Chapter 4. Acute neurological problems
Prioritising the initial management: GCS and ABCDE 101
Managing ‘medical’ and ‘neurological’ coma 107
Responding to neurological deterioration 195
Meningococcal meningitis 130
Introduction
The major issue in the patient who presents as a neurological problem is to decide whether the patient needs supportive care or whether the patient needs active intervention. Many cases who present as neurological emergencies will recover fully, given time and good supportive treatment, because their underlying disorder is fully reversible. Examples include coma due to self-poisoning and the confusional states that follow an epileptic fit. Other cases require a much more active approach: typical examples are antibiotics for acute meningitis, glucose for hypoglycaemia and neurosurgery for subarachnoid haemorrhage. Therefore management decisions can only be made safely if the correct diagnosis has been established. Time is critical in neurological cases, as the nervous system has limited powers of recovery – the diagnostic path has to be followed with an appropriate degree of urgency. The Acute Medical Unit has a duty to ensure the safety of the patient during this time and the nursing staff have the key role in this process (→Box 4.1).
Box 4.1
1. Ensure the safety of the patient
2. Prioritise the initial management
3. Differentiate and manage ‘medical’ and ‘neurological’ coma
4. Identify and respond to neurological deterioration
5. Reach appropriate outcomes
Role of the Acute Medical Unit in Acute Neurological Problems
Ensuring the Safety of the Patient
There must be confidence in the ability to support the patient while the correct diagnosis is being reached. The role of the Acute Medical Unit at this stage is to prioritise the initial management. It may, for example, be necessary to stabilise the patient’s condition before attempting to take a history.
There are four critical initial observations:
• Are the vital signs stable?
• Is the airway secure and is ventilation adequate?
• Is there a non-neurological condition that needs immediate attention?
• Is there anything neurological that needs urgent active intervention?
It is critical that the patient reaches the appropriate service in the best possible condition, be it the acute neurosurgical unit, the stroke unit, the general medical wards or the ITU.
Prioritising the Initial Management
GCS and ABCDE: Assessing and keeping the patient alive
Protection of the airway, ensuring adequate ventilation and maintaining the circulation are the immediate priorities in any patient. At the most basic level this means:
• ensuring the upper airway is patent
• giving sufficient oxygen
• securing i.v. access and maintaining the blood pressure
Warning: At this first stage, if meningococcal meningitis is suspected appropriate i.v. antibiotics must be given straight away.
If there is any suspicion of a neck injury (an example would be a patient who had been found at the bottom of some steps, having had an apparent fall), the neck must be protected and immediately X-rayed. If there is a cervical spine fracture, manipulating an unsupported neck either to establish an airway or to move a patient around in bed can damage the spinal cord beyond repair. At least be aware of the risk, know how to apply a stiff collar and use sand bags to support the neck, and if there is any doubt protect the neck and log-roll the patient until the neck films have been cleared by a radiologist.
Critical nursing observations
Blood sugar
Hypoglycaemia is the most important cause of a fully reversible neurological disorder. At blood sugars below 3mmol/L the patient becomes comatose and may fit, but any neurological picture can be caused by hypoglycaemia. Providing the patient has not suffered irreversible damage from sustained hypoglycaemia, correction of the blood sugar will lead to a full and rapid recovery.
Response of the pupils
The size, symmetry and reactivity of the pupils are fundamental neurological observations. The most common cause of bilaterally fixed and dilated pupils is self-poisoning with tricyclic antidepressants, whereas poisoning with both opiates and diazepam-like drugs produces fixed constricted pupils.
The major reason for following the change in the pupils is to identify ‘uncal herniation’: a shift of the brain because of cerebral oedema that results in the IIIrd cranial nerve being ‘pinched’ between the brain and part of the skull (→Fig. 4.3). As the IIIrd nerve is responsible for pupillary constriction, an early warning of uncal herniation is pupillary dilatation followed by loss of the light reflex.
 |
| Fig. 4.3 |
Any suspicion that a pupil is dilating and losing its light reflex must be reported to the medical staff for action to reassess the neurological status of the patient.
Temperature
In the right context, an increased temperature should be considered strong evidence of intracranial sepsis: meningitis, more rarely meningo-encephalitis, or even a collection of intracranial pus (intracerebral abscess).
Not every fever in a neurological emergency is due to intracranial sepsis, as there may be infection elsewhere such as aspiration pneumonia. High temperatures also characteristically occur in brain stem strokes. Nonetheless, intracranial infection should be the first consideration because of the need for immediate action.
Pulse
The rhythm and rate of the pulse are important. They may draw attention to non-neurological problems that need to be addressed. Extreme bradycardia, for example, may be the cause of an unexplained blackout. Atrial fibrillation is recognised by the irregularity of the pulse and its presence increases the likelihood that an acute stroke is due to a clot of blood finding its way from the left atrium (a fibrillating atrium encourages clot formation) to the brain: an embolic cerebral infarction. Changes in the pulse can be important: progressive slowing can indicate increasing ICP and is often accompanied by an increasing blood pressure.
Blood pressure
Hypertension is commonly seen as an acute response to neurological events such as intracranial haemorrhage or large cerebral infarction. Severe hypertension can also complicate coma caused by self-poisoning with sympathomimetic recreational drugs such as ecstasy, amphetamines and cocaine, and may need urgent correction. However, in most neurological conditions, including stroke, lowering the blood pressure acutely to ‘normalise’ it is fraught with danger. The brain is usually fairly impervious to changes in blood pressure as the cerebral blood flow ‘autoregulates’: if the blood pressure drops, the vessels open up to allow greater flow through them. If there is a major neurological insult this autoregulation is lost, so if the blood pressure falls the blood supply also decreases, which may worsen the neurological deficit. Therefore in acute situations, especially in strokes, the temptation to ‘treat’ a high blood pressure should in most situations be resisted. The only exceptions are in special situations when very high levels, say around 230/140mmHg, are considered dangerous. Nonetheless, any change in blood pressure must be gradual.
As already explained, the combination of a progressively falling pulse and an increasing blood pressure may, in the appropriate circumstances (such as the aftermath of a major intracranial bleed), be a sign of increased ICP.
Respiratory rate
The respiratory rate remains an essential observation in the patient who is critically ill, whatever the cause. It is particularly important to document any changes in respiratory rate. Thus in the neurological emergency a falling respiratory rate may reflect deepening coma. If the change in consciousness level is due to increasing ICP, the slowing respiration is accompanied by a falling pulse and rising blood pressure. It is instructive to contrast this with the signs that are seen in shock, in which a change in consciousness level is accompanied by an increase in the respiratory rate, an increase in the pulse and a decrease in the blood pressure.
Oxygen saturation
Hypoxia remains a common, yet easily correctable, cause of avoidable death on acute emergency wards. The normal oxygen saturation is around 97% and it must be appreciated that to decrease the saturation to 90% or less requires a major disturbance in ventilation that may be due to deepening coma and a worsening neurological state. A fall in oxygen saturation needs careful reassessment.
• Is the coma deepening?
• What about the lungs and the circulation?
• Should blood gases be checked?
• What is the respiratory rate?
It must be remembered that the oxygen saturation does not always reflect the level of ventilation. If the patient is on a high concentration of oxygen, the saturations can be more or less normal even when the patient is hardly breathing. The saturations must not be interpreted in isolation. There would be no reassurance from an oxygen saturation that had stayed constant for 4h at 95% if, during that time, the GCS had dropped from 12 to 4 and the respiratory rate had fallen from 15 to 8 breaths/min. This patient should be on ITU with a view to immediate ventilation.
Glasgow Coma Score
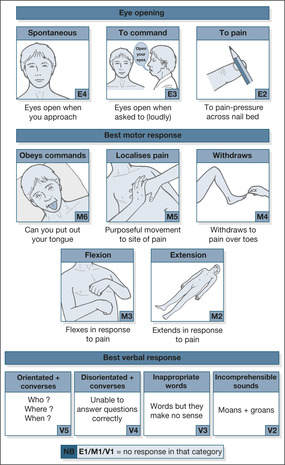
The GCS was described in 1974 as a way to assess any patient with an altered level of consciousness. It is based on three categories of response: eye opening, the best motor response and the best verbal response (→Table 4.1; Fig. 4.1). The GCS is the universal measurement of the depth of coma. It is simple to carry out and score and, provided it is performed properly, the result should be the same whoever makes the measurement.
| Response | Score |
|---|---|
| Eye opening (E) | |
| Spontaneously | E4 |
| To verbal command | E3 |
| To pain | E2 |
| No response | E1 |
| Best motor response (M) | |
| Obeys commands | M6 |
| Localises pain | M5 |
| Withdraws to pain | M4 |
| Abnormal flexion to pain | M3 |
| Extension to pain | M2 |
| No response | M1 |
| Best verbal response (V) | |
| Orientated and converses | V5 |
| Disorientated and converses | V4 |
| Inappropriate words | V3 |
| Incomprehensible sounds | V2 |
| No response | V1 |
| Total score (3 to 15) |
 |
| Fig. 4.1 |
The GCS can be documented on a chart as the grand total from the three categories against time or as E4 M6 V5, etc. As a general rule the scores reflect the severity of the neurological status:
| mild | 13 to 15 |
| moderate | 9 to 12 |
| severe | 3 to 8 |
Coma is defined as a GCS of less than 9.
The initial GCS, often performed by the paramedics outside hospital, acts as a baseline. A low initial GCS score (less than 9) will mean the airway is vulnerable and the patient is at risk from aspiration.
A change in the GCS is the single most important observation in the acute neurological patient. A fall in GCS needs immediate attention. Deepening coma may need increased supportive measures, perhaps involving a move to ITU with a view to ventilation. More critically, a fall in GCS is often the first sign of increasing ICP, which occurs in active intracranial bleeding, intracranial sepsis and during the development of brain oedema following a cerebral infarct.
Some triage systems use an abbreviated assessment of the consciousness level based on the acronym AVPU:
| A | Alert |
| V | Responds to voice |
| P | Responds to pain |
| U | Unresponsive |
This is quick to perform and easy to recall. It is useful as part of a rapid primary assessment, but it is not detailed enough to use for serial observations.
Top to toe examination
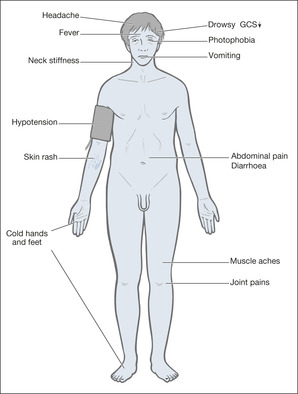
Those closely involved with the care of acute neurological disorders should be aware of the importance of quite subtle physical signs in giving clues, particularly of head injury and infection. A head injury can result in local bogginess or scalp bruising, perhaps a palpable depressed skull and bleeding or loss of CSF from the ears and nose. Fractures to the base of the skull characteristically produce bruising around the eyes (‘raccoon eyes’) and behind the ears (Battle’s sign). All these can and will be missed by clerking doctors, particularly if a ‘stroke’ label has been attached to the patient and the examination is less than thorough because of the pressures of time. Be particularly careful to look at any sites that appear to be the source of pain. Severe sinus sepsis is tender to the touch and very painful; falls from whatever cause lead to fractured hips; and grand mal fits can produce shoulder dislocation. A widespread haemorrhagic skin rash will clinch the diagnosis in meningococcal septicaemia, but it can appear with alarming speed, sometimes even after the patient has been clerked in.
A reliable history
One of the difficulties with neurology is the unreliability of the preadmission diagnosis. Unfortunately, diagnostic labels tend to stick and the consequences can sometimes be disastrous: the ‘cerebrovascular accident’ turns out to be a subdural bleed, ‘off legs’ a spinal cord compression, the ‘well-known epileptic’ a case of recurrent syncope due to intermittent heart block, and so forth. Every neurological patient who is admitted to hospital should be looked at afresh, particularly where there is a ‘known case of…’ Known by whom and with what degree of certainty? Are we sure that the original diagnosis still applies?
It is not advisable to make ‘stroke’ the initial diagnosis in a patient in coma, even if there is a past history of cerebrovascular disease. This is particularly the case in elderly patients. It is a dangerous tendency to attribute all changes in the consciousness level, all funny turns and all episodes of dizziness to a ‘CVA’ just because the patient is 80 years old.
Recent causes of coma initially labelled as stroke on our Acute Medical Unit included:
• post-ictal state
• respiratory failure
• overdose
• subarachnoid bleed
To make an accurate diagnosis, the history and eyewitness accounts are absolutely critical. By their nature, acute neurological problems are difficult to assess. The patients are often confused or uncooperative, making history taking and examination difficult. Nonetheless, these are the very patients in whom a correct diagnosis is paramount. Skills that are needed to assess patients with confusion or impaired consciousness levels must be perfected. Exact histories must be obtained from relatives and eyewitnesses, in particular to establish whether there is a possibility of trauma to the head or cervical spine. The typical sequence of events leading to a post-traumatic brain haemorrhage is of a head injury with transient loss of consciousness followed by amnesia for the event. The patient then becomes confused and aggressive and may fit. The GCS then starts to fall as a result of the haemorrhage. The patient may present at any of these stages. Intracranial infection is also easy to miss: here the critical questions are concerned with recent sinus or middle ear infections. Collections of pus in and around the brain present with stroke-like illnesses and are frequently missed because a proper history has not been taken and the conditions not even considered.
Managing ‘Medical’ and ‘Neurological’ Coma
Coma is defined as a GCS of 8 or less. Broadly there are two types: ‘medical’ coma, which is coma due to conditions such as liver failure, respiratory failure and DKA, in which the management is primarily aimed at the underlying disease; and ‘neurological’ coma due to conditions such as stroke, subarachnoid haemorrhage and meningitis.
The underlying mechanism in coma
Coma that lasts more than 4h is usually due to one of four groups of conditions (→Box 4.2). By far the most common cause is self-poisoning (40%).
Box 4.2
| • Self-poisoning with sedative drugs | 40% |
| • Post-hypoxic cerebral damage | 25% |
| • Stroke | 20% |
| • Metabolic disorders | 15% |
Several features of the patient’s initial observations can be used to identify coma that is due to causes other than a stroke (→Box 4.3). This has a practical purpose, because coma due to stroke has an extremely poor prognosis, whereas some of the other causes may be reversible.
Box 4.3
• Deep coma
• Reactive pupils
• Pyrexial
• No deviation of the eyes to one side
• Moves all four limbs
There are five causes of coma that must not be missed as they are all treatable and relatively easy to diagnose, provided that they are considered at the time of the initial assessment (→Box 4.4).
Box 4.4
• Sugar (high or low)
• Sepsis
• Sub- (and extra-) dural haemorrhage
• Special causes of coma (respiratory failure, renal failure and liver failure)
• Secret overdoses (late presentation of paracetamol/tricyclic overdose)
Practical management of coma
The systematic way to approach the patient with coma is to use the universal system of primary assessment based on the simple mnemonic ABCDE.
A is the airway
‘Are you all right?’ If the patient responds appropriately, there is unlikely to be an airway problem. The airway can be maintained with a jaw thrust or chin lift. Placing the patient in the recovery position may be necessary (providing there is no sign or suspicion of spinal injury). Use a Guedel oral airway if it can be tolerated. Administer high-flow oxygen immediately. Look, listen and feel for expired air.
B is the breathing
• Count the respiratory rate (breaths/min):
| Less than 10 | imminent respiratory arrest |
| more than 20 | ill |
| more than 30 | critically ill |
• Listen for noise (wheeze, upper airway gurgling, ‘croup’)
• Assess the effort
• Perform oximetry
• Continue high-flow oxygen
• Keep the saturations greater than 90%
C is the circulation
• Assess pulse rate and strength
• Assess capillary refill (press finger pulp for 5s and release; refill should be less than 2s). This gives an idea of the peripheral circulation
• Measure the blood pressure
D is the disability
• GCS
• Assess pupil size, equality and reaction
• Measure blood sugar (give sugar for hypoglycaemia and, if there is a suspicion of alcohol abuse, thiamine)
Importance of an eyewitness in coma
Any relatives or eyewitnesses must be kept on the ward and interviewed (or if necessary telephoned). Their information may provide vital clues to the likely diagnosis. It is unacceptable for the doctor or the nurse ever to conclude that a history is either unobtainable or unavailable. Cerebral malaria is the classic example to emphasise this point: fatal if undiagnosed, curable with drugs, but too rare to remember to consider as a cause of coma. Only the patient’s relatives will tell you of the safari holiday taken a week before the onset of headaches, fever and confusion. Patients who are most at risk are the least able to provide a good history for themselves. It is critical to ascertain whether there is a possibility of trauma. Patients with head injuries and trauma to the cervical spine are not usually admitted to the medical wards, but you should not lower your guard: in neurology the most challenging and difficult problems are those in which a history is unavailable and for which preadmission clinical details are scanty. Review all the details in the paramedics’ record. Their information is often comprehensive and systematic and dates from soon after the event. A good paramedic assessment will give you important data on the circumstances in which the patient was found, the initial GCS and their clinical course in the critical early stages of the illness.
Responding to Neurological Deterioration
It is not sufficient to document neurological observations. The reason for performing these time-consuming and exacting assessments is to act as soon as they change. The patient described below (→Case Study 4.1) sustained traumatic intracerebral bleeding and was ‘coning’ (a shift of the brain as a result of raised ICP). The signs of increasing ICP were identified, but immediate action was not taken. A one-sided dilated pupil is often the earliest warning sign of elevated ICP which, if not corrected urgently, leads to a pressure cone.
Case Study 4.1
A 59-year-old man on warfarin for a valve replacement was taken to the AED having collapsed and fallen backwards, striking and bruising his head. He appeared well, but X-rays showed a basal skull fracture. In view of his ‘medical’ problems, he was admitted to the Acute Medical Unit, where he was described as ‘drowsy and vague, complaining of headache’. He was moderately over-anticoagulated (INR 4.9). Half-hourly neurological observations recorded a systolic blood pressure increasing from 130 to 220mmHg and a sluggishly reacting right pupil which, over 3.5h, became more dilated. He was responsive to commands until 04.00h, 6h after admission, when he suddenly vomited copiously and became unresponsive (GCS 3).At this stage his right pupil was fixed and dilated. His anticoagulation was reversed with fresh frozen plasma. He was intubated, ventilated and sent urgently to the neurosurgeons.
How the clinical state can change in the first hours of admission
Case Study 4.2, Case Study 4.3 and Case Study 4.4 illustrate how the clinical state can change rapidly.
Case Study 4.2
A 55-year-old man became acutely confused the day after returning from a Turkish holiday. Over the next 48h his speech deteriorated and he became increasingly difficult to rouse. He then had a series of tonic-clonic convulsions. Investigations showed a severe viral encephalitis.
Case Study 4.3
A 19-year-old student was admitted on Christmas Eve with an acute abdomen. In the recovery room after a negative laparotomy, the anaesthetist noticed a petechial rash. Lumbar puncture was normal, but treatment for meningococcal infection was started. Repeat lumbar puncture 8h later yielded ginger-beer coloured CSF which grew meningococci. He recovered fully.
Case Study 4.4
An 80-year-old man developed dizziness and a mild hemiparesis. On admission his GCS was 15 but he could not swallow at all. He would not tolerate an airway. Late the same night he vomited and dropped his GCS to 5. He became acutely short of breath and pyrexial.The cause of his deterioration was an aspiration pneumonia. In spite of physiotherapy and antibiotics, he deteriorated and died.
Other important examples include:
• deepening coma in an overdose
• deterioration due to missed infection elsewhere (blood, urine, chest, etc.)
Reaching Appropriate Outcomes
The combination of good nursing and medical care should aim to reach an appropriate outcome for every patient. Important outcomes and the principles by which they can be achieved are listed below.
Transfer to the stroke unit in an optimum condition
• Coma management
• Swallowing assessment
• Early aspirin where indicated
Avoiding major stroke after a transient ischaemic attack
• Urgent investigation of suspected TIAs and minor strokes
Surviving intracranial sepsis
• Early treatment of meningitis
• Recognising and managing complications
Reaching the neurosurgeons alive and on time
• Recognising operable intracranial bleeds
• Diagnosing intracerebral masses
• Managing increased ICP
Full recovery from self-harm
• Responding to a changing GCS
Recovery from reversible causes of neurological emergencies
• Rapid and effective primary assessment
• Good coma management
Stroke and Stroke-Like Emergencies
The World Health Organisation’s definition of a stroke is a syndrome of rapidly developing clinical signs of focal (e.g. hemiplegia) or global (e.g. coma) disturbance of function, with symptoms lasting 24h or longer (or leading to death) with no apparent cause other than of vascular origin.
The time frame of 24h is to differentiate a stroke from a TIA, which recovers rapidly but is an important risk factor for developing a subsequent completed stroke. There are around 2.4 strokes per 1000 population per year. As 80% of these will be admitted as emergencies, most Acute Medical Units can expect to admit one to two strokes per day. It is important, however, not to overuse the stroke label on the Acute Medical Unit. Estimates suggest overdiagnosis, with up to a fifth of all ‘strokes’ turning out to be something else: post-epileptic states, toxic states due to infection, and metabolic problems such as respiratory failure.
Two factors have had a major impact on improving the outlook after a stroke in terms of mortality and long-term disability: the introduction of Acute Stroke Teams who become involved at, or soon after, emergency admission, and the increased availability of stroke rehabilitation in specialist Stroke Units. Nonetheless, stroke remains the most frequent cause of major disability in adults.
There is increasing emphasis on drugs that improve the outcome of stroke or prevent further damage. Aspirin, given as soon as a diagnosis of ischaemic stroke has been confirmed, reduces the risk of recurrence. Emergency thrombolysis (which needs to be given within three hours of the onset of symptoms) significantly improves the neurological outcome in cerebral infarction and should now be standard practice in the UK. Recent studies have shown that, after a stroke, lowering the blood pressure (for example with the combination of the ACE inhibitor, perindopril, and the diuretic, indapamide) and lowering the cholesterol with a statin improve the outlook – and that this benefit is obtained even if the initial blood pressure and cholesterol level were normal. High blood sugars after a stroke may also be harmful and careful sugar control is likely to become a routine part of emergency stroke management.
Causes
Most typical strokes are caused either by a cerebral infarction (an area of brain tissue death) or by an intracerebral haemorrhage (bleeding into and around the brain). Stroke-like illnesses are also caused by subarachnoid haemorrhage, subdural haemorrhage and extradural haemorrhage. Subarachnoid haemorrhage is an important cause of sudden severe headache and collapse and requires neurosurgical input. Subdural and extradural haemorrhages are rare but important causes of stroke-like illnesses that can be treated successfully if they are recognised in time.
Cerebral infarction (approximately 80% of all strokes)
A cerebral infarction occurs when the blood supply to part of the brain is cut off, resulting in a focal area of brain-tissue death (an ischaemic stroke). There are two ways in which the blood supply can be interrupted: by a clot that travels through the arterial system and becomes lodged somewhere critical in the cerebral circulation (cerebral embolus), or by a clot that forms within the cerebral arteries themselves (cerebral thrombosis).
Cerebral embolus
Left atrial clot. The most common cause of left atrial clot is atrial fibrillation. In atrial fibrillation, normal regular atrial contraction is replaced by irregular, uncoordinated atrial activity more akin to muscular shivering than proper contraction. As a result blood stagnates and can form a clot within the atrium. There is a particular risk of this happening if there is also heart disease in the form of heart muscle damage (which leads to further stagnation) or diseased valves (which can act as a focus for clot formation). Atrial fibrillation is a common age-related arrhythmia. It is found in around 8% of people older than 80 years and constitutes a major risk factor for stroke. However, the risk can be significantly reduced by anticoagulating patients who have atrial fibrillation so that there is less chance of clot formation, or by trying to restore a normal heart rhythm.
Left ventricular clot. It is quite common for a clot to form on the inside of the heart chamber over the scar of a recent myocardial infarction. In most cases the clot stays put, but there is always a risk of it breaking loose and travelling to the cerebral circulation. This is a particular problem in the first week or two after a myocardial infarction, when the clot is new.
Carotid artery clot. Atheromatous hardening of the carotid arteries has two relevant consequences for stroke formation. It may lead to critical narrowing (carotid artery stenosis) with a reduction in the blood supply to the brain. More importantly, blood clots form on the abnormally roughened arterial wall and can break off to lodge within the cerebral arterial tree. Small emboli produce minor stroke-like symptoms (TIAs), which are warning signs of a subsequent major stroke.
Cerebral thrombosis
Hardening of the cerebral arteries (cerebral arteriosclerosis) leads to narrowing of the vessels and can progress to atheromatous plaque formation and localised thrombosis with blockage. As with atheroma elsewhere in the body, there are several causes: these include smoking, high cholesterol and a positive family history.
The two most important risk factors are:
• hypertension
• diabetes
Of the two, hypertension is the more significant: a diastolic blood pressure of 110mmHg or more increases the stroke risk 15-fold. This compares with an increase of two to three times normal in diabetes.
Clinical picture of a cerebral infarction
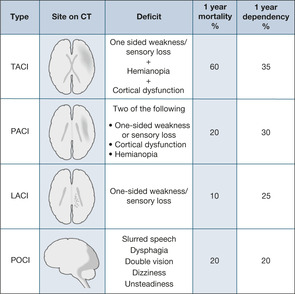
The clinical features depend on the site and extent of the infarct, and range from immediate coma and death to minor short-lived clumsiness of a limb. It is helpful to use clinical features to divide cerebral infarctions into two categories: those affecting the circulation to the front of the brain (anterior circulation infarcts) and those affecting the back (posterior circulation infarcts) (→Fig. 4.2).
 |
| Fig. 4.2 |
Anterior circulation infarcts
Anterior circulation infarcts result in the picture of the ‘classic’ stroke with a variable combination of one-sided weakness (hemiparesis), one-sided sensory loss (hemianaesthesia), visual disturbance in the form of a gaze paralysis (the eyes are deviated away from the weak side) and partial loss of the visual field (the patient cannot see towards the weak side). In addition, there is a variable disturbance of higher functions (termed ‘cortical function’): language disturbance (if the dominant side of the brain is damaged), neglect of the affected side and incontinence.
‘Total’ anterior circulation infarct. TACI is a combination of motor or sensory deficit, visual field loss and cortical dysfunction (e.g. language disturbance).
‘Partial’ anterior circulation infarct. PACI consists of any two of:
• unilateral weakness/sensory loss
• visual field defect (hemianopia)
• cortical dysfunction (language disorder or neglect)
Lacunar infarct. LACI consists of very small infarcts within the anterior circulation that pick off single pathways at critical points in the brain. They are particularly associated with a history of hypertension. The clinical picture is of a ‘pure’ one-sided motor or sensory loss.
Posterior circulation infarcts
POCI, also termed a ‘brain stem CVA’, result in symptoms that often affect both sides of the body (the brain stem transmits information to and from both sides of the brain) and characteristically affect coordination in four vital functions: talking, swallowing, looking and balancing. These patients have combinations of dysarthria (slurred speech), dysphagia (difficulty swallowing), double vision and dizziness.
Site of the cerebral infarction and the outlook
Patients who suffer a TACI have a poor outcome: a high initial mortality and high levels of long-term dependence. Patients who have suffered a PACI have a much better outlook, with a low mortality but a high recurrence rate. A LACI is associated with a good outcome, low mortality and a low recurrence rate.
Posterior circulation strokes have the best overall outlook, although the swallowing difficulties need to be managed carefully to prevent the development of aspiration pneumonia. There is also a high recurrence rate.
Transient ischaemic attacks
Transient ischaemic attacks are minor strokes caused by the passage of small emboli that negotiate the cerebral circulation without producing any permanent damage. The clinical picture depends on the route the emboli take and varies from a paralysed or clumsy limb (→Case Study 4.5) to an isolated speech or visual disturbance. By definition the symptoms and signs of TIA last no longer than 24h, but in practice the clinical picture is of an immediate onset with resolution within 5–20min. It is unusual for any deficit to last longer than 1h. Clearly, such short-lived symptoms can be confused with several other conditions, including epilepsy, migraine, hypoglycaemia, syncope and acute vertigo (giddiness).
Case Study 4.5
A man was admitted with episodic weakness of the right arm and leg lasting 5min and associated with difficulties finding the right words. He returned to normal between attacks. During the previous week there had been four or five episodes. He had treated hypertension and a heart murmur. He was otherwise well. Subsequent urgent investigations showed a near total blockage of the right common carotid artery. His hypertension was controlled, and he was started on aspirin and referred for consideration of surgery to the blocked carotid.
The areas of narrowing and roughening within the carotid arteries from which the emboli usually arise can, in time, give rise to a major stroke. Someone who suffers a TIA is at more than 10 times the normal risk of having a major stroke and the risk is highest immediately after the event. As an approximate guide, the chances of a major stroke after a TIA or minor stroke are 8–12% at 7 days and 11–15% at 1 month. Treatment can significantly reduce these chances, but the situation is urgent and systems should be in place for those with TIAs and minor strokes to have specialist assessment within 24–48h.
The ABCD2 scale (→Table 4.2) is used in patients admitted with a TIA, but who have recovered, to identify those at high risk of an early stroke:
| Score | ||
|---|---|---|
| A | age = or > 60 | 1 |
| B | BP > 140/90 | 1 |
| C | Clinical: unilateral weakness | 1 |
| speech only | 1 | |
| other | 0 | |
| D | Duration: > 60 minutes | 2 |
| 10–60 minutes | 1 | |
| < 10 minutes | 0 | |
| D | Diabetes | 1 |
| Total = |
It should also be noted that patients who have had two or more TIAs within a period of seven days carry a 30% risk of stroke within the subsequent week.
Patients who score 4 or more are at high risk: they require immediate aspirin 300mg and expert assessment within 24 hours. If subsequent investigations show significant blockage (> 70%) of the carotid artery, patients need surgical endarterectomy within a week of the TIA to lessen the risk of an early and disabling stroke.
Furthermore, surgery only benefits patients with anterior circulation-type symptoms, in whom the carotid narrowing is very severe.
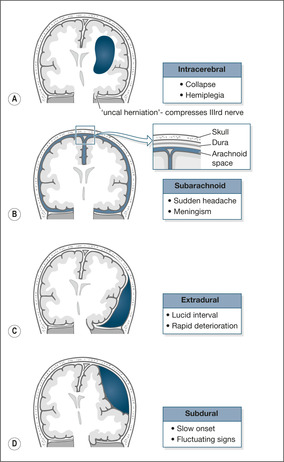
Intracerebral haemorrhage (approximately 10% of all strokes)
Intracerebral haemorrhage occurs when the wall of an abnormally weak cerebral artery suddenly bursts and releases blood into the brain tissue with the formation of a clot (intracerebral haematoma) (→Fig. 4.3). The weakened areas consist of small outpouchings of the vessels, termed microaneurysms, which are the result of damage from chronic hypertension. As with cerebral thrombosis, controlling hypertension has a major role in primary prevention by reducing arterial damage.
Clinical features
A severe intracerebral haemorrhage presents as a dramatic collapse with headache and vomiting, leading rapidly to coma and then death. This is by no means the rule, however, and in many cases there are no clinical features that reliably differentiate a stroke caused by haemorrhage from that due to a cerebral infarct.
The distinction is critical because aspirin and, increasingly, thrombolytic drugs are being used to treat cerebral infarction and they would be absolutely contraindicated in cerebral haemorrhage. For this reason, early CT scanning has become an important part of acute stroke management, as it will show a haemorrhage within minutes of it happening. The longer the CT scan is delayed, the more difficult it is to distinguish a haemorrhage from an infarct. Ideally, patients with acute stroke should have access to a CT scan within hours of admission.
Subarachnoid haemorrhage (approximately 5% of all strokes)
The arachnoid is a double-layered membrane that coats the surfaces of the brain and forms the subarachnoid space through which the cerebral arteries pass. Due to a genetic predisposition, some patients develop berry-like aneurysms on these vessels. The aneurysms are prone to rupture, particularly during periods of physical exertion. If an aneurysm bursts, there is acute haemorrhage that spreads throughout the subarachnoid space, typically causing intense meningeal irritation with severe sudden headache and vomiting (→Fig. 4.3).
The major complications are:
• increased ICP
• spasm of the cerebral arteries, leading to further damage
• re-bleeding
Subdural haemorrhage
This is a low-pressure venous ‘ooze’ that slowly enlarges within the confines of the subdural space. The slowly forming haematoma presses on the brain and causes a progressive or fluctuating neurological deficit. Subdurals are usually caused by minor and frequently forgotten trauma, particularly in patients with other risk factors, most notably anticoagulant therapy or frequent falling (→Case Studies 4.6). The classic presentation is with fluctuating drowsiness and rather vague one-sided weakness. The diagnosis is confirmed by CT scan.
Case Studies 4.6
CASE 1
An 84-year-old woman was admitted from a nursing home where she was normally mobile, conversant and continent. Four days previously, she had fallen out of bed and banged her head. She was discharged from AED with a GCS of 14.The following day she became drowsy and incoherent. Her GP diagnosed a urinary infection. She deteriorated and was admitted. Her GCS was 8.There was bruising around the left eye. Her CT scan showed a large subdural haematoma. She was referred to neurosurgery to aspirate the haematoma.
CASE 2
A 75-year-old man was knocked down by a slowly moving bus and sustained a glancing blow to the side of his head, with transient loss of consciousness. On admission to the surgical ward his GCS was 13. He remained ‘mildly’ confused, but was discharged after 3 days.The relatives rang the surgical ward to say his mobility and confusion were worsening, but no action was taken. A week after discharge he was re-admitted to the medical ward. He was difficult to rouse: he had a GCS of 8 and was still confused. A CT scan showed a large subdural haemorrhage, with shift of the brain to the opposite side. Despite neurosurgical intervention, he made very poor progress.
Extradural haemorrhage
This is a high-pressure arterial bleed virtually always associated with a fractured skull (→Fig. 4.3). The rapid development of a large extradural clot compresses and shifts the cerebral hemisphere, producing coma and pupillary changes. Characteristically, but not always, there is a lucid interval after the head injury before the patient suddenly starts to deteriorate. It is the fear of a delayed extradural haemorrhage that keeps patients in hospital after head injuries.
In theory, extradural haemorrhages should not be seen on the medical wards, but an occasional patient may be admitted with an unrecognised head injury who suddenly deteriorates as a result of an extradural bleed. These patients survive only if the condition is recognised and they are sent for urgent neurosurgery.
The patients themselves may simply appear confused and aggressive after a head injury of which they have no recollection. The key, as with many of these neurological disorders, is to remember the possibility of the diagnosis, particularly where there is a dramatic change in the level of consciousness. It is, of course, critical to take a full history from the patient and witnesses with regards to possible head injury. It is also helpful to remember that vomiting after a head injury is a warning symptom of a skull fracture and these are the patients who are at risk of an extradural haemorrhage.
Nursing the Patient with a Stroke: The First 24h
The nature of the emergency initial assessment of a patient with a stroke is determined by his condition. When there is impairment of the consciousness level, the immediate assessment and management is that of any patient in coma, with the focus naturally being on securing the airway and performing resuscitation (ABCDE). When there appears to be a less severe disability such as a weak arm or speech disturbance in an alert and stable patient, the assessment may concentrate on the functional capacity of the patient, in particular vision, communication, balance and swallowing.
Critical nursing observations and immediate management
The immediate management of the stroke is that of coma and has already been described. The primary aim is to maintain oxygen delivery to the brain. The initial examination should concentrate on the vital signs, the consciousness level (GCS) and the swallowing:
• assess security of the airway (recovery position ensures the safety of the airway)
• maintain an oxygen saturation > 95%
• pulse (atrial fibrillation?)
• temperature (aspiration pneumonia? hypothermia?)
• blood pressure
Avoiding aspiration pneumonia and assessing the swallow
About one in three stroke patients will have at least temporary swallowing problems after a stroke and will be at risk from aspiration; if they are also kept ‘nil by mouth’, there is the added problem of avoiding dehydration and even malnutrition. The fact that the consciousness level is normal does not reliably predict that the swallow is safe (→Case Study 4.7). There must be a formal swallowing assessment in every patient with acute stroke before oral fluids are allowed. The initial assessment will identify some of the features that are seen in patients who are at risk from aspiration:
• low GCS (less than 8)
• the voice sounds wet and rough and the gag reflex is reduced
• the cough is weak
• the larynx (Adam’s apple) does not move upwards on swallowing
• the patient is elderly and frail
• previous stroke
• patients who are unable to sit upright and who have loss of balance
Case Study 4.7
A 74-year-old man was admitted to hospital because he awoke and found that he could not swallow. He tried to get to the phone, but was unable to stand because of unsteadiness. On admission he had slurring of his speech and was unable to sit unsupported without toppling over. His arm movements were very uncoordinated; when he tried to touch his nose he was in danger of damaging his eyes. His blood pressure was 180/120mmHg and he had loud bruits over both carotid arteries, suggesting arterial disease. He insisted he was well enough to go home but could not stand. On testing his swallow there was immediate choking and coughing. Investigations confirmed a POCI.
A simple water test must be carried out to identify disordered swallowing:
• support the patient in the sitting position
• give 5ml of water and look for choking, coughing, dribbling, wet voice
• if swallowing is satisfactory, repeat the test with 50ml of water
• if the test fails, keep the patient nil by mouth, start i.v. fluids and refer for advice
If the swallow is unsafe, maintain the fluid intake with i.v. fluids. Refer the patient to the specialist team (either the dietician or the speech therapist) and consider re-testing in 24h. As there is a risk of inhalation of oropharyngeal contaminants, particular care should be taken to ensure good oral hygiene. Adequate hydration and nutrition are critical to ensuring the patient’s recovery: if the swallow is not secure the decision on naso-gastric tube feeding should not be delayed beyond 24 hours after admission.
History
The basis for making the correct diagnosis is the history from the patient and their relatives. It is important to evaluate pre-existing dependency using measures such as the Barthel ADL index (→Box 4.5) and to find out the home situation e.g. carers, independent housing, warden control, residential care or nursing home.
Box 4.5
| Bowels | Mobility |
| Bladder | Transfer |
| Grooming | Dressing |
| Toilet use | Stairs |
| Feeding | Bathing |
If there has been a previous stroke, it will have to be established how much of the present problem is due to residual disability and how much is new. Risk factors for stroke can be identified from the history: hypertension, atrial fibrillation, diabetes and heart disease.
Managing urinary incontinence
Urinary incontinence suggests disordered higher (cortical) function and is common in anterior circulation strokes. Catheterisation causes infection and leads to a reduction in the bladder capacity; it should therefore be avoided, as it reduces the chance of the spontaneous recovery in bladder function that usually occurs after a stroke. Urge incontinence is a common early problem and is not helped by problems with communication and mobility. The focus for the nursing staff should be on the use of simple communication aids and in trying to anticipate or respond as rapidly as possible to the patient’s toileting needs. The return of independent mobility is often associated with improved continence.
Communication
During the initial assessment, it will be apparent if there is a communication problem other than that due to an impaired consciousness level. The three basic aspects to communication must be evaluated and documented at an early stage: this must be interpreted in the light of the relatives’ description of the patient’s pre-existing cognitive function.
Vision. The more extensive strokes are often complicated by visual disturbance. The pattern of visual loss that is usually seen is termed a hemianopia. This is a loss of the left or right visual field and involves both eyes. As a rule the visual loss is towards the paralysed side so, for example, a patient with a left hemiplegia would not be able to see towards the left. A person standing and talking from the patient’s left-hand side will therefore not be seen and will often be ignored by the patient. Similarly, food and fluid placed to the left of the patient will not be seen. A hemianopia has to be specifically looked for, because many stroke patients will be unaware of its presence. It is also important to note if the patient is unable to move his eyes to one or other side (gaze palsy).
Speech and language. Slurring of the speech may be a problem, particularly after a POCI, but it is usually not a major barrier to communication. Much more importantly, stroke may be complicated by a problem with language: finding, using and understanding words, known as dysphasia (dysphasia is difficulty with speech; aphasia is absence of speech). There are two types of dysphasia:
1. Motor dysphasia. There is a block in getting the words out, despite the patient understanding everything and knowing what he wants to say. This is most frustrating for the patient. Motor dysphasia is often accompanied by a hemiplegia.
2. Sensory dysphasia. The patient has difficulty finding the right words and in understanding what is being said. The patient’s speech is jumbled and senseless, although the patient may not appear distressed by it. Sensory dysphasia is often accompanied by a hemianopia.
Interventions in the first 24h
Diagnosis
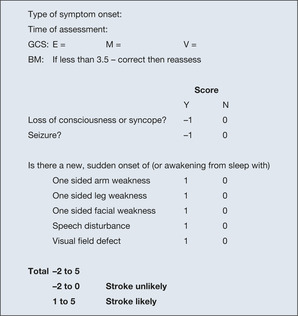
The FAST test (Facial droop – Arm weakness – Slurred speech Test) has been promoted to speed up the referral of stroke victims to hospital. Initial hospital assessment is dependent on the Rosier Scale (→Fig. 4.4) to differentiate stroke from ‘look-alikes’ including hypoglycaemia, syncope and epilepsy.
 |
| Fig. 4.4 |
Imaging
Emergency stroke care is dependent on a head CT scan to show whether the patient has had a cerebral infarction, a cerebral haemorrhage or a stroke ‘look-alike’ – subarachnoid, subdural or extradural haemorrhage. There are therefore specific situations when the scan must be done immediately (within an hour of admission) where:
• thrombolysis is a potential therapy
• the patient is on anti-coagulants, and they may need reversing
• the GCS is less than 13 or the picture is progressing or fluctuating
• the onset was with a sudden severe headache (subarachnoid?)
• there is fever, neck stiffness or raised intracerebral pressure (papilloedema)
The remaining stroke patients should all be scanned within 24 hours.
Acute drug intervention
Acute stroke services are now organised so that immediate thrombolysis with intravenous alteplase can be given to those cases with a non-haemorrhagic stroke who would benefit in terms of reduced disability and better survival. The critical issue is that an improved outlook has only been shown if alteplase is given within three hours of the onset of symptoms to patients with a proven ischaemic stroke. Outside this timeframe and until further data are available, the risk of causing intracerebral bleeding outweighs the benefit of reducing ischaemic brain damage. Stroke patients not suitable for alteplase also include: those over 80 years of age, those with a minor or improving neurological deficit, those with a very serious stroke, those on warfarin, those with diabetes and a previous stroke and anyone with a stroke within the previous three months. Initiatives around early recognition, referral and accelerated assessment are aimed at achieving thrombolysis rates of at least 10% of stroke patients. For patients with ischaemic strokes unsuitable for thrombolysis the only immediate active intervention that is universally acknowledged to reduce the recurrence rate and decrease mortality after a stroke is long-term aspirin treatment for proven cerebral infarcts. Long-term aspirin reduces the risk of further major vascular events in stroke patients by about 25%. The correct dose of aspirin remains controversial, but 300mg per day is usually given. Anticoagulation with warfarin reduces the recurrence of stroke in patients with atrial fibrillation. However, because of the risk of bleeding into the softened and damaged area affected by the stroke, most clinicians prefer to wait 14 days at least before starting warfarin therapy after a thrombotic stroke and, as with aspirin, only after cerebral haemorrhage has been excluded with a CT.
Blood pressure
The blood pressure often increases acutely after a stroke, only to settle without treatment over a matter of days. Reducing blood pressure with intervention may reduce the blood supply to an area of the brain already threatened by ischaemia. Unless extreme hypertension (e.g. diastolic blood pressure of 140mmHg) is encountered, it is safest to monitor the level and allow the blood pressure to settle. It is safe to discontinue usual antihypertensives for a week or so after an acute stroke, provided that the blood pressure is monitored.
Temperature
Pyrexia during the first 72h of hospital admission is associated with early worsening of the neurological status. Apart from excluding infection, paracetamol can be used to bring the temperature down to normal (less than 37.2).
Cerebral oedema
Cerebral oedema may develop after a severe stroke and produce a deterioration in the consciousness level within the first day or so. Provided that alternative causes of deterioration such as aspiration pneumonia or urinary infection have been excluded and the CT scan does not indicate a surgically treatable lesion, the management is supportive, as there is little evidence that measures to reduce ICP such as mannitol and steroids are helpful in this situation after a stroke.
Reversing warfarin
Patients on warfarin, particularly those where the INR is too high, are at risk of an intracerebral (or subdural) haemorrhage. Should this occur the warfarin may need to be reversed urgently. Prothrombin Complex Concentrate (PCC) 20IU/kg combined with intravenous vitamin K 5mg will normalise the INR within ten minutes and (because of the vitamin K) the INR will remain normal for at least 24 hours.
Blood sugar
Maintaining a blood sugar between 4 and 11mmol/L during the acute phase is associated with an improved outcome even in the non-diabetic. The approach is similar to that following a myocardial infarct (a DIGAMI regime).
Avoiding pressure sores
Many elderly patients who suffer a stroke do so alone and they may have been lying in one position for a number of hours, putting pressure on the skin and setting the scene for skin breakdown. This can be alleviated with appropriate physical measures such as the correct pressure-relieving mattress, combined with attention to nutrition and hydration.
The philosophy in the Acute Medical Unit should be based on initiating preventative measures and on starting to address nutritional issues as soon as the patient is admitted. In the past, hydration, nutrition and their effects on the risk of complications have been neglected. Not uncommonly, patients can be admitted and find their way to a rehabilitation unit over the course of two weeks or more, without having received any proper food. In these cases, patients lose weight and are at a major risk from pressure sores. As a rule, although fluids are appropriate for 24h while the swallowing is assessed, after that nutrition is required. This often means passing a fine-bore nasogastric tube.
Resuscitation status
A judgement must be made and explicitly stated on the resuscitation status of the patient. This is based on a number of important factors that include:
• the wishes of the patient (in practice, these will seldom be known, and in the patients in whom the decision is most relevant – those who are critically ill – it is unlikely to be ascertainable)
• the chances of survival (as an example, patients who are comatose 24h after a stroke have only a 20% chance of survival)
• the pre-existing dependency of the patient (the Barthel score will give an accurate picture of the patient’s level of independence)
• the perceptions of close relatives and carers (due weight is given to their views, although ultimately the decision rests with the clinical staff in the absence of the patient’s own views)
• coexisting diseases, e.g. advanced malignant disease
• the opinion of the relevant medical and nursing staff
Any resuscitation decision must be reviewed at predetermined intervals and on an Acute Medical Unit it would be appropriate to re-visit the question of resuscitation at least 12-hourly during the first 2–3 days.
Avoiding venous thromboembolism
Stroke victims are at risk from venous thromboembolism (VTE), DVT and pulmonary embolus. Of the possible preventative measures, anti-embolus stockings probably do more harm than good but LMWH should be considered if:
• cerebral haemorrhage has been excluded
• the risk of bleeding into the cerebral infarct is thought to be low and the patient is severely immobile/dehydrated/has co-morbidities
Answering Relatives’ Questions on Stroke
Will he recover? The answer to this depends on the severity of the stroke. Patients who are comatose on admission or who persistently ignore or neglect their paralysed side do badly. Stroke patients who start to recover within hours, particularly in terms of sitting balance, will do well. A ‘cover-all’ answer for all cases soon after admission would be to say that two out of every three stroke victims regain more or less full independence after a stroke. The patients who do badly are those with severe motor and sensory impairment, especially combined with hemianopias and urinary incontinence.
Why cannot we simply dissolve the clot as in a heart attack? The evidence that clot busters work in acute stroke is controversial, and in any case, if they are given more than three hours after the onset, they probably do more harm than good. At present they are not part of our usual care unless we see patients within three hours of the onset of symptoms.
Why have you not done MRI? We need to tell whether the stroke is caused by a bleed or a blockage. The CT is the easiest test to do this. The MRI is reserved for particular types of stroke, but it is much less easy to do and the patient can find it quite an unpleasant test.
Why can’t you operate? Surgery can only rarely help a stroke patient – if there has been a large cerebral haemorrhage which has blocked the internal circulation of spinal fluid or if a young patient has a massive cerebral thrombosis and there are signs of increasing pressure inside the brain. Even in these cases there is a risk that surgery can make the situation worse.
What has happened? Part of the brain has been damaged and the function of that area has been affected. This explains why there is paralysis/speech problems/confusion. The cause is a blockage to the blood supply or a sudden leak of blood into the brain tissue from a leaking artery.
Is it a heart attack – is the heart affected? Although sometimes a clot causing the stroke comes from the heart, the heart itself is not affected. Nonetheless, the risk factors for stroke and for heart attack are the same, so any preventative measures we recommend, such as smoking cessation, will have a dual benefit.
When will we know the amount of damage? The main recovery takes place over the first days or weeks after a stroke, but some further more limited recovery is possible over a much longer period.
Why is he not in a specialist unit? All patients who are acutely ill and unstable are admitted to the acute ward. However, we work closely with the stroke/rehabilitation unit and we will transfer the patient there at the first opportunity. Physiotherapy and other specific treatment has, however, started already.
What physiotherapy will he need? The immediate emphasis is on positioning the limbs and body correctly and on assessing balance, rather than on simply seeing if the patient can walk. If you wish, you can talk to the physiotherapists about it.
What about feeding? At present his swallowing is not particularly good, so we are using a drip and restricting any oral intake for a day or so to allow time for it to improve.
What is in the drip? This is dextrose, which provides fluid and some calories. If we cannot feed the patient orally within 24h, we will need to consider a thin feeding tube, possibly as a short-term measure, to give some nutrition.
Was it stress that caused the stroke? No, there is no relationship between the two.
Will it happen again? There is a chance of a further stroke if he has suffered one already, but we will do whatever we can to lessen the risks of recurrence. This will involve aspirin/cessation of smoking/control of blood pressure, etc.
Were there any warning signs we/the GP could have seen? Unless there were mini-strokes, there are few if any warnings of this sort of thing.
Will he always be disabled/handicapped? The degree of disability cannot be accurately predicted at this early stage. (However, if the patient is recovering already or has retained good power in the legs and has a clear understanding of the situation, you can be reasonably encouraging, particularly if he was not previously dependent for his care.)
Is there anything we can do? Once we know the level of ability with feeding and personal needs, you can assist with meals and other care if you wish.
Documentation of:
▪ Consciousness level
▪ Eye movements
▪ Swallowing
▪ Communication
▪ Understanding
▪ Vision (field and gaze)
▪ Sensation
▪ Neglect of the affected side
▪ Classification of the type of stroke
▪ Pre-stroke function
Early:
▪ CT scan and assessment for thrombolysis
▪ Physiotherapist assessment
▪ Speech therapy assessment
Meningococcal Meningitis
There are several strains of meningococcal bacteria, but in the UK groups B and C cause virtually all meningococcal infections (→Table 4.3). This is not the case world-wide: for example in the Middle East and in the tropics, group A has been responsible for major outbreaks of meningitis.
| Strain | Frequency | Max. age group | Vaccination? | Case clusters |
|---|---|---|---|---|
| GROUP B | 60–65% | 0.5–4 years | Ineffective | No |
| GROUP C | 35–40% | 15–19 years | Effective | Yes |
Group B, which causes 60–65% of the cases in the UK, affects babies and toddlers, while group C causes the remaining 35–40% and particularly affects young adults around university age. In recent years, the proportion of cases caused by group C (specifically the C2a serotype) has reduced due to the introduction in the UK of an effective vaccine.
There are important public health implications when a case of meningococcal infection is admitted. It is an infectious disease that can be spread to close contacts, particularly in semi-closed communities such as university halls of residence. Close contacts of isolated cases receive prophylactic antibiotics to kill any meningococcal bacteria colonising the oropharynx. In meningitis outbreaks, whole groups may require prophylaxis and, in the case of group C infections, vaccination.
Clinical Picture
Meningococcal bacteria normally live quite naturally in the oropharynx of about 10% of the healthy population. This figure rises to 25% in those living in close proximity, particularly when they are of school and university age. In meningococcal disease, for some unexplained reason the bacteria become virulent and invade the bloodstream. The result is meningococcal septicaemia and, if the nervous system is also invaded, meningococcal meningitis (→Fig. 4.5).
There are therefore two components to meningococcal infections, either of which may dominate the clinical picture:
• septicaemia alone (mortality 30%) 10% of cases
• meningitis alone (mortality 5%) 50% of cases
• mixed features of meningitis and septicaemia 40% of cases
The key features of meningococcal infection are (→Box 4.6):
• fever
• haemorrhagic rash
• altered level of consciousness
Box 4.6
| Septicaemia | Meningitis |
|---|---|
| • influenza | • photophobia |
| • vomiting | • headache |
| • muscle pain | • neck stiffness |
| • drowsiness | • coma |
However, the early symptoms can be non-specific ones of fever, lethargy, abdominal pain, vomiting, joint pains and diarrhoea. The rash is caused by meningeal infection within the skin and is the major feature of septicaemia, but it can be difficult to recognise at first. It may start off as a red discolouration that blanches on pressure and only later develops into the non-blanching haemorrhagic spots that are so characteristic of meningococcal infection. Patients often have an extensive rash and an impaired consciousness level during the septicaemic phase, with headache, photophobia and neck stiffness developing with the onset of meningitis. Patients with meningitis alone may not have a rash at all.
Clinical deterioration can be extremely rapid – of patients who die, almost a fifth die at home or on the way to hospital and, overall, 50% of the deaths occur in the first 48h. Antibiotics must be given at the first suspicion of meningococcal infection – 2g of i.v. cefotaxime or 2g of i.v. ceftriaxone. Over a few hours, the patient with septicaemia can become confused, tachycardic, peripherally shut down and acutely hypotensive. The respiratory rate increases, the consciousness level falls and the patient develops profound shock.
The patient with meningitis can develop acute elevation of the Intracranial Pressure (ICP), with deepening coma, fitting, hemiparesis, pupillary signs and the combination of a bradycardia and hypertension. The causes of death in meningitis are:
• septicaemic shock
• increased ICP
Nursing the Patient with Suspected Meningococcal Meningitis
If a patient is admitted to hospital with suspected meningococcal meningitis, there are a number of immediate critical nursing tasks that must be carried out. There are also a number of important nursing tasks.
▪ Assessment of ABCDE
▪ Give oxygen
▪ Vital signs
▪ Secure i.v. access
▪ Administer immediate i.v. antibiotics (ceftriaxone or cefotaxime)
▪ Measurement of GCS: record serial data on a flow chart
▪ Note any rash
▪ Discuss isolation for 24 hours with Control of Infection
▪ Detain any relatives for further history and advice on contacts
▪ Ensure there are no drug allergies
▪ Ensure immediate specimens are taken for bacteriology
— blood cultures
— EDTA blood sample for urgent PCR (a special test to confirm infection)
— clotted sample for meningococcal serology
— oral or per nasal posterior pharyngeal wall swab (immediate culture)
▪ Preparations should be made for a lumbar puncture, should it be needed to confirm a diagnosis of meningitis.There are several contraindications to a lumbar puncture, most notably progressive neurological deterioration such as fits or a GCS less than 13. Lumbar puncture is also not advisable if the patient is severely shocked or if increased ICP is suspected.
Nursing care
The prime aims of nursing management are:
• to support the patient while the antibiotics take effect
• to be vigilant in recognising complications
Changes in the observations, particularly in the GCS, the pulse and the blood pressure, are vitally important. If the patient is doing badly, the GCS drops below 13, the pulse increases and the blood pressure falls (shock), or the pulse falls and the blood pressure increases (increased ICP; →Box 4.7). These patients need to be moved to an ITU.
Box 4.7
Clinical features
• persistent vomiting
• falling GCS
• falling pulse
• rising blood pressure
• unilateral dilated pupil
Management
• raise the head 30°
• intravenous mannitol 20% solution 250–500ml
• dexamethasone 4mg i.v. qds
• reduce external stimuli, adequate pain relief
• ventilation to correct oxygen and blow off carbon dioxide
• CT scan and urgent neurosurgical opinion
Shock needs to be managed with inotropic support (dopamine or adrenaline (epinephrine)), invasive monitoring and, probably, ventilation. Increased ICP caused by severe meningitis requires interventions to bring the pressure down, including raising the head of the bed by 30°, reducing external stimuli, i.v. mannitol, corticosteroids and ventilation (→Box 4.7). The role of urgent use of steroids (dexamethasone 0.15mg/kg qds for four days, starting with the first dose of antibiotics) remains controversial, but will be used in severe cases. In common with other patients with septicaemia there is increasing evidence that good control of the blood sugar during the critical stage improves the outlook.
Critical nursing observations in the first 24h
The frequency of observations should be modified according to the patient’s condition.
▪ Half-hourly GCS (fluctuation or deterioration are bad signs)
▪ Half-hourly pupillary responses (increased ICP)
▪ Half-hourly pulse, blood pressure, state of extremities (capillary filling) (shock/increased ICP)
▪ Half-hourly respiratory rate and saturations (shock/pulmonary oedema)
▪ Hourly urine volumes and strict fluid balance (shock/correcting hypotension)
▪ 2-hourly temperature (response to antibiotics)
▪ ECG monitoring (arrhythmias due to septicaemic shock/heart muscle toxicity)
▪ Progression of any rash (worsening septicaemia)
• Rapidly progressing rash
• Poor peripheral circulation
— capillary refill > 4 seconds
— systolic blood pressure < 90mmHg
— falling urine output
• Respiratory rate < 8 or > 30 breaths/min
• Pulse < 40 or > 140 beats/min
• Falling (less than 12) or fluctuating (by more than 2) GCS
• Development of a focal limb weakness
• Fitting
• Falling pulse and climbing blood pressure
Nursing management apart from the critical tasks and observations
• Managing a confused, restless or aggressive patient
• Comforting and reassuring a critically ill young patient
• Antiemetic treatment
• Antipyrexia measures: tepid sponging, fan, adequate fluids
• Explaining the various observations and interventions
• Assessing headache, joint pains, neck stiffness, muscle pain
• Using appropriate handling manoeuvres to minimise patient’s discomfort (e.g. log rolling)
• Minimising external stimuli: light, noise, visitors
• Ensuring a fully informed nursing handover, including a shared GCS assessment
• Ensure the consultant for CCDC knows about the case; he or she will be responsible for dealing with contacts
• Appropriate management plan for anticipated complications (e.g. fitting)
Answering Relatives’ Questions in Meningitis
The relatives of a young patient with meningococcal infection are understandably among the most distraught that will be encountered on the Acute Medical Unit. The lay perception is of young people struck down en masse at the threshold of their adult life by a ‘killer infection’ (there are currently around 260 deaths per year from meningococcal meningitis in the UK). The first task will be to reassure the family that 90% of all cases of meningococcal infection make a complete recovery.
Should we have acted any sooner? The early symptoms of meningitis are notoriously difficult to spot, and can be confused with flu or gastroenteritis, even by experienced doctors.
When will we know whether he will pull through? Complications, by which we mean shock and worsening of meningitis with increased pressure on the brain, usually occur within the first 48h. We are aiming to head off complications by giving potent antibiotics to clear the infection before it spreads.
What are the signs of improvement we should expect? The patient will become less drowsy and the observations will stabilise, with a return to normal of the pulse, breathing rate and blood pressure.
What are the signs that things are not going so well? Drowsiness will increase and the patient may become more restless and confused. We may have to move the patient to ITU if we think ventilation (life support machine) and other forms of intensive support may be needed to get the patient through the next few days while the antibiotics get to work.
Could there be any permanent brain damage? Permanent damage is unusual but not unknown. The most common damage is hearing disturbance, but this is seen more commonly in children. Most adults make a full recovery. We can do further assessments if necessary, with special tests such as a CT scan at a later stage if we suspect complications.
What about his friends? Household contacts and ‘kissing contacts’ will be given preventative antibiotics and vaccination. We will need their details.
Should we all be vaccinated? Vaccinations against meningitis are now effective in preventing meningococcal infections and close domestic contacts (of group C cases) will be offered a vaccine.
What vaccination is available? Vaccination is available for groups A, C and two strains that cause occasional infections in travellers: W135 and Y. The original C vaccination is not suitable for children under 18 months and its protective effect is short lived. The recent introduction of a new group C conjugate vaccine (MenC), which gives long-term protection and is suitable for babies and toddlers, heralds the start of a routine vaccination programme against meningitis in the general population, in contrast to previous vaccinations that were used to protect household contacts and to control outbreaks when there were clusters of group C cases. As yet there is still no established vaccination for group B infections, the most common cause of meningitis in the UK, although in some countries, Cuba for example, a group B vaccination is given routinely to every child. Trials in the UK are under way, however, and it is likely that a group B vaccination will be available here soon.
What about the university/school? If this is a single case, preventative antibiotics are only recommended for close contacts (flat-mates, girlfriends/boyfriends, etc.). If the case proves to be part of a cluster (more than one case from the same group or class within 4 weeks), then preventative treatment may have to be given to a wider population. This is a matter for the public health specialists.
Are the nurses and doctors at risk? Nurses and doctors are only at risk if they have performed prolonged mouth-to-mouth resuscitation or have inhaled oral secretions during, say, attempts at intubation.
Acute Severe Headache
Patients are admitted to the Acute Medical Unit with headache because of two primary concerns:
• Is this meningitis?
• Is this a subarachnoid haemorrhage?
However, the stakes are high, and most of us remember at least one case of ‘missed subarachnoid’ and ‘missed meningitis’ in our clinical practice. As with most neurological problems, the history is all-important.
Bacterial meningitis has already been described in detail. Viral meningitis has a similar, though less severe, picture and the patients are not usually critically ill unless the inflammation has spread to the brain tissue (encephalitis).
Subarachnoid Haemorrhage
Subarachnoid haemorrhage occurs with the speed of a lightning strike: ‘like a brick’, ‘like a sudden blow’, ‘like an explosion inside my skull’. It does not build up over an hour or so – it drops the patient in seconds. The headache is extremely severe and often spreads to the neck. There may be immediate loss of consciousness followed by immediate recovery or prolonged coma. The patient does not wake up with pain, struggles to work and has to come home to lie in the dark: that is migraine.
There are catches and exceptions: occasionally, patients with a subarachnoid haemorrhage have little warning bleeds in the days leading up to the major event, whereas some patients without a subarachnoid suffer so-called ‘thunderclap headaches’ that can be as severe as a subarachnoid but are entirely harmless. As a rule, however, a description of the exact onset will be the main way in which a provisional diagnosis is reached, to be confirmed or refuted by CT scan and lumbar puncture. Of the true explosive headaches, around one in eight patients will have had a subarachnoid haemorrhage. Interestingly, up to a fifth of all cases of meningitis are initially thought to be a subarachnoid haemorrhage.
The CT and lumbar puncture in subarachnoid haemorrhage
It is critical not to miss a subarachnoid haemorrhage. The 30-day mortality rate is 45%, and most of this is due to re-bleeding. In view of the uncertainty of making a firm clinical diagnosis, a CT scan is always required to confirm a subarachnoid bleed. If the scan is done within 12h, around 98% of the bleeds will be found and in 2% the scan will look normal. If the CT is delayed by 72h, only 75% of the bleeds will be found and in 25% the scan will look normal, even though the patient has bled. Thus, if there is a suggestive history but a negative scan, the patient will need a lumbar puncture to look for blood and xanthochromia in the CSF.
Nursing the patient with subarachnoid haemorrhage
Headache and neck stiffness
These symptoms are severe in patients with a subarachnoid and need powerful regular analgesia, conventionally using intramuscular dihydrocodeine and an antiemetic such as cyclizine. Opiates tend to be avoided because of their effects on the pupils, but may be required occasionally. Patients will be severely photophobic and will be most comfortable in a quiet, darkened room with a minimum of visitors.
Neurological observations
After a subarachnoid haemorrhage, the main complications and their warning signs are:
• Bleeding into the brain tissue (intracerebral haematoma)
— falling GCS
— development of stroke-like features (unilateral weakness, etc.)
— seizures
• Spasm of the cerebral arteries
— falling GCS
• Re-bleeding
— further headache
— falling GCS
• Increased ICP
— falling pulse, rising blood pressure
— falling GCS
— pupillary changes
Sequential neurological assessment
GCS and pupillary responses, pulse and blood pressure and general nursing observations will identify the warning signs of complications. Any change in these parameters should be reported so that the overall clinical state can be reassessed with a view to repeat CT scanning and perhaps accelerated transfer to the neurosurgeons.
Answering Relatives’ Questions in Subarachnoid Haemorrhage
Where is the bleeding? The blood has escaped from a ruptured artery on the surface of the brain. It has spread around the brain and into the spinal fluid.
Why is he unconscious? The bleeding can cause the brain to go into ‘shock’, which we are hoping will be temporary. We are supporting the breathing at present, but we are hoping there will be signs of recovery within the next 24–48h.
Why him? The usual cause is an unexplained weakness in the artery wall that suddenly ruptures.
Could the doctor not have seen the warning signs? Occasionally there is a warning bleed, but these are often like migraine attacks. In most patients there is no warning at all.
Was it all stress? There is no relationship to stress.
What about the well-man clinic visits, blood pressure, etc.? There are no warning signs of a subarachnoid haemorrhage and it is not directly related to high blood pressure.
Will there be brain damage? That depends on whether there is bleeding into the brain substance itself and also whether the treatment we are using (nimodipine) is successful in preventing spasm of the leaking artery.
Can you operate? If the situation stabilises, we will transfer the patient to the neurosurgeons, who will assess whether surgery is needed to prevent a further bleed. The surgery is preventative and is unlikely to improve the patient’s present condition.
Will it happen again? Sometimes there is more than one area of weakness or the weakened spot can itself leak again. About 10% of patients with aneurysms re-bleed within hours and, if untreated, up to 30% re-bleed within a few weeks.
Can he drive again? Non-vocational driving can be resumed in due course, providing recovery is complete. There will, however, need to be a certain period before driving can resume. You may need to inform the DVLA, and check with your insurance company if you are in any doubts.
Can’t you just send him to a specialist unit straight away and they can operate? The best time to operate is when the patient is in the best possible medical condition, but before the peak time for re-bleeding. We are working in conjunction with the specialist unit and have taken their advice on the best time for a transfer.
Lumbar Puncture
The lumbar puncture has a justified reputation as an unpleasant and potentially dangerous procedure. The main danger is from inadvertently doing a lumbar puncture when there is acute increase in the ICP, so that CSF is forced out of the breach in the subarachnoid space. The sudden release of increased CSF pressure sucks the brain in a downwards direction and can cause a dramatic deterioration in the neurological state (termed a ‘pressure cone’).
Contraindications to a lumbar puncture
Indications on the Acute Medical Unit
• Suspected meningitis and encephalitis
• Suspected subarachnoid haemorrhage (after a negative CT)
• Suspected Guillain–Barré syndrome
Suspected meningitis and encephalitis. Although the lumbar puncture is important in the diagnosis and management of meningitis, it should not lead to a delay in giving antibiotics. In patients who are drowsy or with clinical signs of increased ICP or a possible cerebral abscess, the lumbar puncture is only performed once a normal CT scan has been obtained.
Suspected subarachnoid haemorrhage. The lumbar puncture is done to find the few cases of subarachnoid bleeding that the CT has missed. In a suspected subarachnoid bleed with a normal scan, fewer than 1 in 20 lumbar punctures will be positive.
Suspected Guillain–Barré. The CSF is examined to look for the characteristic changes seen in the Guillain–Barré syndrome (a high protein and few or no cells).
Procedure
A full explanation of the reason for doing the lumbar puncture and a description of what discomfort to expect will help to allay the anxiety of most patients. The very restless and agitated patient may need cautious sedation with i.v. diazepam (i.v. flumazenil, which will reverse diazepam immediately, must be on hand in case of oversedation).
The patient should be warned of the following:
• the position
• the cold skin-preparation
• the poking around to find the correct entry spot over the back
• the burning of the local anaesthetic
• the pushing sensation in their back
• the possibility of an acute shooting pain in either leg
• the possibility of post-lumbar-puncture headache due to a low-pressure CSF leak
The position
It cannot be overstated that the correct positioning of the patient makes the difference between success and failure in performing a lumbar puncture.
The patient must be lying right on the edge of the bed curled up (comfortably, not forcibly) in the fetal position with the back and both shoulders perpendicular to the mattress. This position is easiest to achieve with a pillow under the head and two pillows between the knees and pushed into the abdomen, across which the patient can rest his upper arm (→Fig. 4.6).
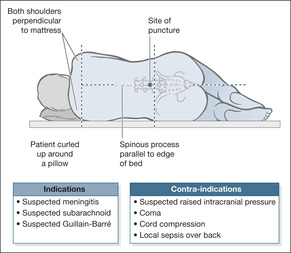 |
| Fig. 4.6 |
The two common mistakes are for the top shoulder to roll over rather than remain vertical, and for the spine to be curved rather than straight.
The equipment
The advice is to use a small (22G) atraumatic (Sprotte) lumbar puncture needle to reduce the risk of post-lumbar-puncture CSF leak and headache. A CSF leak is less likely if the stylet is re-introduced before the needle is removed. The manometer is used to record the CSF pressure (normally 6–20cmH2O; if it is above 30cmH2O there is significantly increased ICP).
In suspected subarachnoid haemorrhage, three sequential bottles are needed (labelled 1, 2, 3) to identify the progressive decrease in blood staining of a traumatic tap. In addition, one specimen is sent for microbiology, one for biochemistry, one for cytology, and a fluoride oxalate (yellow label) tube is used for sugar. Blood is also drawn for determination of sugar level. In cases in which subarachnoid bleeding is not an issue, the three sequential bottles may not be used but should always be on hand.
Normally, approximately five drops (1ml) is sufficient in each bottle. Analysis of the results is summarised in Table 4.4.
| Condition | CSF | Cell count (cells/ml) | Protein (g/l) | CSF sugar/ blood sugar |
|---|---|---|---|---|
| Normal | Clear | Less than 3 cells | 0.2–0.5 | >50% |
| Bacterial meningitis | Cloudy | Neutrophils up to 10 000 | 0.5–2.0 | <50% |
| Viral meningitis | Clearish | Lymphocytes up to 500 | <0.8 | >50% |
| Subarachnoid | Bloody | More than 100 red cells | >0.2 | >50% |
| Guillain–Barré | Clear | Fewer than 3 cells | 0.5–3.5 | >50% |
Aftercare
Patients should stay flat on one pillow for 3–4h after a lumbar puncture. Any post-lumbar-puncture headache should be recorded, analgesia will be needed, and the patient should be reassured but kept flat until the headache improves.
If the CSF pressure is found to be very high, urgent neurosurgical transfer may be required. In these circumstances, frequent neurological observations (pupils, GCS, pulse and blood pressure) should be started as soon as the lumbar puncture has been completed. Measures to lower the pressure may be instituted before transfer. In the meantime, any changes in the neurological status must be recorded and reported.
Xanthochromia: a practical guide to interpreting the CSF result in subarachnoid haemorrhage
• Blood in the CSF from a recent subarachnoid haemorrhage can only be distinguished from a traumatic tap caused by the lumbar puncture needle by the finding of red/yellow pigmentation in the CSF specimen, due to breakdown within the CSF of the red blood cells which releases bilirubin. This process takes 12 hours to occur. Bilirubin appears as a yellow tinge (xanthochromia) after the CSF has been spun down in the laboratory. Xanthochromia can be seen with the naked eye or, in more subtle cases, by spectrophotometry. In traumatic bleeding caused by the lumbar puncture itself, this breakdown does not have time to occur and xanthochromia is absent.
• To allow breakdown to occur, 12h should elapse between the subarachnoid haemorrhage and the lumbar puncture. Before then, especially within 4h of a subarachnoid, it may be difficult to differentiate between a bleed and a traumatic tap. (One consequence of this is that the LP does not usually need to be done during the night). If there is no xanthochromia, three sequential CSF specimens from the lumbar puncture will show a progressive fall off in the amount of blood if the tap was traumatic. However, this is not as reliable as finding xanthochromia.
• The CSF (at least 1ml of it) must be spun immediately, otherwise blood will start to break down in the tube, giving a false positive result; this means that the specimen must be dealt with urgently, day or night. Lumbar punctures are a major intervention and handling of the specimens cannot be left to chance. Careful liaison is needed with the porters and the laboratory staff.
• If the CSF is ‘gin-clear’ but the history was very suspicious, the specimen should be stored in darkness until it can be checked by the laboratory for xanthochromia using a spectrophotometer.
• Xanthochromia will be found in the CSF at lumbar puncture, even if it is performed 2 weeks or more after a subarachnoid haemorrhage.
Sudden Loss of Consciousness: Faints and Fits
Sudden loss of consciousness followed by a recovery is usually due to a faint (syncope) or a fit (tonic-clonic convulsion). These patients are frequently admitted to the Acute Medical Unit with a variety of labels (→Case Study 4.8). Provided that the underlying mechanisms and clinical features of epilepsy and syncope are understood, a correct diagnosis can usually be made without too many investigations, once a good eyewitness account has been obtained.
Case Study 4.8
A 50-year-old woman was admitted with a ‘fit’. She had awoken with severe abdominal colic and while being comforted by her husband turned ashen grey, started to sweat profusely and collapsed on the bed ‘as though she had died’. She stopped breathing for a few seconds and then slowly came round over 2–3min. On close questioning of the husband, there had been no convulsive movements, no confusion after the event, no incontinence and no tongue biting. A similar episode had occurred during a severe attack of calf cramp some years before.The diagnosis was of a vasovagal attack triggered by pain (a simple faint).
The history will differentiate fits and faints in 80% of cases. A witnessed account is vital and may have to be obtained by a phone call from the ward if necessary.
Onset. Fits have an aura: often a feeling of anxiety or fear building up in the pit of the stomach. Faints start gradually and share a number of typical warning features: nausea; dimming of the vision; roaring in the ears; sweating and a feeling of heat; a sense of slipping away.
The attack. Generalised tonic–clonic attacks are accompanied by rapid, grunting respirations, foaming at the mouth and self-injury. In syncope the patient is cold, grey and clammy. There may be isolated convulsive jerkings, but not as the main feature of the attack.
Recovery. Recovery is slow after a convulsion, with drowsiness and confusion lasting at least 30min. This is followed by a headache and generalised limb pains. Syncope recovers in a few minutes with no confusion, but often accompanied by a bout of vomiting. The patient can faint a second time if they sit up or stand up too soon. Self-harm is rare in syncope, in contrast to the tongue biting seen with many convulsions.
The Basic Mechanisms: Syncope
Syncope (fainting) is due to a sudden reduction in the blood supply to the brain. In most cases, provided that the patient is allowed to stay horizontal, there is a full and rapid recovery. Sometimes the blood supply does not return rapidly – typically this is seen if the patient is wedged upright during a faint. As a result, the syncope may progress to an anoxic convulsion (termed convulsive syncope).
The reduction in cerebral blood supply has two possible causes:
• Asudden change in heart rate, e.g.
— heart block
— ventricular tachycardia
• Asudden change in blood pressure, e.g.
— reflex syncope
— acute gastrointestinal haemorrhage
— acute pulmonary embolus
— drug side-effect (postural hypotension)
Cardiac syncope
Syncope due to heart disease is a medical emergency: it has a poor prognosis if it is not recognised – the next attack may be fatal. A history of sudden death in family members under 40 is an important clue to inherited cardiac abnormalities. In cardiac syncope, unlike reflex syncope, there is either no warning at all or it occurs in a setting of recent onset ‘cardiac’ symptoms – chest pain, breathlessness and palpitations. Syncope which occurs during exertion or while lying down strongly suggests a cardiac cause. There may be conduction problems on the 12-lead ECG (abnormal prolongation of the Q-T interval is usually missed as a cause for serious cardiac arrythmias and yet the measurement, which should be less than 450msec, is automatically calculated and printed out on the traces from most modern ECG machines). Further tests of the heart rhythm are vital if a cardiac cause is suspected. These can include bedside monitoring, 24 hour tapes (Holter monitor), external event recorders (1–2 weeks) or implanted loop recorders (months) depending on how often the events are occurring.
Reflex syncope (also termed ‘Neurally mediated syncope’)
This is increasingly recognised as a cause for both sudden faints and misdiagnosed epilepsy. There are two forms of reflex syncope: vasovagal syncope and the carotid sinus syndrome. Vasovagal syncope includes the ‘classic’ faint commonly triggered by the sight of blood, intense pain and emotion and the less common form of vasovagal syncope in which the pulse or blood pressure drops acutely in an upright individual. The carotid sinus syndrome is syncope due to pulse or blood pressure changes in response to stimulation of the carotid sinus (an area of the carotid artery) resulting from sudden head movements or pressure from a tightly fitting collar.
Tilt-table testing, which examines the effect of posture and sublingual GTN on the pulse and blood pressure, has revolutionised the diagnosis of these various forms of syncope and is an important part of the follow-up of unexplained blackouts and ‘drug-resistant’ epilepsy (→Case Study 4.9).
Case Study 4.9
A man of 75 years was admitted to hospital with blackouts. He had suffered a number of fainting attacks in recent months. His ears would start buzzing and he would black out ‘for a few seconds’. In the past he had suffered two mild heart attacks and a small stroke. He had had negative tilt-table studies when the blackouts had been investigated previously.
On admission he had fully recovered but his pulse was 34 beats/min. His ECG showed that the atria and ventricles were beating independently (complete heart block). After settling into bed, he called the nurses suddenly and said he was ‘going’. His ECG monitor showed ventricular standstill.
An urgent temporary pacemaker was inserted before his referral for a permanent device.
Situational syncope
This is syncope triggered by a spasm of coughing (cough syncope) or by passing water, usually in men arising in the night from a warm bed to urinate (micturition syncope). These patients can be re-assured and given simple preventative advice.
The Basic Mechanisms: Epileptic Seizures
Partial seizures
Partial seizures arise from a single electrical focus in the brain and produce a localised focal fit during which the patient is usually conscious (→Case Study 4.10). There is usually a ‘march’ of localised twitching, starting from a single area and spreading up a limb or from the face onto the same side of the body. At times the fit can become generalised into a classic tonic-clonic attack.
Case Study 4.10
A 45-year-old woman was admitted following two transient episodes of numbness and tingling of the right side of her face and right arm.There was slight slurring of her speech. Each episode lasted 15min and cleared completely. She was an insulin-dependent diabetic with good control and had normal blood sugars on admission. A CT scan showed a focal abnormality in the left cerebral hemisphere in the area concerned with movement to the face and arm. At surgery, a large meningioma was found and successfully removed.
The clinical picture can be very similar to, and must be distinguished from, those seen in TIAs and in the initial stage of a migraine attack.
Generalised convulsions (tonic–clonic fitting)
In adults over 25 years, first-time generalised fits usually have an underlying cause – either a structural abnormality such as vascular disease or a brain tumour, or a metabolic cause such as alcoholism or hypoglycaemia (→Case Studies 4.11).
Case Studies 4.11
CASE 1: Epilepsy due to old cerebrovascular disease
A man aged 55 with a known previous stroke was admitted to hospital having collapsed at home.The assumption was that this was another stroke. On admission, his GCS was 6. He had bitten his tongue and both plantars were up-going (suggesting some disturbance to both sides of the brain). He made a full recovery after a period of confusion over the next 24h, but had a witnessed grand mal fit 3 days after admission.The diagnosis was epilepsy triggered off by the scar of an old stroke. After a stroke, 10% of patients will have a seizure within the first five years.
CASE 3:The importance of a drug history
A 60-year-old woman with a residual hemiplegia from a past cerebral haemorrhage was admitted with confusion. Several hours after admission she had a generalised convulsion followed by a series of rapidly recurring convulsions, with no period of recovery between them. She was intubated and ventilated, but continued to fit on weaning despite diazepam, chlormethiazole and phenytoin. Five days after admission, her GP informed us that she had recently discontinued long-term phenobarbitone. A diagnosis of barbiturate withdrawal-induced fits was made and she was commenced on phenobarbitone.
CASE 4: Unexplained convulsion
A 45-year-old man collapsed at the wheel of his car while driving to the local shops, and collided with a roundabout. He had no memory of the event and was still confused when he was seen in AED.When he was admitted, it was noted he had a fine petechial rash over his upper torso and had bitten his tongue.These changes were consistent with the effects of a major convulsion.There was a past history of ischaemic heart disease and mild angina.
A diagnosis of epilepsy was made. He was also investigated to exclude a cardiac cause, in particular an abnormal heart rhythm, for the collapse.
CASE 5: Epilepsy due to alcohol?
A 68-year-old woman was admitted with a 4-day history of rigors and confusion.There had been a single convulsion immediately before admission. On examination, she had a GCS of 14 and a fever.The suspicion was of heavy alcohol consumption, with fitting precipitated by abrupt alcohol withdrawal. Twenty-four hours after admission, she remained pyrexial and mildly confused. She then had a series of tonic-clonic convulsions.The patient was sedated and investigated by urgent CT scan and LP. The scan was normal and the CSF showed a protein of 0.8g/L. Urgent tests on the CSF showed a positive result for the herpes simplex virus, confirming a viral encephalitis. She was treated successfully with acyclovir.
Nursing the Patient Who Has Sudden Loss of Consciousness
Nursing tasks
Patients who are fitting (→Fig. 4.7)
• Primary assessment: ABCDE
— with the emphasis on the airway and good venous access (wide bore)
— do not insert anything in the mouth
• Ensure the patient cannot injure himself; stay with him, summon help
• Give high concentration oxygen with a non-rebreathing mask at 15L/min
• Place the patient in the recovery position, cushion the head, do not restrain
• Carry out blood sugar measurement, bloods for drug levels, biochemistry, clotting, etc.
• Initiate anticonvulsant drugs: i.v. diazepam 10mg
• If there is a history of alcohol abuse, consider i.v. thiamine as Pabrinex®
• Take a full history, especially concerning drugs and alcohol, from a relative
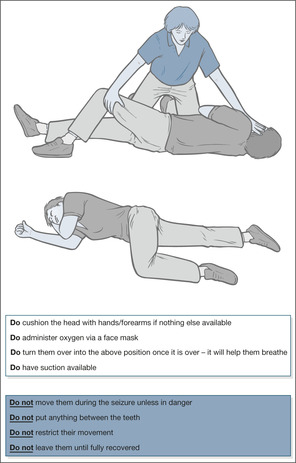 |
| Fig. 4.7 |
Patients with status epilepticus
Status epilepticus is defined as fits that continue for 30min or more without intervening periods of recovery and is a medical emergency. Without treatment there is a risk of brain damage, hypoxia, shock, other major complications and death. Two groups of patients with pre-existing epilepsy are at particular risk of status epilepticus: those with severe learning disability (who often have structural brain damage and are on complex therapeutic regimens) and those undergoing anticonvulsant withdrawal.
The management is the same as for a single fit – resuscitation and preventing harm to the patient. However, because more intensive drug therapy is required, there is a risk of drug-induced respiratory depression. Close monitoring is therefore needed, particularly of the respiratory rate. If the patient’s convulsions cannot be stopped within an hour, the patient needs ITU care with a view to ventilation. If alcohol withdrawal or malnutrition is suspected give high potency Pabrinex® i.v. with or without 50ml of 50% glucose.
Drugs commonly used in status epilepticus
Lorazepam
• This is now the drug of choice for status epilepticus
• 4mg i.v. to be repeated after 10min
• If there is no response, load with phenobarbitone or phenytoin with diazepam
Diazepam
• 10mg i.v. bolus, repeat 5–15min later
• Immediate effect
• Repeated dosing can result in unpredictable response (respiratory depression)
• Can be given rectally (10 to 20mg) if no i.v. access (not active intramuscularly)
Phenytoin
• Slow onset, so give with a bolus of midazolam
• 1000mg intravenously (18mg/kg) over 20min is a typical dose for an adult
• Must be given with ECG monitoring
Chlormethiazole (Heminevrin®)
• Rapid onset, therefore the infusion rate can be tailored to the patient’s needs
• 0.8% solution: 5–15ml/min for 100ml as the loading dose
• 0.5–4ml/min as maintenance infusion for 12h
Phenobarbitone
• Effective, but slow onset, can be combined with phenytoin
• 700mg (10mg/kg) over 7min i.v. is a typical dose for an adult
Paraldehyde
• Safe but smelly
• Asingle dose can be effective for hours
• Can be administered intramuscularly but only by deep intramuscular injection
• 10–20ml of paraldehyde diluted with an equal volume of 0.9% saline
• Glass syringes unnecessary for injections drawn up and given immediately
Common reasons why management is unsuccessful
Sudden Unexplained Death in Epilepsy
Sudden unexplained death in epilepsy (SUDEP) is of increasing concern, because it reflects an overall picture of suboptimal epilepsy care and may therefore be avoidable. SUDEP is particularly associated with long-standing, early-onset epilepsy in patients with multiple disabilities who are not under epilepsy specialists and whose drug regimen is disorganised, outdated, or both.
Pseudoseizures (Non-Epileptic Seizures)
Pseudoseizures are similar to, and can be confused with, true tonic-clonic fits. The attacks represent conscious or unconscious manipulative behaviour, simple malingering or are a manifestation of an underlying psychiatric disorder. Pseudoseizures are commonly misdiagnosed, particularly by inexperienced staff in the emergency situation. The diagnosis can be particularly difficult in patients who have a combination of true and pseudo-fits. However, there are several characteristics that help to identify pseudoseizures:
• pseudoseizures can be of gradual onset with a fluctuating course
• the movements are semi-purposeful and include kicking out
• the head thrashes from side to side; there are often pelvic thrusts
• the posture is often a hyperextended back with the patient lying on his side
• examination is resisted and the eyes remain tightly screwed up
• incontinence and tongue biting can occur in pseudoseizures
• some patients with pseudoseizures just collapse limply to the ground
• cyanosis occurs only in true tonic-clonic convulsions
• true convulsions last between 50 and 90s
• pseudoseizures last between a few seconds and many minutes
• patients with pseudoseizures can recall the period of unconsciousness
Management
Once pseudoseizures are suspected, anticonvulsant medication should be cautiously tailed down and the patient referred to a neurologist with a special interest in epilepsy. It is not helpful to confront the patient at this stage. A simple explanation that further tests are needed is sufficient, particularly as the diagnosis may not be straightforward.
Acute Paralysis of the Lower Limbs
Spinal cord compression
Acute diseases of the spinal cord are compressive (e.g. spinal secondaries or spinal abscess) and need urgent surgery; inflammatory (e.g. multiple sclerosis; →Case Study 4.12) and may need specific drug therapy; or vascular (spinal cord stroke; →Case Study 4.13). Urgent MRI will differentiate between the three groups.
Case Study 4.12
A 30-year-old woman awoke with non-specific lumbar pain that progressed over a few hours to involve numbness of both legs and difficulty passing urine. Within 48h of the onset, she had painless retention and weakness of both legs.
On examination, the legs were weak and she could not stand. Her bladder was palpable and there was loss of sensation from her upper abdomen down to her feet. An emergency MRI was normal, so the provisional diagnosis was not cord compression but an inflammatory lesion, probably multiple sclerosis.
Case Study 4.13
A 65-year-old obese Type II diabetic was admitted with acute ‘left-sided stroke’. On the consultant’s round, it became clear that the weakness involved the extensor muscles of both arms as well as the left leg. She could not stand, and the pattern of the change in her reflexes suggested a high cord lesion. An urgent MRI scan showed a vascular lesion (acute spinal cord ischaemia) in the mid-cervical cord. She remained immobile, and was referred for rehabilitation.
The key for the Acute Medical Unit is to recognise that a patient who comes in ‘off his legs’ could well have a progressive neurological problem in both lower limbs. The nursing staff are well-placed to assess lower limb movement, power and sensation when they first expose the patient during the primary assessment. Patients who have acutely weak legs, bladder dysfunction and escalating back pain (characteristically worse on lying flat) need to be taken particularly seriously, for they are the ones with probable cord compression. The site of the problem can often be identified from the site of the back pain and any points of maximum tenderness over the spine. It is important to look at bladder function in such cases, as painless urinary retention may be one of the early warning signs and always needs urgent investigation.
Guillain–Barré syndrome
Guillain–Barré syndrome is an ascending paralysis that starts in the feet and moves progressively up the body, to an extent that the respiratory muscles are often involved. The disease can progress rapidly over a matter of hours and put the patient’s breathing at risk.
Respiratory monitoring is particularly important in Guillain–Barré syndrome. Impending critical respiratory muscle involvement is suggested by breathlessness and an inability to cough and perhaps swallow effectively. The best way to monitor for deterioration is to take serial FVC measurements with a spirometer (the peak flow rate is not a useful test in this particular situation). As a general rule, once the FVC decreases to 1L the patient needs ventilation, although a downward trend on hourly readings should prompt a transfer to an HDU or ITU.
Recognising Acute Illness in Patients with Chronic Neurological Disability
Acute medical illnesses in the presence of chronic neurological disability can be difficult to diagnose. Cognitive impairment and aphasia can impair history taking – it is therefore vital to interview family and professional carers. Frequently acute illness presents, not with classic symptoms, but with a sudden worsening of the underlying neurological condition. This takes the form of a sudden reduction in functional ability, increased spasticity and muscle spasms, sudden cognitive changes and acute agitation/confusion. The source of the sudden change ranges from a UTI to infected bed-sores or even to the sudden withdrawal of a medication. The classic case of the neurological condition masking an acute illness occurs in chronic paraplegia in which an intra-abdominal catastrophe (e.g. perforated ulcer) presents with sympathetic over-activity: headache, sweating, high blood pressure and vomiting. The abdomen, however remains soft and non-tender.
Further Reading
Coma
Addison, C.; Crawford, B., Not bad, just misunderstood – Glasgow Coma Scale, Nursing Times 95 (43) (1999) 52–53.
Shah, S., Neurological assessment, Nursing Standard 13 (22) (1999) 49–56.
Stroke
Bath, P.M.W.; Lees, K.R., Acute stroke, British Medical Journal 320 (2000) 920–923.
Gubitz, G.; Sandercock, P., Acute ischaemic stroke, British Medical Journal 320 (2000) 692–696.
National clinical guidelines for stroke, (March 2000) Royal College of Physicians, London .
Meningitis
Cohen, J., Management of bacterial meningitis in adults, British Medical Journal 326 (2003) 996–997.
Epilepsy
Heafield, M.T.E., Managing status epilepticus, British Medical Journal 320 (2000) 953–954.
Parry, S.W.; Tan, M.P., An approach to the evaluation and management of syncope in adults, British Medical Journal 340 (2010) c 880.
Chronic Neurological Disability
Bakheit, A.M.O., Recognition of acute illness in people with chronic neurological disability, Postgraduate Medical Journal 82 (2006) 267–269.
Websites
Guidelines for acute stroke management: http://www.rcplondon.ac.uk/pubs/books/stroke/stroke_guidelines_2ed.pdf.
Guidelines for managing meningitis in adults: http://www.britishinfectionsociety.org/meningitis.html.
NICE guidelines, Stroke. (2008) ; http://guidance.nice.org.uk/CG68.
NICE GUIDELINES, Metastatic spinal cord compression. (2008) ; http://guidance.nice.org/CG75.