132 Acute Bloodstream Infection
Acute bloodstream infection, which may be primary or secondary and community-acquired or nosocomial, is one of the most severe forms of infection. Frequently observed among immunocompromised and critically ill patients, bloodstream infection is rarely asymptomatic and may be associated with multiple organ failure.1–3
 Definitions
Definitions
The term bloodstream infection includes all forms of confirmed or unconfirmed bacteremia and fungemia. Acute bloodstream infections should be distinguished from septicemia, clinical sepsis, and sepsis, which refer to clinical syndromes. Definitions are summarized in Table 132-1.
| Type of Bloodstream Infection | Criteria |
|---|---|
| Positive blood culture | Recognized pathogens* identified from one or more blood cultures and not related to an infection at another body site |
| Laboratory-confirmed bloodstream infection | Positive blood culture with at least one of the following signs or symptoms: fever (>100.4°F [38°C]) or hypothermia (<98.6°F [37°C]); chills; low blood pressure (systolic blood pressure ≤ 90 mm Hg or a decrease > 40 mm Hg from baseline) |
| Primary | Laboratory-confirmed bloodstream infection or clinical sepsis occurring without a documented distal source of infection, including those resulting from catheter-related or catheter-associated infections |
| Secondary | Laboratory-confirmed bloodstream infection occurring in the presence of another documented site of infection |
| Catheter-associated | Primary bloodstream infection and presence of an intravascular access device |
| Catheter-related | Laboratory-confirmed bloodstream infection in a patient with an intravascular access device and at least one positive blood culture obtained from a peripheral vein, clinical manifestations of infection (fever, chills, hypotension), and no apparent source of bloodstream infection except for vascular access plus one of the following: positive semiquantitative culture (>15 CFU/catheter segment) with the same organism,103 positive quantitative culture (>103 CFU/catheter segment) with the same organism,104 simultaneous quantitative blood cultures with a ≥ 5 : 1 ratio CVC versus peripheral,105 and differential period of CVC culture versus peripheral blood culture positivity of > 2 h106 |
CFU, colony-forming unit; CVC, central venous catheter.
* One of the following: common skin contaminant (diphtheroids, Bacillus spp., Propionibacterium spp., coagulase-negative staphylococci, or micrococci) cultured from two or more blood cultures drawn on separate occasions; common skin contaminant cultured from one or more blood cultures from a patient with vascular access, and the physician institutes appropriate antimicrobial therapy; positive antigen test on blood and signs and symptoms with positive laboratory results not related to infection at another site.
 Epidemiology
Epidemiology
The epidemiology of bloodstream infection varies according to its source. Bloodstream infections represented 12% of all nosocomial infections reported in 10,038 patients from 1417 intensive care units (ICUs) in the European Prevalence of Infection in Intensive Care (EPIC) study,4 and similar data were found in other clinical studies.5 A worldwide prevalence study among 1265 ICUs (EPIC 2) reported bloodstream infections representing 15% of all healthcare-associated infections among 1265 participating ICUs from 75 countries.6 Almost half of all positive blood cultures obtained in a hospital are due to nosocomial bloodstream infections.7 Of these, most are primary and associated with central catheters.8
Most surveillance systems today such as the U.S. National Healthcare Safety Network (NHSN), the German Krankenhaus Infektions Surveillance System (KISS), or the International Nosocomial Infection Control Consortium (INICC) focus on catheter-associated, laboratory-confirmed, primary bloodstream infections, with reporting of bloodstream infections as episodes per 1000 device-days. Surveillance of clinical sepsis has been mostly abandoned because the definition of this infection leaves much room for interpretation and is resource demanding.8 The exception to this rule are studies among neonates; as blood cultures are often unreliable in this population.9 However, even among adults, clinical sepsis may represent up to two-thirds of central line–associated bloodstream infections (CLABSI), but focusing on microbiologically documented bloodstream infections on the other hand may underestimate true CLABSI rates.8
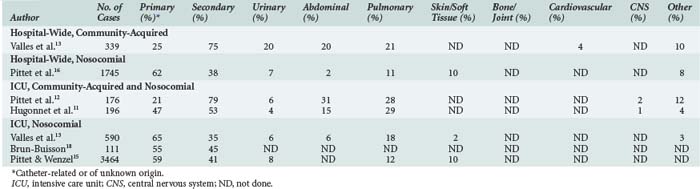
Most community-acquired bloodstream infections are secondary and due to documented infections such as pneumonia and urinary tract or soft-tissue infections (Table 132-2).10–13 Similar to nosocomial bloodstream infections, many primary bloodstream infections are associated with intravascular access devices.14–16
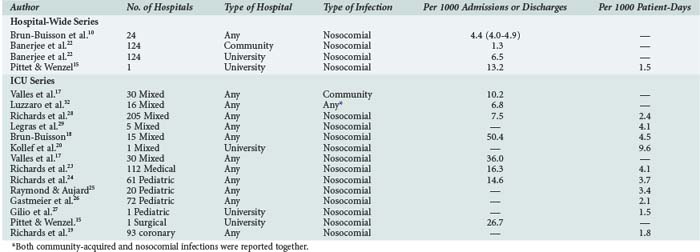
The incidence of bloodstream infection in various patient populations is presented in Table 132-3.10,13,14,17–29 The large observed differences may be related to variable definitions and reporting systems. Thus, comparisons and benchmarking should be done with caution.30,31
 Microbiology
Microbiology
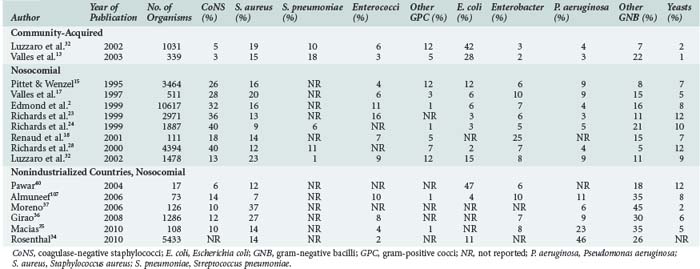
The distribution of microorganisms causing bloodstream infections varies according to source, age category (neonates, children, adults), and resources available for healthcare (Table 132-4).* In most institutions, a shift in predominant organisms from gram-negative bacilli to gram-positive cocci has been observed over the past 2 decades.11,15,28 However, in countries with limited resources, gram-negative pathogens and, among these, non-fermentative organisms such as Pseudomonas spp. and Acinetobacter spp., are still predominant.34–38 The predominance of non-fermentative organisms may be ascribed to contamination of infusates and thus to breaches in basic infection control procedures.35,42 Such breaches are likely due to the multiple use of infusates or single-use vials and a lack of respect of aseptic conditions. The shift towards gram-positive cocci seen in high-resource countries is largely due to the use of intravascular devices and the fact that the proportion of patients with risk factors such as neutropenia, solid organ and bone marrow transplantation, or the use of immunosuppressive agents has increased. The current high density of medical facilities and unrestricted access to medical care for the majority of the population in most developed countries have played major roles in the prescription of antibiotics very early in the course of most infections. In addition, the widespread use of broad-spectrum antibiotics, either for therapy or surgical prophylaxis, may be partially responsible for the increase in the relative proportions of coagulase-negative staphylococci (CoNS) and enterococci. The proportion of Candida spp., especially infections with non-albicans spp., has considerably increased in many institutions, although recent studies suggest a trend toward fewer Candida infections, at least in North America.43,44 Prolonged treatments with multiple antibiotics, the use of intravascular devices, total parenteral nutrition, and prolonged neutropenia in patients with cancer have been identified as independent risk factors in this context.45–52
CoNS are the most common pathogens isolated from blood cultures, especially in primary bloodstream infections.7 Often considered contaminants, the detection of CoNS may not always be harmless; associated mortality up to 18% has been reported.7 In contrast, mortality from Staphylococcus aureus bloodstream infection ranges between 13% and 25%, with higher rates for nosocomial than for community-acquired infection.53,54 Detection of S. aureus on catheter tips is a predictor for subsequent bacteremia, even in the absence of clinical signs and negative blood cultures at the time of catheter removal.55–57 Likewise, bloodstream infections due to Candida spp. have a poor prognosis. Mortality with this microorganism ranges between 15% and 55%, especially when antifungal treatment is delayed by 3 or more days.45,58 An important shift in the epidemiology of Candida bloodstream infections has occurred over the past decades, with decreasing infections due to Candida albicans, but increasing numbers of infections due to non-albicans isolates. In particular, fungemia due to Candida glabrata has increased.59 The emergence of this species presents clinical problems insofar as it is often resistant to fluconazole.60
 Impact
Impact
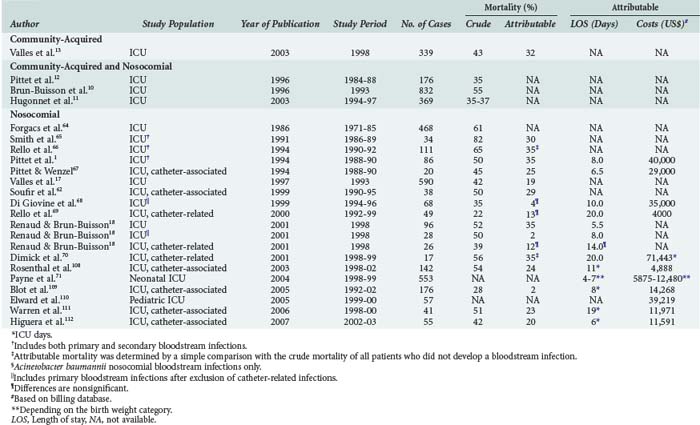
Patients with bloodstream infections are at risk for increased mortality.16,18 A meta-analysis by Siempos and colleagues found attributable mortality rates for CLABSI between 2% and 35%.61 Nosocomial bloodstream infections and, in this context CLABSI in particular, are associated with increased morbidity, prolonged length of hospital stay, and resource utilization in almost all groups of patients studied (Table 132-5).† Attributable costs and length of stay among neonates depend largely on the birthweight category, with extremely low-birthweight infants generating more expense than very low to normal-birthweight infants.71 Interestingly, mortality from secondary bloodstream infections is higher compared to primary bloodstream infections (29%–45% versus 18%–29%, respectively). Furthermore, mortality from CLABSI is lower than mortality from other primary bloodstream infections (15%–26% versus 18%–29%, respectively).16,18 Although the reason for this difference is unclear, delayed antibiotic therapy for community-acquired bloodstream infections and serious comorbidity in the context of secondary bloodstream infections may partially explain such trends.
†References 1, 10, 12–14, 17, 18, and 61–70.
Microbiological factors have been found to be important in the context of mortality among patients with nosocomial bloodstream infection, even after adjustment for major confounders intrinsic to patients’ underlying conditions.16 Pathogens that are independently associated with mortality are Candida spp. and Pseudomonas aeruginosa. CoNS are less associated with mortality compared to other pathogens, although these pathogens are isolated most frequently.16
 General Principles of Management
General Principles of Management
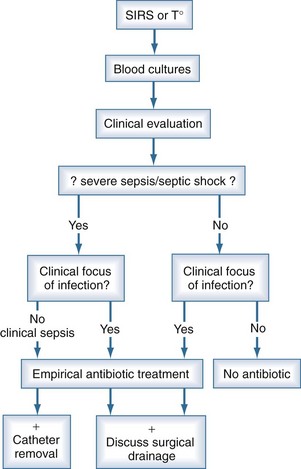
When patients are suspected to have bacteremia or fungemia, blood cultures are performed. The clinical threshold to draw blood cultures should be low, and such testing is often justified in the presence of isolated fever. This may explain why only 10% to 15% of blood cultures performed turn positive. Even in the presence of systemic inflammatory response syndrome, blood cultures are negative in 40% to 60% of cases13; however, severe sepsis and septic shock are associated with increased morbidity, mortality, and end-organ dysfunction.72 Accordingly, when sepsis is suspected, it is generally not possible to wait for results of blood cultures, and empirical antimicrobial treatment is prescribed in most cases (Figure 132-1). Owing to the low quality of blood culture sampling, the situation among neonates is even more pronounced. In one study, only 46% of blood cultures obtained from neonates contained an adequate blood volume, and only 35% were adequate submissions on the basis of collection into the correct blood culture bottle type.9 The overall positive yield of blood cultures was low, and cultures with adequate blood volume were more likely to be positive than those with inadequate blood volumes (5.3% versus 2.1%). The quality of blood culture sampling is better among older children. Of all positive cultures, 32% were contaminants, and 68% grew significant pathogens. However, only 35% of the contaminant cultures had adequate weight-adjusted blood volume, while this rate was 60% in the true bacteremia group (P < 0.001).73 Thus, inappropriate blood culture sampling is more likely to produce pseudobacteremia than correct sampling.
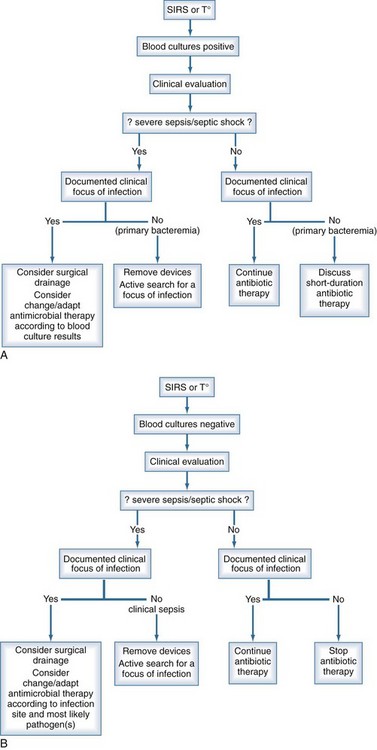
The management of bloodstream infection should combine early antimicrobial treatment and the active search for a source of infection that might require specific therapeutic measures for eradication or therapy (Figure 132-2). It has been repeatedly shown that either delayed or inappropriate antibiotic treatment is associated with higher mortality rates.11,20,74–76 Similar results were observed for candidemia, where mortality was significantly higher when antifungal therapy was delayed.58,77,78 Conversely in some studies, inappropriate antibiotic treatment was not found to be a risk factor for developing septic shock in patients with positive blood cultures,13 but the mortality of those requiring inotropic drugs was significantly higher—85% versus 75% and 58% versus 24%, respectively.
The choice of antibiotics to start empirical therapy should be based on knowledge of the local epidemiology, susceptibility of pathogens, and source of the infection. A multidisciplinary approach, including close collaboration between the physician in charge of the patient, the infectious disease specialist, and the microbiology laboratory, is of paramount importance. Such collaboration improves the accuracy of empirical therapy. Once susceptibility testing from microorganisms identified from blood cultures has been obtained, antibiotic treatment should be adjusted accordingly. In some conditions, pathogens identified from other body sites also have to be considered for treatment. In addition to antimicrobial therapy, specific measures such as drainage of abscesses, adequate surgical management of peritonitis, and removal of infected prosthetic material are necessary to control the infection. Procalcitonin-based deescalation of antibiotic therapy has been reported to reduce exposure to antibiotics by almost 30%.79–81
In the case of primary bloodstream infection or sepsis, central lines should be removed if in place at time of infection. Catheter retention may result in a several-fold increase in risk for recurrence of bloodstream infection. However, recent data suggest that antibiotic locks in addition to systemic antibiotic therapy can be used as a salvage strategy if CLABSI involves long-term catheters, signs of exit site or tunnel infection are absent, and blood cultures reveal the presence of CoNS or enterococci.82,83 Removal of the catheter is mandatory in severe or complicated infections, in the presence of shock, in case of recurrent bloodstream infection, and when microorganisms such as S. aureus, gram-negative bacilli or Candida spp. are isolated.84 Relapse, continuous fever, or bacteremia despite catheter removal requires an active search for complications such as metastatic abscess, septic thrombophlebitis, or endocarditis. Following the completion of antimicrobial therapy, careful follow-up is mandatory owing to the frequent occurrence of late complications.85,86 Recovery of S. aureus on a catheter tip may suggest the initiation of therapy even in the absence of clinical signs and negative blood cultures.55
 Prevention
Prevention
As for any other infection, prevention of bloodstream infection relies on strict respect for the basic rules of hygiene, particularly hand hygiene practices.87–89 It has been shown that improved hand hygiene and good work organization prevents transmission of pathogens.90 For prevention of device-associated infections, there is good evidence that multimodal strategies combining procedural and technical interventions are effective.91–95 Procedural interventions include introducing standardized written procedures for catheter insertion and catheter care. Technical interventions include using chlorhexidine for skin antisepsis; devices (catheters, connectors, sponges) impregnated with chlorhexidine, chlorhexidine/silver sulfadiazine, silver, and antibiotics; using closed rather than open systems; and using lock solutions with agents such as taurolidine, citrate, EDTA, and ethanol. Alcohol-based, chlorhexidine-containing skin antiseptics have now become the standard of care. Use of a chlorhexidine-impregnated sponge was found effective in two randomized controlled trials.96,97 Interestingly, daily bathing with a chlorhexidine-containing solution in the ICU was found effective in reducing bacteremia due to vancomycin-resistant enterococci (VRE) as well as VRE-colonization and methicillin-resistant S. aureus (MRSA) acquisition.98 Two meta-analyses show that products impregnated with chlorhexidine/silver sulfadiazine are effective in reducing catheter colonization but not CLABSI; rifampicin/minocycline-coated catheters are effective in reducing both catheter colonization and CLABSI.99,100 Most studies with central venous catheters are conducted in the ICU, including catheters with a relatively short dwell time. For longer insertion times, there are no data about the efficacy of antibiotic-coated devices, and there is evidence that chlorhexidine/silver sulfadiazine–coated catheters are ineffective.101 The efficacy of lock solutions remains undetermined at present, although some studies show promising results.102 Educational programs or global preventive strategies based on strict application of specific preventive measures and careful control of all factors associated with infection have been shown to be very effective in reducing infection rates. Specific devices such as antiseptic- or antibiotic-coated catheters or chlorhexidine-impregnated sponges are considered to be of advantage when procedural interventions are already successfully in place.96
Key Points
Hugonnet S, Harbarth S, Ferrière K, Ricou B, Suter P, Pittet D. Bacteremic sepsis in intensive care: temporal trends in incidence, organ dysfunction, and prognosis. Crit Care Med. 2003;31:390-394.
Pittet D, Tarara D, Wenzel RP. Nosocomial bloodstream infection in critically ill patients: excess length of stay, extra costs, and attributable mortality. JAMA. 1994;271:1598-1601.
Nobre V, Harbarth S, Grafs JD, Rohner P, Pugin J. Use of procalcitonin to shorten antibiotic treatment duration in septic patients. Am J Respir Crit Care Med. 2008;177:498-505.
Timsit JF, Schwebel C, Bouadma L, Geffroy A, Garrouste-Orgeas M, Pease S, et al. Chlorhexidine-impregnated sponges and less frequent dressing changes for prevention of catheter-related infections in critically ill adults. JAMA. 2009;301:1231-1241.
1 Pittet D, Tarara D, Wenzel RP. Nosocomial bloodstream infection in critically ill patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1994;271:1598-1601.
2 Edmond MB, Wallace SE, McClish DK, Pfaller MA, Jones RN, Wenzel RP. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin Infect Dis. 1999;29:239-244.
3 Marshall JC, Vincent JL, Fink MP, et al. Measures, markers, and mediators: toward a staging system for clinical sepsis. A report of the Fifth Toronto Sepsis Roundtable, Toronto, Ontario, Canada, October 25-26, 2000. Crit Care Med. 2003;31:1560-1567.
4 Vincent JL, Bihari DJ, Suter PM, et al. The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. EPIC International Advisory Committee. JAMA. 1995;274:639-644.
5 Lyytikainen O, Kanerva M, Agthe N, Mottonen T, Ruutu P. Healthcare-associated infections in Finnish acute care hospitals: a national prevalence survey, 2005. J Hosp Infect. 2008;69:288-294.
6 Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323-2329.
7 Favre B, Hugonnet S, Correa L, Sax H, Rohner P, Pittet D. Nosocomial bacteremia: clinical significance of a single blood culture positive for coagulase-negative staphylococci. Infect Control Hosp Epidemiol. 2005;26:697-702.
8 Hugonnet S, Sax H, Eggimann P, Chevrolet JC, Pittet D. Nosocomial bloodstream infection and clinical sepsis. Emerg Infect Dis. 2004;10:76-81.
9 Connell TG, Rele M, Cowley D, Buttery JP, Curtis N. How reliable is a negative blood culture result? Volume of blood submitted for culture in routine practice in a children’s hospital. Pediatrics. 2007;119:891-896.
10 Brun-Buisson C, Doyon F, Carlet J. Bacteremia and severe sepsis in adults: a multicenter prospective survey in ICUs and wards of 24 hospitals. French Bacteremia-Sepsis Study Group. Am J Respir Crit Care Med. 1996;154:617-624.
11 Hugonnet S, Harbarth S, Ferriere K, Ricou B, Suter P, Pittet D. Bacteremic sepsis in intensive care: temporal trends in incidence, organ dysfunction, and prognosis. Crit Care Med. 2003;31:390-394.
12 Pittet D, Thievent B, Wenzel RP, Li N, Auckenthaler R, Suter PM. Bedside prediction of mortality from bacteremic sepsis. A dynamic analysis of ICU patients. Am J Respir Crit Care Med. 1996;153:684-693.
13 Valles J, Rello J, Ochagavia A, Garnacho J, Alcala MA. Community-acquired bloodstream infection in critically ill adult patients: impact of shock and inappropriate antibiotic therapy on survival. Chest. 2003;123:1615-1624.
14 Lyytikainen O, Lumio J, Sarkkinen H, Kolho E, Kostiala A, Ruutu P. Nosocomial bloodstream infections in Finnish hospitals during 1999-2000. Clin Infect Dis. 2002;35:e14-e19.
15 Pittet D, Wenzel RP. Nosocomial bloodstream infections. Secular trends in rates, mortality, and contribution to total hospital deaths. Arch Intern Med. 1995;155:1177-1184.
16 Pittet D, Li N, Woolson RF, Wenzel RP. Microbiological factors influencing the outcome of nosocomial bloodstream infections: a 6-year validated, population-based model. Clin Infect Dis. 1997;24:1068-1078.
17 Valles J, Leon C, Alvarez-Lerma F. Nosocomial bacteremia in critically ill patients: a multicenter study evaluating epidemiology and prognosis. Spanish Collaborative Group for Infections in Intensive Care Units of Sociedad Espanola de Medicina Intensiva y Unidades Coronarias (SEMIUC). Clin Infect Dis. 1997;24:387-395.
18 Renaud B, Brun-Buisson C. Outcomes of primary and catheter-related bacteremia. A cohort and case-control study in critically ill patients. Am J Respir Crit Care Med. 2001;163:1584-1590.
19 Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in coronary care units in the United States. National Nosocomial Infections Surveillance System. Am J Cardiol. 1998;82:789-793.
20 Kollef MH, Sherman G, Ward S, Fraser VJ. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest. 1999;115:462-474.
21 Elhanan G, Raz R, Pitlik SD, et al. Bacteraemia in a community and a university hospital. J Antimicrob Chemother. 1995;36:681-695.
22 Banerjee SN, Emori TG, Culver DH, et al. Secular trends in nosocomial primary bloodstream infections in the United States, 1980-1989. National Nosocomial Infections Surveillance System. Am J Med. 1991;91:86S-89S.
23 Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Crit Care Med. 1999;27:887-892.
24 Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in pediatric intensive care units in the United States. National Nosocomial Infections Surveillance System. Pediatrics. 1999;103:e39.
25 Raymond J, Aujard Y. Nosocomial infections in pediatric patients: a European, multicenter prospective study. European Study Group. Infect Control Hosp Epidemiol. 2000;21:260-263.
26 Gastmeier P, Hentschel J, de Veer I, Obladen M, Ruden H. Device-associated nosocomial infection surveillance in neonatal intensive care using specified criteria for neonates. J Hosp Infect. 1998;38:51-60.
27 Gilio AE, Stape A, Pereira CR, Cardoso MF, Silva CV, Troster EJ. Risk factors for nosocomial infections in a critically ill pediatric population: a 25-month prospective cohort study. Infect Control Hosp Epidemiol. 2000;21:340-342.
28 Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol. 2000;21:510-515.
29 Legras A, Malvy D, Quinioux AI, et al. Nosocomial infections: prospective survey of incidence in five French intensive care units. Intensive Care Med. 1998;24:1040-1046.
30 Eggimann P, Hugonnet S, Sax H, Touveneau S, Chevrolet JC, Pittet D. Ventilator-associated pneumonia: caveats for benchmarking. Intensive Care Med. 2003;29:2086-2089.
31 Richards C, Emori TG, Edwards J, Fridkin S, Tolson J, Gaynes R. Characteristics of hospitals and infection control professionals participating in the National Nosocomial Infections Surveillance System 1999. Am J Infect Control. 2001;29:400-403.
32 Luzzaro F, Vigano EF, Fossati D, et al. Prevalence and drug susceptibility of pathogens causing bloodstream infections in northern Italy: a two-year study in 16 hospitals. Eur J Clin Microbiol Infect Dis. 2002;21:849-855.
33 Garden OJ, Sim AJ. A comparison of tunnelled and nontunnelled subclavian vein catheters: a prospective study of complications during parenteral feeding. Clin Nutr. 1983;2:51-54.
34 Rosenthal VD, Maki DG, Jamulitrat S, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary for 2003-2008, issued June 2009. Am J Infect Control. 2010;38:95-104. e2
35 Macias AE, Huertas M, de Leon SP, et al. Contamination of intravenous fluids: a continuing cause of hospital bacteremia. Am J Infect Control. 2010;38:217-221.
36 Girao E, Levin AS, Basso M, et al. Trends and outcome of 1121 nosocomial bloodstream infections in intensive care units in a Brazilian hospital, 1999-2003. Int J Infect Dis. 2008;12:e145-e146.
37 Moreno CA, Rosenthal VD, Olarte N, et al. Device-associated infection rate and mortality in intensive care units of 9 Colombian hospitals: findings of the International Nosocomial Infection Control Consortium. Infect Control Hosp Epidemiol. 2006;27:349-356.
38 Agarwal R, Gupta D, Ray P, Aggarwal AN, Jindal SK. Epidemiology, risk factors and outcome of nosocomial infections in a Respiratory Intensive Care Unit in North India. J Infect. 2006;53:98-105.
39 de Brito CS, de Brito DV, Abdallah VO, Gontijo Filho PP. Occurrence of bloodstream infection with different types of central vascular catheter in critically neonates. J Infect. 2010;60:128-132.
40 Pawar M, Mehta Y, Kapoor P, Sharma J, Gupta A, Trehan N. Central venous catheter-related blood stream infections: incidence, risk factors, outcome, and associated pathogens. J Cardiothorac Vasc Anesth. 2004;18:304-308.
41 Su BH, Hsieh HY, Chiu HY, Lin HC. Nosocomial infection in a neonatal intensive care unit: a prospective study in Taiwan. Am J Infect Control. 2007;35:190-195.
42 Macias AE, de Leon SP, Huertas M, et al. Endemic infusate contamination and related bacteremia. Am J Infect Control. 2008;36:48-53.
43 Fridkin SK, Kaufman D, Edwards JR, Shetty S, Horan T. Changing incidence of Candida bloodstream infections among NICU patients in the United States: 1995-2004. Pediatrics. 2006;117:1680-1687.
44 Labbe AC, Pepin J, Patino C, Castonguay S, Restieri C, Laverdiere M. A single-centre 10-year experience with Candida bloodstream infections. Can J Infect Dis Med Microbiol. 2009;20:45-50.
45 Bassetti M, Trecarichi EM, Righi E, et al. Incidence, risk factors, and predictors of outcome of candidemia. Survey in 2 Italian university hospitals. Diagn Microbiol Infect Dis. 2007;58:325-331.
46 Boo TW, O’Reilly B, O’Leary J, Cryan B. Candidaemia in an Irish tertiary referral hospital: epidemiology and prognostic factors. Mycoses. 2005;48:251-259.
47 Cheng YR, Lin LC, Young TG, Liu CE, Chen CH, Tsay RW. Risk factors for candidemia-related mortality at a medical center in central Taiwan. J Microbiol Immunol Infect. 2006;39:155-161.
48 Dimopoulos G, Ntziora F, Rachiotis G, Armaganidis A, Falagas ME. Candida albicans versus non-albicans intensive care unit-acquired bloodstream infections: differences in risk factors and outcome. Anesth Analg. 2008;106:523-529. table of contents
49 Klevay MJ, Horn DL, Neofytos D, Pfaller MA, Diekema DJ. Initial treatment and outcome of Candida glabrata versus Candida albicans bloodstream infection. Diagn Microbiol Infect Dis. 2009;64:152-157.
50 Labelle AJ, Micek ST, Roubinian N, Kollef MH. Treatment-related risk factors for hospital mortality in Candida bloodstream infections. Crit Care Med. 2008;36:2967-2972.
51 Odds FC, Hanson MF, Davidson AD, et al. One year prospective survey of Candida bloodstream infections in Scotland. J Med Microbiol. 2007;56:1066-1075.
52 Yapar N, Uysal U, Yucesoy M, Cakir N, Yuce A. Nosocomial bloodstream infections associated with Candida species in a Turkish University Hospital. Mycoses. 2006;49:134-138.
53 Lyytikainen O, Ruotsalainen E, Jarvinen A, Valtonen V, Ruutu P. Trends and outcome of nosocomial and community-acquired bloodstream infections due to Staphylococcus aureus in Finland, 1995-2001. Eur J Clin Microbiol Infect Dis. 2005;24:399-404.
54 Turnidge JD, Kotsanas D, Munckhof W, et al. Staphylococcus aureus bacteraemia: a major cause of mortality in Australia and New Zealand. Med J Aust. 2009;191:368-373.
55 Ekkelenkamp MB, van der Bruggen T, van de Vijver DA, Wolfs TF, Bonten MJ. Bacteremic complications of intravascular catheters colonized with Staphylococcus aureus. Clin Infect Dis. 2008;46:114-118.
56 Park KH, Kim SH, Song EH, et al. Development of bacteraemia or fungaemia after removal of colonized central venous catheters in patients with negative concomitant blood cultures. Clin Microbiol Infect. 2010;16:742-746. Epub 2009 Sep 11
57 Ruhe JJ, Menon A. Clinical significance of isolated Staphylococcus aureus central venous catheter tip cultures. Clin Microbiol Infect. 2006;12:933-936.
58 Garey KW, Rege M, Pai MP, et al. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis. 2006;43:25-31.
59 Trick WE, Fridkin SK, Edwards JR, Hajjeh RA, Gaynes RP. Secular trend of hospital-acquired candidemia among intensive care unit patients in the United States during 1989-1999. Clin Infect Dis. 2002;35:627-630.
60 Lee I, Zaoutis TE, Fishman NO, Morales KH, Nachamkin I, Lautenbach E. Risk factors for fluconazole resistance in patients with Candida glabrata bloodstream infection: Potential impact of control group selection on characterizing the association between previous fluconazole use and fluconazole resistance. Am J Infect Control. 2010;38:456-460.
61 Siempos II, Kopterides P, Tsangaris I, Dimopoulou I, Armaganidis AE. Impact of catheter-related bloodstream infections on the mortality of critically ill patients: a meta-analysis. Crit Care Med. 2009;37:2283-2289.
62 Soufir L, Timsit JF, Mahe C, Carlet J, Regnier B, Chevret S. Attributable morbidity and mortality of catheter-related septicemia in critically ill patients: a matched, risk-adjusted, cohort study. Infect Control Hosp Epidemiol. 1999;20:396-401.
63 Leibovici L, Drucker M, Konigsberger H, et al. Septic shock in bacteremic patients: risk factors, features and prognosis. Scand J Infect Dis. 1997;29:71-75.
64 Forgacs IC, Eykyn SJ, Bradley RD. Serious infection in the intensive therapy unit: a 15-year study of bacteraemia. Q J Med. 1986;60:773-779.
65 Smith RL, Meixler SM, Simberkoff MS. Excess mortality in critically ill patients with nosocomial bloodstream infections. Chest. 1991;100:164-167.
66 Rello J, Ricart M, Mirelis B, et al. Nosocomial bacteremia in a medical-surgical intensive care unit: epidemiologic characteristics and factors influencing mortality in 111 episodes. Intensive Care Med. 1994;20:94-98.
67 Pittet D, Wenzel RP. Nosocomial bloodstream infection in the critically ill. JAMA. 1994;272:1819-1820.
68 Digiovine B, Chenoweth C, Watts C, Higgins M. The attributable mortality and costs of primary nosocomial bloodstream infections in the intensive care unit. Am J Respir Crit Care Med. 1999;160:976-981.
69 Rello J, Ochagavia A, Sabanes E, et al. Evaluation of outcome of intravenous catheter-related infections in critically ill patients. Am J Respir Crit Care Med. 2000;162:1027-1030.
70 Dimick JB, Pelz RK, Consunji R, Swoboda SM, Hendrix CW, Lipsett PA. Increased resource use associated with catheter-related bloodstream infection in the surgical intensive care unit. Arch Surg. 2001;136:229-234.
71 Payne NR, Carpenter JH, Badger GJ, Horbar JD, Rogowski J. Marginal increase in cost and excess length of stay associated with nosocomial bloodstream infections in surviving very low birth weight infants. Pediatrics. 2004;114:348-355.
72 Rangel-Frausto MS, Pittet D, Hwang T, Woolson RF, Wenzel RP. The dynamics of disease progression in sepsis: Markov modeling describing the natural history and the likely impact of effective antisepsis agents. Clin Infect Dis. 1998;27:185-190.
73 Gonsalves WI, Cornish N, Moore M, Chen A, Varman M. Effects of volume and site of blood draw on blood culture results. J Clin Microbiol. 2009;47:3482-3485.
74 Harbarth S, Garbino J, Pugin J, Romand JA, Lew D, Pittet D. Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. Am J Med. 2003;115:529-535.
75 Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. 2000;118:146-155.
76 Harbarth S, Ferriere K, Hugonnet S, Ricou B, Suter P, Pittet D. Epidemiology and prognostic determinants of bloodstream infections in surgical intensive care. Arch Surg. 2002;137:1353-1359. discussion 9
77 Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of Candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother. 2005;49:3640-3645.
78 Parkins MD, Sabuda DM, Elsayed S, Laupland KB. Adequacy of empirical antifungal therapy and effect on outcome among patients with invasive Candida species infections. J Antimicrob Chemother. 2007;60:613-618.
79 Bouadma L, Luyt CE, Tubach F, et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375:463-474.
80 Harbarth S, Albrich WC, Muller B. When once is not enough–further evidence of procalcitonin-guided antibiotic stewardship. Crit Care. 2009;13:165.
81 Nobre V, Harbarth S, Graf JD, Rohner P, Pugin J. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med. 2008;177:498-505.
82 Fernandez-Hidalgo N, Almirante B, Calleja R, et al. Antibiotic-lock therapy for long-term intravascular catheter-related bacteraemia: results of an open, non-comparative study. J Antimicrob Chemother. 2006;57:1172-1180.
83 Fortun J, Grill F, Martin-Davila P, et al. Treatment of long-term intravascular catheter-related bacteraemia with antibiotic-lock therapy. J Antimicrob Chemother. 2006;58:816-821.
84 Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1-45.
85 Maki DG, Mermel LA, Bennett JV, Brachman PS. Hospital Infections, 4th ed. Philadelphia: Lippincott-Raven; 1998.
86 Rosen AB, Fowler VGJr, Corey GR, et al. Cost-effectiveness of transesophageal echocardiography to determine the duration of therapy for intravascular catheter-associated Staphylococcus aureus bacteremia. Ann Intern Med. 1999;130:810-820.
87 Pittet D, Hugonnet S, Harbarth S, et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Infection Control Programme. Lancet. 2000;356:1307-1312.
88 Hugonnet S, Perneger TV, Pittet D. Alcohol-based handrub improves compliance with hand hygiene in intensive care units. Arch Intern Med. 2002;162:1037-1043.
89 World Health Organization. WHO guidelines for hand hygiene in health care. Geneva: World Health Organization; 2009.
90 Pessoa-Silva CL, Hugonnet S, Pfister R, et al. Reduction of health care associated infection risk in neonates by successful hand hygiene promotion. Pediatrics. 2007;120:e382-e390.
91 Eggimann P, Harbarth S, Constantin MN, Touveneau S, Chevrolet JC, Pittet D. Impact of a prevention strategy targeted at vascular-access care on incidence of infections acquired in intensive care. Lancet. 2000;355:1864-1868.
92 Eggimann P, Hugonnet S, Sax H, Harbarth S, Chevrolet JC, Pittet D. Long-term reduction of vascular access-associated bloodstream infection. Ann Intern Med. 2005;142:875-876.
93 Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725-2732.
94 Pronovost PJ, Goeschel CA, Colantuoni E, et al. Sustaining reductions in catheter related bloodstream infections in Michigan intensive care units: observational study. BMJ. 2010;340:c309.
95 Zingg W, Imhof A, Maggiorini M, Stocker R, Keller E, Ruef C. Impact of a prevention strategy targeting hand hygiene and catheter care on the incidence of catheter-related bloodstream infections. Crit Care Med. 2009;37:2167-2173. quiz 80
96 Timsit JF, Schwebel C, Bouadma L, et al. Chlorhexidine-impregnated sponges and less frequent dressing changes for prevention of catheter-related infections in critically ill adults: a randomized controlled trial. JAMA. 2009;301:1231-1241.
97 Ruschulte H, Franke M, Gastmeier P, et al. Prevention of central venous catheter related infections with chlorhexidine gluconate impregnated wound dressings: a randomized controlled trial. Ann Hematol. 2009;88:267-272.
98 Climo MW, Sepkowitz KA, Zuccotti G, et al. The effect of daily bathing with chlorhexidine on the acquisition of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and healthcare-associated bloodstream infections: results of a quasi-experimental multicenter trial. Crit Care Med. 2009;37:1858-1865.
99 Niel-Weise BS, Stijnen T, van den Broek PJ. Anti-infective-treated central venous catheters for total parenteral nutrition or chemotherapy: a systematic review. J Hosp Infect. 2008;69:114-123.
100 Ramritu P, Halton K, Collignon P, et al. A systematic review comparing the relative effectiveness of antimicrobial-coated catheters in intensive care units. Am J Infect Control. 2008;36:104-117.
101 Walder B, Pittet D, Tramer MR. Prevention of bloodstream infections with central venous catheters treated with anti-infective agents depends on catheter type and insertion time: evidence from a meta-analysis. Infect Control Hosp Epidemiol. 2002;23:748-756.
102 Raad II, Fang X, Keutgen XM, Jiang Y, Sherertz R, Hachem R. The role of chelators in preventing biofilm formation and catheter-related bloodstream infections. Curr Opin Infect Dis. 2008;21:385-392.
103 Brun-Buisson C, Abrouk F, Legrand P, Huet Y, Larabi S, Rapin M. Diagnosis of central venous catheter-related sepsis. Critical level of quantitative tip cultures. Arch Intern Med. 1987;147:873-877.
104 Sherertz RJ, Raad II, Belani A, et al. Three-year experience with sonicated vascular catheter cultures in a clinical microbiology laboratory. J Clin Microbiol. 1990;28:76-82.
105 Kite P, Dobbins BM, Wilcox MH, McMahon MJ. Rapid diagnosis of central-venous-catheter-related bloodstream infection without catheter removal. Lancet. 1999;354:1504-1507.
106 Blot F, Nitenberg G, Chachaty E, et al. Diagnosis of catheter-related bacteraemia: a prospective comparison of the time to positivity of hub-blood versus peripheral-blood cultures. Lancet. 1999;354:1071-1077.
107 Almuneef MA, Memish ZA, Balkhy HH, Hijazi O, Cunningham G, Francis C. Rate, risk factors and outcomes of catheter-related bloodstream infection in a paediatric intensive care unit in Saudi Arabia. J Hosp Infect. 2006;62:207-213.
108 Rosenthal VD, Guzman S, Migone O, Crnich CJ. The attributable cost, length of hospital stay, and mortality of central line-associated bloodstream infection in intensive care departments in Argentina: A prospective, matched analysis. Am J Infect Control. 2003;31:475-480.
109 Blot SI, Depuydt P, Annemans L, et al. Clinical and economic outcomes in critically ill patients with nosocomial catheter-related bloodstream infections. Clin Infect Dis. 2005;41:1591-1598.
110 Elward AM, Hollenbeak CS, Warren DK, Fraser VJ. Attributable cost of nosocomial primary bloodstream infection in pediatric intensive care unit patients. Pediatrics. 2005;115:868-872.
111 Warren DK, Quadir WW, Hollenbeak CS, Elward AM, Cox MJ, Fraser VJ. Attributable cost of catheter-associated bloodstream infections among intensive care patients in a nonteaching hospital. Crit Care Med. 2006;34:2084-2089.
112 Higuera F, Rangel-Frausto MS, Rosenthal VD, et al. Attributable cost and length of stay for patients with central venous catheter-associated bloodstream infection in Mexico City intensive care units: a prospective, matched analysis. Infect Control Hosp Epidemiol. 2007;28:31-35.