Acquired Disorders
Gastric Volvulus
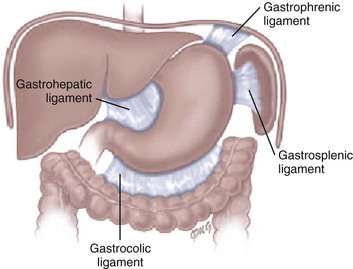
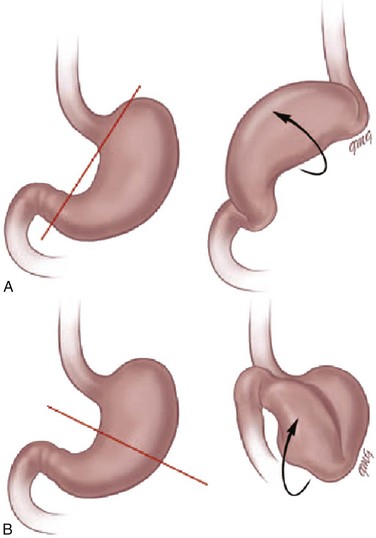
Etiology: Normally, the stomach is relatively fixed in the peritoneal cavity at the esophagogastric junction, with four additional ligaments: (1) gastrohepatic, (2) gastrosplenic, (3) gastrocolic, and (4) gastrophrenic (Fig. 102-1) Gastric volvulus is defined as an abnormal rotation of the stomach of more than 180 degrees around its long (organoaxial) or short (mesenteroaxial) axes (Fig. 102-2, A and B), causing a closed loop obstruction, with consequences such as incarceration, strangulation, and perforation.1 Predisposing factors for gastric volvulus include congenital or acquired absence of one or more ligaments as isolated abnormalities or conditions such as asplenia and diaphragmatic defects.
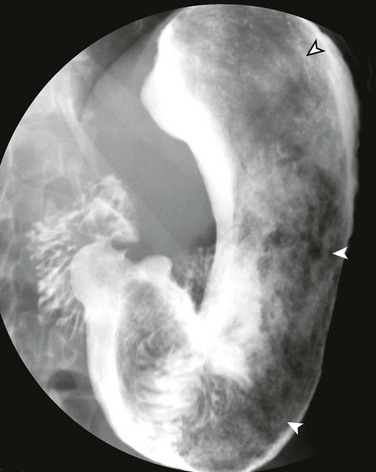
In organoaxial gastric volvulus, an inversion of the position of the greater and lesser curves of the stomach occurs, with the greater curvature positioned to the right and superior to the lesser curvature. In mesenteroaxial gastric volvulus, the stomach folds on its short axis; this leads to reversal of the relationship between the gastroesophageal junction and the pylorus. Clinically, two primary scenarios exist. The first is the acute fulminant presentation, most often encountered in the mesenteroaxial type, with sudden and persistent vomiting and acute abdominal pain.1 The chronic intermittent presentation is more often associated with the organoaxial type, with less specific symptoms, including recurrent abdominal pain, vomiting, and gastric distension.2
Imaging: Abdominal radiographs in patients with gastric volvulus typically show marked gastric distension. The stomach becomes spherical, with paucity of distal bowel gas, indicating gastric outlet obstruction (Fig. 102-3, A). Other findings include diaphragmatic elevation and the presence of two air-fluid levels in the stomach. Occasionally, the type of gastric volvulus can be inferred by the gastric configuration: a pylorus projecting over the gastric fundus and an unusual nasogastric tube course are suggestive of the mesenteroaxial type, whereas an inversion of the relationship of the greater and lesser curvatures is suggestive of the organoaxial type; mixed types also occur, with combined imaging findings.
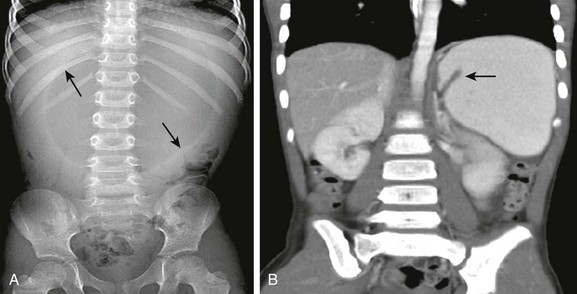
Figure 102-3 Mesenteroaxial volvulus in a 6-year-old girl presenting with acute unremitting vomiting.
A, Supine abdominal radiograph shows a dilated, spherical gastric bubble (arrows) with paucity of distal bowel gas. B, The coronal image of a contrast-enhanced computed tomography scan shows a reversal of the axis of the distended stomach with the pylorus superiorly located and inverted (arrow).
Although, in most cases, plain radiographs are highly suggestive of the diagnosis, the upper gastrointestinal (UGI) series remains the diagnostic procedure of choice, demonstrating the type of volvulus and evidence of gastric outlet obstruction. If performed, other imaging modalities such as CT can also be useful in demonstrating the abnormal orientation of the stomach (see Fig. 102-3, B) as well as associated anomalies such as heterotaxy or the presence of pneumatosis.2
Spontaneous Gastric Perforation
Etiology: Spontaneous perforation of the stomach is an uncommon event mainly seen in the neonatal period as a cause of pneumoperitoneum.5 The etiology is unknown, but possibilities include sudden gastric distension with a degree of ischemia attributable to perinatal hypoxia, a more distal bowel obstruction, and congenital focal absence of the muscle of the gastric wall.6–8 Beyond the neonatal period, perforation is rare and usually secondary to trauma (tubes, catheters), surgery (fundoplication), caustic ingestion, or peptic ulcer.1 The most common presenting manifestations of perforation include sudden onset of abdominal distension, ileus, respiratory distress, and, less frequently, cyanosis, fever, vomiting, and bloody stool.9
Imaging: Abdominal radiography is the imaging method of choice when perforation of the gastrointestinal (GI) tract is suspected. As in any other type of GI perforation, abdominal radiographs will typically demonstrate free intraperitoneal air. A reported suggestive sign of gastric perforation is the lack of an air-fluid level in the stomach in a horizontal beam view, and relative paucity of gas in the distal bowel.10
Peptic Ulcer Disease
Etiology: Peptic ulcer disease represents ulceration of the gastric or duodenal mucosa resulting from, on the one hand, an imbalance between the mucosal protective mechanisms and, on the other, the aggressive factors of acid and pepsin production, injury, and infection.11 The gel layer, a protective bicarbonate and mucous barrier lining the stomach, is approximately 0.2 to 0.5 mm in thickness and consists of 95% water and 5% mucin glycoprotein. Breaches in this gel layer, secondary to H. pylori or antiinflammatory drugs, result in a continuum of damage to the underlying mucosa, with ulceration occurring when damage extends to the muscular layer.
Peptic ulcer disease in children may be primary or secondary (induced by drugs, alcohol, stress, or metabolic disease), with each form having different manifestations and prognostic implications. H. pylori has been recognized as a common human pathogen associated with both inflammatory and malignant conditions of the upper gastrointestinal tract, and affects nearly all children with peptic ulcer disease.12 Primary peptic ulcers are associated with H. pylori infection.
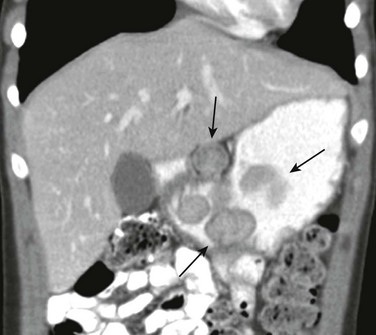
In addition to being classified as primary and secondary, peptic ulcers can also be classified according to the site of involvement (gastric or duodenal). Gastric ulcers are mostly seen in neonates and young children, whereas duodenal ulcers are more common after the neonatal period and tend to be secondary to systemic illness or chronic intake of medications such as non-steroidal antiinflammatory agents. Zollinger-Ellison syndrome causes secondary peptic ulcer disease, often with multiple ulcerations caused by increased acid generated by a gastrin-producing tumor (Fig. 102-4).11,13
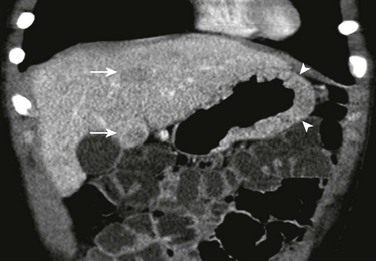
Figure 102-4 Zollinger-Ellison syndrome secondary to a pancreatic gastrinoma in a 10 year-old-boy.
The coronal image of a contrast-enhanced computed tomography shows marked segmental thickening of the gastric fundus and body (arrowheads). Two hypodense lesion in the liver indicate metastases (arrows).
Symptoms of ulcer disease vary with age; infants and young children present with feeding problems and vomiting. In some patients, the first sign of peptic ulcer disease may be upper or lower GI hemorrhage or acute severe abdominal pain due to perforation. Pain can be nocturnal or occur early in the morning. Unlike in adults, the pain is neither precipitated nor relieved by meals or antacid use.11
Imaging: Endoscopy has assumed the primary role in the diagnosis of ulcer disease over the past two decades, while the role of the radiologist has dramatically decreased and is now limited to the incidental case, as UGI contrast studies have been demonstrated to have a high false-negative rate for ulcer detection.14,15 However, these studies, as an initial tool to evaluate the child with abdominal pain and vomiting, may incidentally demonstrate the ulcer. Perforated ulcers may be incidentally identified on CT in the evaluation of a child with acute abdominal pain (Fig. 102-5).
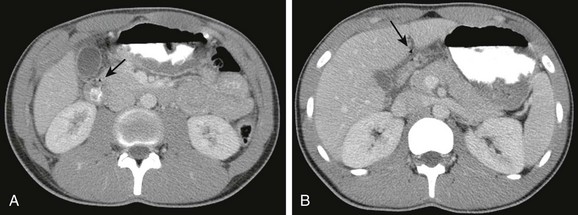
Figure 102-5 Perforated duodenal ulcer in 16-year-old boy on nonsteroidal antiinflammatory regimen for previous knee injury and surgery, presenting with acute onset of abdominal pain while in school.
A, A contrast-enhanced computed tomography scan shows marked thickening of the duodenal wall and small amount of free air (arrow). B, The slightly more cephalad image shows fluid about the duodenum and additional free air extending toward the area of the falciform ligament (arrow).
Treatment and Follow-up: Current therapy has been proven to be effective and is based on medications that decrease acid production. In the cases of H. pylori infection, a combination therapy, including a histamine-2 blocker or proton pump inhibitor, antibiotics, and, bismuth, may be necessary to eradicate the causative organism and prevent both recurrence and malignant complications.11
Hypertrophic Gastropathy (Ménétrier Disease)
Etiology: Hypertrophy of the gastric rugal folds, in association with protein-losing enteropathy, in childhood is labeled Ménétrier disease, or hypertrophic gastropathy of childhood.16 The clinical, pathologic, and etiologic factors of this disease in children differ from those of the adult form. In adults, the disease is chronic and premalignant. In children, the disease is self-limiting, with a peak age of presentation of 5 years. Presentation includes acute vomiting, diarrhea, upper abdominal pain, and anorexia. Peripheral edema is usually present and may be associated with ascites and pleural effusions. Rarely, signs of GI bleeding occur with coexisting ulceration of the gastric rugae. The etiology of the disease remains unknown; however, it has been previously associated with several infectious agents, including cytomegalovirus, H. pylori, mycoplasma, herpes virus, and Giardia lamblia.
Imaging: Diagnosis is most commonly made with UGI contrast studies demonstrating thickened gastric mucosal folds in the fundus and body, sparing the antrum and pylorus, with normal appearance of the small bowel.17 Ultrasound has also been successfully used in diagnosis.18 On the CT scan, similar findings of thickened rugal folds in the fundus and body of the stomach can be seen, with sparing of the antrum (Fig. 102-6). Endoscopy confirms the diagnosis. Differential diagnosis includes eosinophilic gastritis, primary gastric lymphoma, gastric carcinoma, inflammatory pseudotumor, gastric varices, Zollinger-Ellison syndrome, lymphangiectasia, and anisakiasis if there is a history of ingestion of raw fish.16
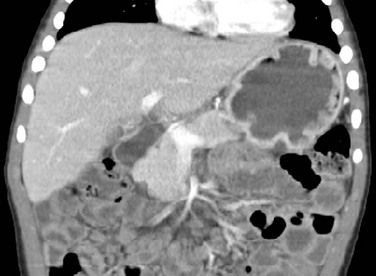
Figure 102-6 Ménétrier disease.
Contrast-enhanced coronal computed tomography reformation shows the typical thickening of the gastric folds in the fundus of the well distended stomach in a 4-year-old boy presenting with 10 days of vomiting with streaks of blood, palpebral edema, and hypoalbuminemia. Ménétrier disease confirmed with endoscopy and gastric biopsy.
Chronic Granulomatous Disease
Etiology: Chronic granulomatous disease (CGD) of childhood is a hereditary disorder of neutrophil function, which is typically inherited as an X-linked recessive disorder, but three autosomal recessive defects have also been identified. The genetic alteration leads to a defect in activation of the NADPH (nicotinamide adenine dinucleotide phosphate-oxidase) molecule within the phagocyte, preventing the formation of free radical superoxide in the “respiratory burst,” and resulting in survival of catalase-positive organisms within the phagocytes, with chronic inflammatory reaction and granuloma formation.20 In the stomach, narrowing of the gastric antrum is a distinctive manifestation of CGD, occurring in 16% of cases.21 Gastric outlet obstruction occurs in the X-linked recessive form more commonly than in the autosomal recessive form and presents at a mean age of 44 months, usually with severe vomiting.22 Histologically, a granuloma forms within the involved antral wall; however, the etiology of the antral wall thickening is unclear, as an infectious agent is not typically isolated.23
Imaging: A patient with CGD and signs of gastric outlet obstruction should be initially evaluated with either an ultrasound or a UGI contrast study. The patient’s medical history is vital to obtain the correct diagnosis. Ultrasound demonstrates the circumferential antral wall thickening. The UGI contrast study shows concentric antral narrowing and evaluates the degree of gastric obstruction.21 To detect the gastric wall thickening on CT, the stomach should be distended. Although CT is not the primary modality to assess gastric involvement, it may be helpful in identifying disease in other areas of involvement such as mesenteric adenopathy and hepatic or splenic involvement. The differential diagnosis of gastric antral involvement includes peptic ulcer disease, Crohn disease, (Fig. 102-7), and eosinophilic gastritis.24,25
Eosinophilic Gastritis
Etiology: Eosinophilic gastroenteritis is a cluster of rare and poorly understood illnesses that share as their hallmark gastric and intestinal eosinophilic infiltration, peripheral eosinophilia, and elevated serum immunoglobulin E and may involve other parts of the GI tract, including the esophagus, duodenum, and colon. Patients may present between infancy and adolescence, with symptoms of abdominal pain, anorexia, failure to thrive, anemia, gastric obstruction, protein-losing enteropathy, and ascites with eosinophilia.26 Foods most often associated with exacerbations include cow’s milk, eggs, and soy. The disease is likely to remit before adulthood in approximately 70% of pediatric patients.
Imaging: On UGI or CT a lacy or nodular mucosal pattern is seen in the antrum, with sparing of the body and fundus, corresponding to the eosinophilic infiltration of the layers of the bowel wall, as seen on histology (Fig. 102-8).27 On ultrasound, the predominantly antral abnormality can mimic findings seen in hypertrophic pyloric stenosis, and this condition should be suspected if such findings are seen in conjunction with eosinophilia.28
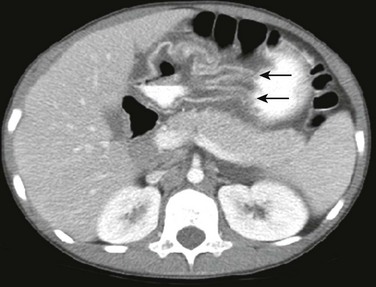
Figure 102-8 Eosinophilic gastritis in a 14-year-old with chronic abdominal pain, failure to thrive, and nausea.
Contrast-enhanced computed tomography shows marked thickening of the gastric rugae predominantly in the antrum (arrows). There is mural stratification indicating edema. Note the lack of subcutaneous fat in this child with failure to thrive.
Gastric Tumors and Tumor-Like Conditions
Gastrointestinal Foreign Bodies
The ingestion of foreign objects is relatively common in pediatric patients. This is a potentially serious problem, with peak occurrence between 6 months and 3 years of age. However, significant morbidity occurs in only approximately 1% of the patients, with many patients remaining asymptomatic.30,31 Since some foreign bodies may remain in the stomach, it is important to identify them and be aware of complications that could occur if these foreign bodies are not removed.
Etiology: Coins and smooth, blunt objects account for most ingested foreign bodies; however, the materials commonly ingested differ, according to geography and cultural group.32 In addition to the complications related to the size and shape of the foreign object, some ingested foreign bodies such as batteries can lead to toxicity because of their chemical composition (Fig. 102-9) or to mechanical problems and pressure necrosis, as can occur after ingestion of multiple magnets.
Imaging: Approximately 64% of foreign bodies are radiopaque and can be identified on plain films.30 Non-radiopaque ingested objects include those made of wood or plastic. Images of the neck, chest, and abdomen are indicated when foreign body ingestion is suspected; the child must be imaged from the mouth to the anus. Fluoroscopic studies using water-soluble contrast may be useful in identifying cases of suspected non-opaque foreign body ingestion.32
Treatment and Follow-up: Although 90% of foreign bodies that have passed through the esophagus do so spontaneously, removal of sharp objects before they enter the duodenum is recommended.32,33 Once the foreign body has passed through the stomach, caregivers of the child are instructed to review the stool to verify that the object has been expelled. If, after a week, the child has not excreted the object, a radiograph is necessary to locate it; if it is still in the duodenum, endoscopic removal is indicated.34 Disk or button batteries require special attention and should be endoscopically removed because of the damage caused by their direct corrosive effects. If they are located within the esophagus, button batteries should be removed emergently because of the potential for burns and strictures. Removal from the stomach is more important with larger batteries that are less likely to pass spontaneously.33,35
Bezoars
Bezoars are foreign bodies within the stomach or other portions of the GI tract that form from the accretion of nondigestible materials and increase in size over time. The term bezoar originates from the Arabic word “badzehr.” The original meaning of the Arabic word was “antidote for poisons” because bezoars from animals were thought to have healing or magical powers and were used as homeopathic treatment for a wide variety of maladies such as seizure disorders and bubonic plague.36
Etiology: Patients with developmental delay and psychiatric illnesses such as anorexia nervosa are at increased risk for bezoars. Predisposing factors include prior gastric surgery, diabetes mellitus with gastroparesis, cystic fibrosis, intrahepatic cholestasis, and renal failure.
The three most common bezoar types are (1) trichobezoars composed of ingested hair, (2) phytobezoars composed of plant matter, and (3) lactobezoars composed of undigested milk curds (Box 102-1). Trichobezoars usually result from swallowing of multiple small amounts of hair plucked from the head or fibers from fur, rugs, or garments. The hairs or fibers become lodged in the gastric mucosal folds and over a period, an intraluminal mass develops. The bezoar forms a cast of the lumen of the stomach and a tail may extend into the duodenum as well. Additional bezoars may occur more distally in the bowel (Fig. 102-10) and occasionally may extend throughout the intestine, in which case, it is termed “Rapunzel syndrome.”36
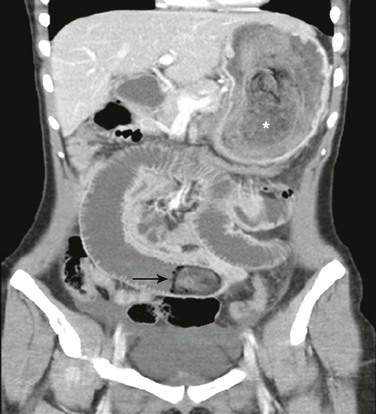
Figure 102-10 Trichobezoar in an adolescent girl with anorexia nervosa.
A coronal image of a contrast-enhanced computed tomography scan shows a trichobezoar (asterisk) of multiple densities forming a cast within the gastric lumen. An additional trichobezoar in the midjejunum (arrow) is causing proximal small bowel obstruction.
Lactobezoars (Fig. 102-11) occur in pediatric patients with a history of prematurity and who are given a highly concentrated formula; additional risk factors include poor neonatal gastric motility and dehydration.
Imaging: In the case of bezoars, the plain radiograph demonstrates a filling defect outlined by gas in the region of the stomach, with gastric distension if gastric outlet obstruction is also present. Fluoroscopic contrast studies will show the mass when the contrast material coats the bezoar and infiltrates within its interstices, producing the characteristic mottled appearance (see Fig. 102-11). A CT scan may also help identify a bezoar, typically without the need for ingestion of contrast material; CT may also help identify additional bezoars (see Fig. 102-10) as well as potential complications such as obstruction or perforation.1
Treatment and Follow-up: Therapeutic options for treating amenable bezoars include fragmentation and dissolution with enzymatic therapy or, in cases of lactobezoars, gastric lavage with saline, followed by dietary modification. In cases of motility disorders, the administration of prokinetic agents is important to prevent recurrence. Surgical or endoscopic extraction is reserved for trichobezoars or for those instances where the above measures fail.36
Gastric Tumors
Etiology: Tumors of the stomach, whether primary or metastatic, are rare in children. The differential diagnosis of a gastric mass includes polyps, lymphoma, GI stromal tumor (GIST), leiomyosarcoma, teratoma, and inflammatory pseudotumor.
Polyps, which may occur anywhere along the GI tract, are the most common gastric tumors in children. Isolated gastric polyps are typically benign hyperplastic polyps or are related to pancreatic heterotopia. Polyps can be part of a syndrome such as hamartomatous polyps in Peutz-Jeghers syndrome or adenomatous polyps in Gardner syndrome and familial adenomatous polyposis. Gastric polyps may be seen in up to 60% of patients with familial polyposis syndrome, and lifelong endoscopic surveillance of these patients is warranted because of the potential for malignant transformation of adenomatous polyps.37 An increased risk of malignancy is also seen in patients with Peutz-Jeghers syndrome.38–41
Primary gastric lymphoma can be divided into mucosa-associated lymphoid tissue (MALT) lymphoma and non-MALT lymphoma.42,43 MALT lesions typically arise in response to a stimulus such as H. pylori infection and are rare in children.44,45 Non-MALT primary gastric lymphomas are also rare and are usually high-grade non-Hodgkin lymphomas of B-cell origin, usually of the Burkitt type.46
GIST are mesenchymal neoplasms derived from the muscle wall of hollow viscera in the GI tract and are thought to perhaps be derived from the interstitial cells of Cajal. These tumors are very rare in pediatric patients and are thought to represent a majority of tumors previously carrying the diagnoses of leiomyomas, leiomyosarcomas, and leiomyoblastomas.47 However, unlike those tumors, GIST neoplasms are positive for c-KIT and PDGFRA kinase protein and gene mutations, although the pathology in pediatric patients may not be as clear.48–50 These tumors may be found in the gastric antrum or body, are more commonly found in adolescent girls, and may be associated with pulmonary chondroma and extra-adrenal paraganglioma (Carney triad), or neurofibromatosis type-1.47,50
Leiomyoma and leiomyosarcoma are also mesenchymal neoplasms; unlike GIST, these tumors are c-KIT and PDGFRA negative but do demonstrate smooth muscle markers. They are uncommon in children, the peak age at presentation being the sixth decade. Although polypoid leiomyomas are the most common smooth muscle neoplasm arising in the GI tract, they are very rare outside of the esophagus and rectosigmoid; likewise, the malignant leiomyosarcoma in the stomach is very rare.51
Gastric teratomas comprise less than 1% of teratomas in pediatric patients and occur much more frequently in the sacrococcygeal region, the mediastinum, and gonads.52 Gastric teratomas show a striking male predominance and present early in life in neonates and infants.53,54
Inflammatory pseudotumor, also known as plasma cell granuloma, is composed of myofibroblasts, fibroblasts, histiocytes, plasma cells, and lymphocytes. It occurs most commonly in the lung but can occur rarely in the stomach. It is associated with microcystic anemia, hypergammaglobulinemia, and elevated sedimentation rate.55
Imaging: Polyps can be seen on UGI contrast study series as pedunculated or sessile smooth mucosal lesions arising from the gastric wall. Adenomatous polyps are usually antral and multiple. The appearance is similar on CT, requiring appropriate gastric distension to secure the diagnosis (Fig. 102-12).56
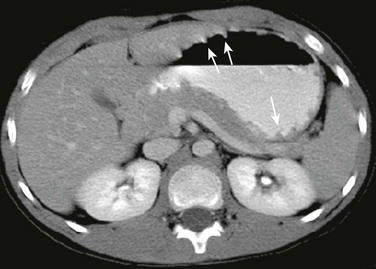
Figure 102-12 Multiple gastric polyps in a patient with Gardner syndrome, status post total colectomy.
Contrast-enhanced computed tomography shows multiple tiny polyps arising diffusely from the gastric wall protruding into the lumen (arrows), confirmed at endoscopy.
In both the rare primary and the more common secondary gastric lymphomas, CT reveals focal or diffuse mural masses that can protrude into the gastric lumen (Fig. 102-13) or cause mass effect on adjacent structures. Multifocal intestinal involvement can be present, as well as hepatosplenomegaly and regional or distant adenopathy. On UGI, mucosal nodularity, rugal thickening, and masses with or without associated ulceration can be seen.45 CT–positron emission tomography (CT-PET) is used in the contemporary staging of lymphoma in children and to assess disease activity and involvement of distant sites, particularly in lymph nodes that do not meet size criteria for pathologic involvement.57,58
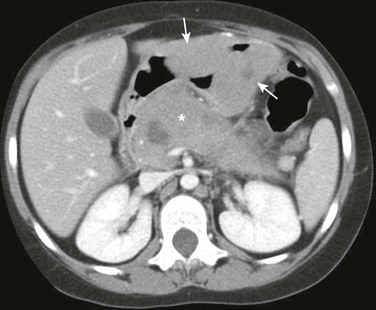
Figure 102-13 Burkitt’s lymphoma in a14-year-old girl presenting with vomiting and fever.
Axial image of a contrast enhanced computed tomography scan shows two large smooth and homogeneous masses arising from the gastric wall protruding into the gastric lumen (arrows). The masses are isodense to muscle. A large more heterogeneous mass involving the pancreas is also present (asterisk).
Other tumors such as GIST are seen as masses extending into the gastric lumen (Fig. 102-14) or as mass effect on adjacent structures. Extension beyond the stomach is best appreciated on cross-sectional imaging such as CT or magnetic resonance imaging. CT-PET is useful in the assessment of distant metastases.50 When calcifications are present, teratoma may be suspected.52
Treatment and Follow-up: Once the diagnosis of a mass has been suggested on imaging studies, it should prompt endoscopic evaluation and biopsy for confirmation. The treatment of gastric lymphoma, which still remains controversial, is usually chemotherapy, but H. pylori–eradicating therapy has been also advocated.59,60 CT-PET is currently used to monitor response to treatment.57,58
Resection is the first-line treatment and the only treatment that may lead to full remission in patients with primary GIST and other solid gastric tumors. It may be possible to remove smaller lesions endoscopically. In very advanced inoperable or metastatic disease, chemotherapy with targeted c-KIT tyrosine kinase inhibitors has proven very effective in GIST. CT-PET is the imaging modality of choice for monitoring the response to chemotherapy.61,62
Chen, MK, Beierle, EA. Gastrointestinal foreign bodies. Pediatr Ann. 2001;30:736–742.
Lin, C, Lee, H, Hung, H, et al. Neonatal gastric perforation: report of 15 cases. Pediatr Neonatol. 2008;49(3):65–70.
Oh, SK, Han, BK, Levin, TL, et al. Gastric volvulus in children: the twists and turns of an unusual entity. Pediatr Radiol. 2008;38:297–304.
Wang, L, Lee, H, Yeung, C, et al. Gastrointestinal polyps in children. Pediatr Neonatol. 2009;50(5):196–201.
References
1. Nijs, E. Radiological imaging of the digestive tract in infants and children. Med Radiol. 2008;3:109–132.
2. Oh, SK, et al. Gastric volvulus in children: the twists and turns of an unusual entity. Pediatr Radiol. 2008;38(3):297–304.
3. Elhalaby, EA, Mashaly, EM. Infants with radiologic diagnosis of gastric volvulus: are they over-treated? Pediatr Surg Int. 2001;17(8):596–600.
4. Al-Salem, AH. Acute and chronic gastric volvulus in infants and children: who should be treated surgically? Pediatr Surg Int. 2007;23(11):1095–1099.
5. Buonomo C, Taylor GA, Share JC, eds. Idiopathic gastric perforation, in practical pediatric imaging, 3rd ed, Lippicot-Raven, New York, 1998:893.
6. Rosser, SB, Clark, CH, Elechi, EN. Spontaneous neonatal gastric perforation. J Pediatr Surg. 1982;17(4):390–394.
7. Ibach, JR, Jr., Inouye, WY. Neonatal gastric perforation secondary to annular pancreas. Am J Surg. 1965;110(6):985–987.
8. Holgersen, LO. The etiology of spontaneous gastric perforation of the newborn: a reevaluation. J Pediatr Surg. 1981;16(4 suppl 1):608–613.
9. Lin, CM, et al. Neonatal gastric perforation: report of 15 cases and review of the literature. Pediatr Neonatol. 2008;49(3):65–70.
10. Pochaczevsdy, R, Bryk, D. New roentgenographic signs of neonatal gastric perforation. Radiology. 1972;102:145–147.
11. Gryboski, JD. Peptic ulcer disease in children. Pediatr Rev. 1990;12(1):15–21.
12. Wallis-Crespo, MC, Crespo, A. Helicobacter pylori infection in pediatric population: epidemiology, pathophysiology, and therapy. Fetal Pediatr Pathol. 2004;23(1):11–28.
13. Wolfe, MM, Soll, AH. The physiology of gastric acid secretion. N Engl J Med. 1988;319(26):1707–1715.
14. Tedesco, FJ, et al. Upper gastrointestinal endoscopy in the pediatric patient. Gastroenterology. 1976;70(4):492–494.
15. Drumm, B, et al. Helicobacter pylori and peptic ulcer: Working Group Report of the second World Congress of Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2004;39(suppl 2):S626–S631.
16. Jacobe, S, Lam, A, Elliott, E. Transient hypertrophic gastropathy. J Pediatr Gastroenterol Nutr. 1998;26(2):211–215.
17. Knight, JA, Matlak, ME, Condon, VR. Menetrier’s disease in children: report of a case and review of the pediatric literature. Pediatr Pathol. 1983;1(2):179–186.
18. Gassner, I, et al. Sonographic appearance of Menetrier’s disease in a child. J Ultrasound Med. 1990;9(9):537–539.
19. Stillman, AE, et al. Transient protein-losing enteropathy and enlarged gastric rugae in childhood. Am J Dis Child. 1981;135(1):29–33.
20. Granot, E, et al. Functional gastrointestinal obstruction in a child with chronic granulomatous disease. J Pediatr Gastroenterol Nutr. 1986;5(2):321–323.
21. Griscom, NT, et al. Gastric antral narrowing in chronic granulomatous disease of childhood. Pediatrics. 1974;54(4):456–460.
22. Dickerman, JD, Colletti, RB, Tampas, JB. Gastric outlet obstruction in chronic granulomatous disease of childhood. Am J Dis Child. 1986;140(6):567–570.
23. Khanna, G, et al. Imaging of chronic granulomatous disease in children. Radiographics. 2005;25(5):1183–1195.
24. Manson, DE, et al. Primary immunodeficiencies: a pictorial immunology primer for radiologists. Pediatr Radiol. 2000;30(8):501–510.
25. Kenney, PJ, Brinkso, RE, Patel, DV. Gastric involvement in CGD. J Comput Assist Tomogr. 1985;9(3):563–565.
26. Garcia-Careaga, M, Jr., Kerner, JA, Jr. Gastrointestinal manifestations of food allergies in pediatric patients. Nutr Clin Pract. 2005;20(5):526–535.
27. Teele, RL, et al. Radiographic features of eosinophilic gastroenteritis (allergic gastroenteropathy) of childhood. AJR Am J Roentgenol. 1979;132(4):575–580.
28. Hummer-Ehret, BH, et al. Eosinophilic gastroenteritis mimicking idiopathic hypertrophic pyloric stenosis. Pediatr Radiol. 1998;28(9):711–713.
29. Justinich, C, et al. Elemental diet improves steroid-dependent eosinophilic gastroenteritis and reverses growth failure. J Pediatr Gastroenterol Nutr. 1996;23(1):81–85.
30. Uyeimura, MC. Foreign body ingestion in children. Am Fam Physician. 2005;72:287–291.
31. Arana, A, et al. Management of ingested foreign bodies in childhood and review of the literature. Eur J Pediatr. 2001;160(8):468–472.
32. Chen, MK, Beierle, EA. Gastrointestinal foreign bodies. Pediatr Ann. 2001;30(12):736–742.
33. Kay, M, Wyllie, R. Pediatric foreign bodies and their management. Curr Gastroenterol Rep. 2005;7(3):212–218.
34. Wunsch, R, et al. Foreign body ingestion (translation). Radiologe. 1999;39(6):472–477.
35. Kuhns, DW, Dire, DJ. Button battery ingestions. Ann Emerg Med. 1989;18(3):293–300.
36. Sanders, MK. Bezoars: from mystical charms to medical and nutritional management. Pract Gastroenterol. 2004;13:37–50.
37. Goedde, TA, et al. Gastroduodenal polyps in familial adenomatous polyposis. Surg Oncol. 1992;1(5):357–361.
38. Bethel, CA, et al. Alimentary tract malignancies in children. J Pediatr Surg. 1997;32(7):1004–1008. [discussion 1008–1009].
39. Buck, JL, et al. Peutz-Jeghers syndrome. Radiographics. 1992;12(2):365–378.
40. Defago, MR, et al. Carcinoma in situ arising in a gastric hamartomatous polyp in a patient with Peutz-Jeghers syndrome. Endoscopy. 1996;28(2):267.
41. Wang, LC, et al. Gastrointestinal polyps in children. Pediatr Neonatol. 2009;50(5):196–201.
42. Isaacson, PG. Gastrointestinal lymphoma. Hum Pathol. 1994;25(10):1020–1029.
43. Mahour, GH, Isaacs, H, Jr., Chang, L. Primary malignant tumors of the stomach in children. J Pediatr Surg. 1980;15(5):603–608.
44. Bouzourene, H, et al. The role of Helicobacter pylori in primary gastric MALT lymphoma. Histopathology. 1999;34(2):118–123.
45. Kurugoglu, S, et al. Radiological features in paediatric primary gastric MALT lymphoma and association with Helicobacter pylori. Pediatr Radiol. 2002;32(2):82–87.
46. Donaldson, SS, Link, MP. Childhood lymphomas: Hodgkin’s disease and non-Hodgkin lymphoma. In: Comprehensive textbook of oncology. Baltimore: Williams and Wilkins; 1986:1176–1179.
47. Prakash, S, et al. Gastrointestinal stromal tumors in children and young adults: a clinicopathologic, molecular, and genomic study of 15 cases and review of the literature. J Pediatr Hematol Oncol. 2005;27(4):179–187.
48. Heinrich, MC, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299(5607):708–710.
49. Hirota, S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577–580.
50. Miettinen, M, Lasota, J, Sobin, LH. Gastrointestinal stromal tumors of the stomach in children and young adults: a clinicopathologic, immunohistochemical, and molecular genetic study of 44 cases with long-term follow-up and review of the literature. Am J Surg Pathol. 2005;29(10):1373–1381.
51. Agaimy, A, Wunsch, PH. True smooth muscle neoplasms of the gastrointestinal tract: morphological spectrum and classification in a series of 85 cases from a single institute. Langenbecks Arch Surg. 2007;392(1):75–81.
52. Chandrasekharam, VV, Gupta, AK, Bhatnagar, V. Infantile gastric teratoma. Trop Gastroenterol. 2000;21(4):192–193.
53. Sharma, A, et al. Immature gastric teratoma in an infant: report of a case and review of the literature. Indian J Pathol Microbiol. 2010;53(4):868–870.
54. Gupta, DK, et al. Gastric teratoma in children. Pediatr Surg Int. 2000;16(5-6):329–332.
55. Taratuta, E, et al. Pediatric inflammatory pseudotumor of the stomach: contrast-enhanced CT and MR imaging findings. AJR Am J Roentgenol. 1996;167(4):919–920.
56. Buck, JL, Harned, KH. Polyposis syndromes. In: Textbook of gastrointestinal radiology. Philadelphia: Saunders; 2000:1075–1088.
57. Perry, C, et al. Diagnostic accuracy of PET/CT in patients with extranodal marginal zone MALT lymphoma. Eur J Haematol. 2007;79(3):205–209.
58. Kaste, SC, Shulkin, BL. 18F-Fluorodeoxyglucose PET/CT in childhood lymphoma. PET Clin of N Am. 2006;1:265–273.
59. Kupeli, S, et al. Association of Helicobacter pylori and childhood lymphoma. J Pediatr Hematol Oncol. 2007;29(5):301–304.
60. Moschovi, M, et al. Primary gastric Burkitt lymphoma in childhood: associated with Helicobacter pylori? Med Pediatr Oncol. 2003;41(5):444–447.
61. Tarn, C, Godwin, AK. Molecular research directions in the management of gastrointestinal stromal tumors. Curr Treat Options Oncol. 2005;6(6):473–486.
62. Paral, J, et al. Gastrointestinal stromal tumors: review on morphology, molecular pathology, diagnostics, prognosis and treatment options. Acta Gastroenterol Belg. 2010;73(3):349–359.