Acquired Lesions of The Lung and Pleura
Empyema
Epidemiology
Although overall rates of bacterial pneumonia have been declining in children, the incidence of complications such as parapneumonic effusions and empyema has increased.1 In the USA, pneumonia in children occurs at an estimated rate of 30–40 per 100,000.2 Parapneumonic effusions (PPE) can complicate pediatric pneumonia in 28–53% of patients.3,4 In children less than two years of age, the incidence of empyema doubled over a 10-year span, increasing from 3.5/100,000 in 1996–1998 to 7/100,000 in 2005–2007.5 Similarly, in patients between 2 to 4 years age, empyema rates nearly tripled from 3.7/100,000 to 10.3/100,000 during the same time period. While empyema in children is less serious compared to adults where mortality can approach 20%, it still poses a considerable burden on hospitals and families.
Pathogenesis
The natural progression of parapneumonic pleural disease has been outlined with three to four stages of increasing complexity.6–8 The pre-collection stage involves pleuritis and inflammation. This is followed by the exudative stage, which is a simple PPE, and is characterized by clear, free-flowing pleural fluid with a low white cell count. The fibrinopurulent stage is a complicated PPE (empyema) marked by the deposition of fibrin and purulent material in the pleural space and an increase in the leukocyte count of the fluid. Septations and fibrin strands begin to develop. These result from decreased fibrinolytic activity allowing increased fibrin deposition. The result is a procoagulant environment that leads to the development of solid material in the form of septations followed by loculations of purulent fluid (Fig. 23-1).9 The most advanced state is termed the organized stage when a thick peel is established. This peel can entrap the lung and result in chronic restrictive lung disease. While these stages are outlined in sequential progression, there is no certainty that each stage will progress to the next. Although outlined as a simple progression, the degree of patient illness may not correspond with these stages, depending on the extent of the concomitant parenchymal disease or the inflammatory response to the infectious processes.
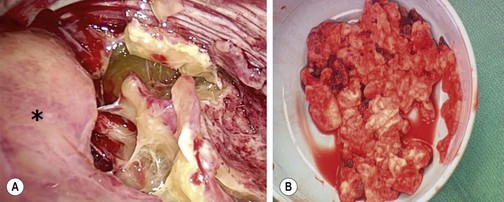
FIGURE 23-1 (A) The thoracoscopic view shows the inflammatory septations that can develop with empyema. The collapsed lung is marked with an asterisk. (B) Note the thick, solid purulent material that is often found in these patients.
As the stages advance, the chemistry of the parapneumonic fluid changes: glucose decreases, pH decreases, and lactate dehydrogenase (LDH) rises. The Light criteria for complicated PPE include pH <7.2, lactate dehydrogenase >1000 units, glucose <40 mg/dL or < 25% of the blood glucose, Gram stain or culture positive, and loculations or septations documented with imaging.10 Retrospective data suggests that a prolonged fever, a low pleural fluid pH and glucose along with a high LDH pleural/serum ratio are associated with more severe disease.11 Another study, using multivariate logistic analysis of a retrospective dataset, found that a pleural fluid pH less than 7.27 was the only significant factor for the formation of fibrin with/without septations.12 A third study suggested that a tumor necrosis factor level greater than 80 pg/mL in the pleural fluid suggests a complicated effusion.13
In a 1995 review, Light proposed a detailed classification which defines seven different stages from nonsignificant PPEs to a complex empyema. Recommendations on appropriate therapy were also described, which ranged from observation to thoracoscopic debridement/decortication (Table 23-1).14 A consensus statement from the American College of Chest Physicians in 2000 noted that more interventional therapy was needed as the stage of effusion progressed.15 Similarly, in another retrospective study, a pleural fluid pH less than 7.1 was found to result in a sixfold increase in the likelihood of operative intervention.16 While all these criteria may document the physiologic progression of disease, the clinical relevance of a patient’s pleural fluid analysis may not be that important because once symptoms develop and fluid with or without septations are found, intervention is needed.
TABLE 23-1
Classification Scheme for Parapneumonic Effusions and Empyema
| Categorization | Pleural Fluid Findings |
| Class 1 | |
| Nonsignificant parapneumonic effusion | Small <10 mm thick on decubitus X-ray |
| Class 2 | |
| Typical parapneumonic effusion | >10 mm thick Glucose >40 mg/dL, pH>7.20 Gram stain and culture negative |
| Class 3 | |
| Borderline complicated parapneumonic effusion | 7.00<pH<7.20 and/or LDH>1,000 and glucose >40 mg/dL Gram stain and culture negative |
| Class 4 | |
| Simple complicated parapneumonic effusion | pH<7.00 and/or glucose <40 mg/dL and/or Gram stain or culture positive Not loculated, no frank pus |
| Class 5 | |
| Complex complicated parapneumonic effusion | pH<7.00 and/or glucose <40 mg/dL and/or Gram stain or culture positive Multiloculated |
| Class 6 | |
| Simple empyema | Frank pus present Single locule or free flowing |
| Class 7 | |
| Complex empyema | Frank pus present Multiple locules |
Modified from Light RW. A new classification of parapneumonic effusions and empyema. Chest 1995;108:299–301.
Diagnosis
The diagnosis is usually a progressive clinical picture beginning with pneumonia. Patients with an empyema almost always demonstrate some degree of respiratory distress, malaise, persistent fever, or pleuritic chest pain.17–20 Diminished breath sounds with dullness to percussion on the affected side are found on examination. An ileus is common as is a lack of appetite.
Initial imaging is a chest radiograph (CXR) which shows poor penetration on the affected side. However, it is often difficult to distinguish between parenchymal consolidation and pleural fluid on a plain film.21 In a retrospective review of over 300 adult patients, CXR missed all effusions that were significant enough to warrant drainage when compared with subsequent computed tomography (CT) scans (Fig. 23-2A).22 Decubitus films may be helpful in distinguishing between nonloculated and loculated effusions.21
FIGURE 23-2 Ultrasonography and/or CT of the chest are helpful during the initial evaluation of children with a pleural effusion and possible empyema. (A) In the ultrasound study, note the loculations identified in the pleural fluid. (B) On the CT scan, a large pleural effusion (asterisk) is noted. Also there is collapse of the underlying lung parenchyma as well as septations (arrow).
Ultrasonography (US) is portable, relatively inexpensive, and does not involve radiation (Fig. 23-2B). It is very sensitive in diagnosing loculated fluid and can be used to guide percutaneous drainage and catheter placement.23,24 Some authors suggest that ultrasound is superior to CT in the identification of pleural debris or loculations.25,26 ultrasound can reliably differentiate between parenchymal and pleural based processes.6 A post hoc review of a prospective trial in children found that in 31 patients in whom both CT and ultrasound were performed, there was no advantage of CT over ultrasound in most cases.1 Two independent series reviewed the implementation of an algorithm using initial ultrasound in children with complicated pneumonia.27,28 Both demonstrated a significant reduction in hospitalization and a decrease in the use of CT without an increase in the rate of operative management or pleural drainage. A small retrospective review comparing ultrasound and CT found that CT had no advantage in most cases and suggested that CT should be used in complex cases only, such as patients undergoing operation or thought to have parenchymal abscesses or a bronchopleural fistula.26 CT has been found to be inferior to ultrasound at demonstrating fibrin strands or septations within the pleural fluid.25 Neither CT nor ultrasound is completely reliable in differentiating the specific stages of parapneumonic disease.1,25,26 The main disadvantages of ultrasound appear to be lack of 24-hour ultrasound availability and being operator dependent.
With pleural space disease, CT with intravenous contrast can differentiate between parenchymal and pleural processes, identifying pleural thickening and loculations.25 CT radiation exposure has raised the concern for a long-term cancer risk.29 Currently, chest CT scans can be performed effectively with the use of automatic dose modulation software that limit the radiation dose.25 Despite this ability to limit radiation exposure with CT scan, consensus statements are clear in their recommendations for performing CT only when needed, such as preoperative planning in some cases.6,7
Management
Parapneumonic Effusion
After the diagnosis of PPE is made, the first branch in the management algorithm depends on the nature of the fluid. With a free-flowing effusion and no solid components or signs of frank pus, the nature of the intervention will depend on the size of the effusion and the symptoms. Classifying the size is difficult to define precisely. However, in general, small effusions are defined as having 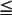 1 cm rim of fluid, moderate effusions have a 1–2 cm rim, and large effusions have
1 cm rim of fluid, moderate effusions have a 1–2 cm rim, and large effusions have 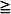 2 cm rim on decubitus films. A recently published twelve year retrospective study in children classified small effusions as less than a quarter hemithorax opacification, moderate effusions as a quarter to a half opacification, and large effusions as more than half opacification based on upright films.30 In this study, the authors found that small and most moderately sized effusions could be effectively managed without drainage and without an increase in the length of hospitalization or other complications. The authors suggest that interventions should be based on symptoms, not the size of the effusion alone.
2 cm rim on decubitus films. A recently published twelve year retrospective study in children classified small effusions as less than a quarter hemithorax opacification, moderate effusions as a quarter to a half opacification, and large effusions as more than half opacification based on upright films.30 In this study, the authors found that small and most moderately sized effusions could be effectively managed without drainage and without an increase in the length of hospitalization or other complications. The authors suggest that interventions should be based on symptoms, not the size of the effusion alone.
Symptoms leading to intervention generally are feeding intolerance, tachypnea, and an increasing oxygen requirement. A retrospective case series in children found respiratory distress on presentation was related to prolonged stay and a higher likelihood for intervention.31
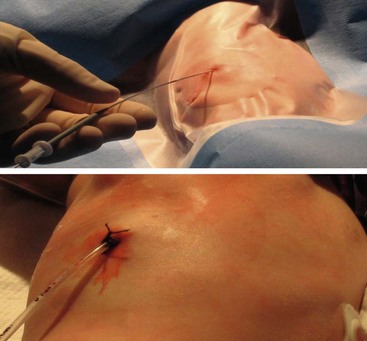
After deciding to drain the effusion, options include single or multiple thoracentesis versus tube thoracostomy or catheter drainage. A prospective, nonrandomized pediatric series compared treatment with repeated ultrasound-guided needle aspirations vs tube thoracostomy. There was a mean 2.4 drainages/patient in the aspiration group, but similar length of stay.32 While this approach may be reasonable in an older child who can tolerate the procedure with local anesthesia, it would likely not be appropriate in younger children. The British Thoracic Society guidelines recommend a chest tube for cases in which the first thoracentesis fails to adequately drain the effusion in order to avoid multiple thoracentesis attempts.6 A retrospective series compared 33 children who underwent chest tube placement on the basis of effusion size and/or thoracentesis fluid analysis vs 32 who were treated conservatively with tube thoracostomy only for progressive symptoms or mediastinal shift.33 The authors found no difference in duration of hospitalization and suggested frugal use of chest tubes.3 In a series of 405 adult patients, 266 had a chest tube smaller than 14 French compared to 139 with larger tubes.34 There was no difference in the ability to drain the effusion. Furthermore, smaller caliber tubes did not hinder the use of fibrinolytics. In a retrospective series of 20 children treated with standard chest tubes compared to 12 treated with pigtail tubes, no outcome differences were found.35 In patients with pleural effusions or empyema, we exclusively use 12 French Thal-Quick chest tubes (Cook Critical Care, Bloomington, Indiana, USA) that are inserted using the Seldinger technique (Fig. 23-3). We have not felt it necessary to use image guidance in the majority of patients.
Empyema
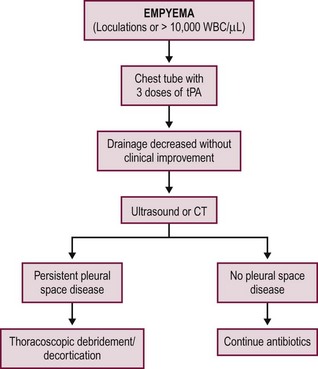
Empyema is diagnosed by identifying solid components in the pleural fluid on imaging studies or pus during thoracentesis or tube catheter placement. The definitive management for empyema has traditionally been operative debridement, which is currently performed via video-assisted thoracoscopy surgery (VATS).36–40 VATS has resulted in earlier and more complete resolution of empyema than chest tube drainage alone in both retrospective and prospective studies, translating into shorter hospitalization with VATS as the initial therapy.41–44 A retrospective series of 89 children undergoing primary thoracoscopic debridement/decortication found a 12% risk for needing another procedure to address ongoing disease or a complication.45 However, the superiority of operative mechanical debridement as a definitive management strategy has been increasingly challenged by chemical debridement with fibrinolysis.
Examples of fibrinolytics include urokinase, streptokinase, and tissue plasminogen activator (tPA). Since fibrin is a predominant component of the extracellular matrix upon which septations and solid debris develop, fibrinolysis has been shown to be superior to tube thoracostomy alone in retrospective and prospective studies.46–52 These studies include direct comparisons between the two treatment options as well as the use of fibrinolytic therapy after failure of tube thoracostomy.
Two prospective, randomized trials have been conducted independently comparing fibrinolysis to VATS for empyema in children.53,54 The studies were conducted in the UK and USA in 60 and 36 patients, respectively. Both studies compared the instillation of three intrathoracic doses of fibrinolytic agents to thoracoscopy as the initial therapy for empyema. The first fibrinolytic dose in both studies was given at the time of diagnosis and/or chest tube insertion followed by two additional doses in 24-hour increments to complete the fibrinolytic course over a 48-hour period. The results were highly concordant as both studies found no difference in duration of hospitalization between the two interventions. The common parameters between the two studies are outlined in Table 23-2. The USA study found no difference in days of tube drainage, days of fever, doses of analgesics, or oxygen requirements.54 Both studies documented thoracoscopic debridement was more expensive and both utilized an intention-to-treat analysis so the length of stay and total charges included the patients who failed fibrinolysis and subsequently required VATS. The failure rate for fibrinolysis was 16.6% in both studies. This failure rate was similar to previous studies investigating the utility of fibrinolysis.48,55–59 An example of a first-line fibrinolytic approach is outlined Figure 23-4. A similar algorithm has been proposed based on a review of the literature.60
TABLE 23-2
Common Outcome Variables Reported between the Two Prospective Trials Comparing Fibrinolysis to VATS in Children

VATS, video-assisted thoracoscopic surgery; tPA, tissue plasminogen activator.
aCharges are in thousands of British pounds and thousands of ultrasound dollars.
One of the groups that performed the prospective randomized trial comparing fibrinolysis to thoracoscopic debridement recently reported their data using an evidence-based algorithm with primary fibrinolytic therapy. One hundred and two consecutive patients were treated with fibrinolysis following completion of the prospective randomized trial and 16 patients (15.7%) required subsequent thoracoscopic debridement and decortication. The length of hospitalization with fibrinolysis was 6.1 ± 2.5 days. In those patients that failed fibrinolytic therapy, the length of hospitalization after thoracoscopic debridement was 5.9 ± 3.7 days. Factors correlating with the need for thoracoscopy included age, gender, and initial drain output. However, none of these variables were independent predictors.61
Lung Abscess
Pulmonary abscess is often assumed to develop as a primary process in a previously normal lung, usually as a result of a necrotizing pneumonia. However, a pulmonary abscess in a child without an antecedent history ought to be considered as a secondary abscess which arises in an infected pulmonary anomaly such as a cystic pulmonary adenomatoid malformation, bronchogenic cyst, or foreign body. Most primary lung abscesses are located in the posterior segment of the right upper lobe and the superior segments of the right and left lower lobes (Fig. 23-5). In contrast, secondary and/or recurrent collections may be found in multiple locations with no specific anatomic predilection.
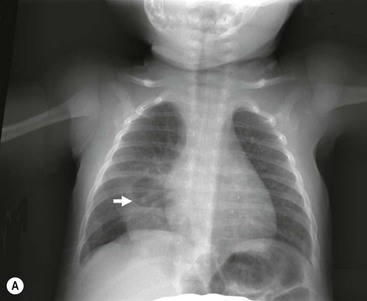
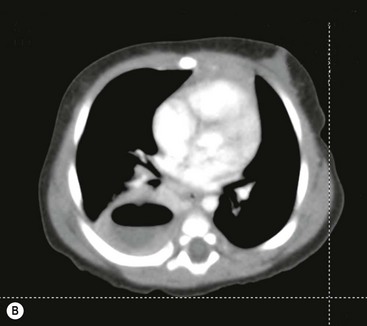
FIGURE 23-5 (A) This young infant was found to have this lung abscess in the right lower lobe on chest radiograph. Note the air-fluid level (arrow). (B) The lesion is seen on the CT as well. Workup did not reveal an associated congenital pulmonary airway malformation and she recovered with antibiotic therapy.
In the patient with known pneumonia, the non-responding patient who develops a suspicious lesion on chest film should undergo CT to evaluate for an abscess. The treatment of a lung abscess in an infant or child follows the same basic principles of postural drainage and pulmonary toilet used in adults, but it is more often ineffective, secondary to the small size of the airway.62,63
In general, an operation should be avoided as abscesses can usually be successfully treated with antibiotics alone.64,65 CT-guided drainage or catheter placement is usually needed if the lesion is peripheral and not connected to the airway, .66–68 Retrospective data suggests that drainage shortens hospitalization and facilitates earlier recovery.69 A recent series of 11 children with pulmonary abscess treated with thoracoscopic drainage fared well without complications which may be a good option when less invasive maneuvers fail.70 Alternatively, pulmonary resection may be required for abscesses that are more centrally located and resistant to medical management.62,71 Patients with fungal isolates and immunocompromised patients generally require early and aggressive pulmonary resection. However, as a general statement, patience should be employed in managing pulmonary abscesses.
Pneumatocele
Pneumatoceles are thin-walled, air-filled, intraparenchymal pulmonary cysts. They typically occur secondary to an underlying bacterial pneumonia, a treated abscess, or as a result of trauma. Pneumatoceles are the result of a severe inflammatory reaction and the subsequent destruction of the alveolar and interstitial architecture. With an infectious etiology, the release of bacterial exotoxins has been postulated.72 Although pneumatoceles have been associated with a variety of underlying bacterial organisms, the majority appear to be the result of staphylococcal pneumonia.72–74 Other pathogens that have been identified as being associated with pneumatocele formation include Streptococcus, Haemophilus influenzae, Klebsiella, Escherichia coli, and Pseudomonas. In addition, pneumatoceles have been found in cases of pulmonary tuberculosis and measles.73
Additional complications associated with pneumatoceles include the development of secondary infections, empyema, and bronchopleural fistulas.75
Management
Most pneumatoceles will involute over time, and do not require any specific therapy other than supportive care and appropriate antibiotic coverage. In the case of a rapidly enlarging and/or tension pneumatocele, resulting in respiratory compromise, urgent decompression may be needed. In addition to closed-tube thoracostomy or cystostomy, percutaneous catheter drainage using fluoroscopy and ultrasound, has been reported to be an effective means of decompression.76,77 Open drainage with decortication and oversewing of the cyst wall is rarely necessary.
Bronchiectasis
Bronchiectasis is defined as a permanent dilatation of segmental airways, initially described almost 200 years ago.78 A significant number of deaths from respiratory insufficiency due to bronchiectasis continue to occur. Bronchiectasis is not a pathophysiologic process, but is an architectural abnormality resulting from any pathologic process that causes persistent pulmonary inflammation and airway damage. Decreased epithelial and mucociliary integrity results in poor airway clearance leading to a predisposition for further infections. Three pathologic forms have been described: saccular, cylindrical, and fusiform or varicose.79 Saccular bronchiectasis tends to occur in third- and fourth-order bronchioles, whereas cylindrical bronchiectasis occurs in sixth- to seventh-order bronchioles. The fusiform variety is an intermediate type.
Bronchiectasis can be categorized based on the source of the respiratory injury (Table 23-3).80 As might be expected, congenital abnormalities are more likely to result in diffuse distribution with bilateral disease whereas acquired bronchiectasis is more likely to be focal. Focal disease is more common in the left lower lobe, lingula, or right middle lobe. Many cases remain idiopathic without an explanation for the source of the parenchymal or airway damage. Retrospective reports indicate that about 50% of patients, in whom a specific cause is determined, experience their first pulmonary insult before age 14.81,82
TABLE 23-3
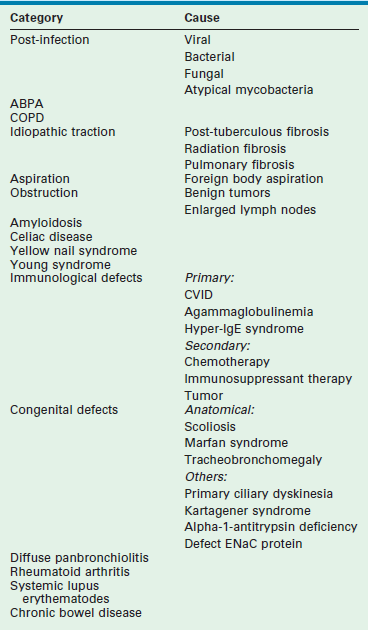
Modified from: Rademacher J, Welte T. Bronchiectasis-Diagnosis and Treatment. Dtsch Arztebl Int 2011;108:809–15.
Presentation and Diagnosis
Patients typically present with nonspecific symptoms such as cough, sputum production, and lethargy. Classically, patients develop a three-layer sputum consisting of a foamy outer layer, a mucous middle layer, and a viscous purulent bottom layer. This three-layer sputum is considered pathognomonic, but is not often seen. Patients with more advanced disease present with hemoptysis, chest pain, weight loss, bronchospasm, dyspnea, and impaired physical performance.83 Patients can be symptom free most of the time, but can become symptomatic during exacerbations. Physical examination usually reveals coarse inspiratory crackles and expiratory wheezes. The diagnosis is often delayed due to the lack of few specific signs and the low incidence of this disease.
A chest radiograph may show focal density and crowding of interstitial markings (Fig. 23-6). Historically, bronchography was the best diagnostic test, but high-resolution CT is now the preferred study.81,82 There is a high correlation between bronchographic and CT findings,84 particularly bronchi within 1 cm of the visceral pleural surface (Fig. 23-7).85 Ventilation/perfusion scans may be helpful to delineate the presence of ventilation-perfusion mismatching.
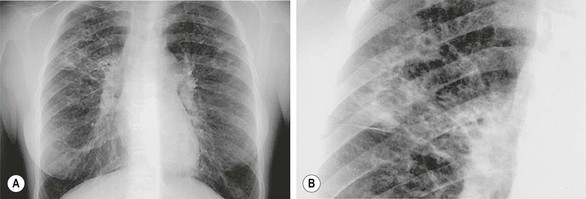
FIGURE 23-6 (A) Chest radiograph demonstrating the classic appearance of bronchiectasis in a pediatric patient with cystic fibrosis. (B) Magnified view of the patient demonstrating honeycombed appearance.
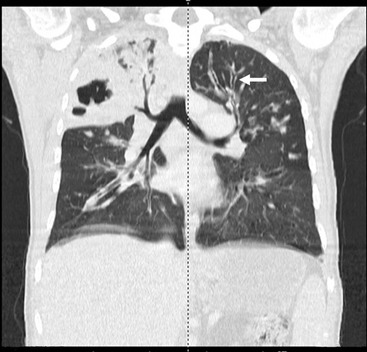
FIGURE 23-7 High-resolution CT demonstrates severe bronchiectasis. On the patient’s left side, dilated bronchi (arrow) are seen throughout the lung. Apically, they extend to near the pleural surface. On the patient’s right, the dilated bronchi are seen extending into an upper lobe complicated by infection and consolidation which can be a recurring problem in patients with bronchiectasis.
Management
The surgeon is not typically involved until irreversible bronchiectasis develops. Clearly the best outcomes are obtained when the underlying process is controlled to minimize disease progression. If a foreign body is found, it may be possible to remove it at bronchoscopy. However, for a long-standing foreign body, a resection likely will be needed. Resection is also needed in children with repeated localized involvement despite nonoperative therapy. The absence of vascular perfusion indicates end-stage disease and resection will likely be needed.85 Occasionally, massive hemoptysis will be the indication for resection, although embolization of bronchial vessels may be a viable option if hospital resources are available.
The principles of operative treatment should be to preserve as much uninvolved pulmonary parenchyma as possible using segmental resections.86 In a retrospective series of open resection in 35 children with bronchiectasis, two-thirds had cylindrical bronchiectases.87 Sixty-five per cent of the children became asymptomatic and 24% were clinically improved. A little over 10% did not have clinical improvement. The authors stress the importance of careful selection of patients and complete resection of the disease. Another series of 58 resections in 54 children included lobectomy (63%), pneumonectomy (18.5%), lobectomy with segmentectomy (11.1%), segmentectomy (3.7%), and bilobectomy (3.7%).88 There were 23 patients (43%) who were clinically well, another 23 (43%) who were improved, and 5 (10%) who were the same or worse. Three patients died. In a recent series of 19 thoracoscopic lobectomies for bronchiectasis, there were no conversions and minimal complications.89
Chylothorax
The cause of a lymphatic leak into the pleural space can be broadly classified as traumatic or atraumatic. Nontraumatic causes include congenital abnormalities such as lymphatic malformations or lymphangiomatosis. Venous thromboses have been associated with chylous effusions. Infiltration of the chest and mediastinum by infection or malignancy can also result in a chyle leak. Gorham syndrome, which is a primary osteolytic process where spontaneous bone resorption occurs, is often associated with a refractory and debilitation chyle leak when ribs are involved. Congenital chylothorax occurs spontaneously in the neonatal period. It is most frequently idiopathic but has been described with some dysmorphic syndromes.90,91 These cases are presumed to result from a structural defect in the lymphatic drainage system. By far, the most common cause is an injury to the thoracic duct or a major tributary. The incidence of chyle leak has been reported as high as 1% after cardiac operations in infants and children.92
Presentation and Diagnosis
As with any pleural effusion, chylothorax can present with respiratory symptoms. Congenital cases may be suspected during routine prenatal ultrasound. In postoperative patients, it will present with milky drainage from the chest drain/tube. The drainage may seem like normal pleural fluid until enteral nutrition is resumed and the drainage becomes chylous. Analysis of the fluid confirms the diagnosis. Chyle typically demonstrates a total fat content greater than 400 mg/dL, triglycerides greater than 200 mg/dL, or a specific gravity greater than 1.012. In addition, Gram stain demonstrates the presence of more than 90% lymphocytes and Sudan red staining may reveal the presence of chylomicrons.93
Management
There are physiologic consequences from the chylothorax. Protein and fat losses can result in acute malnutrition. Lymphocyte losses result in immunosuppression. The net fluid losses can cause dehydration. Therefore, it is important to understand these losses so vigorous monitoring and replacement can occur concomitant with maneuvers to stop the leak. Initially, nonoperative maneuvers should be attempted based on the goals of reducing the amount of chyle produced while still providing adequate nutrition. This begins with limiting fat intake. As medium-chain fatty acids are carried through the portal venous system (as opposed to the intestinal lymphatic network), it is reasonable to start with a fat-restricted diet rich in medium-chain fatty acids. The next step would be to stop oral intake and begin total parenteral nutrition. As many as 80% of patients respond to this management strategy.92
In addition to dietary manipulations, several authors have reported success with the use of somatostatin analogs’, such as octreotide, whether the chyle leak is spontaneous or post-traumatic.94–98 Some authors recommend utilizing octreotide early in the plan of care.97
Nonoperative therapy is typically recommended for one to two weeks prior to considering operative options.93 The goal of the operation begins with stopping the leak. This is straightforward if the leak is visualized and can be closed or obliterated. Thoracic duct ligation proximal to the leak is usually curative. If neither of these options can be accomplished, pleurodesis can be attempted.99 These cases can be approached with thoracoscopy. Right thoracoscopy with occlusion of the thoracic duct as it crosses the diaphragm has been shown to be a useful technique in patients with a traumatic leak.100,101 Others have described direct suture of the area of chylous leak followed by the application of fibrin glue.102 In adults, a minimally invasive technique of percutaneous injection of the cisterna chyli with platinum coil embolization of the thoracic duct has been shown to be successful in over two-thirds of patients with high-output chylothorax in whom nonoperative management has failed.103,104 In cases that are refractory after an operation, use of a pleuro-peritoneal shunt has been described (Fig. 23-8).105 In these patients, the chyle in the pleural space is manually pumped into the peritoneal cavity where it is absorbed, presumably into the venous system. These shunts can remain open for several months until the chylous leak seals.
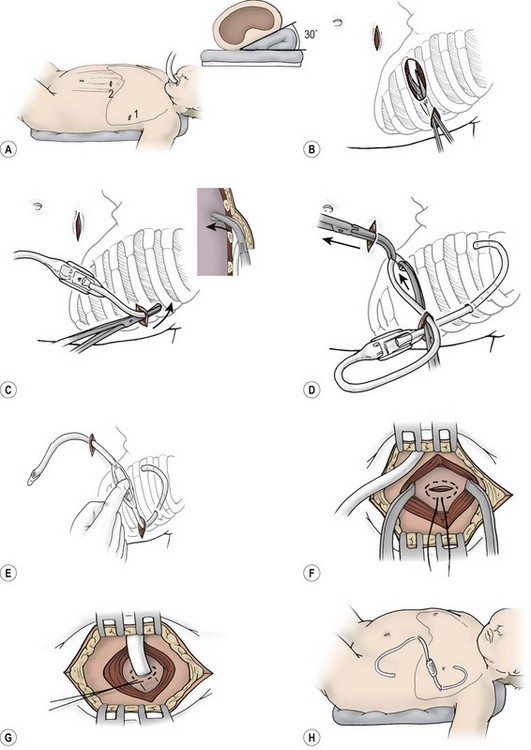
FIGURE 23-8 Insertion of pleuroperitoneal shunt. (A) The affected hemithorax is elevated 30°. The two incisions are planned to allow the pump chamber to rest on the costal margin. (B) A small incision is made over the rib in the anterior axillary line, and a deep subcutaneous pocket is created inferiorly. (C) Insertion of pleuroperitoneal shunt into the pleural space is done with a large curved clamp. The pleural catheter is tunneled 2–3 cm and bluntly passed through the intercostal space. The catheter must be carefully passed through the intercostal muscle at an angle to avoid kinking. (D) A second small incision is made overlying the rectus muscle, and the peritoneal catheter is tunneled through this incision. The distal end of the shunt device is delivered to the second incision, as shown. The pumping chamber is drawn into the subcutaneous pocket by traction on the peritoneal catheter. (E) The flow of chyle is confirmed before the distal catheter is inserted into the peritoneum. (F,G) A purse-string suture is used to secure the peritoneal catheter at the level of the posterior rectus fascia. (H) Both incisions should be closed with an absorbable suture, leaving a totally implanted system. (Adapted from Murphy M, Newman B, Rodgers B. Pleuroperitoneal shunts in the management of persistent chylothorax. Ann Thorac Surg 1989;48:195–200.)
Diffuse Interstitial Disease
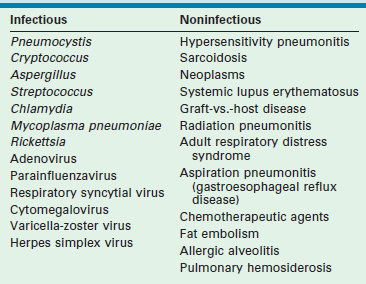
The interstitium of the lung is the tissue around and between the airway and vascular system. A persistent lung injury results in a reparative process which causes scarring and inflammation. The lung may be permanently damaged, with inflamed interstitial tissue replacing the normal capillaries, alveoli, and healthy interstitium. This condition is known as chronic interstitial lung disease (ILD). It may be the result of myriad processes, some of which are listed in Table 23-4.106–108 The clinical consequence is restrictive lung physiology and abnormal gas exchange which produces considerable morbidity and mortality. The clinical disease patterns range from a chronic, slowly progressive picture in a relatively stable patient to one of acute pulmonary decompensation, requiring emergency life-saving maneuvers.
TABLE 23-4
Infectious and Noninfectious Processes Associated with the Development of Chronic Interstitial Lung Disease
The role of the surgeon is to help establish the diagnosis, usually with a lung biopsy, after imaging demonstrates a diffuse pulmonary process (Fig. 23-9). Unfortunately, the results of bronchoalveolar lavage (BAL) are disappointing in this disease. In a prospective analysis of children with ILD undergoing BAL, a definitive primary diagnosis could be made in only 17%.107
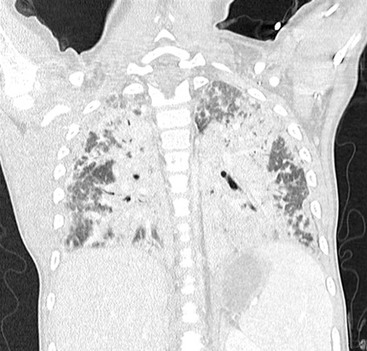
FIGURE 23-9 High-resolution CT demonstrates severe diffuse bilateral parenchyma disease on a patient referred for lung biopsy.
The Childhood Interstitial Lung Disease Pathology Co-operative Group has published guidelines about handling the biopsy tissue.109 The principles of biopsy begin with obtaining tissue from a region of heavy involvement based on imaging. If there is diffuse lung involvement, any site is reasonable, except the tip of the right middle lobe or the tip of the lingula where the changes are often disproportionate and may lead to a faulty impression of the disease severity. If it is not obvious from the imaging studies which is the more diseased area, biopsies should be taken from more than one area. A lung biopsy in a child should yield a specimen at least 1 cm × 1 cm × 1 cm. The biopsy specimen should be a wedge with its apex extending at least 1 cm into the lung parenchyma. An elongated or elliptical shaped biopsy obtained by shaving off the margin of a lobe should be avoided. In an older child where space allows, a stapler may be the most reliable means of parenchymal division and sealing. We have had success in smaller children using the Ligasure (Covidien, Inc., Mansfield, MA). However, this often requires a deflated lung. Also, the diseased lung may not seal well and it may be necessary to oversew the lung edge. The patient on a high-frequency oscillating ventilator can be problematic. The constant distal airway pressure makes it very difficult to obtain a seal by any means. Thus, oversewing the edge of the biopsy is usually needed. We have also used fibrin glue to help prevent an air leak. Once the biopsy is obtained, a piece should be sent for microbiologic analysis under sterile conditions prior to handing the specimen off the operative field.
Right Middle Lobe Syndrome
Right middle lobe (RML) syndrome refers to a picture of persistent atelectasis in the right middle lobe. Similar to bronchiectasis, the syndrome is a clinical diagnosis resulting from a variety of underlying causes. In a large review of the literature encompassing over 900 cases, the etiologic causes of RML syndrome were inflammation in 47%, bronchiectasis in 15%, malignant tumors in 22%, benign tumors in 2%, tuberculosis in 9%, aspiration in 2%, and miscellaneous in the remaining 3%.110 It is felt there is a predisposition for the RML to develop atelectasis because of the narrow diameter and acute take-off of the RML bronchus. Also, the relative anatomic isolation of the RML with poor collateral ventilation reduces the chance of reinflation once atelectasis develops.111
The clinical presentation usually consists of nonspecific respiratory symptoms. On plain chest radiograph, the diagnosis is suggested by a wedge-shaped density that extends anteriorly and inferiorly from the hilum of the lung. This finding is best visualized on the lateral view. However, plain films may be inadequate and a high-resolution CT may be needed to delineate the bronchial anatomy and the presence of bronchiectasis.112 Bronchoscopy is a useful initial step in diagnosis as it can document stenosis or obstruction of the RML bronchus from a variety of causes such as granulation tissue, tumor, or foreign body. Clearing secretions and obtaining specimens for microbiological analysis may also guide management.
Complete bronchial obstruction, the presence of bronchiectasis, and persistent or recurrent disease despite medical management are an indication for lobectomy.113 Given the relatively small contribution of the RML to normal ventilation along with the ineffectiveness of ventilation in the presence of this disease, the operation usually results in little morbidity to the patient and an excellent clinical outcome. In a series of 20 children, 17 were asymptomatic after RML lobectomy with an additional two being improved.113 Therefore, it may be reasonable to consider lobectomy early in the disease course prior to chronic debilitation.
Spontaneous Pneumothorax
The development of a spontaneous pneumothorax is thought to be due to ruptured apical bullae or blebs (Fig. 23-10). The incidence of spontaneous pneumothorax is thought to be 4 per 100,000 males and 1.1 per 100,000 females.114,115 Although the etiology is unclear, this disorder appears to be more prevalent in patients with asthma, cystic fibrosis, or connective tissue disorders.
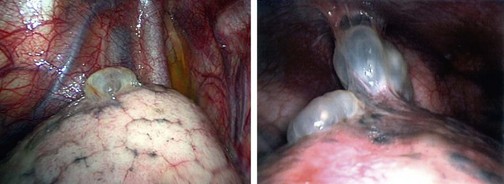
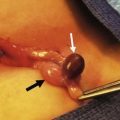
FIGURE 23-10 A spontaneous pneumothorax generally develops from rupture of apical bullae. These bullae were found in two different patients at thoracoscopy for spontaneous pneumothorax.
Patients can develop symptoms both at rest and with any maneuvers that increase intrathoracic pressure, such as lifting or straining. Chest pain and shortness of breath are the most common symptoms. In a small number of patients, there can be an emergency presentation with tension pneumothorax. Tube thoracostomy is needed immediately in these patients.
Some clinicians value a CT scan in the management of patients with a spontaneous pneumothorax. A recent study from our hospital looked at the management of 34 patients over three years.116 The mean age was 16 years and there was an average of 1.7 pneumothoraces per patient before operation. CT scans were performed in 26 cases and blebs were found on eight studies. However, of the 18 negative scans, 14 patients (78%) were found to have blebs at thoracoscopy. In this study, the sensitivity of CT scan for identifying blebs was 36%. The authors concluded that the decisions for operation should be based on clinical judgment without the use of CT scans.
The optimal approach for management in these patients is thoracoscopy. If a bleb is identified, then it can be ligated and excised. Also, pleurodesis can be performed with a variety of measures including the use of the electrocautery scratch pad to abrade the cupola of the lung. Talc poudrage has also been found to have a high success rate in treatment of spontaneous pneumothorax and a very low morbidity.117
References
1. Jaffe, A, Calder, AD, Owens, CM, et al. Role of routine computed tomography in paediatric pleural empyema. Thorax. 2008; 63:897–902.
2. McIntosh, K. Community-acquired pneumonia in children. N Engl J Med. 2002; 346:429–437.
3. Byington, CL, Spencer, LY, Johnson, TA, et al. An epidemiological investigation of a sustained high rate of pediatric parapneumonic empyema: Risk factors and microbiological associations. Clin Infect Dis. 2002; 34:434–440.
4. Tan, TQ, Mason, EO, Jr., Wald, ER, et al. Clinical characteristics of children with complicated pneumonia caused by Streptococcus pneumoniae. Pediatrics. 2002; 110:1–6.
5. Grijalva, CG, Nuorti, JP, Zhu, Y, et al. Increasing Incidence of Empyema Complicating Childhood Community Acquired Pneumonia In The United States. CID. 2010; 50:805–813.
6. Balfour-Lynn, IM, Abrahamson, E, Cohen, G, et al. BTS guidelines for the management of pleural infection in children. Thorax. 2005; 60(Suppl. 1):i1–21.
7. Bradley, JS, Byington, CL, Shah, SS, et al. Executive Summary: The management of community acquired pneumonia in infants and children older than 3 months of age: Clinical practice guidelines by the Pediatric Infectious Disease Society and the Infectious Disease Society of America. Clin Infect Dis. 2011; 53:617–630.
8. Hamm, H, Light, RW. Parapneumonic effusion and empyema. Eur Respir J. 1997; 10:1150–1156.
9. Idell, S, Girard, W, Koenig, KB, et al. Abnormalities of pathways of fibrin turnover in the human pleural space. Am Rev Respir Dis. 1991; 144:187–194.
10. Light, RW. Parapneumonic effusions and empyema. Clin Chest Med. 1985; 6:55–62.
11. Picard, E, Joseph, L, Goldberg, S, et al. Predictive factors of morbidity in childhood parapneumonic effusion-associated pneumonia: A retrospective study. Pediatr Infect Dis J. 2010; 29:840–843.
12. Padman, R, King, KA, Iqbal, S, et al. Parapneumoniceffusion and empyema in children: Retrospective review of the duPont experience. ClinPediatr (Phila). 2007; 46:518–522.
13. Porcel, J, Vives, M, Esquerda, A. Tumor necrosis factor-α in pleural fluid: A marker of complicated parapneumonic effusions. Chest. 2004; 125:160–164.
14. Light, RW. A new classification of parapneumonic effusions and empyema. Chest. 1995; 108:299–301.
15. Colice, GL, Curtic, A, Deslauriers, J, et al. Medical and surgical treatment of parapneumonic effusions: An evidence based guideline. Chest. 2000; 118:1158–1171.
16. Wong, KS, Lin, TY, Huang, YC, et al. Scoring system for empyema thoracis and help in management. Indian J Pediatr. 2005; 72:1025–1028.
17. Buckingham, S, King, M, Miller, M. Incidence and etiologies of complicated parapneumonic effusions in children. Pediatr Infect Dis. 2003; 22:499–504.
18. McLaughlin, F, Goldman, D, Rosenbaum, D, et al. Empyema in children: Clinical course and long-term follow-up. Pediatrics. 1984; 73:587–593.
19. Goeman, A, Kipur, N, Toppare, M, et al. Conservative treatment of empyema in children. Respiration. 1993; 60:182–185.
20. Schultz, KD, Fan, L, Pinsky, J, et al. The changing face of pleural empyemas in children: Epidemiology and management. Pediatrics. 2004; 113:1735–1740.
21. King, S, Thomson, A. Radiological perspectives in empyema. Br Med Bull. 2002; 61:203–214.
22. Brixey, AG, Luo, Y, Skouras, V, et al. The efficacy of chest radiographs in detecting parapneumonic effusions. Respirology. 2011; 16:1000–1004.
23. Balik, M, Plasil, P, Waldauf, P, et al. Ultrasound estimation of volume of pleural fluid in mechanically ventilated patients. Intensive Care Med. 2006; 32:318–321.
24. Eibenberger, KL, Dock, WI, Ammann, ME, et al. Quantification of pleural effusions: Sonography versus radiography. Radiology. 1994; 191:681–684.
25. Calder, A, Owens, CM. Imaging of parapneumonic pleural effusions and empyema in children. Pediatr Radiol. 2009; 39:527–537.
26. Kurian, J, Levin, TL, Han, BK, et al. Comparison of ultrasound and CT in the evaluation of pneumonia complicated by parapneumonic effusion in children. AJR Am J Roentgenol. 2009; 193:1648–1654.
27. Pillai, D, Song, X, Pastor, W, et al. Implementation and impact of a consensus diagnostic and management algorithm for complicated pneumonia in children. J Investig Med. 2011; 59:1221–1227.
28. Shomaker, KL, Weiner, T, Esther, CR, Jr. Impact of an evidence-based algorithm on quality of care in pediatric parapneumonic effusion and empyema. Pediatr Pulmonol. 2011; 46:722–728.
29. Brenner, DJ, Hall, EJ. Computed tomography–an increasing source of radiation exposure. N Engl J Med. 2007; 357:2277–2284.
30. Carter, E, Waldhausen, J, Zhang, W, et al. Management of children with empyema: Pleural drainage is not always necessary. Pediatr Pulmonol. 2010; 45:475–480.
31. Soares, P, Barreira, J, Pissarra, S, et al. Pediatric parapneumonic pleural effusions: Experience in a university central hospital. Rev Port Pneumol. 2009; 15:241–259.
32. Shoseyov, D, Bibi, H, Shatzberg, G, et al. Short-term course and outcome of treatments of pleural empyema in pediatric patients: Repeated ultrasound-guided needle thoracocentesis vs chest tube drainage. Chest. 2002; 121:836–840.
33. Epaud, R, Aubertin, G, Larroquet, M, et al. Conservative use of chest tube insertion in children with pleural effusion. Pediatr Surg Int. 2006; 22:357–362.
34. Rahman, NM, Maskell, NA, Davies, CW, et al. The relationship between chest tube size and clinical outcome in pleural infection. Chest. 2010; 137:536–543.
35. Lin, CH, Lin, WC, Chang, JS. Comparison of pigtail catheter with chest tube for drainage of parapneumonic effusion in children. Pediatr Neonatol. 2011; 52:337–341.
36. Wurnig, PN, Wittmer, V, Pridun, NS, et al. Video-assisted thoracic surgery for pleural empyema. Ann Thorac Surg. 2006; 81:309–313.
37. Hope, WW, Bolton, WD, Stephenson, JE. The utility and timing of surgical intervention for parapneumonic empyema in the era of video-assisted thoracoscopy. Am Surg. 2005; 71:512–514.
38. Olgac, G, Fazlioglu, M, Kutlu, CA. VATS decortication in patients with stage 3 empyema. Thorac Cardiovasc Surg. 2005; 53:18–20.
39. Cheng, G, Vintch, JR. A retrospective analysis of the management of parapneumonic empyemas in a county teaching facility from 1992 to 2004. Chest. 2005; 128:3284–3290.
40. Tsao, K, St Peter, SD, Sharp, SW, et al. Current application of thoracoscopy in children. J Laparoendosc Adv Surg Tech A. 2008; 18:131–135.
41. Kurt, BA, Winterhalter, KM, Connors, RH, et al. Therapy of parapneumonic effusions in children: Video assisted thoracoscopic surgery versus conventional thoracostomy drainage. Pediatrics. 2006; 118:e547–e553.
42. Gates, RL, Hogan, M, Weinstein, S, et al. Drainage, fibrinolytics, or surgery: A comparison of treatment options in pediatric empyema. J Pediatr Surg. 2004; 39:1638–1642.
43. Aziz, A, Healey, JM, Qureshi, F, et al. Comparative analysis of chest tube thoracostomy and video-assisted thoracoscopic surgery in empyema and parapneumonic effusion associated with pneumonia in children. Surg Infect (Larchmt). 2008; 9:317–323.
44. Chiu, CY, Wong, KS, Huang, YC, et al. Echo-guided management of complicated parapneumonic effusion in children. Pediatr Pulmonol. 2006; 41:1226–1232.
45. Freitas, S, Fraga, JC, Canani, F. Thoracoscopy in children with complicated parapneumonic pleural effusion at the fibrinopurulent stage: A multi-institutional study. J Bras Pneumol. 2009; 35:660–668.
46. Yao, CT, Wu, JM, Liu, CC, et al. Treatment of complicated parapneumonic pleural effusion with intrapleural streptokinase in children. Chest. 2004; 125:566–571.
47. Ekingen, G, Guvenc, BH, Sozubir, S, et al. Fibrinolytic treatment of complicated pediatric thoracic empyemas with intrapleural streptokinase. Eur J Cardiothorac Surg. 2004; 26:503–507.
48. Misthos, P, Sepsas, E, Konstantinou, M, et al. Early use of intrapleural fibrinolytics in the management of postpneumonic empyema. A prospective study. Eur J Cardiothorac Surg. 2005; 28:599–603.
49. Kiliç, N, Celebi, S, Gürpinar, A, et al. Management of thoracic empyema in children. Pediatr Surg Int. 2002; 18:21–23.
50. Cochran, JB, Tecklenburg, FW, Turner, RB. Intrapleural instillation of fibrinolytic agents for treatment of pleural empyema. Pediatr Crit Care Med. 2003; 4:39–43.
51. Ulku, R, Onat, S, Kiliç, N. Intrapleural fibrinolytic treatment of multiloculated pediatric empyemas. Minerva Pediatr. 2004; 56:419–423.
52. Bouros, D, Antoniou, KM, Chalkiadakis, G, et al. The role of video-assisted thoracoscopic surgery in the treatment of parapneumonic empyema after the failure of fibrinolytics. Surg Endosc. 2002; 16:151–154.
53. Sonnappa, S, Cohen, G, Owens, CM, et al. Comparison of urokinase and video-assisted thoracoscopic surgery for treatment of childhood empyema. Am J Respir Crit Care Med. 2006; 174:221–227.
54. St. Peter, SD, Tsao, K, Spilde, TL, et al. Thoracoscopic decortication vs tube thoracostomy with fibrinolysis for empyema in children: A prospective, randomized trial. J Pediatr Surg. 2009; 44:106–111.
55. Zuckerman, DA, Reed, MF, Howington, JA, et al. Efficacy of intrapleural tissue-type plasminogen activator in the treatment of loculated parapneumonic effusions. J Vasc Interv Radiol. 2009; 20:1066–1069.
56. Cohen, E, Weinstein, M, Fisman, DN. Cost-effectiveness of competing strategies for the treatment of pediatric empyema. Pediatrics. 2008; 121:e1250–e1257.
57. Kalfa, N, Allal, H, Lopez, M, et al. Thoracoscopy in pediatric pleural empyema: A prospective study of prognostic factors. J Pediatr Surg. 2006; 41:1732–1737.
58. Bouros, D, Schiza, S, Tzanakis, N, et al. Intraplerual urokinase versus normal saline in the treatment of complicated parapneumonic effusions and empyema. A randomized, double-blind study. Am J Respir Crit Care Med. 1999; 159:37–42.
59. Diacon, AH, Theron, J, Schuurmans, MM, et al. Intrapleural streptokinase for empyema and complicated parapneumonic effusions. Am J Respir Crit Care Med. 2004; 170:49–53.
60. Proesmans, M, De Boeck, K. Clinical practice: Treatment of childhood empyema. Eur J Pediatr. 2009; 168:639–645.
61. Gasior, AC, Knott, EM, Sharp, SW, et al. Experience with an evidence-based protocol using fibrinolysis as first-line treatment for empyema in children. J Pediatr Surg. 2013; 48:1312–1315.
62. Kosloske, AM, Ball, WS, Butler, C, et al. Drainage of pediatric lung abscess by cough, catheter or complete resection. J Pediatr Surg. 1986; 21:596–600.
63. Tseng, Y, Wu, M, Lin, M. Surgery for lung abscess in immunocompetent and immunocompromised children. J Pediatr Surg. 2001; 36:470–473.
64. Estera, AS, Platt, MR, Mills, LJ, et al. Primary lung abscess. J Thorac Cardiovasc Surg. 1980; 79:275–282.
65. Chidi, CC, Mendelsohn, HJ. Lung abscess. A study of the results of treatment based on 90 consecutive cases. J Thorac Cardiovasc Surg. 1974; 68:168–172.
66. Lorenzo, RL, Bradford, BF, Black, J, et al. Lung abscesses in children: Diagnostic and therapeutic needle aspiration. Radiology. 1985; 157:79–80.
67. Ball, BS, Bisset, GS, Towbin, RB. Percutaneous drainage of chest abscesses in children. Radiology. 1989; 171:431–434.
68. Hoffer, FA, Bloom, DA, Colin, AA, et al. Lung abscess versus necrotizing pneumonia: Implications for interventional therapy. Pediatr Radiol. 1999; 29:87–91.
69. Patradoon-Ho, P, Fitzgerald, DA. Lung abscesses in children. Paediatr Respir Rev. 2007; 8:77–84.
70. Nagasawa, KK, Johnson, SM. Thoracoscopic treatment of pediatric lung abscesses. J Pediatr Surg. 2010; 45:574–578.
71. Ayed, AK, Al-Rowayeh, A. Lung resection in children for infectious pulmonary diseases. Pediatr Surg Int. 2005; 21:604–608.
72. Quigley, MJ, Fraser, RS. Pulmonary pneumatocele: Pathology and pathogenesis. AJR Am J Roentgenol. 1988; 150:1275–1277.
73. Oviawe, O, Ogundipe, O. Pneumatoceles associated with pneumonia: Incidence and clinical course in Nigerian children. Trop Geogr Med. 1985; 37:264–269.
74. Glustein, JZ, Kaplan, M. Enterobacter cloacae causing pneumatocele in a neonate. Acta Pediatr. 1994; 83:990–991.
75. Zuhdi, MK, Bradley, JS, Spear, RM, et al. Fatal air embolism as a complication of staphylococcal pneumonia with pneumatoceles. Pediatr Infect Dis J. 1995; 14:811–812.
76. Zuhdi, MK, Spear, R, Worthen, M, et al. Percutaneous catheter drainage of tension pneumatocele, secondarily infected pneumatocele and lung abscess in children. Crit Care Med. 1996; 42:330–333.
77. Kogutt, M, Lutrell, C, Pulau, F, et al. Decompression of pneumatocele in a neonate by percutaneous catheter placement. Pediatr Radiol. 1999; 29:488–489.
78. Laënnec, RTH. De l’Auscultation Médiaté on Traite du Diagnostic des Maladies des Poumons et du Coeur, Fondé Principalement sur ce Noveau Moyer d’Exploration. Paris: Brosson et Claude; 1819.
79. Reid, LM. Reduction in bronchial subdivision in bronchiectasis. Thorax. 1950; 5:233–247.
80. Rademacher, J, Welte, T. Bronchiectasis-Diagnosis and Treatment. Dtsch Arztebl Int. 2011; 108:809–815.
81. McGuinnea, G, Naidich, DP. CT of airways disease and bronchiectasis. Radiol Clin North Am. 2002; 40:1–19.
82. Agasthian, T, Deschamps, C, Trastek, VF, et al. Surgical management of bronchiectasis. Ann Thorac Surg. 1996; 62:976–980.
83. Goeminne, P, Dupont, L. Non-cystic fibrosis bronchiectasis: Diagnosis and management in 21st century. Postgrad Med J. 2010; 86:493–501.
84. Kumar, NA, Nguyen, B, Maki, D. Bronchiectasis: Current clinical and imaging concepts. Semin Roentgenol. 2001; 36:41–50.
85. Smevik, B. Complementary investigations in bronchiectasis in children. Monaldi Arch Chest Dis. 2000; 55:420–426.
86. Ashour, M, Al-Kattan, K, Rafay, MA, et al. Current surgical therapy for bronchiectasis. World J Surg. 1999; 23:1096–1104.
87. Haciibrahimoglu, G, Fazliogu, M, Olcmen, A, et al. Surgical management of childhood bronchiectasis due to infectious disease. Gen Thorac Surg. 2004; 127:1361–1365.
88. Otgün, I, Karnak, I, Tanyel, FC, et al. Surgical treatment of bronchiectasis in children. J Pediatr Surg. 2004; 39:1532–1536.
89. Rothenberg, SS, Kuenzler, KA, Middlesworth, W. Thoracoscopic lobectomy for severe bronchiectasis in children. J Laparoendosc Adv Surg Tech A. 2009; 19:555–557.
90. Dubin, P, King, I, Gallagher, P. Congenital chylothorax. Curr Opin Pediatr. 2000; 12:505–509.
91. Beghatti, M, La Scala, G, Belli, D, et al. Etiology and management of pediatric chylothorax. J Pediatr. 2000; 8:136–138.
92. Allen, EM, Van Heeckeren, DW, Spector, ML, et al. Management of nutritional and infectious complications of postoperative chylothorax in children. J Pediatr Surg. 1991; 26:1169–1174.
93. Bond, SJ, Guzzetta, PC, Snyder, ML, et al. Management of pediatric postoperative chylothorax. Ann Thoracic Surg. 1993; 56:469–473.
94. Sharkey, AJ, Rao, JN. The successful use of octreotide in the treatment of traumatic chylothorax. Tex Heart Inst J. 2012; 39:428–430.
95. Luca, R, Bini, R, Chessa, M, et al. The effectiveness of octreotide in the treatment of postoperative chylothorax. Eur J Pediatr. 2002; 161:149–150.
96. Rodgers, B, Michalsky, MP, Kattwinkel, J. The use of octreotide to treat congenital chylothorax. J Pediatr Surg. 2006; 41:845–847.
97. Au, M, Weber, TR, Flemmin, RE. Successful use of octreotide in a case of neonatal chylothorax. J Pediatr Surg. 2003; 38:1106–1107.
98. Benedix, F, Schulz, HU, Scheidbach, H, et al. Successful conservative treatment of chylothorax following oesophagectomy – a clinical algorithm. S Afr J Surg. 2010; 48:86–88.
99. Valentine, VG, Raffin, TA. The management of chylothorax. Chest. 1992; 102:586–591.
100. Buchan, K, Amir-Reza, H, Ritchie, A. Thoracoscopic thoracic duct ligation for traumatic chylothorax. Ann Thorac Surg. 2001; 72:1366–1367.
101. Graham, DD, McGahren, ED, Tribble, CG, et al. Use of video-assisted thoracic surgery in the treatment of chylothorax. Ann Thorac Surg. 1994; 57:1507–1511.
102. Fahimi, H, Casselman, F, Mariani, M, et al. Current management of postoperative chylothorax. Ann Thorac Surg. 2001; 71:448–451.
103. Cope, C. Management of chylothorax via percutaneous embolization. Curr Opin Pulmon Med. 2004; 10:311–314.
104. Cope, C, Kaiser, LR. Management of unremitting chylothorax by percutaneous embolization and blockage of retroperitoneal lymphatic vessels in 42 patients. J Vasc Interv Radiol. 2002; 13:1139–1148.
105. Wolff, AB, Silen, ML, Kokoska, ER, et al. Treatment of refractory chylothorax with externalized pleuroperitoneal shunts in children. Ann Thorac Surg. 1999; 68:1053–1057.
106. Turner-Warwick, M. Interstitial lung disease. Semin Respir Med. 1984; 6:1–102.
107. Fan, L, Langston, C. Chronic interstitial lung disease in children. Pediatr Pulmonol. 1993; 16:184–196.
108. Bokulic, RE, Hilman, BC. Interstitial lung disease in children. Pediatr Clin North Am. 1994; 41:543–567.
109. Langston, C, Patterson, K, Dishop, MK, et al. A protocol for the handling of tissue obtained by operative lung biopsy: Recommendations of the child pathology co-operative group. Child Pathology Co-operative Group. Pediatr Dev Pathol. 2006; 9:173–180.
110. Wagner, RB, Johnston, MR. Middle lobe syndrome. Ann Thorac Surg. 1983; 35:679–686.
111. Saha, SP, Mayo, P, Long, GA, et al. Middle lobe syndrome: Diagnosis and management. Ann Thorac Surg. 1982; 33:28–31.
112. Yung, K, Aspestrand, F, Kolbenstvedt, A. High-resolution CT and bronchography in the assessment of bronchiectasis. Acta Radiol. 1991; 32:439–441.
113. Sehitoqullari, A, Sayir, F, Cobanaglu, U, et al. Surgical treatment of right middle lobe syndrome in children. Ann Thorac Med. 2012; 7:8–11.
114. Healthcare Cost and Utilization Project (HCUP). Kids’ Inpatient Database (KID). 1997, 2000, 2003, 2006. Available at: http://www.hcup-us.ahrq.gov/kidoverview.jsp. [Accessed October 15, 2010].
115. United States Census Bureau. Your Gateway to Census. Available at http://www.census.gov/main/www/cen2000.html, 2000. [Accessed October 15, 2010].
116. Laituri, CA, Valusek, PA, Rivard, DC, et al. The utility of computed tomography in the management of patients with spontaneous pneumothorax. J Pediatr Surg. 2011; 46:1523–1525.
117. Cardillo, G, Carleo, F, Giunti, R, et al. Videothoracoscopic talc poudrage in primary spontaneous pneumothorax: A single-institution experience in 861 cases. J Thorac Cardiovasc Surg. 2006; 131:322–328.