Acromioplasty
Steven R. Tippett and Mark R. Phillips
Before the broad topic of acromioplasty is addressed, the topic of subacromial impingement syndrome must be explored. In 1972 Neer1 described subacromial impingement as a distinct clinical entity. He correlated the anatomy of the subacromial space with the bony and soft tissue relationships and described the impingement zone. Neer2 also described a continuum of three clinical and pathologic stages. This study provides a basis for understanding the impingement syndrome, which ranges from reversible inflammation to full-thickness rotator cuff tearing. The relationships among the anterior third of the acromion, the coracoacromial ligament, and the acromioclavicular (AC) joint and the underlying subacromial soft tissue—including the rotator cuff—remain the basis for most of the subsequent surgery-related impingement studies. Many other researchers have contributed to the current knowledge regarding the subacromial shoulder impingement syndrome. The works of Meyer,3 Codman,4 Armstrong,5 Diamond,6 and McLaughlin and Asherman7 provide a historical perspective.
Surgical Indications And Considerations
Anatomic Etiologic Factors
Any abnormality that disrupts the intricate relationship within the subacromial space may lead to impingement. Both intrinsic (intratendinous) and extrinsic (extratendinous) factors have been implicated as etiologies of the impingement process. The role of muscle weakness within the rotator cuff has been described as leading to tension overload, humeral head elevation, and changes in the supraspinatus tendon, which is used most often in high-demand, repetitive overhead activities.8,9 Authors10–12 also have described inflammation and thickening of the bursal contents and their relationship to the impingement syndrome. Jobe13 and Jobe, Kvitne, and Giangarra11 studied the role of microtrauma and overuse in intrinsic tendonitis and glenohumeral instability and their implications for overhead-throwing athletes. Intrinsic degenerative tenopathy also has been discussed as an intrinsic cause of subacromial impingement symptoms.14
Extrinsic or extratendinous etiologic factors form the second broad category of causes of impingement syndrome. Rare secondary extrinsic factors (e.g., neurologic pathology secondary to cervical radiculopathy, supraspinatus nerve entrapment) are not discussed here, but the primary extrinsic factors and their anatomic relationships are of primary surgical concern. The unique anatomy of the shoulder joint sandwiches the soft tissue structures of the subacromial space (i.e., rotator cuff tendons, coracoacromial ligament, long head of biceps, bursa) between the overlying anterior acromion, AC joint, and coracoid process and the underlying greater tuberosity of the humeral head and the superior glenoid rim. Toivonen, Tuite, and Orwin15 have supported Bigliani, Morrison, and April’s description16 of three primary acromial types and their correlation to impingement and full-thickness rotator cuff tears. AC degenerative joint disease also can be an extrinsic primary cause of impingement disease.1,2 Many authors support Neer’s original position on the contribution of AC degenerative joint disease to the impingement process.17,18 The os acromiale, the unfused distal acromial epiphysis, also has been discussed as a separate entity and a potential etiologic factor related to impingement.19 Glenohumeral instability is a secondary extrinsic cause or contribution to impingement. Its relationship to the impingement syndrome is poorly understood, but it helps explain the failure of acromioplasty in the subset of young, competitive, overhead-throwing athletes with a clinical impingement syndrome.11,20,21
Diagnosis and Evaluation of the Impingement Syndrome
History and physical examinations are crucial in diagnosing subacromial impingement syndrome. Findings may be subtle, and symptoms may overlap in the various differential diagnoses; therefore, appreciating the impingement syndrome symptom complex may be difficult. The classic history has an insidious onset and a chronic component that develops over months, usually in a patient over 40 years old. The patient frequently describes repetitive activity during recreation, recreational sports, competitive athletics, and work. Pain is the most common symptom, especially pain with specific high-demand or repetitive away-from-the-chest and overhead shoulder activities. Night pain is seen later in impingement syndrome, after heightening of the inflammatory response. Weakness and stiffness may occur secondary to pain inhibition. If true weakness persists after the pain is eliminated, then the differential diagnoses of rotator cuff tearing or neurologic cervical entrapment type of pathologies must be addressed. If stiffness persists, then frozen shoulder–related conditions (e.g., adhesive capsulitis, inflammatory arthritis, degenerative joint disease) must be ruled out.22 Younger athletic and throwing patients need continual assessment for glenohumeral instability.
The physical examination of a patient with impingement syndrome focuses on the shoulder and neck regions. Physical examination of the neck helps rule out cervical radiculopathy, degenerative joint disease, and other disorders of the neck contributing to referred pain complexes in the shoulder area. The shoulder evaluation includes a general inspection for muscle asymmetry or atrophy, with emphasis on the supraspinatus region. Range of motion (ROM) and muscle strength testing and generalized glenohumeral stability testing are emphasized during the evaluation. The Neer impingement sign2 and Hawkins-Kennedy sign23 are gold standard tests to help diagnose impingement. The impingement test, which includes subacromial injection of a Xylocaine type of compound and repeated impingement sign maneuvers, is most helpful in ascertaining the presence of an impingement syndrome. The AC joint also is addressed during the shoulder evaluation. The clinician should note AC joint pain with direct palpation and pain on horizontal adduction of the shoulder. Selective AC joint injection also may be helpful. Long head biceps tendon pathology, including ruptures, is rare but may occur in this subset of patients. Physical examination will define the tendon’s contribution to the symptom complex. Instability testing, especially in the younger athletic patient, also should be performed. The clinician should assess for classic apprehension signs and perform the Jobe relocation test, recording any positive findings.
Radiographic Evaluation
Standard radiographic evaluation is carried out with special attention to anteroposterior (AP), 30° caudal tilt AP, and outlet views of the shoulder.24,25 These plain studies are helpful in demonstrating acromial anatomy types, hypertrophic coracoacromial ligament spurring, AC joint osteoarthrosis, and calcific tendonitis. These views, in combination with an axillary view, can uncover os acromiale lesions. Magnetic resonance imaging (MRI) also is helpful in revealing relationships in impingement syndrome, especially if rotator cuff tear and other internal derangement pathologies (e.g., glenolabral or biceps tendon pathologies) are suspected.26
Surgical Procedure
Subacromial impingement syndrome that has not responded to rehabilitation techniques and nonoperative means may require surgery. If proven trials of rehabilitation, activity modification, use of nonsteroidal antiinflammatory agents (NSAIDs), and judicious use of subacromial cortisone injections are unsuccessful, then acromioplasty and subacromial decompression (SAD) should be considered.
Historically, open acromioplasties produced excellent results and still have a significant role in surgical treatment.1,19,27 Ellman28 is credited with the first significant arthroscopic SAD techniques and studies, and many surgeons and investigators have developed techniques and arthroscopic SAD advancements for the surgical treatment of subacromial impingement syndrome.29–34 Indications for surgery to correct subacromial impingement syndrome include persistent pain and dysfunction that have failed to respond to nonsurgical treatment, including physician- or therapist-directed physical therapy, trials of NSAIDs, subacromial cortisone or lidocaine injections, and activity modification.
The most controversial surgical indication topic concerns the amount of time that should elapse before nonoperative management is considered a failure.19 Most surgeons and investigators recommend a trial period of approximately 6 months. However, this depends on the individual patient and pathologic condition and should be tailored to the circumstances. For example, a 42-year-old patient with a history of several months of progressive symptoms has an occupation or recreational activity that requires high-demand, repetitive overhead movement. In the absence of instability, with a hooked acromion (type III) and MRI-documented, partial-thickness tearing, this patient need not endure the 6-month trial period to meet surgical indications for the treatment of his condition. On the other hand, a noncompliant patient in a workers’ compensation–related situation who has a flat acromion and equivocal, inconsistent clinical findings may never meet the surgical indications.
Procedure
Both open acromioplasty and the arthroscopic SAD procedure are discussed in the following sections. Open acromioplasty techniques have been well documented, their outcomes have been well researched, and their results have been rated as very good to excellent in numerous studies.1,27,29,30,35 Because of these factors and the high technical demands of arthroscopic decompression, surgeons should never completely abandon this proven technique for the surgical management of persistent shoulder impingement. Surgeons also may resort to these open techniques in the event of arthroscopic procedure failure or intraoperative difficulties. Depending on surgical experience and expertise, an open procedure may be used in deference to an arthroscopic SAD procedure.
Arthroscopic SAD for the surgical treatment of impingement syndrome has a number of advantages. First, the arthroscopic technique allows evaluation of the glenohumeral joint for associated labral, rotator cuff, and biceps pathology, as well as assessment of the AC joint and surgical treatment of any condition contributing to impingement. Second, this technique produces less postoperative morbidity and is relatively noninvasive, minimizing deltoid muscle fiber detachment. However, arthroscopic SAD is a technically demanding procedure with a learning curve that can be higher than for other orthopedic procedures.
Many different arthroscopic techniques have been described, but the authors of this chapter recommend the modified technique initially described by Caspari.36 The patient is usually anesthetized with both a general and a scalene block regional anesthetic. In most community settings this combination has been highly successful in allowing patients to have this procedure done on an outpatient basis. A scalene regional block and home patient-controlled analgesia (PCA) provide acceptable pain control and ensure a comfortable postoperative course.
After the patient has reached the appropriate depth of anesthesia, the shoulder is evaluated in relationship to the contralateral side in both a supine and a semisitting beach chair position. Any concern regarding stability testing can be further assessed at this time, taking advantage of the complete anesthesia. Then, using the standard beach chair positioning, the surgeon begins the arthroscopic procedure. An inflow pressure pump (Davol) is used to maintain appropriate tissue space distention. Epinephrine is added to the irrigation solution to a concentration of 1 mg/L, thus enhancing hemostasis.
Specific portal placement is important to eliminate technical difficulties. Carefully addressing the palpable bony topography of the shoulder and marking the acromion, clavicle, AC joint, and coracoid process greatly facilitate portal placement (Fig. 3-1). First, the sulcus is palpated directly posterior to the AC joint. From this universal landmark, appropriate orientation can be obtained and consistent reproducible posterior, anterior, and lateral portal placement can be achieved.
Using the standard posterior portal, the surgeon inserts the arthroscope into the glenohumeral joint. In a routine and sequential fashion, the glenohumeral joint is evaluated with attention directed to the biceps tendon and the labral and rotator cuff anatomy. Any incidental pathology can be addressed arthroscopically at this point. Subacromial space arthroscopy can now be performed.
For subacromial procedures, a long diagnostic double-cannula arthroscope is recommended. The cannula with a blunt trocar is placed from the posterior portal superior to the cuff, and exits through the anterior portal.
Using this cannula as a switch stick equivalent, the surgeon places a cannula with a plastic diaphragm over the arthroscopic instrument and returns it to the subacromial space. Gently retracting the arthroscopic cannula and inserting the arthroscope allows the inflow and arthroscopic cannulas to be close together. Adequate distention and maintenance of inflow and outflow are crucial for visualization and indirect hemostasis. This technique has been successful in achieving these goals. At this point the lateral portal is fashioned, generally on the lateral aspect of the acromion just posterior and inferior to a line drawn by extending the topographic anatomy of the anterior AC complex (see Fig. 3-1). A spinal needle may assist in the accurate placement of this portal, which is crucial to instrument placement and subsequent visualization.
Starting from the posterior portal and using an aggressive synovial resector with the inflow in the anterior portal, the surgeon uses the lateral portal to perform a bursectomy and débride the soft tissue of the subacromial space. This is done in a sequential manner, working from the lateral bursal area to the anterior and medial AC regions. Spinal needles can be placed in the anterolateral and AC joint region to facilitate visualization and reveal spatial relationships. After the subacromial bursectomy and denudement of the undersurface of the acromion, the superior rotator cuff can be visualized along with the AC joint and anterior acromial anatomy is more easily defined. The surgeon must take care not to violate the coracoacromial ligament during this initial bursectomy procedure.
At this point the surgeon inserts the arthroscope in the lateral portal for visualization. Using the posterior portal and following the posterior slope of the normal acromion, the surgeon performs sequential acromioplasty with an acromionizer instrument. In the technique described by Caspari,36 the shank of the acromionizer is directed flat against the posterior acromial slope and acromioplasty is completed from the posterior to the anterior aspect. This accomplishes two goals. First, it provides a reliable and reproducible template to convert any abnormal hooked, sloped, or curved acromion to the therapeutic goal of a flat, type I configuration. Second, it allows for the removal of the coracoacromial ligament from its bony attachment with minimal chance for coracoacromial artery bleeding, thereby maximizing arthroscopic visualization and minimizing technical difficulties. At this point any further modification or “fine tuning” may be done through both the lateral and the anterior portals. Any residual coracoacromial ligament is removed from its acromial insertion while its bursal extension is excised.
The AC joint also may be assessed at this stage, and minimal inferior osteophytes may be excised. Depending on the results of the preoperative evaluation, distal clavicle procedures can be performed at this point either through directed arthroscopic techniques or, as the authors of this chapter prefer, through a small incision located over the AC joint region. If AC joint symptoms are present with horizontal adduction and direct palpation, if radiographs confirm the pathology, or if both occur, then the surgeon should proceed with a distal clavicle excision. A T-type capsular incision is located over the AC joint region, with the anterior and posterior capsular leaves elevated subperiosteally from the distal clavicle. Using small Homan retractors, the surgeon can excise the distal clavicle (usually 1.5 to 2 cm) with an oscillating saw. The distal clavicle can then be easily palpated and rasped smooth. With a simple digital confirmation, the undersurface of the acromion also can be checked and any residual osteophytes rasped through this minimal-incision technique.
The soft tissue is then closed in anatomic fashion, with essentially no deltoid detachment. A routine subcuticular skin closure is used. The patient is placed in a postoperative pouch sling, and cryotherapy is frequently suggested. The patient is discharged to continue treatment as an outpatient; if insurance or health demands require it, overnight observation is used. Physical therapy may begin immediately on the first postoperative day and should follow the standard program discussed previously.36
Outcomes
The surgical outcomes for arthroscopic SAD, partial acromioplasties, and distal clavicle excisions16,32,33 have been most favorable. Many studies have compared open and closed techniques and obtained similar overall findings.19,35,37 SAD procedures have three general goals:
Challenges and Precautions
The most common causes of surgical failures are associated with incomplete bone resection and not addressing AC joint arthropathy. These common pitfalls can be eliminated by carefully considering surgical techniques and including (if necessary) distal clavicle excision or combined open techniques. Another common reason for failure of arthroscopic SAD surgery is inappropriate diagnosis or patient selection. Again, with careful assessment—especially regarding instability, underlying lesions, and differential diagnoses—these failures can be lessened dramatically.
Rehabilitation Concerns: The Surgeon’s Perspective
Therapists spend more time with postoperative patients than most surgeons do, and their input and direction are important in achieving a successful outcome. Their understanding of the procedure, postoperative pain, patient apprehension, and general medical concerns is vital. Physical therapy–directed early diagnosis of any wound problems (evidenced by erythema) or superficial infection can eliminate potential major complications. Postoperative inflammation also can be assessed with careful observation. Stiffness in frozen shoulder syndrome, although rare, can develop postoperatively and is addressed optimally with early diagnosis and progressive physical therapy.
Therapy Guidelines For Rehabilitation
The goal of the therapeutic exercise program after a SAD procedure is to augment the surgical decompression by increasing the subacromial space. Additional clearance for subacromial structures can be gained by strengthening the scapular upward rotators and humeral head depressors. Exercises to enhance the surgical decompression are straightforward.
![]() The challenge for the physical therapist is to implement the appropriate therapeutic exercise regimen without overloading healing tissue.
The challenge for the physical therapist is to implement the appropriate therapeutic exercise regimen without overloading healing tissue.
The postoperative rehabilitation program can be divided into three phases:
1 Phase one emphasizes a return of ROM.
2 Phase two stresses regaining muscle strength.
3 Phase three stresses endurance and functional progression.
These three phases are not distinct entities and they do overlap. Together they serve as a template on which the physical therapist can build a management protocol for the post-SAD patient. An absence of pain is the primary guideline for progressing to more strenuous activities.38 The phases are simply guidelines and should be adapted to each patient. Patients with significant rotator cuff involvement, articular cartilage defects, significant preoperative motion or strength loss, perioperative or intraoperative complications, and glenohumeral instability require special consideration and may not progress as rapidly as indicated in the standard rehabilitation program, which assumes that no glenohumeral instability exists and that the rotator cuff tendons are intact.
Signs that therapeutic activities are too aggressive include the following:
• Increased levels of referred pain to the area of insertion of the deltoid
• Pain that lasts more than 2 hours after exercising39
• Pain that alters the performance of an activity or exercise39
Evaluation
Every rehabilitation program begins with a thorough evaluation at the initial physical therapy visit. This evaluation provides pertinent information for formulating a treatment program. As the patient progresses through the program, assessment is ongoing. Activities that are too stressful for healing tissue at one point are reassessed when the tissue is ready for the stress. Measures to be included in the physical therapy evaluation are provided in Box 3-1.
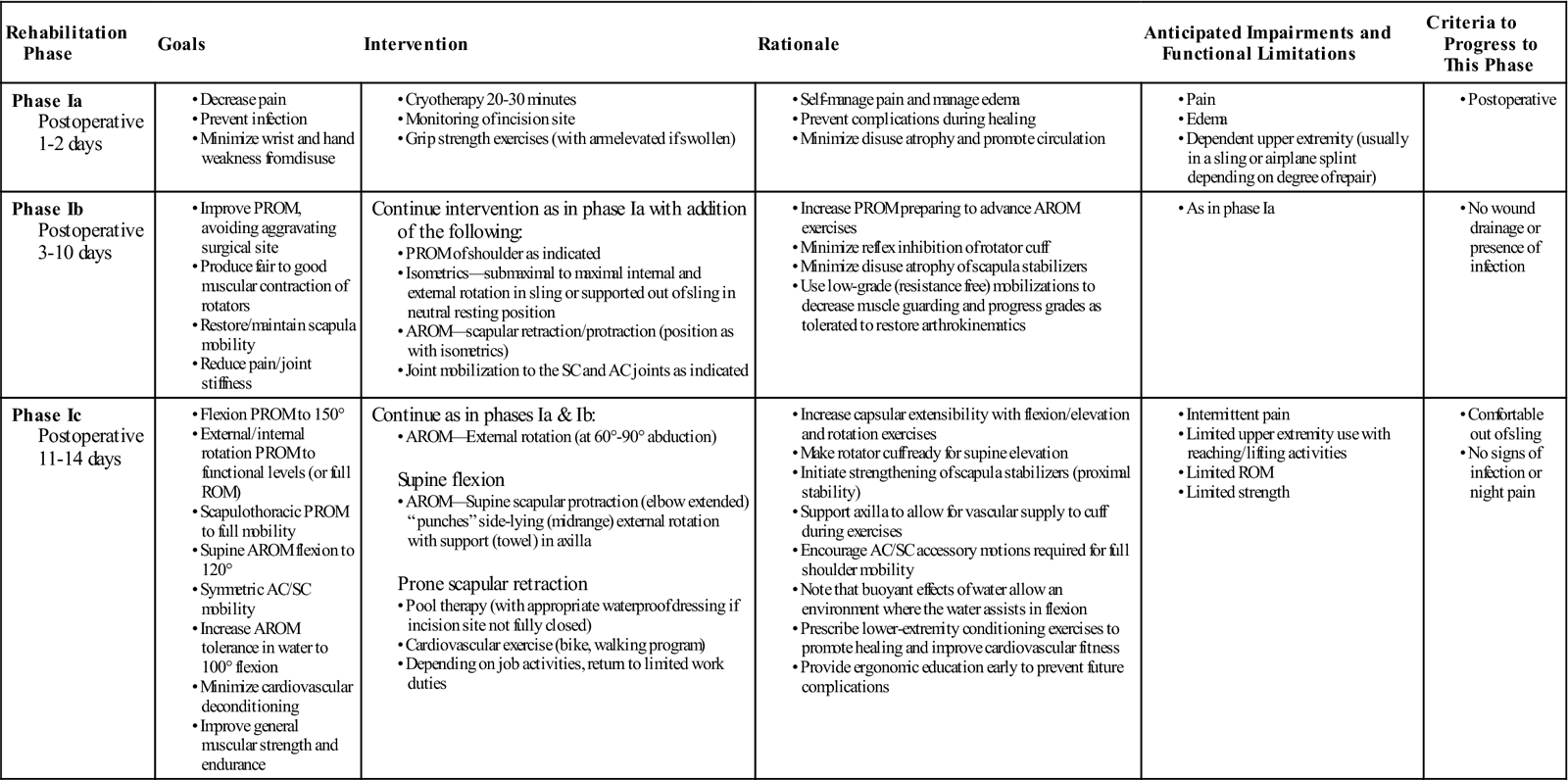
Phase I
TIME: First 2 to 3 weeks after surgery
GOALS: Emphasis on measures to control normal postoperative inflammation and pain, protect healing soft tissue, and minimize the effects of immobilization and activity restriction (Table 3-1)
TABLE 3-1
< ?comst?>
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Control of Inflammation and Pain.
The surgeon may have prescribed NSAIDs to control normal postoperative inflammation and pain. These can be an adjunct to the other means the physical therapist uses to decrease inflammation (i.e., gentle therapeutic exercise, cryotherapy).
The therapist should determine whether a scalene block was performed in addition to the general anesthetic. If a block was performed, then the onset of immediate postoperative pain may be delayed; the patient should be monitored for signs of delayed motor return and prolonged or abnormal hypesthesia. If narcotics are used past the first few postoperative days, then the therapist must undertake the therapeutic exercise program cautiously.
Cryotherapy can be used to help manage postoperative pain. Crushed ice conforms nicely to the shoulder, but commercially available cryotherapy and compression units (PolarCare, Cryocuff), although tedious to use, can be less messy. Sterile postoperative liners allow the source of the cold to be placed under the initial bulky dressing. The physical therapist should be aware of reimbursement practices for these units and use them accordingly.
Protection of Healing Soft Tissues.
Decreased use of the upper extremity is required to protect healing soft tissue after SAD. A sling may be prescribed depending on the surgeon’s protocol and operative findings. The sling helps decrease the forces on the supraspinatus tendon by centralizing the head of the humerus in the glenoid fossa in a dependent position. Use of the sling is encouraged for the first 2 to 3 days after surgery in most cases, with the patient’s level of discomfort dictating the degree of sling use.
Although the sling is used to minimize pain, it can add to the patient’s discomfort. A “critical zone” of hypovascularity in the supraspinatus tendon initially described by Rathbun and McNab40 may contribute to shoulder pain in a resting-dependent position. Some authors debate the existence of this critical zone, but recent work by Lohr and Ulthoff41 corroborates Rathbun and McNab’s initial findings. This critical zone corresponds to the anastomoses between osseous vessels and vessels within the supraspinatus tendon. Vessels in this critical zone fill poorly when the arm is at the side,2 but this wringing out of the supraspinatus tendon is not observed when the arm is abducted.42 If the patient experiences increased shoulder discomfort after prolonged periods with the arm at the side, then he or she should place a small bolster (2 to 3 inches in diameter) in the axilla (resting the arm in a supported, slightly abducted position) to help decrease the pain.
Immobilization and Restricted Activities.
Although the sling protects the healing tissue around the glenohumeral joint, motion should be encouraged at proximal and distal joints. Scapular protraction, retraction, and elevation can be performed in the sling. The patient should remove the arm from the sling at least three to four times daily to perform supported elbow, wrist, and hand ROM exercises.
The patient should always perform warm-up activities. This enhances the rate of muscular relaxation, increases the mechanical efficiency of muscle by decreasing viscous resistance, allows for greater hemoglobin and myoglobin dissociation in the time spent working, decreases resistance in the vascular bed, increases nerve conduction velocity, decreases the risk for electrocardiographic abnormalities, and increases metabolism.43
The physical therapist should educate the patient and help him or her to understand that discomfort experienced with passive stretching into external rotation comes from the capsule and occurs because the supraspinatus muscle is slack. Patients with sedentary occupations who do not have lifting duties typically can return to work during phase one. Those returning to work should perform scapular, elbow, wrist, and hand exercises during working hours.
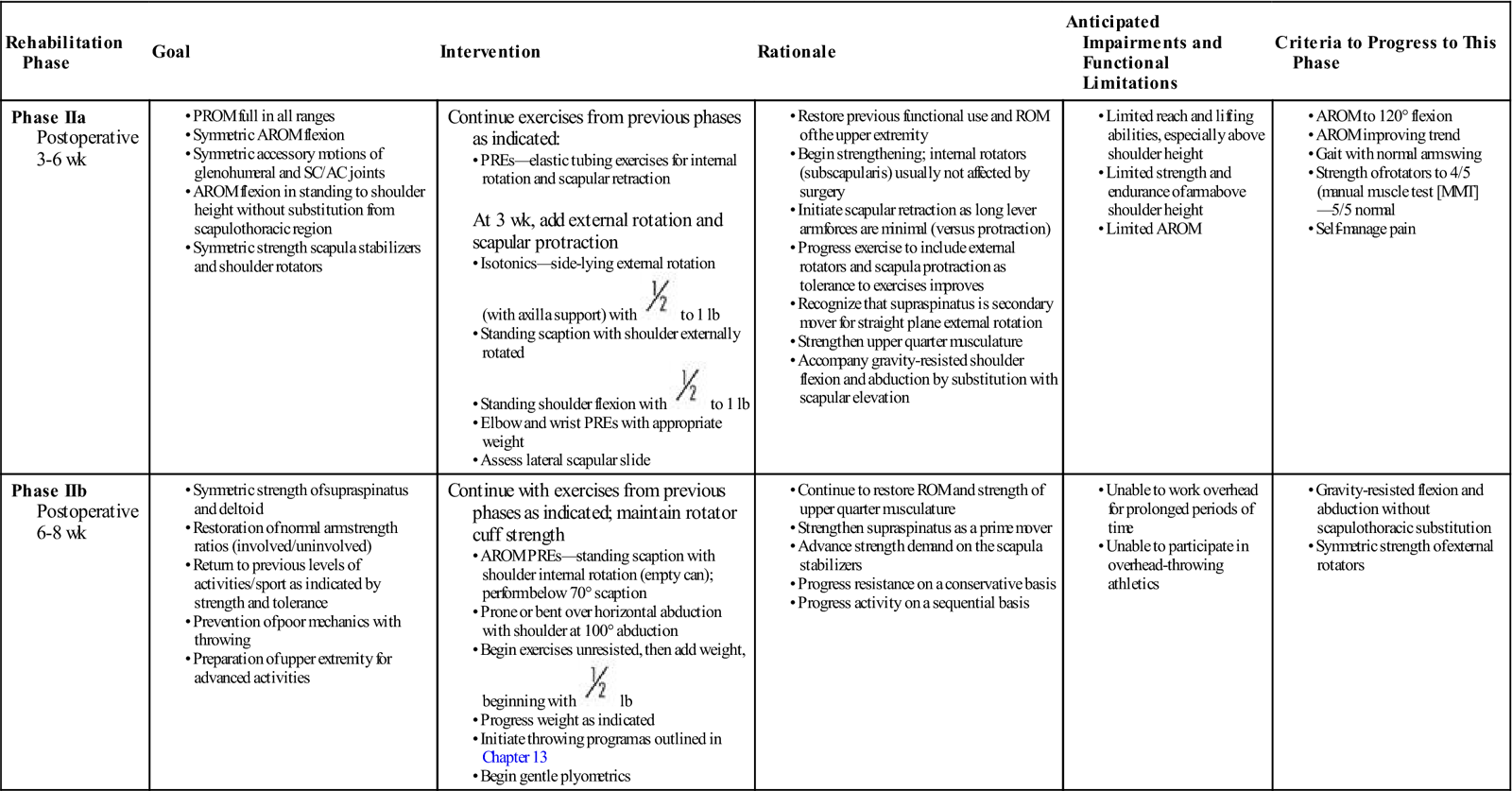
Phase II
TIME: From 3 to at least 6 weeks after surgery
GOALS: Emphasis on muscle strengthening, with continued work on rotator cuff musculature and scapula stabilizer strengthening (Table 3-2)
TABLE 3-2
< ?comst?>
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Many of the exercises used to strengthen the rotator cuff and scapular stabilizers have been assessed by electromyography (EMG).44 EMG (both superficial and fine wire) has been used to document electrical activity in the rotator cuff and intrascapular musculature during the performance of various therapeutic exercises. Strengthening of the rotator cuff muscles can be selectively progressed from supine active exercises to upright resistive exercises.45 Muscles of the rotator cuff (especially the supraspinatus) have relatively small cross-sectional areas and short lever arms. When working with them, the therapist should apply minimal resistance, starting at 8 oz, then increase to 1 lb, and then advance in 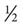 lb or 1 lb increments as tolerated. Weights seldom have to exceed 3 to 5 lb for the supraspinatus. The infraspinatus and subscapularis can be stressed to a greater degree, and weights can be progressed from 5 to 8 lb. The therapist should emphasize scapular stabilizer efforts for proximal stability before addressing distal mobility.
lb or 1 lb increments as tolerated. Weights seldom have to exceed 3 to 5 lb for the supraspinatus. The infraspinatus and subscapularis can be stressed to a greater degree, and weights can be progressed from 5 to 8 lb. The therapist should emphasize scapular stabilizer efforts for proximal stability before addressing distal mobility.
Townsend, Jobe, and Pink46 assessed the EMG output of three slips of deltoid, pectoralis major, latissimus dorsi, and the four rotator cuff muscles during 17 exercises. Findings from this study indicate that the majority of the muscles studied are most effectively recruited with the following:
• Scaption (with internal shoulder rotation)
• Flexion
Because the supraspinatus is the most frequently involved cuff muscle necessitating a subacromial decompression, diligent efforts to return supraspinatus strength are vital. The most effective exercise position to maximally recruit the supraspinatus has been evaluated in numerous studies with varying results. Elevation in the plane of the scapula (i.e., scaption) with the shoulder internally rotated is referred to as the empty-can position (Fig. 3-2).
![]() To decrease the likelihood of compressing the supraspinatus between the greater tuberosity of the humerus and the subacromial structures, care should be taken never to perform the empty-can exercise past 60° to 70° of elevation.
To decrease the likelihood of compressing the supraspinatus between the greater tuberosity of the humerus and the subacromial structures, care should be taken never to perform the empty-can exercise past 60° to 70° of elevation.
Scaption can also be performed with the humerus externally rotated (Fig. 3-3). Another position that is very effective in recruiting the supraspinatus is prone horizontal abduction of the shoulder, with the shoulder abducted to 100° (Fig. 3-4). In cases of secondary impingement related to glenohumeral instability, care must be taken not to position the arm to increase stress on static restraints. Inherent humeral head localization is enhanced during strengthening exercises by performing these activities in the plane of the scapula.47
Box 3-2 summarizes research findings relative to the most effective exercise position to recruit the supraspinatus.48–54
Remaining muscles of the rotator cuff cannot be neglected. The infraspinatus is most actively recruited via external rotation at zero degrees of abduction. This muscle also plays a significant role in activities involving abduction (in the plane of the body and plane of the scapula) especially with combined external rotation and when greater resistance is applied.55 The teres minor assists the infraspinatus in the above activities to a minor extent and is most effective in horizontal abduction of the humerus along with scapular retraction and glenohumeral extension.56 Two practical and comprehensive reviews of shoulder muscle activity and function in common shoulder exercises may be of particular benefit to the reader.57,58
Although isolation of specific muscles is vital to ensure a comprehensive strengthening program, work with muscles contracting in synchrony about a joint also is an important consideration. Wilk59 and Toivonen, Tuite, and Orwin15 note that in overhead activities the subscapularis is counterbalanced by the infraspinatus and teres minor in the transverse plane, whereas the deltoid is opposed by the infraspinatus and teres minor in the coronal plane. Because overhead movements are incorporated in the rehabilitation program, the physical therapist also should address the force couple of the upper and lower trapezius for scapular upward rotation. Upper and lower trapezius recruitment may increase during glenohumeral flexion and abduction exercises when using dynamic reactive instruments as opposed to elastic or cuff weight resistance.60 Multiplanar work can be beneficial by incorporating dynamic trunk, scapulothoracic, and glenohumeral activities simultaneously. Two activities in the standing position that recruit firing of the upper and lower trapezius before the serratus anterior include trunk extension with simultaneous unilateral scapular retraction/upward rotation combined with elbow flexion, glenohumeral extension, and external rotation (lawnmower exercise); and trunk extension with simultaneous bilateral scapular retraction/upward rotation with elbow flexion, glenohumeral extension, and external rotation (robbery exercise).61
When strengthening the shoulder internal rotators, do not work with the patient in a side-lying position. Lying on the involved shoulder often increases shoulder pain; therefore, internal rotation should be performed in the standing or prone position. When working on strengthening the supraspinatus and standing flexion and abduction in the same exercise session, perform the gravity-resisted elevation exercises before the strengthening ones. This sequence allows a nonfatigued supraspinatus to contribute effectively to achieve an adequate force couple. Cadaveric analysis of rotator cuff composition indicates that these muscles consist of a mix of type I and type II fibers.62 Resistance applied to the muscles should be a healthy fix of function-specific velocities and repetitions.
Exercise combinations can be used effectively to strengthen the muscles of the shoulder girdle. Wolf63 describes a “four square” combination of tubing-resisted flexion, extension, external rotation, and internal rotation (IR) followed by stretching of the external rotators and abductors. A combination of “around the world” exercises of flexion, abduction, and horizontal abduction followed by rotator cuff stretching also can be used during phase two. The physical therapist should use care when performing flexibility exercises of the rotator cuff because horizontal adduction can reproduce or cause impingement symptoms.
Strong scapular stabilizers are required to provide a stable base for the glenohumeral joint, elevate the acromion, and provide for retraction and protraction around the thoracic wall.64 Moseley and associates65 studied eight scapular muscles via indwelling EMG and identified a core group of four strengthening exercises that include scaption with external rotation (i.e., full can), rowing, press-ups, and push-ups with a plus. Ludewig and associates66 found a push-up with a plus to be effective in recruiting the serratus anterior with less activity of the trapezius musculature. Lear and Gross67 noted increased serratus anterior activity during a push-up with a plus with the feet elevated. Decker and associates68 noted push-ups with a plus (both traditional and on the knees), punching, scaption, and a dynamic hug all resulted in serratus anterior activity greater than 20% of a maximum voluntary contraction. Ekstrom, Donatelli, and Soderberg69 found a seated shoulder diagonal movement of forward flexion, horizontal adduction, and external rotation to be most effective in recruiting the serratus anterior when compared with nine other open-chain exercises.
In addition to careful observation of scapulohumeral rhythm during overhead motions, scapular stabilizer efficiency can be assessed with the lateral scapular slide test. This test, which was initially described by Kibler,64 involves observing and measuring scapular motion during abduction of the shoulder. The steps of the modified lateral scapular slide test are described in Box 3-3. The lateral scapular slide is a valid tool to assess scapular motion.70 Kibler64 described side-to-side differences of 1 cm as an indicator of scapulohumeral dysfunction. Other authors assessing the reliability of the lateral scapular slide, however, note that a 1 cm difference cannot be used as an indicator of dysfunction and that 1 cm can fall within intertester variability.71
Continue ROM efforts during phase two, especially if limited capsular extensibility detrimentally affects physiologic motion. In addition to aggressive stretching exercises and mobilization of the glenohumeral joint, self-mobilizations also may be of benefit.72 Patients with glenohumeral laxity also require special consideration as ROM and strengthening exercises progress. For patients with anterior instability, exercises should not stress extremes of horizontal abduction and external rotation. Posterior glenohumeral instability requires care with horizontal adduction and IR. Strengthening programs for patients with glenohumeral instability are best performed in the plane of the scapula.
Phase III
TIME: Weeks 9 to 12
GOALS: Emphasis on enhancing kinesthesia and joint position sense, building endurance, strengthening the scapular stabilizer, and performing work-specific and sport-specific tasks (Table 3-3)
TABLE 3-3
< ?comst?>
| Rehabilitation Phase | Goals | Intervention | Rationale | Anticipated Impairments and Functional Limitations | Criteria to Progress to This Phase |
|
Phase III Postoperative 9-12 wk
|
• Unrestricted overhead work and sporting activity
|
• Formal return to throwing and overhead activities
|
• Create a specific training principle to return the patient to the desired activity
|
• Decreased work or sport-specific endurance
|
• Symmetric range of motion and strength of upper quarter
|
< ?comen?>< ?comst1?>

< ?comst1?>
< ?comen1?>
After the patient has progressed through the first two phases, the obvious deficits resulting from surgery (i.e., pain, limited motion, decreased strength) have essentially been eliminated. Deficits in endurance and proprioception are not as readily apparent. Violation of the capsule, decreased use of the shoulder, and abnormal or restricted movement of the shoulder may decrease endurance and proprioception. One study73 has demonstrated decreased proprioception in lax shoulders, with patients able to sense external rotation movements with greater ease than IR, especially at end range. Exercises to improve both passive detection of shoulder movement and active joint repositioning may help enhance kinesthesia and joint position sense, respectively. Voight and associates74 noted decreased glenohumeral joint proprioception with muscle fatigue of the rotator cuff.
Decreasing the weight used with strengthening exercises and increasing the repetitions address endurance training. Scapular stabilizer strengthening has been performed in sets of 30 repetitions to this point, and repetitions can be increased as required. Work- and sport-specific tasks should be used as guidelines to the number of prescribed repetitions. The supraspinatus tendon is the one most frequently involved in the injury, so it should be strengthened last.
The physical therapist also can address proprioception by having the patient perform functional tasks and emphasizing the timing of muscle contraction and movement without substitution. When rehabilitating overhead-throwing athletes, Pappas, Zawacki, and McCarthy75 suggest timing muscle recruitment to correlate with the throwing sequence of active abduction, horizontal extension, and external rotation. Appropriate timing of muscle contraction also can be addressed using proprioceptive neuromuscular facilitation techniques.76 Although the majority of upper extremity function in daily, work, or sport activities occur in the open kinetic chain, closed kinetic chain activities provide stimulation to the glenohumeral joint to enhance joint awareness and kinesthesia (important in secondary impingement). Activities in the closed chain should progress from low ground reaction forces (as a percent of body weight) to higher forces that have been shown to recruit greater shoulder girdle musculature as evidenced by the percentage of maximum volitional isometric contraction.77
A functional progression program can be used to enhance the return of proprioception and endurance. Functional progression involves a series of sport- or work-specific basic movement patterns graduated according to the difficulty of the skill and the patient’s tolerance. Providing a comprehensive functional progression program for every job or sport that a patient is involved in is impossible. Programs to return the patient to throwing, swimming, and tennis activities can be found in other sources.78,79 Plyometric activities help restore endurance, proprioception, and muscle power.80,81
Suggested Home Maintenance For The Postsurgical Patient
Box 3-4 outlines the shoulder rehabilitation the patient is to follow. The physical therapist can use it in customizing a patient-specific program.
Unlike more complex arthroscopic procedures or sophisticated open operative procedures, the need for structured clinic-based rehabilitation of the SAD patient should be the exception rather than the rule. Most of the rehabilitation for the patient after an uneventful SAD procedure can take place through a comprehensive home exercise program. Special cases may warrant a more formal and structured treatment program after the SAD procedure to detect problems. These special situations typically involve patients with the following conditions:
• Full-thickness rotator cuff pathology
• Biceps tendon or labral pathology
• Articular cartilage involvement
• Tendency for excessive scarring
• History of regional complex pain syndrome or reflex sympathetic dystrophy (RSD)
Troubleshooting
2 Appropriate exercise dose. Dye82 has described the envelope of function, which is defined as the range of load that can be applied across a joint in a given period without overloading it. The challenge is to stress the healing tissue to maximize functional collagen cross-linking without exceeding the envelope of function. As functional levels are increased, alter the therapeutic exercise dose. In cases of significant scapulothoracic dysfunction (long thoracic nerve neuropathy), scapulothoracic taping or figure-eight strapping may be used for additional stability.83
5 Prevention. As the old adage goes, an ounce of prevention is worth a pound of cure. Preventing early primary impingement symptoms from becoming chronic may eliminate the need for surgery. Nirschl8 notes the following factors as keys in preventing chronic impingement syndrome: relief of inflammation, strengthening (especially the external rotators, abductors, and scapular stabilizers), flexibility (especially shoulder internal rotators and adductors), general fitness, education, and proper equipment.
Summary
This chapter discusses the surgical procedure of SAD along with principles that govern postoperative rehabilitation. A surgeon with sound diagnostic, management, and surgical skills, along with a physical therapist with the expertise to advance the patient through the postoperative phase, typically produces a favorable result. Of even greater importance is the rapport established between surgeon and therapist, and the relationship between the health care providers and the patient.
Clinical Case Review
1Carl arrives for therapy 5 weeks after a shoulder acromioplasty. He is having difficulty performing shoulder flexion and scaption exercises correctly. He occasionally demonstrates a mild shoulder hike with arm elevation exercises above 70° of elevation. How can the therapist sequence his exercises to maximize his ability to elevate his arm above shoulder height?
If the patient is going to be strengthening the supraspinatus and performing shoulder elevation exercises (i.e., shoulder flexion, abduction, or scaption exercises), he should perform the gravity-resisted elevation exercises first. The supraspinatus works more efficiently, without fatigue, to achieve an adequate force couple, thereby helping Carl to execute the elevation exercises correctly.
2Drew is a 55-year-old plumber. He has a history of shoulder pain over the past 2 years and has a slouched posture. He had an acromioplasty performed 8 weeks ago. He still has minimal deficits with average range of motion (AROM) for reaching overhead objects and cannot reach into his back pocket. On evaluation, Drew demonstrates near full passive range of motion (PROM) for shoulder flexion. PROM for IR and a combined movement of IR with shoulder extension is limited. What are some essential points to address and treatment techniques to use during Drew’s treatment?
After correction of Drew’s posture he was able to reach higher above his head. His slouched posture had previously restricted full active shoulder flexion. The shoulder capsule needs to be assessed immediately for restrictions. Emphasizing capsular mobilization of a restricted capsule allows for better joint arthrokinematics and increased ROM. The anterior, posterior, and inferior capsule were all restricted. General mobilizations were performed for all areas of the capsule, and specific mobilizations were performed for the anterior capsule. Drew then performed ROM and stretching to the shoulder, including stretches with the hand behind the back. A considerable increase in PROM and AROM for the hand behind the back was noted after this treatment.
3Kelly had an acromioplasty on her right shoulder 6 weeks ago. She complains of a pinching pain when reaching above her head, through the last 20° of shoulder flexion and abduction. She also has a pinching pain when actively reaching across her body in horizontal adduction. With PROM for horizontal adduction, shoulder flexion, and abduction, she has pain near the end of the ranges. What type of treatment may be helpful during her next session?
Kelly was treated with joint mobilizations to the glenohumeral joint as usual to increase shoulder flexion and shoulder abduction. AC mobilizations have been performed in the past, with the arm in anatomic position while the patient was supine. However, today AC mobilizations were performed with the shoulder in horizontal adduction and again with the shoulder in flexion above 140°. An assistant was required to hold the extremity of the patient in place while the therapist performed the mobilizations. AROM for shoulder flexion, abduction, and horizontal adduction increased by 10° of pain-free motion. In addition, the complaints of pain intensity were less when experienced. After one more visit for the same treatment, the patient exhibited full ROM.
4Cynthia had an acromioplasty on her right shoulder 5 days ago. She complains of moderate to severe pain intermittently throughout the day. She also has difficulty sleeping secondary to shoulder pain. She is a mother of young children. Patient uses arm for light activities of daily living (ADLs) when possible. ROM is limited in all directions. Strength is not tested secondary to healing tissue and pain levels. How did the therapist advise her and treat her for pain management?
The therapist encouraged her to use a sling for a couple of days to prevent overuse of the healing extremity. She was told to use the sling when she was up and about for protection and for rest. Children as well as others would be less likely to bump or grab her arm. She was also encouraged to use cryotherapy intermittently throughout the day. The therapist advised her to try sleeping in a recliner chair or a semireclined position with her shoulder supported in a loose packed position. Treatment consisted of assessing the cervical area. Gentle mobilizations were done in the cervical area along with massage to the cervical and scapular musculature to decrease muscle guarding and spasms. The patient’s pain level decreased slightly after the treatment. Pain began subsiding over the next couple of days.
5Mike had an acromioplasty on his right shoulder 4 weeks ago. He notes aching in the shoulder after early functional progression consisting of a short toss program. This discomfort is in the posterior aspect of the shoulder. The discomfort is most pronounced during the follow though in the throwing motion. He has no night pain but has minimal discomfort with daily overhead activities. His rotator cuff strength is excellent, and he has a normal scapulohumeral rhythm. What additional concerns should be addressed?
As Mike is a throwing athlete, the cause of his rotator cuff issues may have been due to secondary impingement as a result of anterior glenohumeral instability. Subtle anterior instability may be increased due to a tight posterior capsule. Since this is where Mike’s symptoms are present, stretching of the posterior capsule may be indicated. Manual techniques the therapist can employ include horizontal adduction without stabilization of the axillary border of the scapula progressing to stabilizing the scapula and focusing on glenohumeral motion. The sleeper stretch (side-lying on the involved shoulder with the arm forward flexed while passively pushing the forearm toward the floor) may be helpful, but should be done carefully with the arm flexed to no more than 70°.
6Tim is employed as a truck driver who has responsibilities for overhead lifting and stacking. His SAD was done 5 weeks ago and he is readying to return to work. His rehab has gone well, with the only difficulty being the last 5° to 8° of flexion needed to get to the upper shelves in his delivery truck. The restriction is not painful but is accompanied by stiffness. His motion preoperatively was also slightly restricted due to pain and stiffness. In addition to passive ROM of the shoulder what other areas may be of concern?
The last few degrees of elevation can be troublesome. Many issues can contribute to decreased mobility at the cervical-thoracic junction and throughout the midthoracic spine. Assessment of joint play in these regions may reveal one or more hypomobile segments. Restoring normal accessory motions in these areas may assist in regaining full shoulder elevation.
7John has been referred to you from a surgeon from out of state. He states that he had a shoulder decompression 6 weeks ago and did not have postoperative physical therapy. He found a rotator cuff strengthening program online and has been doing those exercises daily and is using “3 or 4 lb.” In addition to significant rotator cuff weakness, your evaluation demonstrates scapular malposition, inferior medial border prominence, pain at the coracoids, and an abnormal scapulohumeral rhythm. How will you modify John’s program?
Excessive loads can be placed on the rotator cuff with insufficient proximal stability afforded at the scapulothoracic joint. In addition, muscles of the rotator cuff (especially the supraspinatus) may not tolerate excessive external resistance due to short lever arms and a relatively small physiologic cross-sectional area. John should not be performing progressive resistive exercises for the cuff at this juncture. You should emphasize anterior chest muscle flexibility and strengthening of the scapular stabilizers.
8Ann works in a data entry position and has had right shoulder pain off and on for 3 years with occasional complaints of “carpal tunnel.” She had a SAD performed 3 weeks ago and has yet to start rehabilitation because of poorly localized postoperative pain. Two days after your initial evaluation, Ann returned for a follow-up visit with complaints of increased shoulder pain and occasional numbness and tingling down the right arm and into the hand. Is it safe to proceed with rehabilitation?
You should counsel Ann that any increase in activity may result in discomfort. Ann may be experiencing delayed onset muscle soreness and/or her level of activity may be in excess of what she is ready for. You can certainly trouble shoot Ann’s level of activities and exercise. You can also determine if discomfort is delayed-onset muscle soreness in nature or from joint and healing tissue. The issue of occasional numbness and tingling is not a contraindication for exercise, but it also cannot be ignored. If you have not yet done so, Ann’s cervical spine should be cleared, nerves cleared for adverse neural tension/compression, and peripheral nerve entrapments.













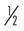 to 1 lb
to 1 lb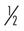 to 1 lb
to 1 lb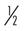 lb
lb



