effective analgesia in a child at risk of compartment syndrome should raise suspicion and prompt investigation before any changes are made to the analgesic regimen (see also Chapter 30).