CHAPTER 52 Acute Coronary Syndrome
Acute coronary syndrome (ACS) is usually diagnosed in the emergency department based on history, physical examination, abnormalities on ECG, and elevations of cardiac serum biomarkers. According to the American Heart Association (AHA) and American College of Cardiology (ACC), high-risk patients recognized as having ACS should undergo early invasive coronary angiography and angiographically directed revascularization.1 The ultimate goal is to reperfuse and salvage any viable myocardium immediately. Other imaging modalities play minimal role in the evaluation of these patients. ACS is just one of many possible diagnoses when patients present to the emergency department with acute chest pain, however. Other considerations range from acute life-threatening conditions, such as pulmonary embolism, tension pneumothorax, or aortic dissection, to conditions with less morbidity and mortality, such as gastroesophageal reflux or costochondritis.
Acute chest pain accounts for approximately 6 million emergency department visits annually, and is the second most common reason for patients to present to the emergency department in the United States.2 Although most emergency department physicians and cardiologists have a low threshold for diagnosing ACS, 2% to 5% of patients are misdiagnosed and discharged inappropriately.3 In these patients, the mortality rate is 1.7 to 1.9 times greater than in patients who are hospitalized. Most patients who are inappropriately discharged with ACS have a nondiagnostic initial assessment. Various noninvasive imaging modalities are available to evaluate patients with acute, but indeterminate, chest pain in the emergency department.
PREVALENCE AND EPIDEMIOLOGY
In the most recent National Hospital Discharge Survey by the National Center for Health Statistics, there were approximately 1.4 million unique hospitalizations because of ACS in the United States in 2005.4 ACS is a major cause of morbidity and mortality in Western countries. The prevalence of ACS increases with age and predominates in men among patients younger than 70 years. In patients older than 70 years, the incidence occurs with equal frequency in men and women. Other risk factors that predispose patients to ACS include diabetes mellitus, smoking, hypertension, hypercholesterolemia, hyperlipidemia, a prior cerebrovascular event, inherited metabolic or connective tissue disorder, cocaine or amphetamine use, and occupational stress.
MANIFESTATIONS OF DISEASE
Imaging Technique and Findings
Radiography
Conventional chest radiography is the imaging modality used initially for screening noncardiac etiologies of acute chest pain, including pneumonia, pneumothorax, or rib fractures. Chest pain of cardiac origin also can be indirectly evaluated using chest radiography. Coronary arterial stenosis may be hinted by coronary artery calcification, which is seen in the “coronary triangle”—the mid-upper portion of the left cardiac silhouette on the frontal chest radiograph. In addition, circumferential calcifications may be seen within the expected location of myocardial wall, suggesting prior MI (Fig. 52-1). On the lateral chest radiograph, “tram-track” hyperdensities along the aortic arch correspond to atherosclerotic calcifications in the aorta, which can be independently correlated with coronary heart disease.5
Atherosclerotic calcifications overlying the borders of the heart may also indicate previous episodes of MI.6,7 Specifically, hyperdensity or calcifications of the left heart border, often curvilinear, may signify prior infarction of the left ventricular anterolateral wall. Focal outpouching of the left heart border may also represent postinfarct myocardial pseudoaneurysm or aneurysm.8
Ultrasonography
In risk stratifying and evaluating patients who present to the emergency department with acute chest pain, stress echocardiography is a versatile imaging modality. The prognostic information obtained is equivalent to radionuclide perfusion imaging and may be used to exclude ACS.9,10 Stress echocardiography provides functional and anatomic information by evaluating baseline ventricular function (e.g., ejection fraction, wall motion), valvular function, aortic root morphology, and pericardial anatomy. If a study shows reduction in ejection fraction or wall motion abnormality, especially as an interval change from previous assessment, the findings suggest myocardial ischemia.
Computed Tomography
In recent years, CT has become a noninvasive alternative to invasive coronary angiography in addition to its long-standing role in more general evaluation of the thorax. As the number of detectors has increased from 4 to 320, and gantry rotation speeds have increased, the spatial and temporal resolution for cardiac imaging acquisition has dramatically improved. The current generation of multidetector CT scanners provides accurate anatomic assessment of the coronary vasculature, allowing visualization to at least the third generation of coronary vessels. Compared with invasive coronary angiography, multiple studies have found multidetector CT to have sensitivity of 83% to 99%, specificity of 86% to 98%, positive predictive value of 66% to 93%, and negative predictive value of 97% to 99% in the evaluation of significant coronary arterial stenosis.11
Cardiac multidetector CT approaches the capability of invasive coronary angiography, the gold standard, in its ability to evaluate coronary arterial stenosis. Multidetector CT has several additional capabilities that can be valuable to emergency department physicians and cardiologists (Figs. 52-2 to 52-5). Cardiac multidetector CT can be used to assess the quantity and composition of atherosclerotic plaques and, potentially, to differentiate stable from unstable plaques.12,13 Cardiac multidetector CT also can evaluate myocardial function by assessing left ventricular wall motion and ejection fraction on the basis of images obtained throughout the different phases of the cardiac cycle.14 Dynamic imaging can be performed to assess perfusion of the myocardium.
Images of a standard multidetector CT scan of the chest are acquired from the thoracic inlet to the upper portion of the abdomen without the use of ECG gating and with a wide field of view. The dedicated coronary CT angiography protocol requires the use of ECG gating and a limited field of view. A major challenge of the triple rule-out protocol is the fusion of these two methods. Currently in our institution, the triple rule-out protocol is obtained using ECG gating and an unrestricted field of view. Images are acquired in a caudocephalic direction, the reverse of dedicated coronary CT angiography, with the goal of minimizing artifacts caused by respiration. For optimal image quality, a slightly larger volume of intravenous contrast agent is administered to maintain simultaneous peak contrast levels in the coronary and pulmonary arteries (Table 52-1).
TABLE 52-1 Sample Protocol Parameters for 64-Detector Multidetector CT for Acute Chest Pain Evaluation
| Protocol | Dedicated CT Angiography | Triple Rule-Out |
|---|---|---|
| kVp | 120 | 120 |
| mAs/slice | 800 | 600 |
| Field of view | 220 | 400 |
| Thickness (mm) | 0.67 | 0.9 |
| Increment (mm) | 0.33 | 0.45 |
| Rotation time (sec) | 0.4 | 0.4 |
| Direction | Cephalocaudal | Caudocephalad |
| Time (sec) | 9-10 | 15-18 |
| Injection protocol | 6 mL/sec (80 mL contrast), then 5 mL/sec (50 mL contrast/saline), then 5 mL/sec (50 mL saline) | 5 mL/sec (80 mL contrast), then 2 mL/sec (50 mL contrast), then 2 mL/sec (50 mL saline) |
| Bolus tracker | Ascending aorta | Descending aorta |
Several preliminary studies have shown dedicated coronary CT angiography to be a useful noninvasive imaging modality in the evaluation of patients with acute chest pain in the emergency department. In one study, all patients received standard of care assessment to rule out ACS, including invasive coronary angiography.15 Patients also underwent CT angiography to assess the presence of coronary atherosclerotic plaque and significant coronary artery stenosis (≥50%). Compared with standard of care, coronary CT angiography had 100% sensitivity and 82% specificity. The absence of coronary plaque and significant arterial stenosis excluded ACS in 100% of patients.
The use of the comprehensive or triple rule-out multidetector CT protocol has also been studied in patients with acute chest pain in the emergency department. In a separate study of this algorithm, all patients underwent a triple rule-out multidetector CT scan immediately after a serum creatinine value and an initial history, physical examination, and ECG were obtained.16 After CT, patients proceeded to receive the standard of care evaluation and treatment. One month after discharge, medical records were reviewed by consensus. Overall, the sensitivity, specificity, positive predictive value, and negative predictive value for CAD were 83%, 96%, 83%, and 96%, respectively. If noncardiac etiologies were also assessed, the sensitivity and positive predictive value increased to 87%. Comprehensive multidetector CT has the potential to evaluate cardiac and noncardiac causes of acute chest pain.
Another study has shown cardiac multidetector CT to be just as accurate in detecting and excluding ACS as the current standard of care using radionuclide perfusion imaging.17 In addition, the median diagnostic time and cost of care were significantly reduced. Cardiac multidetector CT can be as safe and efficacious as the standard of care, and the efficiency (diagnostic time and cost of care) is improved in evaluating emergency department patients with acute chest pain.
To date, there are several limitations to the widespread adoption of cardiac multidetector CT in the emergency department. First, the postprocessing of data after image acquisition is time-consuming and labor-intensive, restricting its use during off hours. Second, the radiation burden is still high (often >10 mSv), even with the use of current technologies, such as ECG-controlled tube current modulation, which can potentially reduce the exposure. Radionuclide perfusion imaging (8 to 10 mSv) and invasive coronary angiography also have substantial radiation doses, however. Third, although the triage of a negative result in cardiac multidetector CT is straightforward, the course of a positive result is less certain and remains to be defined (Table 52-2). Finally, a larger, multi-institutional clinical study using cardiac multidetector CT to evaluate ACS is still required to establish the role of multidetector CT in acute chest pain assessment.
TABLE 52-2 University of Maryland Triage for Cardiac Multidetector CT Studies
| Risk Category for ACS | CT Interpretation | Clinical Guideline |
|---|---|---|
| High | Coronary calcification (Agatston score) >400 | Admission |
| Hard or soft plaque | ||
| Stenosis >70% in any vessel | ||
| Stenosis >50% in left main artery | ||
| Medium | Coronary calcification 100-400 | Cardiology consultation |
| Stenosis 30%-70% in any vessel | ||
| Low | Coronary calcification <100 | Follow-up with preventive cardiology |
| Negative | Normal study | Follow-up with primary care provider |
Magnetic Resonance Imaging
MRI can be used to assess myocardial function, perfusion, and viability.18 Myocardial function assessment is based on the evaluation of myocardial wall motion. Assessments of myocardial perfusion and viability require intravenous administration of gadolinium contrast material and acquisition of dynamic images during the immediate and delayed (10 to 15 minutes) phases. These findings can facilitate the detection of myocardial ischemia, postischemic stunning or hibernation, and MI. In one study, cardiac MRI detected and diagnosed ACS with a sensitivity of 84% and specificity of 85%.19
Cardiac assessment using MRI is primarily based on myocardial wall motion and perfusion and viability images obtained after contrast administration. If wall motion abnormality is observed, but the perfusion and viability (delayed) images are normal, the myocardium may have endured an ischemic insult, a situation termed myocardial stunning. If wall motion and the perfusion (immediate postgadolinium) images are abnormal, but no abnormal uptake of the contrast material is seen in the viability (delayed) images, postischemic hibernation is diagnosed. If viability images show uptake of gadolinium within the myocardium, as shown by high signal intensity, the finding is consistent with MI (Fig. 52-6).
Nuclear Medicine
In patients with low to intermediate risk of ACS, radionuclide or myocardial perfusion imaging is typically a part of a cardiac evaluation protocol for risk stratification.20 Either during or immediately after an acute episode of chest pain, Tc 99m tetrofosmin or sestamibi is injected. Image acquisition is obtained using single photon emission computed tomography (SPECT) 45 to 60 minutes after injection of the radionuclide. Two sets of images are acquired to reflect myocardial blood flow at the time of the injection: one set of images with adenosine infusion or exercise stress and a second set of images at rest.
Radionuclide perfusion imaging is a powerful imaging modality in the diagnosis of ACS. The sensitivity, specificity, and negative predictive value are 92%, 71%, and 99%, respectively.21 As a result, a negative radionuclide perfusion study in a patient with acute chest pain in the emergency department suggests minimal or no risk of an adverse cardiac event.22 Myocardial perfusion imaging can also be used for prognosis and risk stratification for future cardiac events.23 If a perfusion defect is shown, the patient is at higher risk of having a cardiac event during hospitalization or at follow-up.
The main purpose of myocardial perfusion study is to assess the myocardial function by evaluating inducible perfusion abnormality, myocardial viability, and regional and global myocardial wall motion. Inducible perfusion abnormality is diagnosed if the uptake of radiotracer in a certain region of the myocardium is reduced during stress imaging. During the rest images, the myocardium takes up the radiotracer normally, suggesting the ischemic territory is reversible, and the perfusion abnormality is inducible by stress (Fig. 52-7). Conversely, if the radiotracer is not taken up by the myocardium during the rest images, the myocardium is considered nonviable. Abnormality in wall motion can also be evaluated by visualizing the decrease or lack of contractility in the region of myocardial ischemia and infarct.
Angiography
Percutaneous coronary intervention (PCI) using angioplasty and stent placement to revascularize the myocardium may be performed in the same setting. Compared with thrombolytic therapy, PCI is associated with a lower mortality, lower reinfarction rate, and lower frequency of stroke.24,25 PCI also restores flow to the occluded coronary artery with greater speed and frequency and with a high success rate.26
TREATMENT OPTIONS
Medical
ACC/AHA guidelines recommend all initial therapy be implemented in the emergency department based on an institution-specific, written protocol.27 Patients presenting to the emergency department with symptoms suggestive of an acute myocardial event should be evaluated within 10 minutes after arrival. Early assessment includes confirmation of hemodynamic stability and securing the airway, breathing, and circulation; obtaining a 12-lead ECG; and administering oxygen to maintain saturation greater than 90%, intravenous analgesia (most commonly morphine sulfate), oral aspirin (162 to 325 mg), and sublingual nitroglycerin (0.4 mg per 5 minutes for a total of three doses) if not contraindicated. Initial history, physical examination, and laboratory work are also necessary.
Surgical/Interventional
The ACC/AHA Task Force recommends the use of PCI with possible stent placement for any patient with acute STEMI who presents within 12 hours of onset of symptoms, and who can undergo the procedure within 90 minutes of presentation.27 For unstable angina and NSTEMI, only high-risk patients should undergo early PCI management. High-risk indicators include (1) recurrent angina at rest or with low-level activity despite intensive medical therapy; (2) elevated cardiac troponin levels; (3) new, or presumably new, ST segment depression; (4) recurrent angina or ischemia with heart failure; (5) high-risk findings on noninvasive stress testing; (6) depressed left ventricular function (ejection fraction <40%) on noninvasive study; (7) hemodynamic instability; (8) sustained ventricular tachycardia; (9) PCI within the previous 6 months; and (10) previous coronary artery bypass graft (CABG) surgery.28 Otherwise, medical management is recommended.
As described in the most recent ACC/AHA practice guideline, CABG surgery may offer a survival advantage compared with medical management in patients with unstable angina and left ventricular dysfunction, especially in patients with three-vessel CAD.29 There is as yet no medical evidence, however, to suggest CABG surgery is better than PCI in patients with unstable angina. No study has shown that CABG surgery should be performed in patients with NSTEMI or STEMI in the acute setting.
INFORMATION FOR THE REFERRING PHYSICIAN
Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50:e1.
Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). Circulation. 2004;110:588.
Hoffmann U, Pena AJ, Cury RC, et al. Cardiac CT in emergency department patients with acute chest pain. RadioGraphics. 2006;26:963.
Jeudy J, White CS. Evaluation of acute chest pain in the emergency department: utility of multidetector computed tomography. Semin Ultrasound CT MR. 2007;28:109.
Kuo D, Dilsizian V, Prasad R, et al. Emergency cardiac imaging: state of the art. Cardiol Clin. 2006;24:53.
Thilo C, Auler M, Zwerner P, et al. Coronary CTA: indications, patient selection, and clinical implications. J Thorac Imaging. 2007;22:35.
White CS, Kuo D. Chest pain in the emergency department: role of multidetector CT. Radiology. 2007;245:672.
1 Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA 2002 guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction—summary article: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee on the Management of Patients With Unstable Angina). J Am Coll Cardiol. 2002;40:1366.
2 McCaig LF, Nawar EW. National Hospital Ambulatory Medical Care Survey: 2004 emergency department summary. Adv Data. 2006;372:1.
3 Pope JH, Aufderheide TP, Ruthazer R, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342:1163.
4 Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25.
5 Iribarren C, Sidney S, Sternfeld B, et al. Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA. 2000;283:2810.
6 MacGregor JH, Chen JT, Chiles C, et al. The radiographic distinction between pericardial and myocardial calcifications. AJR Am J Roentgenol. 1987;148:675.
7 Margolis JR, Chen JT, Kong Y, et al. The diagnostic and prognostic significance of coronary artery calcification: a report of 800 cases. Radiology. 1980;137:609.
8 Higgins CB, Lipton MJ, Johnson AD, et al. False aneurysms of the left ventricle: identification of distinctive clinical, radiographic, and angiographic features. Radiology. 1978;127:21.
9 Sicari R, Pasanisi E, Venneri L, et al. Stress echo results predict mortality: a large-scale multicenter prospective international study. J Am Coll Cardiol. 2003;41:589.
10 Kontos MC, Arrowood JA, Jesse RL, et al. Comparison between 2-dimensional echocardiography and myocardial perfusion imaging in the emergency department in patients with possible myocardial ischemia. Am Heart J. 1998;136:724.
11 Raff GL, Gallagher MJ, O’Neill WW, et al. Diagnostic accuracy of noninvasive coronary angiography using 64-slice spiral computed tomography. J Am Coll Cardiol. 2005;46:552.
12 Kunimasa T, Sato Y, Sugi K, et al. Evaluation by multislice computed tomography of atherosclerotic coronary artery plaques in non-culprit, remote coronary arteries of patients with acute coronary syndrome. Circ J. 2005;69:1346.
13 Leber AW, Knez A, von Ziegler F, et al. Quantification of obstructive and nonobstructive coronary lesions by 64-slice computed tomography: a comparative study with quantitative coronary angiography and intravascular ultrasound. J Am Coll Cardiol. 2005;46:147.
14 Yamamuro M, Tadamura E, Kubo S, et al. Cardiac functional analysis with multi-detector row CT and segmental reconstruction algorithm: comparison with echocardiography, SPECT, and MR imaging. Radiology. 2005;234:381.
15 Hoffmann U, Nagurney JT, Moselewski F, et al. Coronary multidetector computed tomography in the assessment of patients with acute chest pain. Circulation. 2006;114:2251.
16 White CS, Kuo D, Kelemen M, et al. Chest pain evaluation in the emergency department: can MDCT provide a comprehensive evaluation? AJR Am J Roentgenol. 2005;185:533.
17 Goldstein JA, Gallagher MJ, O’Neill WW, et al. A randomized controlled trial of multi-slice coronary computed tomography for evaluation of acute chest pain. J Am Coll Cardiol. 2007;49:863.
18 Al-Saadi N, Nagel E, Gross M, et al. Noninvasive detection of myocardial ischemia from perfusion reserve based on cardiovascular magnetic resonance. Circulation. 2000;101:1379.
19 Kwong RY, Schussheim AE, Rekhraj S, et al. Detecting acute coronary syndrome in the emergency department with cardiac magnetic resonance imaging. Circulation. 2003;107:531.
20 Heller GV, Stowers SA, Hendel RC, et al. Clinical value of acute rest technetium-99m tetrofosmin tomographic myocardial perfusion imaging in patients with acute chest pain and nondiagnostic electrocardiograms. J Am Coll Cardiol. 1998;31:1011.
21 Kontos MC, Jesse RL, Schmidt KL, et al. Value of acute rest sestamibi perfusion imaging for evaluation of patients admitted to the emergency department with chest pain. J Am Coll Cardiol. 1997;30:976.
22 Wackers FJ, Brown KA, Heller GV, et al. American Society of Nuclear Cardiology position statement on radionuclide imaging in patients with suspected acute ischemic syndromes in the emergency department or chest pain center. J Nucl Cardiol. 2002;9:246.
23 Bulow H, Schwaiger M. Nuclear cardiology in acute coronary syndromes. Q J Nucl Med Mol Imaging. 2005;49:59.
24 A clinical trial comparing primary coronary angioplasty with tissue plasminogen activator for acute myocardial infarction. The Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes (GUSTO IIb) Angioplasty Substudy Investigators. N Engl J Med. 1997;336:1621.
25 Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13.
26 Berger PB, Bell MR, Holmes DRJr, et al. Time to reperfusion with direct coronary angioplasty and thrombolytic therapy in acute myocardial infarction. Am J Cardiol. 1994;73:231.
27 Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). Circulation. 2004;110:588.
28 Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50:e1.
29 Eagle KA, Guyton RA, Davidoff R, et al. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery). Circulation. 2004;110:e340.

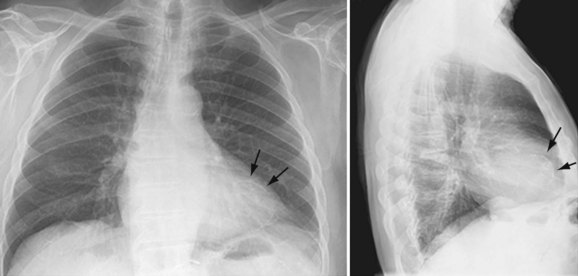
 FIGURE 52-1
FIGURE 52-1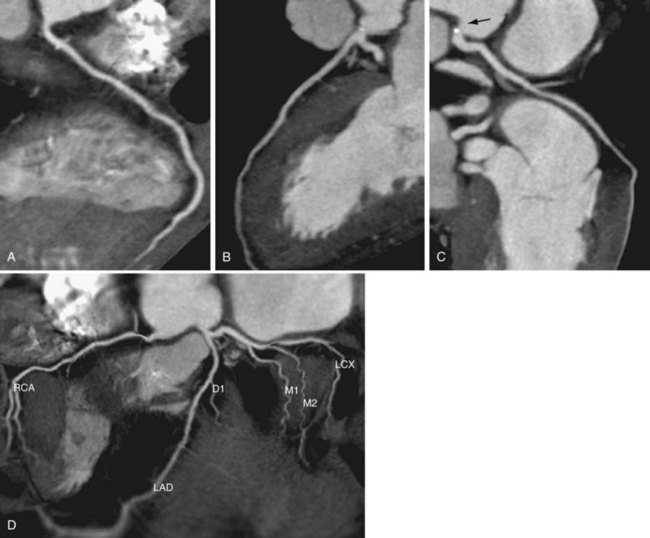
 FIGURE 52-2
FIGURE 52-2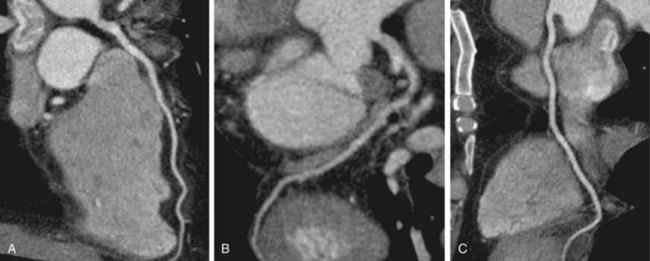
 FIGURE 52-3
FIGURE 52-3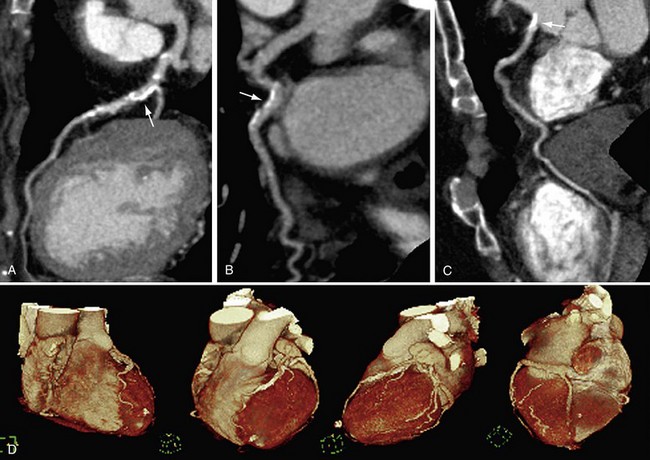
 FIGURE 52-4
FIGURE 52-4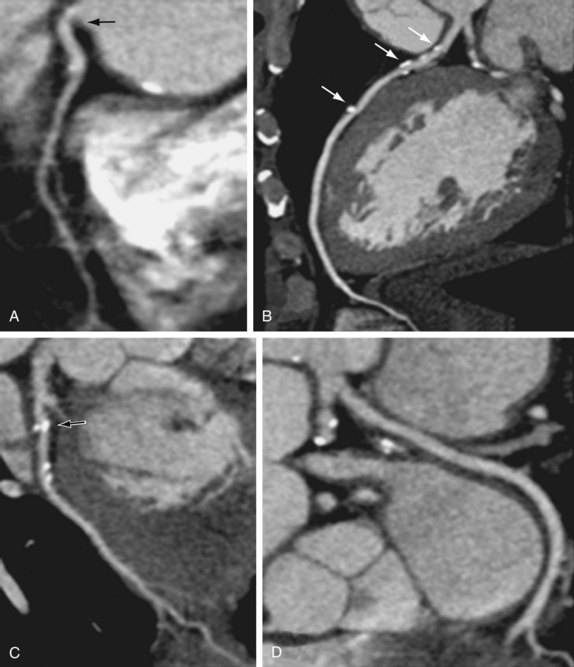
 FIGURE 52-5
FIGURE 52-5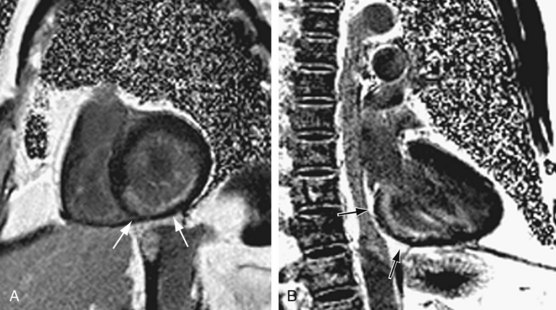
 FIGURE 52-6
FIGURE 52-6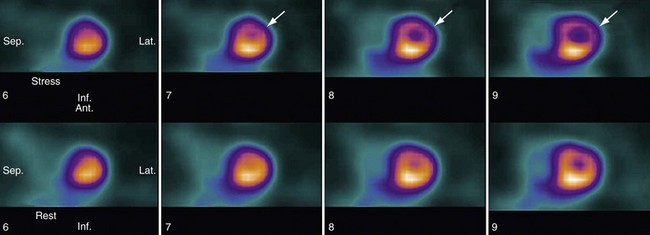
 FIGURE 52-7
FIGURE 52-7


