6.3 Acute asthma
Introduction
The National Asthma Campaign for Australia updated the 2002 guidelines in 20061 and this forms the basis of the chapter. However, there are other best practice guidelines2,3 and national guidelines4–7 and these are important resources for cross-reference and comparison to highlight the controversies.
Acute asthma is one of the commonest reasons for presentation to an emergency department and admission to a hospital. A recent review of admissions to nine paediatric emergency departments in Australia and New Zealand, examining over 300 000 presentations, demonstrated that acute asthma was the fourth most common presentation, accounting for 3.5% of the total number of presentations.8 It is well recognised that in many cases admission to hospital may be preventable9 if managed effectively by the family and medical team involved with a child’s care. There are still great gaps between best practice guidelines and what actually happens in practice.10–12 Practice is highly variable, particularly for severe to critical acute asthma.13
History
Consider acute asthma when a child presents with signs of increase work of breathing, widespread wheezing and shortness of breath. There are other causes to consider such as mycoplasma pneumonia, aspiration, inhaled foreign body, and cardiac failure (Table 6.3.1). In the setting of a child with a previous history of asthma or where asthma seems the most likely diagnosis, one can perform a primary assessment of severity and institute the initial treatment at the onset of history taking.
It is important to understand the patterns of asthma in children – infrequent episodic, frequent episodic, and persistent.1 The pattern of asthma determines the need for preventive therapy. When a child is discharged from the emergency department (ED) or ward, consideration of the child’s preventative treatment is essential.
Infrequent episodic asthma
Infrequent episodic asthma (IEA) is the most common pattern, accounting for 70 to 75% of children with asthma. In this pattern, children have isolated episodes of asthma lasting from 1 to 2 days up to 1 to 2 weeks, usually triggered by an upper respiratory tract infection (URTI) or an environmental allergen. The episodes are usually more than 6 to 8 weeks apart and these children are asymptomatic in the interval periods. They require management of the individual episode only and regular preventive therapy is unnecessary. Within this group there is a wide range of severity. Most are mild, but this group accounts for up to 60% of paediatric hospital admissions for asthma.1
Frequent episodic asthma
Frequent episodic asthma (FEA) accounts for approximately 20% of childhood asthma. This pattern is similar to IEA but the interval between episodes is shorter, less than 6 to 8 weeks, and the children have only minimal symptoms, such as exercise-induced wheeze, in the interval period. These children may benefit from regular preventive therapy such as low dose (not greater than 400 mcg per day) inhaled corticosteroids or leukotriene antagonist. Commonly, these children are troubled through the winter months only and may require preventive treatment for that part of the year.1
Persistent asthma
Persistent asthma (PA) accounts for 5–10% of childhood asthma. These children can have acute episodes like the categories above, but they also have symptoms on most days in the interval periods. These symptoms commonly include: sleep disturbance due to wheeze or cough, early morning chest tightness, exercise intolerance and spontaneous wheeze. Again, there is a wide range of severity in this group, ranging from those with mild symptoms 4 to 5 days per week readily controlled with low-dose corticosteroid preventive therapy, to those with frequent severe symptoms and abnormal lung function requiring intensive therapy.1
Acute episode
Examination
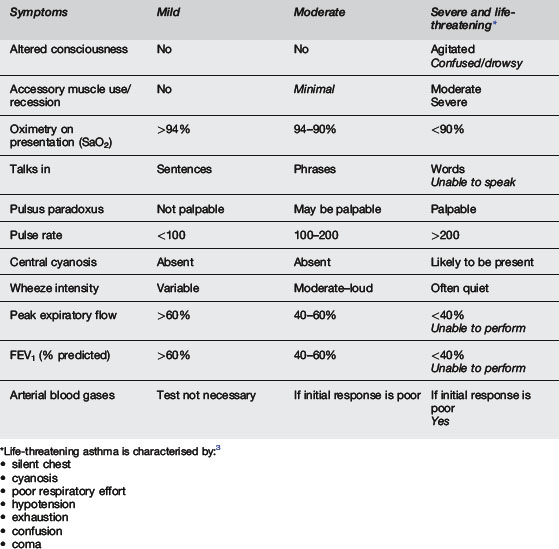
The most important parameters in the assessment of the severity of acute childhood asthma are general appearance/mental state and work of breathing (accessory muscle use, recession), as indicated in Table 6.3.2. Initial SaO2 in air, heart rate and ability to talk are helpful but less reliable additional features. Wheeze intensity, pulsus paradoxus, and peak expiratory flow rate are not reliable.2 Clinical signs of acute asthma correlate poorly with the severity of the asthma attack and none of the signs in isolation are predictive of severity.4 Classification of an acute attack, using the NAC Australia guidelines1 is as follows:
Differential diagnosis
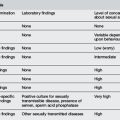
During an acute episode of wheezing, asymmetry on auscultation is often found due to mucous plugging, but warrants consideration of foreign body. Consider other causes of wheeze (e.g. bronchiolitis, mycoplasma, aspiration, heart failure, or foreign body). Chronically wheezy children may have a diagnosis other than asthma, such as cystic fibrosis, cilial dyskinesia, immune dysfunction, developmental/congenital abnormality, upper airway problems or bronchiectasis. There may be clues in the family or perinatal history or symptoms and signs that may suggest an alternative diagnosis to asthma.4
Treatment
Treatment – mild
Treatment – moderate
Treatment – severe
Treatment – life threatening
Disposition
Each child should have a written action plan on discharge from the ED. Observe the child’s inhaler technique before discharge. Advise parents to seek further medical review should their child’s condition deteriorate or if there is no significant improvement within 48 hours. At discharge all patients should have an outpatient appointment or appropriate follow up arranged with a paediatrician or local doctor as appropriate. Parents should be informed of other sources of information about asthma such as the Asthma Foundation. The concept of an asthma discharge pack is useful to ensure all aspects of discharge are considered. Adult data suggest that self-monitoring, regular review and written action plans can improve outcomes.17 Two paediatric studies suggest that an intensive nurse-led discharge concentrating on education, written action plans and inhaler technique, appropriate follow up with discharge prescription for steroids can reduce readmissions and following morbidity.18,19 A child should be ready for discharge when it is considered that they can be stable on 3–4-hour inhaled bronchodilators.20 This is often a subjective decision.
Discharge pack
 Review need for preventative treatment.
Review need for preventative treatment.
Initial preventative treatment for frequent episodic asthma is inhaled steroids.
Prognosis
Those with episodic asthma tend to improve throughout childhood, with asthma resolving by their adult years in approximately two-thirds. Those who continue to wheeze tend to have very mild asthma and maintain normal lung function. On the other hand, those with persistent asthma in childhood are more likely to continue to wheeze in their adult years (about two-thirds), with some impairment of lung function. Available evidence suggests that treatment does not influence the natural history of childhood asthma.1
Prevention
There are two areas of prevention to consider but the evidence is mostly inconclusive and confusing.4
Primary prophylaxis
Secondary prophylaxis
If an organised discharge from hospital is completed as per recommendations (as above), then this is more likely to prevent readmission or re-presentation and reduce morbidity. A number of preventable factors associated with admission have been identified and these issues should be addressed at discharge: adherence issues, prophylactic treatment, action plan use and advice to prevent delay in seeking medical advice.9
 Bronchodilator aerosol delivery
Bronchodilator aerosol delivery
Pressurised metered dose inhalers (pMDI) and spacers are an effective way of delivering inhalation medication to treat mild to moderate and probably severe attacks of asthma.21 Successful implementation22,23 and sustained use of spacers after changeover from nebulisers has been demonstrated.24 There are fewer side effects such as tachycardia, vomiting and hypoxia compared to when a drug is given via a nebuliser.21 There is still debate as to cost in different countries and whether the pMDI/spacer combination use in adult acute asthma is as effective as nebulisers.21 The pMDI/spacer combination is used less frequently in adult patients.10 For mild and moderate asthma all of the eleven Clinical Practice Guidelines (CPG) in the PREDICT study recommended MDI and spacers; for severe asthma half of the CPG recommended spacer delivery and for critical asthma spacers were not recommended at all.13 In the UK there has been a significant increase in the use of spacers in the hospital setting despite the asthma severity remaining stable over the last decade.12 There are no data on the use of spacers in severe to life threatening asthma but clearly they are being used in the severe group.13
It probably doesn’t make much difference what doses are given; if there is a response, cut down the dose, if there is no response, increase it! There are similar dosing recommendations for nebulised bronchodilator; between 2.5 mg and 5 mg of salbutamol per nebuliser every 20 minutes1–7 or frequent doses of 5–10 mg of nebulised teburtaline.4 There is little evidence for any benefit for continuous nebulised (0.5% undiluted salbutamol) treatment compared to frequent intermittent doses of treatment4 although many CPG recommend use in life-threatening exacerbations.1–3 Indeed all the sites in the PREDICT study used continuous nebulised bronchodilator treatment in life-threatening asthma and 64% of sites recommended use in severe attacks.13 There are some interesting data in the adult literature addressing the use of long acting β agonists in acute asthma, e.g. formoterol, which has a rapid onset of action and long duration of effect6 (see Research/Future Directions section). Current BTS recommendation are, however, to stop long acting β2 agonists if short acting β2 agonists are required more than 4-hourly.4
 Ipratropium bromide
Ipratropium bromide
Repeated early doses of ipratropium bromide during the first 1–2 hours of presentation are associated with improved outcomes;25 efficacy and safety have been demonstrated. It is recommended for children with severe and life-threatening attacks1–7 but often overused in children with moderate and mild attacks’10 with 37% of physicians reporting its use in moderate asthma exacerbations and 3% use in mild.13 Although doses of inhaled ipratropium bromide are recommended for use with a metered dose inhaler and spacer1–3,5 there are no data demonstrating that these doses are appropriate and this is a consensus statement recommendation. Different doses are recommended.
Standard doses of 250–500 mcg per dose for nebulised treatment seems universal.1–7 Repeated doses of ipratropium bromide should be given early in children who are not responsive to β2 agonists.4 It is not clear whether there are any benefits from continued use after the attack has shown response to treatment. Once hospitalised, the addition of ipratropium to salbutamol and steroids appears to add no benefit.26 BTS recommend that the dose should be weaned down to 4–6-hourly or stopped once admitted.4
 Intravenous bronchodilators
Intravenous bronchodilators
The main problem with deciding which intravenous bronchodilator to use is that there are no good direct comparisons with the three intravenous treatments commonly used. There are certainly drug versus placebo studies but no head to head studies to endorse recommendations. Thus the referenced guidelines vary with recommendations.1–7 The role of intravenous bronchodilators in addition to nebulised treatment remains unclear.27
Salbutamol – continuous intravenous infusions are recommended following on from a bolus or initial infusion, if the child does not respond, but different doses are recommended:28
The guidelines from the Global Initiative for Asthma (GINA) do not recommend intravenous β2 agonists, stating there is no evidence for their benefit25 and the AAP do not mention intravenous therapy.7
Aminophylline – there is no evidence that there is a role for aminophylline in children with mild to moderate exacerbations of their asthma. However, if a child is unresponsive to maximal inhaled therapy as above, then in the more severe and life-threatening attacks, aminophylline has been shown to have an effect on outcome (intubation).29,30
This is clearly a controversial area. There is no doubt that vomiting is a serious side effect from the higher doses of aminophylline and this may be the reason not to use it.29
Magnesium – intravenous magnesium relaxes smooth muscle and causes bronchodilation. Its exact place in the treatment of acute asthma in children has yet to be established. Doses of 40–100 mg kg–1 as a 20-minute infusion have been used with varying effects on lung function and asthma severity scores when compared to placebo.31 NAC recommend using 50% 0.1 mL kg–1 (50 mg kg–1) IV over 20 minutes for severe and life-threatening episodes, followed by an infusion of 0.06 ml kg–1 hr–1 (30 mg kg–1 hr–1): target serum levels 1.5–2.5 mmol L–1.1 The use of intravenous magnesium sulfate in severe and critical exacerbations is discussed in the BTS guidelines but not fully endorsed4 and GINA5 does not recommend its use routinely in children but mentions it may be of benefit in severe asthma (no dose discussed). Magnesium is not mentioned in the other guidelines2,3,6,7 for use in children. It is clearly being used in EDs in Australia and New Zealand in 50% of critical and 18% of severe exacerbations.13
Nebulised magnesium may have a role in adult asthma but there are few data on its role in children.6 None of the guidelines recommend its use. A multicentre double blind RCT study of nebulised magnesium is currently under way in the UK.32
Adrenaline – this bronchodilator is not mentioned in the paediatric guidelines.1–5 However, the GINA guidelines suggest it may be of use.6 There is no doubt that some acute asthma may be anaphylaxis 33 and intramuscular adrenaline (epinephrine) may well have a role.6 The dose suggested is 0.01 mg kg–1 up to 0.3–0.5 mg of 1:1000 adrenaline (epinephrine) every 20 minutes for three doses for acute asthma.5 This treatment is very rarely used in acute asthma in a paediatric population.10
 Corticosteroids
Corticosteroids
There is no doubt that early use of steroids for an acute attack reduces not only the need for admission but also morbidity.4,34 If a child can take a dose orally there is no benefit to administering it intravenously.33,35 There are a number of different dosing regimens suggested: A 2-day course of dexamethasone has been shown to be as effective as a 5-day course of prednisolone and there may be some suggestions that this may help adherence.36
Inhaled steroids – there is insufficient evidence in children that inhaled steroids should replace oral or systemic steroids in acute exacerbations.4 There are some adult data to suggest that high-dose inhaled steroids may have a value.5
 Leukotriene antagonists
Leukotriene antagonists
Initiating oral leukotriene antagonists in primary care early at the onset of an exacerbation can result in reduced symptoms, time off school, healthcare resource utilisation and parental time off work in children with intermittent asthma.37 There is no clear evidence that there is a role as intravenous therapy in acute asthma exacerbations; only adult data exist.38 Current studies are reviewing the use of leukotriene antagonists in the management of acute asthma in children.
 Children less than 2 years of age
Children less than 2 years of age
This age group can be difficult to assess and the different phenotypes of acute wheezing and indeed different labels used in different countries can cause problems when examining the literature on appropriate treatment.4 In the recent update of the BTS guidelines where asthma is considered to be the likeliest diagnosis, β2 agonists delivered by spacer and pMDI is the optimum delivery device; oral β2 agonists are not recommended. There is little evidence that β2 agonists or ipratropium bromide have an impact on wheezy children of this age in regard to their need for hospitalisation or length of stay.4,39 Steroid therapy may have a role but this is still not clear.4 There are problems differentiating between bronchiolitis and asthma in younger infants and this is outside the scope of this chapter.
 Intensive care management
Intensive care management
The NAC and BTS recommendation is that once a child has severe enough asthma to require intravenous treatment they should be referred to a PICU or an PHDU even if they do not require intubation.1,4 There are a number of indications for intensive care admission: deteriorating lung function, persisting or worsening hypoxia, hypercapnia, exhaustion, drowsiness, confusion, coma or respiratory arrest.4 These features are clearly all part of a spectrum and the overall picture, plus lack of response to treatment, should indicate that the child should be admitted to a high dependency or intensive care area. They may not necessarily require ventilation. There are no absolute criteria and the decision needs to be made by an experienced physician/anaesthetist.4,6 Non-invasive ventilation has had some success in adults but none of the paediatric guidelines recommend its use. Detailed discussion about ventilating a child with asthma is beyond the scope of this chapter. Helium and oxygen mixtures have been used in adults but again are not endorsed in the paediatric guidelines on current evidence.6
1 National Asthma Council Australia Asthma Management Handbook. http://www.nationalasthma.org.au/, 2007.
2 Royal Children’s Hospital, Melbourne. clinical guidelines. 2010. Available from http://www.rch.unimelb.edu.au/clinicalguide/ [accessed 13.10.10]
3 Sydney Children’s Hospital Randwick NSW. Australia. Available from http://www.sch.edu.au, 2010. [accessed 13.10.10]
4 British Thoracic Society and Scottish Intercollegiate Guidelines Network updated. Available from http://www.sign.ac.uk/guidelines/published/, 2009. [accessed 13.10.10]
5 Canadian Association for Emergency Physicians. Available from http://www.cps.ca/, 2009. [accessed 13.10.10]
6 Global Strategy for Asthma Management and Prevention. Available from http://www.ginasthma.com, 2008. [accessed 13.10.10]
7 Hegenbarth M.A. American Academy of Pediatrics Preparing for Pediatric Emergencies. Pediatrics. 2008;121:433-443.
8 Acworth J., Babl F., Borland M., et al. Patterns of presentation to the Australian and New Zealand Pediatric Research Network. Emerg Med Australas. 2009;21:59-66.
9 Ordonez G.A., Phelan P.D., Olinsky A., Robertson C.F. Preventable factors in hospital admissions for asthma. Arch Dis Child. 1998;78(2):143-147.
10 Kelly A.M., Powell C.V.E., Kerr D. Snapshot of acute asthma: treatment and outcome of patients with acute asthma treated in Australian emergency departments. Intern Med J. 2003;33:406-413.
11 Powell C.V., Raftos J., Kerr D., et al. Asthma in emergency departments: combined adult versus paediatric only centres. Paediatr Child Health. 2004;40:433-437.
12 Davies G., Payton J.Y., Beaton S.J., et al. Children admitted with acute wheeze/asthma during November 1998–2005: a national audit. Arch Dis Child. 2008;93:952-958.
13 Babl F.E., Sherriff N., Borland M., et al. Paediatric asthma management in Australia and New Zealand: practice patterns in the context of clinical guidelines. Arch Dis Child. 2008;93:307-312.
14 Robertson C.F., Rubinfield A.R., Bowes G. Pediatric asthma deaths in Victoria: the mild are at risk. Pediatr Pulmonol. 1992;12:95-100.
15 Martin A.J., Campbell D.A., Gluyas P.A., et al. Characteristics of near fatal asthma in children. Pediatr Pulmonol. 1995;20:1-8.
16 Strunk R.C., Mzrazek D.A., Wolfson Fuhrmann G.S., LaBrecque J.F. Physiologic and psychological characteristics associated with deaths due to asthma in childhood. JAMA. 1985;254:1193-1198.
17 Gibson P.G., Powell H., Wilson A., et al. Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst Rev. (3):2002. Art. No.:CD001117. doi:10.1002/14651858.CD001117
18 Madge P., McColl J., Payton J. Impact of a nurse led home management training programme in children admitted to hospital with acute asthma. A randomized controlled study. Thorax. 1997;3:223-228.
19 Wessldine L.J., McCarthy P., Silverman M. Structured discharge procedure for children admitted to hospital with acute asthma. A randomized controlled trail of nursing practice. Arch Dis Child. 1999;80:110-114.
20 Storman M.O., Mellis C.M., Van Asperen P.P., et al. Outcome evaluation of early discharge of asthmatic children from hospital: a randomized control trial. J Qual Clin Pract. 1999;19:149-154.
21 Cates C.J., Crilly J.A., Rowe B.H. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. (2):2006. Art. No.:CD000052. doi:10.1002/14651858.CD000052.pub2
22 Powell C.V., Maskell G.R., Marks M., et al. Successful implementation of spacer treatment guidelines for acute asthma. Arch Dis Child. 2001;84:142-146.
23 Gazarian M., Henry R.L., Wales S.R., et al. Evaluating the effectiveness of evidence-based guidelines for the use of spacer devices in children with acute asthma. Med J Aust. 2001;174:394-397.
24 Cheng N.G., Browne G.J., Lam L.T., et al. Spacer compliance after discharge following a mild to moderate asthma attack. Arch Dis Child. 2002;87(4):302-305.
25 Plotnick L.H., Ducharme F.M. Combined inhaled anticholinergic agents and beta-2 agonists for initial treatment of acute asthma in children (Cochrane Review). In: The Cochrane Library. Oxford: Update Software; 2001.
26 Goggin N., Macarthur C., Parkin P.C. Randomized trial of the addition of ipratropium bromide to albuterol and corticosteroid therapy in children hospitalized because of an acute asthma exacerbation. Arch Pediatr Adolesc Med. 2001;155:1329-1334.
27 Travers A.A., Jones A.P., Kelly K.D., et al. Intravenous beta2-agonists for acute asthma in the emergency department. Cochrane Database Syst Rev. (1):2001. Art. No.: CD002988. doi:10.1002/14651858.CD002988 . Cochrane Database Syst Rev 2010 Issue 1, Copyright © 2010 The Cochrane Collaboration
28 Browne G.J., Penna A.S., Phung X., Soo M. Randomised trial of intravenous salbutamol in early management of acute severe asthma in children. Lancet. 1997;49(9048):301-305.
29 Yung M., South M. Randomised controlled trial of aminophylline for severe asthma. Arch Dis Child. 1998;79:405-410.
30 Ream R.S., Loftus L.L., Albers G.M., et al. Efficacy of IV theophylline in children with severe status asthmaticus. Chest. 2001;119:1480-1488.
31 Rowe B.H., Bretzlaff J., Bourdon C., et al. Magnesium sulfate for treating exacerbations of acute asthma in the emergency department. Cochrane Database Syst Rev. (1):2000. Art. No.: CD001490. doi:10.1002/14651858.CD001490. Cochrane Database Syst Rev 2010 Issue 1, Copyright © 2010 The Cochrane Collaboration
32 Powell C.V.E. A randomised, placebo controlled study of nebulised magnesium in acute severe asthma in children. Available from http://www.controlled-trials.com/ISRCTN81456894 [accessed 13.10.10]
33 Rainbow J., Browne G.J. Fatal asthma or anaphylaxis? Emerg Med J. 2002;19(5):415-417.
34 Smith M., Iqbal S.M.S.I., Rowe B.H., N’Diaye T. Corticosteroids for hospitalised children with acute asthma. Cochrane Database Syst Rev. (1):2003. Art. No.:CD002886 . doi: 10.1002/14651858.CD002886. Cochrane Database Syst Rev 2010 Issue 1, Copyright © 2010 The Cochrane Collaboration
35 Barnett P., Caputo G.L., Baskin M., et al. Intravenous versus oral corticosteroids in acute pediatric asthma. Ann Emerg Med. 1997;29:212-217.
36 Qureshi F., Zaritsky A., Poirier M.P. Comparitive efficacy of oral dexamethasone versus oral prednisolone in acute pediatric asthma. J Paediatr. 2001;139:20-26.
37 Robertson C.F., Price D., Henry R., et al. Short course Montelukast for intermittent asthma in children: A randomize controlled trial. Am J Respir Critical Med. 2007;175:323-329.
38 Camargo C.A., Smithline H.A., Marie-Pierre M., et al. A randomized controlled trial of intravenous montelukast in acute asthma. Am J Respir Crit Care Med. 2003;167:528-533.
39 Everard M., Bara A., Kurian M., et al. Anticholinergic drugs for wheeze in children under the age of two years. Cochrane Database Syst Rev. (3):2005. Art. No.: CD001279. doi: 10.1002/14651858.CD001279.pub2