84 Acquired and Congenital Heart Disease in Children
 Physiology
Physiology
Circulatory Changes at Birth
During the transition from intrauterine to extrauterine life, major circulatory changes occur which have important implications for the clinical care of the newborn.1,2 At birth in the normal newborn, the low-resistance placenta is eliminated from the circulation, resulting in an immediate increase in systemic vascular resistance (SVR). The pulmonary vascular resistance (PVR) falls when the lungs become responsible for gas exchange, and the fetal channels, foramen ovale, and arterial duct become redundant and close. In addition to altered hemodynamics in babies born with congenital heart disease, some babies with structurally normal hearts have a persistent right-to-left shunt after birth due to failure of the transition from fetal to postnatal circulation. Babies with this circulatory pattern, which is characterized by failure of the PVR to fall, have persistent pulmonary hypertension of the newborn (PPHN).3 PPHN is one of the two principal causes of nonpulmonary cyanosis in the neonate, the other being cyanotic congenital heart disease.
Physiology of the Neonatal Myocardium
The neonatal myocardium is functionally immature.4 Age-dependent changes in intrinsic function and integration with a maturing circulation determines its response to insults such as hypoxia and ischemia.5
Healthy infants have higher plasma concentrations of catecholamines and higher-density cardiac sympathetic innervation than older children and adults. This may partly explain the reduced ability of neonates to increase cardiac output in response to endogenous or exogenous catecholamines. Children in heart failure also have higher plasma catecholamine concentrations6 but reduced densities of β-adrenergic receptors compared to age-matched controls.7 The effects of this are similar to those seen with exogenous agonist-induced desensitization. Children with severe heart failure show evidence of uncoupling of β1-adrenergic receptors from the enzyme adenylcyclase7 and other maladaptive responses that result in reduced response to receptor agonists. In addition to heart failure, chronic hypoxia, such as is seen in cyanotic congenital heart disease, induces activation of the sympathetic nervous system, with resultant adrenergic receptor desensitization. Developmental aspects of myocardial support have recently been reviewed.8 The characteristics of the neonatal ventricle are listed in Table 84-1.
| Comparison to Mature Ventricle | |
|---|---|
| Contractility | Contractility of the neonatal ventricle is reduced compared to the mature ventricle. |
| Compliance | Neonatal ventricle inherently noncompliant compared to mature ventricle |
| Augmentation cardiac output | Little stoke volume reserve due to low compliance. Therefore cardiac output is highly heart-rate dependent in neonates. |
| Afterload | Neonatal ventricle tolerates increased afterload poorly. |
| Energy substrate | Lactate is primary substrate of neonatal ventricle under aerobic conditions. Glucose metabolized under anaerobic conditions. By 1-2 years, changes over to primary adult substrate, free fatty acids. |
Congestive Heart Failure
Although the basic pathophysiologic mechanisms of heart failure have age-independent common mechanisms, the presentation and management of heart failure changes with age. The overwhelming cause of heart failure in the first year of life is congenital heart disease, usually with an intracardiac left-to-right shunt or a ventricular obstructive lesion (Table 84-2). By contrast, the primary abnormality in adult heart failure is usually left ventricular dysfunction. Heart failure in adults is often gradual in onset; the neonate has little functional reserve, resulting in rapid decompensation and an emergent presentation.
| Neonate < 2 Weeks Age | Neonate > 2 Weeks Age, Infant | Older Child |
|---|---|---|
| Congenital Heart Disease | ||
| left-sided obstructive Lesions | left-to-right shunt lesions | any lesion |
The clinical features9 of heart failure in infants are listed in Table 84-3. A prominent sign of cardiac failure in infancy is difficulty in feeding secondary to increased respiratory rate and effort. This equates to exertional dyspnea in the older child or adult. Failure to thrive results and leads to the classic “wizened” appearance. Although hepatomegaly is a common sign of heart failure in infants (resulting from an increase in total circulating volume and hepatic venous congestion), peripheral edema, ascites, and pericardial or pleural effusions are much less commonly seen than in adults. One relatively common feature of severe heart failure in infancy is the occurrence of compression of the bronchial tree—particularly the left mainstem or lower lobe bronchus—secondary to extrinsic compression by an enlarged left atrium or pulmonary artery. This can cause airway obstruction and associated lobar collapse, or localized hyperinflation due to distal air-trapping. Long-standing extrinsic compression may rarely cause tracheobronchomalacia, resulting in long-term respiratory difficulties even after resolution of heart failure.
TABLE84-3 Clinical Features of Heart Failure in Infants
| Respiratory Signs |
| Other Signs |
Cyanosis
Cyanosis is the visible manifestation of greater than 5 g/dL of reduced deoxygenated hemoglobin in cutaneous blood vessels, and is a prominent feature in many types of congenital heart disease. Peripheral cyanosis results from high oxygen extraction ratios across the tissue vascular bed, reflecting low tissue blood flow or high tissue oxygen demand. Central cyanosis results from desaturation of arterial blood, which may be due to pulmonary disease or right-to-left shunting of deoxygenated systemic venous blood in association with a congenital heart defect. Pulmonary and cardiac causes of central cyanosis can usually be differentiated by allowing the child to breathe 100% oxygen (a “hyperoxic test”), which will result in a substantial improvement in oxygen saturation the case of cyanosis of pulmonary origin but have little effect on the child with cyanosis due to right-to-left shunt.10 During administration of 100% oxygen, arterial oxygen tensions (PaO2) above 160 mm Hg are highly suggestive of a noncardiac diagnosis, and a PaO2 over 250 mm Hg excludes it. Occasionally, differential cyanosis is seen where one or both of the upper limbs are normally saturated and the lower limbs cyanosed. The cause is deoxygenated blood traversing the arterial duct to enter the aorta distal to the origin of one or both subclavian arteries and supplying the lower limbs, while oxygenated blood from the left ventricle predominantly supplies the upper limbs.
Pulmonary Vasculature and Pulmonary Hypertension
The pulmonary vascular bed is of central importance to the manifestations of congenital heart disease from the first hours of life. Pulmonary vascular resistance usually falls dramatically in response to aeration of the lungs with the first breaths. Thereafter, the smooth muscle of the pulmonary vascular bed thins gradually during the first months of life, with associated fall in PVR to adult values by approximately 2 months of age. In infants with congenital heart lesions where an intracardiac communication between the systemic and pulmonary circulations is present, such as a ventricular septal defect (VSD), the fall in PVR encourages flow into the low-resistance pulmonary vascular bed, and a left-to-right shunt develops. In response to the increased flow and subsequent shear stress this induces, progressive structural changes occur in the pulmonary arteries and arterioles. Initially these changes consist of accelerated extension of muscle to the distal “non-muscular” pulmonary arteries and medial muscular hypertrophy in the proximal muscular arteries. Later changes involve gradual hypertrophy of the arterial intima, with deposition of collagen and elastin leading to gradual luminal obstruction and eventual occlusion. Associated with this is the development of plexiform lesions, the histologic hallmark of pulmonary vascular disease. Mild pulmonary vascular changes are of little significance to the cardiac intensivist; however, children with more extensive medial muscular hypertrophy of the pulmonary arteries are at risk of labile pulmonary hypertension (PHT) in the postoperative period (see later). The extent of pulmonary hypertensive changes frequently determine the feasibility of surgical options. Children with established fixed high PVR are unsuitable for corrective surgery, as surgical separation of the two circulations in the face of fixed high PVR will result in immediate right ventricular failure. Smaller elevations in PVR determine operability in the single-ventricle Fontan circulation (discussed later). Calculation of PVR and the response to varying vasodilators can be achieved following a pulmonary reversibility study in the cardiac catheter laboratory.11–13
 Circulatory Support in Children
Circulatory Support in Children
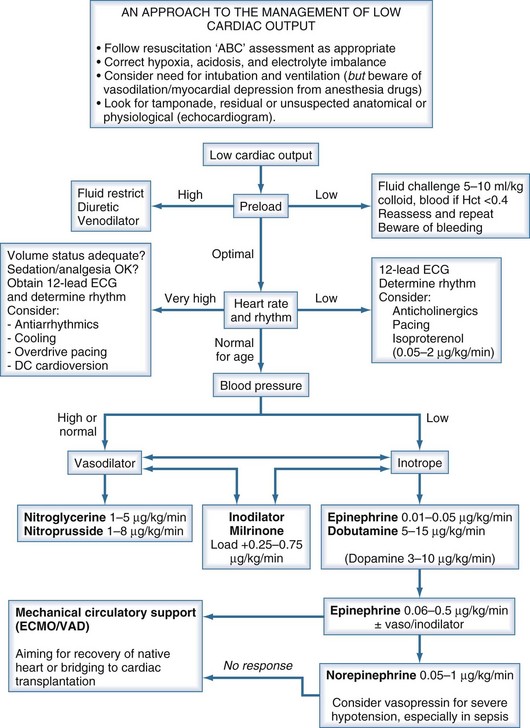
Children presenting with circulatory failure14 must initially be assessed and managed according to standard resuscitation algorithms. These require that adequate oxygenation and circulating volume be achieved. If cardiac output remains low, cardiovascular drug therapy is usually indicated. The developmental differences previously noted serve to emphasize the need to adopt age-appropriate pharmacologic strategies when supporting the failing myocardium of the neonate and infant.15–17 If cardiac output remains low despite application of such measures, mechanical circulatory support should be considered (Figure 84-1).
Pharmacologic Support
β-Adrenergic Agonists
Clinical and experimental studies have demonstrated marked age-related differences in the hemodynamic response to inotropic therapy. Although some of the observed differences may be accounted for by differences in drug pharmacokinetics, the variable maturation of the sympathetic nervous system, its receptors, and the cardiac myocytes mitigate against the recommendation of narrow specific dose ranges for the use of catecholamines in neonates and children.8
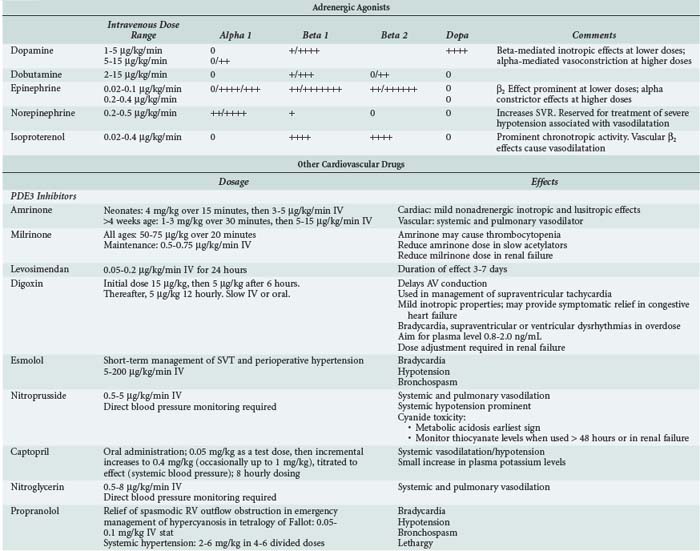
In clinical pediatric practice, adrenergic agonists are titrated to hemodynamic effect much as they are in adults (Table 84-4). When systolic ventricular function is impaired, low-dose epinephrine is commonly used as the first-line inotrope, although dobutamine and dopamine still have their advocates. Dopamine was formerly preeminent but is now less favored because of its noncardiac adverse effects.18 Additional agents should be administered according to assessment of response, judged clinically and from available hemodynamic monitoring. Higher-dose epinephrine, norepinephrine, or vasopressin can be used in refractory circulatory failure, particularly if vasodilation is present, such as occurs occasionally after cardiopulmonary bypass in children.19,20 Isoproterenol is a nonspecific β-adrenergic agonist whose principal cardiovascular effects are vasodilation and increasing heart rate. The drug is rarely used in intensive care except as a chronotropic agent where heart rate is critically low and cardiac pacing not yet established. Caution is needed when higher-dose catecholamine support is used in the neonate, as these can induce a rise in ventricular end-diastolic pressure (EDP) in a ventricle already developmentally noncompliant. Catecholamine-induced myocardial necrosis has been identified in neonatal animal models.21,22
Phosphodiesterase Inhibitors
Phosphodiesterase (PDE) inhibitors have emerged as important agents in the management of neonates and children with cardiac failure. The cardiovascular actions of the clinically available PDE3 inhibitors, amrinone,23 milrinone,24 and enoximone, are similar (Table 84-5). By inhibiting breakdown of cyclic adenosine monophosphate (cAMP), intracellular calcium accumulation is promoted and augments the contractile state of the myocyte. In addition, reuptake of calcium—a cAMP-dependent process—is also augmented, and these agents may therefore enhance diastolic relaxation, a particularly important aspect of neonatal cardiac function. In a recent multicenter randomized controlled study of neonates and young children following cardiac surgery, prophylactic administration of milrinone reduced the incidence of low cardiac output.25 Clinical studies in infants and children have demonstrated a synergistic effect when β1-agonists and PDE inhibitors such as amrinone, milrinone, or enoximone are coadministered, and this effect may be greater in neonates than in adults. In clinical use, the vasodilating action of the PDE3 inhibitors is prominent, a useful property given the usual well-documented pattern of low cardiac output associated with rising SVR and PVR in young patients following cardiac surgery.26
TABLE84-5 Strategies to Prevent and Treat Pulmonary Hypertension
| Strategy | Comment |
|---|---|
| Anatomic investigation | Rule out residual or undiagnosed anatomic abnormalities |
| Permit right-to-left decompression | Deliberate residual ASD acts as “pop-off” in at-risk situations |
| Analgesia/sedation | Facilitate ventilation; minimize sympathetic influences |
| Avoid acidosis | Respiratory and metabolic acidosis raise PVR |
| Maintain oxygenation | Normal/high alveolar and mixed venous PO2 lower PVR |
| Optimize hematocrit | Ensures optimal oxygen delivery and higher mixed venous PO2 |
| Optimize cardiac output | Ensures optimal oxygen delivery and higher mixed venous PO2 |
| Pulmonary vasodilators | Selectively reduce PVR |
Systemic Vasodilators
In children, sodium nitroprusside is frequently the systemic vasodilator of choice because of its powerful arteriolar dilating properties and short half-life which render it both effective and highly titratable. Nitroglycerin is an alternative short-acting drug which acts as an arteriolar dilator at higher doses but is an effective venodilator at lower doses. Phentolamine, a long-acting α-adrenergic blocker, is used in some centers for children undergoing surgery for congenital heart disease.27,28
For longer-term vasodilator therapy in children able to absorb enterally administered drugs, angiotensin-converting enzyme (ACE) inhibitors such as captopril and enalapril are used.29 They have peripheral vascular and neurohormonal effects, as well as direct effects on the myocardium through activation of intracellular signaling pathways involved in growth and apoptosis of cardiac myocytes and fibroblasts. Studies in adults have established that ACE inhibitors improve survival and symptoms in heart failure, in part because of their favorable effects on cardiac remodeling. Evidence for the use of ACE inhibitors in children is much less clear. Acute hemodynamic benefits have been demonstrated in children with heart failure due to left-to-right shunts and systolic dysfunction of the systemic ventricle. Prolonged treatment with ACE inhibitors has been shown to be effective in reducing not only LV volume overload but also LV hypertrophy in the hearts of growing children with chronic LV volume overload.30,31 The results of a randomized controlled trial of the use of ACE inhibitors in infants with single-ventricle circulations is awaited.32
Digoxin
Digoxin may have weak inotropic actions through its inhibitory effect on Na+/K+-ATPase and may also have peripheral effects that attenuate the actions of the neurohormonal system. Several adult studies have shown that digoxin improves symptoms in heart failure.33 Although no studies have shown survival improvement,33,34 there is a resurgence of interest in defining the role of digoxin in the management of heart failure. Digoxin is widely used to treat heart failure in children, although as in adults there are few data supporting or refuting its use.17
Diuretics
Standard practice is to use diuretics in virtually all children with heart failure.17 There are no pediatric studies showing that diuretic therapy reduces morbidity or mortality, but a recent adult study has shown that the diuretic, spironolactone, improves survival in adults with heart failure.35
Potent diuretics such as furosemide are widely used in heart failure treatment in childhood36; in the perioperative period, controlling fluid balance is crucial, and renal function may be impaired. The intravenous (IV) route is preferred in these situations. Studies have shown that continuous infusion leads to smoother control of fluid and electrolyte shifts than intermittent IV bolus administration.36
Beta-Blockers
Although there is increasing evidence of survival benefits accruing from beta-blocker therapy in adults with moderate and severe heart failure,37,38 evidence of similar benefits in children with heart failure is limited.29,39,40 A recent publication suggests that the benefit of adding beta blockade to ACE inhibition is minimal.41 While it might be reasonable to extrapolate adult survival advantages to older children with heart failure, extreme caution should be exercised in seeking to apply such therapy in the neonatal period.
Levosimendan
Levosimendan offers new therapeutic possibilities in the management of patients with severe ventricular dysfunction by improving cardiac contractility and vasodilatation without affecting intracellular free calcium.46 This drug enhances the sensitivity of cardiac myofilaments to calcium. The myocardial effects of levosimendan show improvement not only in systolic function but also in improved diastolic function, which is significantly impaired in severe heart failure. Anecdotes about the efficacy of levosimendan continue to be reported47 to add to the small previously published studies such as that of Namachivayam et al.48 It is, however, disappointing not to be able to report the results of more substantive pediatric trials. One of the problems with understanding the clinical utility of levosimendan has been to quantify the magnitude of its lusitropic effects, separating this from inotropic and chronotropic effects. Recently Jorgensen et al.49 published an elegant study of the use of levosimendan in a carefully monitored group of adult patients with aortic valve disease. This study demonstrated unequivocally that levosimendan exerts a direct positive lusitropic effect, shortening isovolumic relaxation time and improving LV filling.
The potential for tight control of blood glucose to improve cardiac outcomes in children has recently been highlighted.50 Further evidence from clinical trials such as the CHiP trial51 are required before tight control is routinely adopted in pediatric critical care.
Other Inotropic Agents
Triiodothyronine (T3) plays an important role in the regulation of heart metabolism,42 up-regulating β-adrenoceptors and increasing cardiac myocyte contractility.43 Clinical studies have shown that T3 supplementation can produce elevation in heart rate without concomitant decrease in systemic blood pressure44 and may enhance cardiac function reserve in infants after cardiopulmonary bypass. A recent double-blind placebo-controlled trial investigated the use of triiodothyronine supplementation in children younger than 2 years of age undergoing cardiopulmonary bypass. Although some indices of cardiac function assessed by echocardiography were judged better in the T3 group, no significant differences were found in clinical endpoints including time to extubation or intensive care unit (ICU) discharge.45
Pulmonary Vasodilators and Other Strategies to Prevent and Treat Pulmonary Hypertension11
Oxygen alone is a potent dilator of the pulmonary vascular bed, with both alveolar oxygen concentration and systemic oxygen saturation having a favorable influence. Pulmonary vascular resistance is also influenced by lung volume, being raised at both low and very high lung volumes. Avoiding atelectasis, alveolar hypoxia, and pulmonary arteriolar hypoxia are simple strategies to minimize PVR and pulmonary artery pressure. Historically, most IV drugs used to treat PHT had nonselective effects, dilating both the pulmonary and systemic vascular beds. Tolazoline, prostaglandin E1, and prostacyclin are among many agents which have been used as pulmonary vasodilators. Prostacyclin is a short-acting vasodilator which acts via increasing levels of the intracellular messenger, cAMP, which has been widely used in the treatment of primary PHT in children.52 The pulmonary effects of such nonselective agents are frequently limited by their nonspecific action leading to clinically important systemic hypotension. In contrast, nitrates, sodium nitroprusside, and indeed nitric oxide act via the activation of guanylate cyclase and hence increase cellular levels of cyclic guanosine monophosphate (cGMP) which is then inactivated by PDE5.
Elevation of PVR is seen in all children following cardiopulmonary bypass (CPB),26 with reactive postoperative pulmonary hypertensive episodes typically occurring in children following correction of left-to-right shunt lesions or in those with preoperative pulmonary venous hypertension.53 These crises are particularly associated with long CPB durations and late presentation for surgery. In the current era, early corrective surgery has dramatically reduced the numbers of infants in whom PHT is a major perioperative issue. Postoperative PHT is still seen in neonates and infants in association with lesions such as obstructed total anomalous pulmonary venous drainage, truncus arteriosus, and mitral valve replacement for congenital mitral stenosis. Children with lesser elevations in PVR may also benefit from pulmonary vasodilatation, including children with predominant RV dysfunction, for instance following cardiac transplantation54 and in Fontan circulations and relatively high PVR.55 General measures associated with the prevention and treatment of PHT should be considered before deploying specific pulmonary vasodilators (see Table 84-5). In patients at high risk of PHT following cardiac surgery, left ventricular filling can be maintained by right-to-left shunting through a small, surgically created atrial septal defect (ASD). Right-to-left shunt acts as a safety valve, and while some systemic desaturation occurs, LV filling and hence cardiac output are maintained.
Nitric oxide is an endogenous endothelial-derived vasodilator and a gas at room temperature. If added to inhaled gas mixtures in children with reactive PHT, it induces selective pulmonary vasodilation.56 It is distributed to ventilated alveoli, from where it diffuses into the adjacent pulmonary arteriolar smooth muscle. Inhaled nitric oxide (iNO) has been shown in randomized controlled trials to be effective and safe therapy in neonates with PPHN. Although the evidence for outcome benefit is limited to one randomized controlled study,57 there is a substantial body of evidence to show that iNO is effective in pediatric cardiac patients, including those with acute postoperative PHT following congenital heart surgery58,59 and following pediatric heart transplantation. Inhaled nitric oxide can also be used in preoperative assessment of patients with PHT.13,60
Other candidate selective pulmonary vasodilators undergoing investigation in children include inhaled prostacyclin61; the PDE5 inhibitor, sildenafil62–64; and bosentan, an endothelin-1 receptor blocker.65–67
Mechanical Circulatory Support
Extracorporeal membrane oxygenation (ECMO) is a mature technology which has been used to support over 27,000 neonates with respiratory failure, in whom survival rates of 70% to 80% are expected. Its use in this indication is supported by randomized controlled trials that demonstrate good short- and medium-term outcomes.68 ECMO and ventricular assist devices (VADs) have subsequently been used to provide temporary circulatory support in children with intractable circulatory failure (see Chapter 93). Indications for mechanical circulatory support include selected children with problems including severe ventricular failure, refractory arrhythmias, and cardiac arrest.69,70 The aim of mechanical circulatory support in such circumstances is to provide optimal cardiac output while resting the heart, awaiting its recovery, or to achieve survival by successful support of the child to cardiac transplantation. Single-center series71 and collaborative registry figures of ECMO72 or VAD for acute postoperative indications report similar figures for survival to hospital discharge (~40%) in children who (it is assumed) would not have survived without mechanical support. Rapid-deployment ECMO has recently been reported as an effective intervention for the management of cardiac arrest in the pediatric cardiac ICU and cardiac catheter laboratory.73 Hospital survival figures for CPR-ECMO seem encouraging,74 but long-term neurodevelopmental follow-up studies are urgently needed before such strategies can be recommended unequivocally.75,76
 Cardiomyopathies
Cardiomyopathies
The two most common causes of heart failure in children are congenital heart disease and cardiomyopathy. Cardiomyopathies are primary myocardial diseases of either known or unknown cause, characterized by left or biventricular dilatation and impaired contractility; they occur in children and adults of all ages. Additional information on cardiomyopathy in adults is provided in Chapter 83. Key aspects germane to pediatrics are provided in the following discussions.
Nugent et al. reported the incidence of pediatric cardiomyopathy in a 10-year population-based study in Australian children as 1.24 cases per 100,000 children younger than 10 years of age,77 a remarkably similar finding to a recently reported U.S. study.78 Of 314 cases of cardiomyopathy reported by Nugent et al., 184/314 (59%) were dilated cardiomyopathy, 80 (25%) hypertrophic cardiomyopathy, 8 (2.5%) restrictive cardiomyopathy, and 42 (13%) unclassified, of which 29 (69%) exhibited LV non-compaction. In this study, 20% of cardiomyopathies were classified as familial, and in 8.9%, specific mitochondrial or metabolic disease etiologically linked to cardiomyopathy were identified. Of the children in Nugent’s study who underwent myocardial biopsy, 40.3% had histologic evidence of lymphocytic myocarditis according to the Dallas criteria,79 which contrasts with an incidence of lymphocytic myocarditis in adult studies of only 10%.80
Presentation
Most children present with signs and symptoms of heart failure including dyspnea, upper abdominal discomfort, nausea, and vomiting. Abdominal symptoms are often misdiagnosed as indicative of gastroenteritis, although the astute clinician will note the absence of diarrhea. It is presumed that these abdominal symptoms result from hepatic congestion and gut edema as a result of right heart failure or ischemia (from splanchnic vasoconstriction). A history of an antecedent flulike illness is strongly suggestive of a diagnosis of myocarditis. Some children with myocarditis follow a fulminant course typified by rapid onset of cardiogenic shock.81,82
Prognosis
Recent studies have reported 5-year survival rates in childhood cardiomyopathy of between 64% and 84%, although the impact of cardiac transplantation on survival rates is not clear in all studies. In contrast to myocarditis, sudden death is uncommon in children with other forms of dilated cardiomyopathy. Children with cardiomyopathies who fail to respond to conservative treatment, and especially those with ongoing requirement for IV inotropic support, ventilatory support, or mechanical circulatory support and children with recurrent arrhythmias are candidates for early cardiac transplantation.83 Late recovery of ventricular function is, however, possible.84 The prognosis for cardiomyopathy due to myocarditis in children appears to differ from adults, with survival of up to 80% among children who reach the hospital alive.85,86 Many children who survive the acute phase go on to recover normal cardiac function—in marked contrast to adults, in whom mortality rates of 20% at 1 year increased to 56% at 5 years.80
ICU Management of Dilated Cardiomyopathy and Myocarditis
In children presenting with acute heart failure, hypotension, or cardiogenic shock, β-adrenergic agonists may improve systolic ventricular function. PDE3 inhibitors such as milrinone are of hemodynamic benefit in acute heart failure, although large trials in adult heart failure have failed to show clear benefit from chronic administration.87 While metoprolol and carvedilol may be of benefit in chronic heart failure,29,39,40 they should be avoided in hemodynamically unstable children. Nasal or mask continuous positive airway pressure (CPAP) has been shown to result in symptomatic improvement both by unloading of respiratory muscles and lowering of LV afterload as a consequence of raising intrathoracic pressure.88 Children in severe heart failure have high SVRs and no ventricular reserve. Great care is therefore needed if sedative agents are administered to facilitate tracheal intubation or ICU procedures. Agents with the least effects on the cardiovascular system should be chosen and allowance made for slow circulatory times when titrating sedative doses.
The use of mechanical circulatory support with ECMO or ventricular-assist systems can be life saving in children with myocarditis or cardiomyopathy who develop cardiogenic shock.89,90 A high proportion of children who receive mechanical support for fulminant myocarditis will recover ventricular function. Those who do not may be bridged to cardiac transplantation. Clearly, survival with a recovered native ventricle is a better outcome for a child than survival via cardiac transplantation. A multicenter series86 documented a median time to return of ventricular function of 9 days in those who survived without transplantation. The absolute time limits for recovery of native ventricular function have not been established, although pragmatic decisions on whether or not to proceed to cardiac transplantation should probably be made if cardiac recovery has not occurred after 10 to 14 days of support.91
 Congenital Heart Disease
Congenital Heart Disease
Congenital heart disease (CHD) classified as moderate or severe is detected in approximately 6/1000 live births, of whom between 2.5 and 3 will require expert cardiologic care soon after birth. The presence of extracardiac anomalies in children with CHD is associated with poorer outcomes. Syndromes associated with cardiovascular involvement are of particular significance to the pediatric intensivist who must coordinate care of the cardiac and extracardiac aspects of care.92 Trisomy 21 (Down’s syndrome) is associated with a high incidence of congenital heart disease, in particular atrioventricular septal defects. Deletion of the q11 region of chromosome 22 is associated with a spectrum of cardiac conotruncal defects (e.g., truncus arteriosus, tetralogy of Fallot) and extracardiac abnormalities.93 Of the later, thymic aplasia places infants at risk from hypocalcemia secondary to hypoparathyroidism and impaired cellular immunity.
Many classifications of congenital heart lesions have been proposed. A sequential approach to the description of cardiac anatomy is most frequently employed by pediatric cardiologists, but a broader physiologic approach is more useful to the non-specialist. It is beyond the scope of this chapter to present a detailed overview of all aspects of CHD. A brief overview is presented, focusing on common lesions and information of particular importance to intensivists. Readers are directed elsewhere for more detailed coverage of pediatric cardiology,94 pediatric cardiac surgery,95 and pediatric cardiac intensive care.96
Lesions with Predominant Left-To-Right Shunt
Ventricular septal defect is the archetypal lesion associated with left-to-right shunting of blood. VSDs may occur in isolation or in association with other cardiac anomalies. Ventricular output will follow the path of least resistance, resulting in blood shunting across the defect and into the lungs, as the PVR is lower than the SVR. The magnitude of the shunt, usually expressed as the ratio of pulmonary blood flow to systemic blood flow (Qp : Qs), depends on the size of the VSD and the level of the PVR. Small-diameter defects offer resistance at the level of the ventricular septum, limiting flow from the left to right ventricle and maintaining a pressure gradient between the two chambers. Larger-diameter defects are unrestrictive, with no pressure gradient between the two ventricles, and in this situation, flow is solely dependent on the ratio of PVR to SVR. Small, restrictive VSDs rarely result in symptoms in infancy, typically presenting when a cardiac murmur is detected as an incidental finding. Infants with larger unrestrictive VSDs gradually develop congestive cardiac failure due to the increase in pulmonary blood flow which occurs as the developmental fall in PVR falls in the first weeks of life.97 Thus the consequences of a moderate or large unrestrictive VSD are increased pulmonary blood flow (high Qp : Qs) and extra volume work demanded of the left ventricle. The volume overload of the LV results in LV enlargement and failure. If large left-to-right shunts are left untreated, PVR gradually rises. Although the initial rise is the result of pulmonary arteriolar muscular hypertrophy which is reversible, irreversible pulmonary vascular obstructive disease98 eventually ensues and may result in the onset of right-to-left shunt (Eisenmenger syndrome). For this reason, steps must be taken in all children with congenital heart lesions and raised pulmonary blood flow to correct the lesion or protect the lungs by either a corrective procedure or a palliative procedure such as pulmonary artery banding before severe pulmonary vascular changes develop. With the exception of isolated atrial septal defects, most left-to-right shunt lesions which require surgical intervention present in the first year of life, with heart failure and associated development of PHT. The principal lesions are described next.
Ventricular Septal Defect
Anatomy
Ventricular septal defects occur in any part of the interventricular septum and are classified by location.99,100
Pathophysiology
Many small VSDs close spontaneously,101 but if closure does not occur, infants with unrestrictive defects will fail to thrive and develop congestive heart failure as the PVR falls in early infancy. Untreated VSD leads to PHT and eventual progression to fixed pulmonary vascular obstructive disease. Eventually, pulmonary artery pressure and vascular resistance exceeds that of the systemic circulation, leading to shunt reversal and cyanosis (Eisenmenger syndrome). Patients with a fixed high PVR are not suitable for VSD closure, since the right ventricle will not tolerate the excessive afterload of the hypertensive pulmonary vascular bed.
VSD Closure
Most VSDs are repaired as a primary surgical procedure.102 Occasionally, pulmonary artery banding is undertaken to reduce pulmonary blood flow and protect the pulmonary vascular bed in neonates in whom primary repair is high risk. This may be the case with complex defects such as multiple defects or in very small premature infants. These conservative strategies are questioned by some surgeons.103,104 Although most VSDs are closed surgically with a sutured patch during CPB, some defects can be closed at cardiac catheterization with an occlusion device.105
Postoperative Management
Most children undergoing elective VSD closure progress rapidly to extubation. Patients with severe cardiac failure or high pulmonary artery pressures preoperatively benefit from a more cautious approach in the early postoperative period, as do those with complex associated lesions. Low cardiac output or pulmonary edema may be noted in the early postoperative period as a consequence of generalized myocardial hypocontractility or due to the presence of a residual VSD. Pulmonary hypertension is relatively rare in the current era of early primary repair of VSD. Late-presenting cases may have PHT, and life-threatening pulmonary hypertensive crises can occur in the postoperative period. Surgically placed pulmonary artery catheters greatly assist in the early detection and management of such episodes.106 Junctional ectopic tachycardia (JET)107,108 and complete heart block are generic risks of surgery in the vicinity of the ventricular septum. Compete heart block may be transient, but if AV synchrony has not returned by 7 to 10 days, a permanent pacing system is required.109
Atrial Septal Defect
Anatomy
Anatomically, interatrial communications99,110 are of four types. Ostium secundum defects are the most common form of ASD and are centrally located in the atrial septum. Ostium primum defects are part of the atrioventriculoseptal defect spectrum (see later). Sinus venosus defects occur close to the RA-SVC or RA-IVC junction and are commonly associated with partial anomalous pulmonary venous drainage. Coronary sinus defects describe a type of ASD associated with absence of the wall between the left atrium and coronary sinus, which allows left atrial blood to reach the right atrium via the coronary sinus.
Pathophysiology
Left-to-right shunting of blood at the atrial level leads to right atrial and ventricular dilatation with increased pulmonary blood flow. Congestive heart failure occurs in up to 5% of children with ASD in the first year of life. Pulmonary hypertension in association with ASD is relatively rare in childhood, with an incidence of 13% in unoperated children younger than 10 years of age, although if defects are not closed, patients may progress to irreversible PHT.111 Occasionally infants or young children with primary PHT, pulmonary hypoplasia, or similar conditions present with apparently symptomatic ASD with right-to-left shunting. In these situations, the ASD is beneficial, decompressing the right heart, and symptoms being a consequence of PHT rather than simply the presence of an ASD.
ASD Closure
Centrally located secundum ASDs are frequently closed by placement of an ASD closure device at cardiac catheterization.112,113 Occasionally, surgery is required in association with immediate or long-term complications of ASD device closure.114 Large defects and nonsecundum defects are closed surgically using CPB. Defects are typically closed if a child becomes symptomatic or electively at between 3 and 5 years of age. There is essentially no mortality risk associated with closure of an isolated ASD, and good long-term morbidity-free survival is expected.115
Atrioventriculoseptal Defect
Anatomy
Atrioventriculoseptal defects (AVSDs)116 result from failure of the lower part of the atrial septum to fuse with the upper part of the ventricular septum. The hallmark of all atrioventricular septal defects is the presence of a common atrioventricular (AV) junction and valve (AVV) with two bridging and three smaller leaflets. The common AVV has varying degrees of competence. There are three potential components of this defect, an ostium primum atrial septal defect, a ventriculoseptal defect, and abnormal formation of the AVVs. The condition presents as partial AVSDs (sometimes referred to as primum ASDs), where an ASD and cleft AV valve are present, and complete AVSDs, which in addition have a VSD. AVSD spectrum lesions commonly occur in children with Down’s syndrome.
Surgery
Partial or complete AVSDs are repaired under cardiopulmonary bypass. Partial defects are usually repaired electively at between 1 and 5 years, whereas complete defects are usually repaired between 3 and 6 months to avoid severe pulmonary hypertensive complications.116
Postoperative Management
Afterload reduction with sodium nitroprusside or milrinone is useful if mild AV valve regurgitation is present following repair. If residual valve incompetence persists or increases, the operation should be revised. Problems seen after AVSD surgery include PHT,117 which is, however, uncommon in the current era of early surgical repair. Residual lesions such as residual left AVV regurgitation or residual VSD will slow postoperative recovery and require prompt diagnosis and aggressive management including re-operation if necessary. Elevated LAP following AVSD repair can occur for reasons including the presence of residual left AVV regurgitation, left AVV stenosis, left ventricular outflow tract obstruction, residual VSD, and left ventricular myocardial dysfunction. The precise cause of elevated LAP must be diagnosed and appropriate management instituted.
Patent Ductus Arteriosus
Pathophysiology
The key pathophysiologic abnormality in patent ductus arteriosus (PDA), as in VSD, is left-to-right shunting leading to increased pulmonary blood flow, PHT, and left ventricular volume overload.118 Neonates with this condition usually present with congestive heart failure, apneas, or respiratory problems. In term infants and older children, isolated PDA may present incidentally or with the onset of cardiac failure or problems with recurrent pulmonary infections. Pulmonary hypertension progressing to pulmonary vascular obstructive disease can occur within the first year of life, the rate of onset of symptoms depending on the size of the duct.
Management
Indomethacin or ibuprofen are used to induce closure of patent ductus in premature neonates, acting through inhibition of the vasodilatory prostaglandin production, with success in approximately 70% of cases.119 Transcutaneous catheter occlusion can be effective in suitable cases, with a low incidence of associated complications.120 Surgical ligation or division are required in very small subjects and in older children with large ducts in whom occlusion devices cannot be safely deployed. Surgical closure is carried out via a lateral thoracotomy or as a video-assisted thoracoscopic procedure.121
Postprocedural Issues
The principal complications of conservative treatment of PDA with indomethacin or ibuprofen in preterm neonates are failure to induce closure and renal failure.119 Surgical approaches may be complicated by occlusion failure and complications of thoracotomy, including infection and hemorrhage. Adjacent structures including the thoracic duct, phrenic nerve, and the recurrent laryngeal nerve may be damaged during surgery. Complications following transcatheter closure include residual shunt, embolization of closure device, and hemolysis.
Truncus Arteriosus
Anatomy
Aorto-pulmonary window is a rare lesion in which an abnormal vascular communication exists between the ascending aorta and the main pulmonary artery. Like truncus arteriosus, this lesion is associated with 22q11 chromosomal deletion.122,123
Surgery.124,125
The pulmonary arteries are removed from the arterial trunk, leaving a vessel which becomes the “neo-aorta.” A valved conduit is then placed from the right ventricle to the pulmonary arteries, and the VSD is closed. Mortality risk is less than 10% if the truncal valve is functionally normal, no other lesions are present, and the child is of an acceptable weight. Long-term results are encouraging, although the valved conduit will require upsizing during childhood.126
Left Heart Obstruction
Valvar Aortic Stenosis
Anatomy
Aortic stenosis (AS) at valve level is the most common form of aortic stenosis and may be associated with other left heart abnormalities (e.g., supravalvular AS, mitral valve anomalies, aortic coarctation), aortic insufficiency (AI), and endocardial fibroelastosis. In neonatal AS,127 the LV and other left-sided structures may be hypoplastic.
Surgery
A number of treatment options are available, with the choice of procedure dependent on age, clinical status of the child at presentation, associated anomalies, and anatomic complexity. The simplest procedure, percutaneous balloon valvotomy, is appropriate in patients with mild to moderate stenosis and favorable aortic valve anatomy.128 Open aortic valve surgery is an alternative to balloon valvoplasty and may be favored if additional procedures such as duct ligation are required. If the native aortic valve cannot be salvaged or reconstructed, surgical choices include replacement of the aortic valve with a homograft or valved conduit, or placement of the patient’s own pulmonary valve into the aortic position with associated pulmonary homograft autograft (the Ross procedure).129–131 A variant of the Ross procedure, the Ross-Konno procedure, is indicated for complex LV outflow tract obstruction; in addition to the Ross operation, annular enlargement or aortoventriculoplasty are undertaken.132
Postoperative Management
Most neonates presenting in heart failure or shock who undergo urgent procedures remain critically ill postoperatively and require ongoing multiorgan support.133 If low cardiac output persists following repair, residual aortic stenosis or regurgitation must be excluded. Inotropic and vasodilator support of the failing myocardium should be guided by serial hemodynamic and echocardiographic evaluations. Relief of aortic stenosis in older children may be associated with systemic hypertension secondary to the unrestrained force of contraction of the hypertrophied LV. Children undergoing prosthetic valve replacement require long-term anticoagulation therapy.129,134
Subvalvar Aortic Stenosis
Subaortic stenosis135 is seen in various forms including a fibrous diaphragm-like ring with a central orifice, a fibromuscular tunnel (frequently associated with hypoplasia of ascending aorta and LV anomalies), or simply as dynamic obstruction due to hypertrophy of LV outflow.
Surgery
The choice of surgical procedure depends on the anatomic substrate. Membranous sub-AS requires simple resection. The tunnel form may be suitable for resection or require a more extensive Konno or Ross-Konno procedure. Finally, the hypertrophic form of sub-AS requires a Ross-Konno operation with resection of LV myocardium.129,132 Some children with a small-diameter aortic valve and endocardial fibroelastosis of the LV with poor function may not be suitable for biventricular repair and are palliated by creation of cavopulmonary circulations.
Supravalvular Aortic Stenosis
Supravalvular aortic stenosis occurs in isolation and in association with Williams syndrome (supravalvar AS, RV outflow tract obstruction, peripheral pulmonary stenoses, renal artery stenoses).136,137 It may be a localized or diffuse narrowing above the sinotubular junction. The stenosis is occasionally associated with a hypoplastic ascending aorta, and there may be compromise to coronary filling.
Aortic Coarctation
Anatomy
Aortic coarctation is a constriction of the thoracic aorta in the region of the left subclavian artery where the ligamentum arteriosum originates. The complexity of the lesion varies from a discrete narrowing to more extensive aortic-arch hypoplasia extending back to the proximal aortic arch.138 Coarctation commonly coexists with VSD139 and can also be associated with other left-sided lesions including aortic and mitral valve stenosis.
Pathophysiology
In the neonatal presentation of aortic coarctation, a normal circulation is maintained until ductal tissue contracts, at which point distal aortic flow is severely reduced, leading to a clinical presentation of heart failure or shock and characteristic loss of lower limb pulses.140 Prostaglandin E1 or E2 infusion should be started as soon as the diagnosis of a duct-dependent lesion is suspected in order to reopen or maintain patency of the ductus arteriosus. Following initial resuscitation, urinary output and resolution of metabolic acidosis are early indicators of successful reperfusion of the distal aorta. Early surgical repair is indicated.
Surgery
In the newborn period, surgical resection of the narrowed aortic segment and associated ductal tissue and either direct anastomosis or repair with a subclavian flap or similar angioplasty without CPB are performed.141 If aortic arch hypoplasia is more extensive, a homograft or prosthetic tube graft may be incorporated in the repair and CPB may be required.142 Neonatal coarctation associated with VSD can be palliated by resection of the coarctation and banding of the pulmonary artery to restrict pulmonary blood flow, with delayed VSD repair. Alternatively, both lesions can be corrected in the neonatal period.139 The mortality rate for repair of neonatal coarctation is low. Kanter et al. reported 91% survival in a series which included both isolated and complex coarctation.143 In older children it is less than 1%, although paraplegia secondary to interruption of spinal cord perfusion remains a concern.
Balloon angioplasty with or without endovascular stent placement is frequently used to alleviate recurrent aortic coarctation and is increasingly being used, with apparent success, to address native coarctation, particularly in older patients. Balloon angioplasty is not favored in symptomatic neonates.141,144
Postoperative Management
Specific postoperative problems include systemic hypertension, which is thought to be due to multiple factors including altered baroceptor and adrenal catecholamine and renin-angiotensin axes.145,146 Persistent hypertension is less common following neonatal repair, and when present it usually responds to short-term vasodilator therapy.147,145 Additional β-adrenergic blockade (esmolol,148 propranolol, or labetalol) may be required, particularly with late-presenting coarctation, but should be used with caution if ventricular function is impaired. Some children have persistent hypertension following repair149 and require long-term antihypertensive therapy. Post-coarctectomy syndrome150 occurs in older patients and is thought to be the result of restoration of higher-pressure pulsatile flow to the mesenteric arterial tree; this condition presents as abdominal distension, abdominal pain, ascites, or occasionally enteric infarction. The condition is best managed by avoiding enteral feeding for 24 hours following repair and aggressive treatment of systemic hypertension. The necessity of aortic clamping during surgical repair interrupts distal aortic flow and may result in spinal cord ischemia (rare in neonates, 0.4% incidence in older patients) or renal ischemia. The intensivist must seek positive confirmation of lower limb movement and adequate renal function in the early postoperative period. In neonates, low cardiac output due to preexisting ventricular dysfunction may persist, although residual coarctation should be excluded. Structures near the aortic arch prone to surgical injury include the thoracic duct, recurrent laryngeal nerve, and phrenic nerve, leading to postoperative chylothorax, stridor, or hemidiaphragm paralysis.
Interrupted Aortic Arch
Anatomy
In interrupted aortic arch (IAA), the aortic arch is either atretic or interrupted, creating either complete disruption or luminal obstruction (without external interruption). A patent arterial duct is necessary to maintain perfusion of the distal aortic arch, closure of which leads to emergent presentation. A VSD and obstruction of the left ventricular outflow tract commonly coexist. The more common form of IAA (type B) is associated with 22q11 chromosomal deletion122,123 (see earlier).
Pathophysiology
IAA can be regarded as a severe form of aortic coarctation with duct-dependent distal aortic perfusion and requires similar initial management.140
Anomalous Pulmonary Venous Connection
Anatomy
Pulmonary veins drain anomalously into systemic venous structures and subsequently to the right atrium rather than directly into the left atrium. The condition may affect all pulmonary veins (total anomalous pulmonary venous connection [TAPVC]) or fewer, typically one vein (partial anomalous pulmonary venous connection). In supracardiac TAPVC (45% of cases) the pulmonary veins drain via a vertical vein to the innominate vein or connect directly into the SVC. In intracardiac TAPVC (25% of cases), the venous confluence drains via the coronary sinus into the RA; and in infracardiac TAPVC (25% of cases), the veins drain into the IVC or portal veins. Mixed forms also exist (5% of cases).152 TAPVC is associated with an obligate ASD to allow mixing of systemic and pulmonary venous return to access the left ventricle and systemic circulation.
Surgery
The pulmonary veins are anastomosed or baffled into the left atrium. In the current era, the expected operative mortality is less than 5%, although higher risks are reported in complex cases with associated lesions.153
Specific Postoperative Problems
Pulmonary hypertension, which may on occasion be severe or even life threatening, is common in infants following surgery for obstructed anomalous pulmonary veins.117 If high pulmonary artery pressure occurs postoperatively, it is essential to rule out residual pulmonary venous obstruction. Late restenosis is seen in up to 10% of cases and carries a poor prognosis, often related to a progressive fibrotic process occluding the lumen of the pulmonary veins.154
Cyanotic Lesions
Tetralogy of Fallot
Anatomy
Tetralogy of Fallot (TOF)155 was initially described in the 19th century as an association of four anatomical findings: VSD, subpulmonary stenosis, aortic override of the ventricular septum, and right ventricular hypertrophy. The four lesions are actually the result of just one central problem, anterior and superior malalignment of the infundibular septum with respect to the muscular septum, which creates an obstruction in the right ventricular outflow tract and leads to the four features seen. Children presenting with TOF should be investigated for a 22q11 microdeletion (see earlier).
Surgery
The timing and type of surgical intervention in TOF is controversial.156,157 Complete repair is usually undertaken in the first year of life, although some centers adopt a two-stage approach with initial placement of a modified Blalock-Taussig shunt in cyanotic infants. Complete repair is then undertaken when the child is bigger.
Specific Postoperative Problems
Residual VSD is poorly tolerated after TOF repair and requires early surgical closure. Moderate degrees of residual right ventricular outflow tract obstruction may be well tolerated in the early postoperative period, but severe residual obstruction demands early reinvestigation and reoperation, with placement of a larger RV outflow tract patch or valved RV-PA conduit. All patients with a right ventricular incision develop right bundle branch block. Junctional ectopic tachycardia is poorly tolerated after Fallot repair.107 Low cardiac output due to RV dysfunction is relatively common and should be suspected if the child is hypotensive, tachycardic, and has a raised CVP and hepatomegaly. The problem is predominantly one of poor RV compliance, often referred to as RV restriction,158 and typically resolves in 3 to 5 days. Until recovery occurs, the heart should be supported by optimizing RV filling and ensuring atrioventricular synchrony. Negative pressure ventilation has been shown to improve cardiac output where RV restriction exists.14,159
Pulmonary Atresia with Intact Ventricular Septum160
Anatomy
In pulmonary atresia with intact ventricular septum (PA/IVS), there is complete obstruction to the outflow of the right ventricle, along with a variable degree of hypoplasia of the RV and tricuspid valve (TV). The TV may also be incompetent. Pulmonary blood flow occurs via a PDA. Coronary artery sinusoids or fistulae are often found in severe PA/IVS with a small right ventricle. Some 10% of cases will have an RV-dependent coronary circulation, where coronary sinusoids/fistulae are associated with proximal stenosis, and perfusion of areas of myocardium is dependent on flow via the right ventricle. In some patients, the pulmonary arterial supply is abnormal, with segments of the lungs being supplied solely or partially (dual supply) from systemic collateral vessels termed major aortopulmonary collateral arteries (MAPCA).161 Children presenting with this condition should be investigated for a 22q11 microdeletion (see earlier).
Surgery
The goal of treatment is to provide a secure source of pulmonary blood flow balanced to systemic flow, and to permit the right ventricle to develop to its maximal potential, always aiming for a two-ventricle repair where possible.161,162 Interventional procedures are needed in all cases in the fetal163 or neonatal period164,165 because of duct dependency. Subsequent strategies are chosen according to individual anatomic findings.
In severe forms of the condition (severe RV hypoplasia ± coronary fistulae) a two-ventricle repair will never be possible, and a palliative approach is adopted. Initial palliation secures pulmonary blood flow with systemic pulmonary artery shunts (30%-40% PA/IVS), with the ultimate aim being a single-ventricle Fontan circulation (see later). In contrast, babies with a normal-sized RV may be suitable for RV outflow tract reconstruction in the neonatal period, therefore avoiding a shunt and ending up with early anatomic correction (10% of cases). An intermediate group of patients—the majority of cases of PA/IVS—need initial palliation with decompression of the RV by radiofrequency perforation of the atretic pulmonary valve or outflow tract patch, and often require a systemic–pulmonary artery shunt. Progression to either a single, “one-and-a-half,”166 or biventricular repair depends on subsequent development of the RV and pulmonary arteries. Fetal cardiac valvoplasty may have a role in the management of this condition in the future.163
Specific postoperative problems include low cardiac output due to excessive runoff through the shunt, myocardial ischemia due to decompressed coronary fistulae, or low systemic diastolic pressure due to excessive shunt runoff.167
D-Transposition of the Great Arteries
Anatomy
In D-transposition of the great arteries (TGA),168,169 which accounts for 5% to 7% of all congenital heart lesions, the great vessels are transposed so that the aorta arises from the anatomic right ventricle and the pulmonary artery from the left ventricle, so-called ventriculo-arterial discordance. The condition occurs with a VSD in approximately 40% of cases. Other commonly associated lesions include coarctation (10%), left ventricular outflow tract obstruction (5%), and coronary abnormalities (33%).
Pathophysiology
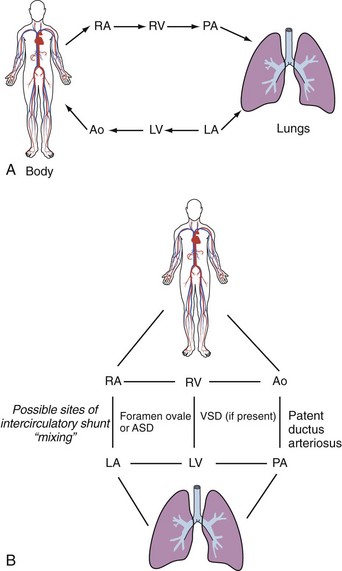
The predominant finding in TGA is cyanosis due to parallel rather than serial function of the pulmonary and systemic circulations, with the greatest proportion of the output of a ventricle being recirculated to that ventricle. Survival is therefore dependent on the presence of mixing between the two circulations (Figure 84-2). The presence of either a PDA or VSD (alone or in combination) without an atrial communication does not ensure adequate mixing of the two circulations. If the diagnosis is suspected in a neonate, an infusion of prostaglandin E1 or E2 should be established to maintain ductal patency, and following echocardiographic confirmation of the diagnosis, a balloon atrial septostomy is sometimes necessary to enlarge the foramen ovale and secure mixing at atrial level, particularly if the foramen ovale is restrictive, leading to high pulmonary venous pressures. Saturations typically increase from very low levels (<50%) to 65% to 85% following these interventions, and it is then usually possible to discontinue the prostaglandin infusion.
Surgery
The preferred surgical option in the current era is the arterial switch (Jatene) operation,168–170 although long-term results following Senning operations also appear to be acceptable.171 The switch operation is usually performed within the first 2 weeks of life, beyond which the left ventricle (functioning as a low-pressure subpulmonary or right ventricle since birth) is less able to cope with systemic pressures.172 Babies with a large VSD have equal ventricular pressures, and repair can be delayed a little longer, although in practice most surgeons repair TGA with VSD within the first month of life. The operation consists of transection of the aorta and pulmonary artery, with reconstruction of the vessels in their anatomic position, which necessitates transfer of the coronary arteries from the PA to the neo-aorta.
Specific Postoperative Problems
Left ventricular dysfunction is common in babies during the first 12 hours following the arterial switch operation.26 It may be a sign of coronary insufficiency,173 acute dysfunction secondary to an unprepared/involuted LV, or simply nonspecific post-CPB low cardiac output. In the absence of ECG or echocardiographic evidence of regional coronary ischemia, low cardiac output is managed conservatively. The postoperative LV of the neonate is poorly compliant. Rapid volume infusion should be avoided, as LV distension and ischemia may result. Preload should be augmented gradually, titrating volume infused against measured left atrial pressure.
Alternative Surgical Techniques
Atrial switch operations (Senning and Mustard procedures) are alternatives to the arterial switch and may be chosen in infants presenting beyond the early neonatal period in whom a one-stage arterial switch is not possible owing to deconditioning of the left ventricle. In atrial switch operations, blood is diverted by an atrial baffle to establish a series circulation, leaving the RV as the systemic ventricle. It is believed that the burden of late complications such as RV failure is greater after atrial switch procedures. An alternative strategy for late-presenting transposition is a two-stage repair, with initial banding of the pulmonary artery to condition the LV, with switch once the ventricle is conditioned.174
Complex Single-Ventricle Circulations
Some defects are such that they can never be corrected to provide two functioning ventricles.175,176 These complex arrangements include any heart in which one ventricle is hypoplastic such that it would be incapable of supporting either the pulmonary or systemic circulation independently. Examples of such situations include tricuspid atresia or double-inlet left ventricle. In these examples, the right ventricle has failed to develop adequately and is connected to a dominant left ventricle via a VSD. Flow to the circulation supplied by the rudimentary ventricle originates from the dominant chamber and is dependent on an adequate VSD. Children with this type of anatomy will always have two ventricles, even if one is hypoplastic, but physiologically they behave as if the heart consists of only a single ventricle.
Complex single-ventricle hearts can be palliated with a series of interventions leading to creation of a Fontan circulation in which the systemic and pulmonary circulations are completely separated.177 Initially, adequate intracardiac communications are established to ensure both systemic and pulmonary venous return have unobstructed access to the dominant ventricle to supply both systemic and pulmonary blood flow. If necessary, pulmonary flow is augmented by the use of a systemic-to–pulmonary artery shunt or right ventricle–to–pulmonary artery conduit. Systemic and pulmonary blood flow are assured at the expense of mixing of pulmonary and systemic venous returns, with consequent cyanosis and volume loading of the single ventricle.
Subsequently, if hemodynamic conditions are favorable, the Fontan circulation is established, usually in two staged procedures. Initially a bidirectional cavopulmonary or a hemi-Fontan anastomosis is created in which the SVC is connected to the proximal right pulmonary artery. This has the benefit of reducing the volume load placed on the systemic ventricle by previously placed systemic/pulmonary shunt. Finally, venous return form the IVC is also directed to the pulmonary circulation. This is achieved by forming a lateral tunnel178 or using a synthetic extracardiac conduit179 to channel blood from the IVC to the inferior aspect of the right pulmonary artery, completing the total cavopulmonary connection or Fontan circulation.
In the Fontan circulation, there is no subpulmonary ventricle, all ventricular tissue having been incorporated into the single ventricle, which receives pulmonary venous return and ejects into the systemic circulation. This establishes a form of series circulation and results in normal systemic oxygenation and equality of pulmonary and systemic blood flow. Pulmonary blood flow in the Fontan circulation is driven by the transpulmonary hydrostatic gradient and is only viable if the PVR and systemic ventricular end-diastolic pressures (pulmonary venous pressures) are low. The presence of good systemic ventricular function and low PVR are crucial determinants of operability. Patients with a Fontan circulation tolerate factors which impede systemic venous return such as dehydration, pneumothorax, pericardial effusion, positive pressure ventilation,88 raised PVR, or compromised ventricular or respiratory180 function very poorly. Perioperative use of ACE inhibitors181 has been shown to reduce the severity and duration of pleural drainage,182 a common problem caused by high postoperative systemic venous pressures. A communication or fenestration between the systemic venous pathway and pulmonary venous atrium may be created in patients thought to be at higher risk of complications such as effusions or perioperative low cardiac output as a result of relatively high PVR.
Long-term follow-up studies have demonstrated that systemic ventricular function remains abnormal after Fontan procedures.183 Ultimately the Fontan circulation may fail, and cardiac transplantation must be considered.
Hypoplastic Left Heart Syndrome
Hypoplastic left heart syndrome (HLHS) is a term encompassing a range of hypoplastic abnormalities of the left-sided cardiac structures and connections including the ascending aorta.184 The condition is usually palliated in three stages, although some authorities prefer to offer cardiac transplantation without prior palliative surgery.185 The first-stage procedure secures systemic and pulmonary blood flow with either a Norwood procedure or similar or a hybrid procedure.186,187 The Norwood approach consists of reconstruction of the aortic arch, with the establishment of pulmonary blood flow via a central systemic/pulmonary artery shunt. Some advocate replacing the systemic/pulmonary artery shunt of the classical Norwood with an RV-PA conduit, which may be easier to manage postoperatively because there is potentially less diastolic runoff, with less risk of coronary ischemia than occurs across the central shunt of the classical Norwood operation.184,188 Balancing the pulmonary and systemic circulations in the immediate postoperative period can be challenging. Interventions such as sudden hyperventilation or increases in oxygen concentration which lower PVR should be avoided. Strategies to manage the postoperative Norwood patient include the use of long-acting vasodilators such as phenoxybenzamine27,28 and close monitoring of cerebral oxygenation, venous oxygen saturation, and plasma lactate. ACE inhibitors are subsequently introduced and very close inter-stage monitoring may be undertaken in an attempt to minimize inter-stage morbidity and mortality.
Following the first-stage procedure, a bidirectional cavopulmonary anastomosis is undertaken, typically between 2 and 6 months of age, and finally a completion to a Fontan circulation follows at 18 to 24 months of age. Fetal diagnosis facilitates early and appropriate management and may contribute to improved outcomes in HLHS,189 although there is known to be significant risk of poor neurodevelopmental status in survivors of neonatal HLHS interventions.190
Surgical Control of Pulmonary Blood Flow
Pulmonary Artery Banding.
Pulmonary artery banding is a surgical procedure in which a constriction is created in the main pulmonary artery, with the aim of limiting pulmonary blood flow to protect the lungs from overcirculation, usually as a primary palliative procedure ahead of a later definitive repair. It is performed without CPB through either a left thoracotomy or median sternotomy. The procedure is undertaken to restrict pulmonary blood flow, aiming to maintain a balance between the systemic and pulmonary circulations and to prevent the onset of PHT in some complex anomalies unsuitable for early anatomic repair.191 PA banding is a palliative procedure and is usually a stepping stone to a more complex repair.
Specific Management Issues
Oxygen saturations may be an issue. Very low SaO2 postoperatively (<70%) may indicate that the band is too tight—that is, the pulmonary blood flow is too restricted. Urgent echo evaluation of the band gradient192 and exclusion of other causes of hypoxemia should be undertaken. If hypoxemia persists, and particularly if significant metabolic acidosis develops, urgent removal of the band may be indicated. PA bands may occasionally be too loose to adequately reduce pulmonary blood flow, resulting in arterial oxygen saturations in excess of 90%. Signs of congestive cardiac failure may be noted and require medical treatment (diuretics) or further surgical intervention (re-banding or correct lesion.)
Other Lesions
Vascular Rings and Slings
Vascular rings and slings193 result from abnormal branching or positioning of the great vessels, which result in encirclement or compression of the trachea and/or esophagus. They are seen in isolation or in association with intracardiac defects.
Anatomy
Anomalous Left Coronary Artery from the Pulmonary Artery
 Specific Issues for the Intensivist
Specific Issues for the Intensivist
Delayed Sternal Closure
Complex cardiac surgery involving cardiopulmonary bypass results in edema of the myocardium and other mediastinal tissues. Under these circumstances, sternal closure at the end of the surgical procedure may cause cardiac compression (“tissue tamponade”) which decreases ventricular compliance and leads to reduced cardiac output and elevated pulmonary venous pressures.198,199 The child may therefore be returned to the ICU, where the sternum may be closed once the hemodynamic situation has improved. Transient deteriorations at delayed closure are usually self-limiting and can be tolerated, but hypotension, oliguria, rising plasma lactate, or falling venous saturations suggest closure will not be tolerated.200
Infective Endocarditis
Infective endocarditis is a condition characterized by microbial infection of the heart valves or other structures and is associated with substantial morbidity and mortality in both children and adults. The subject has recently been extensively reviewed.201 While prophylactic antibiotic treatment prior to non-cardiac procedures is no longer recommended in children and young people,202 intensivists should aim to minimize the risk of line-related bloodstream infection complications by employing best-practice guidelines in the care and surveillance of central venous lines.203
Key Points
1 Rudolph AM, Iwamoto HS, Teitel DF. Circulatory changes at birth. J Perinat Med. 1988;16(Suppl 1):9-21.
2 Fineman JR, Hetmann MA, Morin FCIII. Fetal and Post-natal Circulations: Pulmonary and Persistent pulmonary Hypertension of the Newborn. In: Allen HD, Gutgesell HP, Clark EB, Driscoll DJ, editors. Moss and Adam’s Heart Disease in Infants Children and adolescents. Philadelphia: Lippincott Williams and Wilkins; 2001:24-40.
3 Walsh MC, Stork EK. Persistent pulmonary hypertension of the newborn. Rational therapy based on pathophysiology. Clin Perinatol. 2001;28(3):609-627. vii
4 Mahony L. Development of myocardial structure and function. In: Allen HD, Gutgesell HP, Clark EB, Driscoll DJ, editors. Moss and Adam’s Heart Disease in Infants Children and adolescents. Philadelphia: Lippincott Williams and Wilkins; 2001:24-40.
5 Booker PD. Myocardial stunning in the neonate. BJA: Br J Anaesth. 1998;80(3):371-383.
6 Wu JR, et al. Circulating noradrenaline and beta-adrenergic receptors in children with congestive heart failure. Acta Paediatrica. 1996;85(8):923-927.
7 Wu JR, et al. Reduction in lymphocyte beta-adrenergic receptor density in infants and children with heart failure secondary to congenital heart disease. Am J Cardiol. 1996;77(2):170-174.
8 Booker PD. Pharmacological support for children with myocardial dysfunction. Pediatr Anaesth. 2002;12(1):5-25.
9 Park MK. Pediatric cardiology for practitioners. St. Louis: Mosby; 2002.
10 Lees MH, King DH. Cyanosis in the newborn. Pediatr Rev. 1987;9(2):36-42.
11 Abman SH. Pulmonary hypertension in children: a historical overview. Pediatr Crit Care Med. 2010;11(2 Suppl):S4-S9.
12 Atz AM, et al. Combined effects of nitric oxide and oxygen during acute pulmonary vasodilator testing. J Am Coll Cardiol. 1999;33(3):813-819.
13 Giglia TM, Humpl T. Preoperative pulmonary hemodynamics and assessment of operability: is there a pulmonary vascular resistance that precludes cardiac operation? Pediatr Crit Care Med. 2010;11(2 Suppl):S57-S69.
14 Wessel DL. Managing low cardiac output syndrome after congenital heart surgery. Crit Care Med. 2001;29(10 Suppl):S220-S230.
15 Veldman A, Rupp S, Schranz D. New inotropic pharmacologic strategies targeting the failing myocardium in the newborn and infant. Mini Rev Med Chem. 2006;6(7):785-792.
16 Shaddy RE. Paediatric heart failure trials … and tribulations. Cardiol Young. 2007;17(4):354-355.
17 Margossian R. Contemporary management of pediatric heart failure. Expert Rev Cardiovasc Ther. 2008;6(2):187-197.
18 Debaveye YA, Van den Berghe GH. Is there still a place for dopamine in the modern intensive care unit? Anesth Analg. 2004;98(2):461-468.
19 Rosenzweig EB, et al. Intravenous arginine-vasopressin in children with vasodilatory shock after cardiac surgery. Circulation. 1999:100-II-182.
20 Jerath N, et al. Clinical impact of vasopressin infusion on hemodynamics, liver and renal function in pediatric patients. Intensive Care Med. 2008;34(7):1274-1280.
21 Caspi J, et al. Age-related response to epinephrine-induced myocardial stress. A functional and ultrastructural study. Circulation. 1991;84(5 Suppl):III394-III399.
22 Caspi J, et al. Effects of high plasma epinephrine and Ca2+ concentrations on neonatal myocardial function after ischemia. J Thorac Cardiovasc Surg. 1993;105(1):59-67.
23 Bailey JM, et al. A comparison of the hemodynamic effects of amrinone and sodium nitroprusside in infants after cardiac surgery. Anesth Analg. 1997;84(2):294-298.
24 Chang AC, et al. Milrinone: systemic and pulmonary hemodynamic effects in neonates after cardiac surgery. Crit Care Med. 1995;23(11):1907-1914.
25 Hoffman TM, et al. Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation. 2003;107(7):996-1002.
26 Wernovsky G, et al. Postoperative course and hemodynamic profileafter the arterial switch operation in neonates and infants: a comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation. 1995;92:2226-2235.
27 Guzzetta NA. Phenoxybenzamine in the treatment of hypoplastic left heart syndrome: a core review. Anesth Analg. 2007;105(2):312-315.
28 Tweddell JS, et al. Phenoxybenzamine improves systemic oxygen delivery after the Norwood procedure. Ann Thorac Surg. 1999;67(1):161-167. discussion 167-8
29 Shaddy RE. Optimizing treatment for chronic congestive heart failure in children. Crit Care Med. 2001;29(10 Suppl):S237-S240.
30 Mori Y, et al. Long-term effect of angiotensin-converting enzyme inhibitor in volume overloaded heart during growth: a controlled pilot study. J Am Coll Cardiol. 2000;36(1):270-275.
31 Lewis AB, Chabot M. The effect of treatment with angiotensin-converting enzyme inhibitors on survival of pediatric patients with dilated cardiomyopathy.[comment]. Pediatr Cardiol. 1993;14(1):9-12.
32 Hsu DT, et al. Rationale and design of a trial of angiotensin-converting enzyme inhibition in infants with single ventricle. Am Heart J. 2009;157(1):37-45.
33 Gheorghiade M, van Veldhuisen DJ, Colucci WS. Contemporary use of digoxin in the management of cardiovascular disorders. Circulation. 2006;113(21):2556-2564.
34 Georgiopoulou VV, et al. Digoxin therapy does not improve outcomes in patients with advanced heart failure on contemporary medical therapy. Circ Heart Fail. 2009;2(2):90-97.
35 Pitt B, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators.[comment]. N Engl J Med. 1999;341(10):709-717.
36 Prandota J. Clinical pharmacology of furosemide in children: a supplement. Am J Ther. 2001;8(4):275-289.
37 Sallach JA, Goldstein S. Use of beta-blockers in congestive heart failure. Ann Med. 2003;35(4):259-266.
38 Klapholz M. Beta-blocker use for the stages of heart failure. Mayo Clin Proc. 2009;84(8):718-729.
39 Shaddy RE, et al. The Pediatric Randomized Carvedilol Trial in Children with Heart Failure: rationale and design. Am Heart J. 2002;144(3):383-389.
40 Bruns LA, et al. Carvedilol as therapy in pediatric heart failure: an initial multicenter experience.[comment]. J Pediatr. 2001;138(4):505-511.
41 Kantor PF, et al. The impact of changing medical therapy on transplantation-free survival in pediatric dilated cardiomyopathy. J Am Coll Cardiol. 2010;55(13):1377-1384.
42 Portman MA. Thyroid hormone regulation of heart metabolism. Thyroid. 2008;18(2):217-225.
43 Timek T, et al. Triiodothyronine reverses depressed contractile performance after excessive catecholamine stimulation. Ann Thorac Surg. 1998;66(5):1618-1625.
44 Portman MA, et al. Triiodothyronine repletion in infants during cardiopulmonary bypass for congenital heart disease. J Thorac Cardiovasc Surg. 2000;120(3):604-608.
45 Portman MA, Olson AK, Hastings LA, Larl TR, Patel HT, Mott A, et al. Triiodothyronine for infants and children undergoing cardiopulmonary bypass (TRICC) study: safety and efficacy (Abstract). Circulation. 2008;118:S749-S750.
46 Kivikko M, Lehtonen L, Colucci WS. Sustained hemodynamic effects of intravenous levosimendan. Circulation. 2003;107(1):81-86.
47 Magliola R, et al. [Levosimendan, a new inotropic drug: experience in children with acute heart failure]. [Spanish] Levosimendan, un nuevo agente inotropico: experiencia en ninos con fallo cardiaco agudo. Arch Argent Pediatr. 2009;107(2):139-145.
48 Namachivayam P, et al. Early experience with levosimendan in children with ventricular dysfunction. Pediatr Crit Care Med. 2006;7(5):445-448.
49 Jorgensen K, et al. Effects of levosimendan on left ventricular relaxation and early filling at maintained preload and afterload conditions after aortic valve replacement for aortic stenosis. Circulation. 2008;117(8):1075-1081.
50 Vlasselaers D, et al. Tight glycemic control protects the myocardium and reduces inflammation in neonatal heart surgery. Ann Thorac Surg. 2010;90(1):22-29.
51 Macrae D, et al. Control of hyperglycaemia in paediatric intensive care (CHiP): study protocol. BMC Pediatr. 2010;10:5.
52 Ivy DD. Prostacyclin in the intensive care setting. Pediatr Crit Care Med. 2010;11(2 Suppl):S41-S45.
53 Taylor MB, Laussen PC. Fundamentals of management of acute postoperative pulmonary hypertension. Pediatr Crit Care Med. 2010;11(2 Suppl):S27-S29.
54 Bauer J, et al. Perioperative management of pulmonary hypertension after heart transplantation in childhood. J Heart Lung Transplant. 1997;16(12):1238-1247.
55 Goldman AP, et al. Pharmacological control of pulmonary blood flow with inhaled nitric oxide after the fenestrated Fontan operation. Circulation. 1996;94(9 Suppl):II44-II48.
56 Miller OI, et al. Very-low-dose inhaled nitric oxide: a selective pulmonary vasodilator after operations for congenital heart disease. J Thorac Cardiovasc Surg. 1994;108(3):487-494.
57 Miller OI, et al. Inhaled nitric oxide and prevention of pulmonary hypertension after congenital heart surgery: a randomised double-blind study.[comment]. Lancet. 2000;356(9240):1464-1469.
58 Russell IA, et al. The effects of inhaled nitric oxide on postoperative pulmonary hypertension in infants and children undergoing surgical repair of congenital heart disease. Anesth Analg. 1998;87(1):46-51.
59 Barr FE, Macrae D. Inhaled nitric oxide and related therapies. Pediatr Crit Care Med. 2010;11(2 Suppl):S30-S36.
60 Balzer DT, et al. Inhaled Nitric Oxide as a Preoperative Test (INOP Test I): the INOP Test Study Group. Circulation. 2002;106(12 Suppl 1):I76-I81.
61 Kelly LK, et al. Inhaled prostacyclin for term infants with persistent pulmonary hypertension refractory to inhaled nitric oxide. J Pediatr. 2002;141(6):830-832.
62 Fraisse A, Wessel DL. Acute pulmonary hypertension in infants and children: cGMP-related drugs. Pediatr Crit Care Med. 2010;11(2 Suppl):S37-S40.
63 Huddleston AJ, et al. Sildenafil for the treatment of pulmonary hypertension in pediatric patients. Pediatr Cardiol. 2009;30(7):871-882.
64 Nemoto S, et al. Oral sildenafil for persistent pulmonary hypertension early after congenital cardiac surgery in children. Eur J Cardiothorac Surg. 2010;38(1):71-77.
65 Adatia I, Shekerdemian L. The role of calcium channel blockers, steroids, anticoagulation, antiplatelet drugs, and endothelin receptor antagonists. Pediatr Crit Care Med. 2010;11(2 Suppl):S46-S52.
66 Beghetti M, Tissot C. Pulmonary arterial hypertension and congenital heart disease: targeted therapies and operability. J Thorac Cardiovasc Surg. 2009;138(3):785-786. author reply 786
67 Carter NJ, Keating GM. Bosentan: in pediatric patients with pulmonary arterial hypertension. Paediatr Drugs. 2010;12(1):63-73.
68 Elbourne D, Field D, Mugford M. Extracorporeal membrane oxygenation for severe respiratory failure in newborn infants. Cochrane Database Syst Rev 2002(1): p. CD001340.
69 Cooper DS, et al. Cardiac extracorporeal life support: state of the art in 2007. Cardiol Young. 2007;17(Suppl 2):104-115.
70 Salvin JW, Laussen PC, Thiagarajan RR. Extracorporeal membrane oxygenation for postcardiotomy mechanical cardiovascular support in children with congenital heart disease. Paediatr Anaesth. 2008;18(12):1157-1162.
71 Chaturvedi RR, et al. Cardiac ECMO for biventricular hearts after paediatric open heart surgery. Heart. 2004;90(5):545-551.
72 ELSO, ECLS Registry Report. Ann Arbor: 2010; Extracorporeal Life Support Organization.
73 Allan CK, et al. Emergent use of extracorporeal membrane oxygenation during pediatric cardiac catheterization. Pediatr Crit Care Med. 2006;7(3):212-219.
74 Raymond TT, et al. Outcomes among neonates, infants, and children after extracorporeal cardiopulmonary resuscitation for refractory inhospital pediatric cardiac arrest: a report from the National Registry of Cardiopulmonary Resuscitation. Pediatr Crit Care Med. 2010;11(3):362-371.
75 Topjian AA, Berg RA, Nadkarni VM. Pediatric cardiopulmonary resuscitation: advances in science, techniques, and outcomes. Pediatrics. 2008;122(5):1086-1098.
76 Barrett CS, et al. Neurological injury after extracorporeal membrane oxygenation use to aid pediatric cardiopulmonary resuscitation. Pediatr Crit Care Med. 2009;10(4):445-451.
77 Nugent AW, et al. The epidemiology of childhood cardiomyopathy in Australia.[comment]. N Engl J Med. 2003;348(17):1639-1646.
78 Lipshultz SE, et al. The incidence of pediatric cardiomyopathy in two regions of the United States.[comment]. N Engl J Med. 2003;348(17):1647-1655.
79 Aretz HT, et al. Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol. 1987;1(1):3-14.
80 Mason JW, et al. A clinical trial of immunosuppressive therapy for myocarditis. N Engl J Med. 1995;333:269-275.
81 Batra AS, Lewis AB. Acute myocarditis. Curr Opin Pediatr. 2001;13(3):234-239.
82 Bohn D, Benson L. Diagnosis and management of pediatric myocarditis. Pediatr Drugs. 2002;4:171-181.
83 Canter CE, et al. Indications for heart transplantation in pediatric heart disease: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young; the Councils on Clinical Cardiology, Cardiovascular Nursing, and Cardiovascular Surgery and Anesthesia; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;115(5):658-676.
84 Lewis AB. Late recovery of ventricular function in children with idiopathic dilated cardiomyopathy. Am Heart J. 1999;138(2 Pt 1):334-338.
85 Lee KJ, et al. Clinical outcomes of acute myocarditis in childhood. Heart. 1999;82(2):226-233.
86 Duncan BW, et al. Mechanical circulatory support for treatment of children with acute fulminant myocarditis. J Thorac Cardiovasc Surg. 2001;122:440-448.
87 Dec GW, Fuster V. Idiopathic dilated cardiomyopathy.[comment]. N Engl J Med. 1994;331(23):1564-1575.
88 Shekerdemian L, Bohn D. Cardiovascular effects of mechanical ventilation. Arch Dis Child. 1999;80:475-480.
89 Ibsen LM, Bratton SL. Fulminant myocarditis and extracorporeal membrane oxygenation: what we know, what is there still to learn? Crit Care Med. 2010;38(2):686-688.
90 Goldman A, Cassidy J, de Leval M, et al. The waiting game: bridging to paediatric heart transplantation. Lancet. 2003;362:1967-1970.
91 Brancaccio G, et al. Mechanical assist device as a bridge to heart transplantation in children less than 10 kilograms. Ann Thorac Surg. 2010;90(1):58-62.
92 Brennan P, Young ID. Congenital heart malformations: aetiology and associations. Semin Neonatol. 2001;6(1):17-25.
93 Momma K. Cardiovascular anomalies associated with chromosome 22q11.2 deletion syndrome. Am J Cardiol. 2010;105(11):1617-1624.
94 Allen HD, Driscoll DJ, Feltes TF, Shaddy RE, editors. Moss and Adam’s Heart Disease in Infants Children and adolescents, 7th ed, Philadelphia: Lippincott Williams and Wilkins, 2007.
95 Kouchoukos N, Blackstone E, Doty D, Hanley F, Karp R, editors. Kirklin and Barratt-Boyes cardiac surgery, 3rd ed, New York: Churchill Livingstone, 2003.
96 Nichols DG, Ungerleider RM, Spevak PJ, Greeley WJ, Cameron DE, Lappe DG, Wetzel RC, editors. Critical heart disease in infants and children, 2nd ed, Philadelphia: Mosby, 2006.
97 Rudolph AM. The effects of postnatal circulatory adjustments in congenital heart disease. Pediatrics. 1965;36:763-772.
98 Hoffman JI, Rudolph AM, Heymann MA. Pulmonary vascular disease with congenital heart lesions: pathologic features and causes. Circulation. 1981;64(5):873-877.
99 McDaniel NL. Ventricular and atrial septal defects. Pediatr Rev. 2001;22(8):265-270.
100 Jacobs JP, et al. Congenital Heart Surgery Nomenclature and Database Project: ventricular septal defect. Ann Thorac Surg. 2000;69(4 Suppl):S25-S35.
101 Ramaciotti C, et al. Prevalence, relation to spontaneous closure, and association of muscular ventricular septal defects with other cardiac defects. Am J Cardiol. 1995;75(1):61-65.
102 Hardin JT, et al. Primary surgical closure of large ventricular septal defects in small infants. Ann Thorac Surg. 1992;53(3):397-401.
103 Seddio F, et al. Multiple ventricular septal defects: how and when should they be repaired? J Thorac Cardiovasc Surg. 1999;117(1):134-139. discussion 39-40
104 Reddy VM, Hanley FL. Cardiac surgery in infants with very low birth weight. Semin Pediatr Surg. 2000;9(2):91-95.
105 Crossland DS, et al. Initial results of primary device closure of large muscular ventricular septal defects in early infancy using perventricular access. Catheter Cardiovasc Interv. 2008;72(3):386-391.
106 Flori HR, et al. Transthoracic intracardiac catheters in pediatric patients recovering from congenital heart defect surgery: associated complications and outcomes. Crit Care Med. 2000;28(8):2997-3001.
107 Dodge-Khatami A, et al. Surgical substrates of postoperative junctional ectopic tachycardia in congenital heart defects. J Thorac Cardiovasc Surg. 2002;123(4):624-630.
108 Dodge-Khatami A, et al. Impact of junctional ectopic tachycardia on postoperative morbidity following repair of congenital heart defects. Eur J Cardiothorac Surg. 2002;21(2):255-259.
109 Weindling SN, et al. Duration of complete atrioventricular block after congenital heart disease surgery. Am J Cardiol. 1998;82(4):525-527.
110 Jacobs JP, et al. Congenital Heart Surgery Nomenclature and Database Project: atrial septal defect. Ann Thorac Surg. 2000;69(4 Suppl):S18-S24.
111 Cherian G, et al. Pulmonary hypertension in isolated secundum atrial septal defect: high frequency in young patients. Am Heart J. 1983;105(6):952-957.
112 Fischer G, et al. Catheter-based closure of atrial septal defects in the oval fossa with the Amplatzer device in patients in their first or second year of life. Catheter Cardiovasc Interv. 2009;73(7):949-955.
113 Masura J, Gavora P, Podnar T. Long-term outcome of transcatheter secundum-type atrial septal defect closure using Amplatzer septal occluders. J Am Coll Cardiol. 2005;45(4):505-507.
114 Sarris GE, et al. Surgery for complications of trans-catheter closure of atrial septal defects: a multi-institutional study from the European Congenital Heart Surgeons Association. Eur J Cardiothorac Surg. 2010;37(6):1285-1290.
115 Murphy JG, et al. Long-term outcome after surgical repair of isolated atrial septal defect. Follow-up at 27 to 32 years.[comment]. N Engl J Med. 1990;323(24):1645-1650.
116 Shuhaiber JH, et al. Current options and outcomes for the management of atrioventricular septal defect. Eur J Cardiothorac Surg. 2009;35(5):891-900.
117 Bando K, et al. Pulmonary hypertension after operations for congenital heart disease: analysis of risk factors and management. J Thorac Cardiovasc Surg. 1996;112(6):1600-1607. discussion 1607-9
118 Hermes-DeSantis ER, Clyman RI. Patent ductus arteriosus: pathophysiology and management. J Perinatol. 2006;26(Suppl 1):S14-S18. discussion S22-3
119 Van Overmeire B, et al. A comparison of ibuprofen and indomethacin for closure of patent ductus arteriosus.[comment]. N Engl J Med. 2000;343(10):674-681.
120 Arora R. Transcatheter closure of patent ductus arteriosus. Expert Rev Cardiovasc Ther. 2005;3(5):865-874.
121 Burke RP, et al. Video-assisted thoracoscopic surgery for patent ductus arteriosus in low birth weight neonates and infants. Pediatrics. 1999;104(2 Pt 1):227-230.
122 Perez E, Sullivan KE. Chromosome 22q11.2 deletion syndrome (DiGeorge and velocardiofacial syndromes). Curr Opin Pediatr. 2002;14(6):678-683.
123 Grossfeld PD. The genetics of congenital heart disease. J Nucl Cardiol. 2003;10(1):71-76.
124 Imamura M, et al. Improving early and intermediate results of truncus arteriosus repair: a new technique of truncal valve repair. Ann Thorac Surg. 1999;67(4):1142-1146.
125 Bove EL, et al. Results of a policy of primary repair of truncus arteriosus in the neonate. J Thorac Cardiovasc Surg. 1993;105(6):1057-1065. discussion 1065-6
126 Rajasinghe HA, et al. Long-term follow-up of truncus arteriosus repaired in infancy: a twenty-year experience. J Thorac Cardiovasc Surg. 1997;113(5):869-878. discussion 878-9
127 Drury NE, Veldtman GR, Benson LN. Neonatal aortic stenosis. Expert Rev Cardiovasc Ther. 2005;3(5):831-843.
128 Borghi A, et al. Aortic balloon dilatation for congenital aortic stenosis: report of 90 cases (1986-98). Heart. 1999;82(6):e10.
129 Alsoufi B, et al. Superior results following the Ross procedure in patients with congenital heart disease. J Heart Valve Dis. 2010;19(3):269-277. discussion 278
130 Puranik R, et al. Functional outcomes after the Ross (pulmonary autograft) procedure assessed with magnetic resonance imaging and cardiopulmonary exercise testing. Heart. 2010;96(4):304-308.
131 Shinkawa T, et al. Intermediate-term results of the Ross procedure in neonates and infants. Ann Thorac Surg. 2010;89(6):1827-1832. discussion 1832
132 Piccardo A, et al. Ross and Ross-Konno procedures in infants, children and adolescents: a 13-year experience. J Heart Valve Dis. 2009;18(1):76-82. discussion 83
133 Lofland GK, et al. Critical aortic stenosis in the neonate: a multi-institutional study of management, outcomes, and risk factors. Congenital Heart Surgeons Society. J Thorac Cardiovasc Surg. 2001;121(1):10-27.
134 Reller MD. Congenital heart disease: current indications for antithrombotic therapy in pediatric patients. Curr Cardiol Rep. 2001;3(1):90-95.
135 Jahangiri M, et al. Surgical management of complex and tunnel-like subaortic stenosis. Eur J Cardiothorac Surg. 2000;17(6):637-642.
136 Collins RT2nd, Kaplan P, Rome JJ. Stenosis of the thoracic aorta in Williams syndrome. Pediatr Cardiol. 2010;31(6):829-833.
137 Collins RT2nd, et al. Long-term outcomes of patients with cardiovascular abnormalities and williams syndrome. Am J Cardiol. 2010;105(6):874-878.
138 Abbruzzese PA, Aidala E. Aortic coarctation: an overview. J Cardiovasc Med. 2007;8(2):123-128.
139 Kanter KR. Management of infants with coarctation and ventricular septal defect. Semin Thorac Cardiovasc Surg. 2007;19(3):264-268.
140 Abu-Harb M, et al. Presentation of obstructive left heart malformations in infancy. Arch Dis Child Fetal Neonatal Ed. 1994;71(3):F179-F183.
141 Karl TR. Surgery is the best treatment for primary coarctation in the majority of cases. J Cardiovasc Med. 2007;8(1):50-56.
142 Van Son JA, et al. Repair of coarctation of the aorta in neonates and young infants. J Card Surg. 1997;12(3):139-146.
143 Younoszai AK, et al. Intermediate term follow-up of the end-to-side aortic anastomosis for coarctation of the aorta. Ann Thorac Surg. 2002;74(5):1631-1634.
144 Egan M, Holzer RJ. Comparing balloon angioplasty, stenting and surgery in the treatment of aortic coarctation. Expert Rev Cardiovasc Ther. 2009;7(11):1401-1412.
145 Will RJ, et al. Sodium nitroprusside and propranolol therapy for management of postcoarctectomy hypertension. J Thorac Cardiovasc Surg. 1978;75(5):722-724.
146 Stewart JM, et al. Elevated arginine vasopressin and lowered atrial natriuretic factor associated with hypertension in coarctation of the aorta. J Thorac Cardiovasc Surg. 1995;110(4 Pt 1):900-908.
147 Rouine-Rapp K, et al. Effect of enalaprilat on postoperative hypertension after surgical repair of coarctation of the aorta. Pediatr Crit Care Med. 2003;4(3):327-332.
148 Wiest DB, et al. Esmolol for the management of pediatric hypertension after cardiac operations. J Thorac Cardiovasc Surg. 1998;115(4):890-897.
149 de Divitiis M, et al. Ambulatory blood pressure, left ventricular mass, and conduit artery function late after successful repair of coarctation of the aorta. J Am Coll Cardiol. 2003;41(12):2259-2265.
150 Mays ET, Sergenat CK. Postcoarctectomy syndrome. Arch Surg. 1965;91:58-65.
151 Brand A. Immunological aspects of blood transfusions. Transpl Immunol. 2002;10(2-3):183-190.
152 Delisle G, et al. Total anomalous pulmonary venous connection: Report of 93 autopsied cases with emphasis on diagnostic and surgical considerations. Am Heart J. 1976;91(1):99-122.
153 Kanter KR. Surgical repair of total anomalous pulmonary venous connection. Semin Thorac Cardiovasc Surg Pediatr Card Surg Ann. 2006:40-44.
154 Ricci M, et al. Management of pulmonary venous obstruction after correction of TAPVC: risk factors for adverse outcome. Eur J Cardiothorac Surg. 2003;24(1):28-36. discussion 36
155 Apitz C, Webb GD, Redington AN. Tetralogy of Fallot. Lancet. 2009;374(9699):1462-1471.
156 Van Arsdell GS, et al. What is the optimal age for repair of tetralogy of Fallot? Circulation. 2000;102(19 Suppl 3):III123-III129.
157 Kanter KR, et al. Symptomatic neonatal tetralogy of Fallot: repair or shunt? Ann Thorac Surg. 2010;89(3):858-863.
158 Chaturvedi RR, et al. Acute right ventricular restrictive physiology after repair of tetralogy of Fallot: association with myocardial injury and oxidative stress. Circulation. 1999;100(14):1540-1547.
159 Shekerdemian LS, et al. Negative pressure ventilation as haemodynamic rescue following surgery for congenital heart disease. Intensive Care Med. 2000;26(1):93-96.
160 Bichell DP. Evaluation and management of pulmonary atresia with intact ventricular septum. Curr Opin Cardiol. 1999;14(1):60-66.
161 Reddy VM, et al. Early and intermediate outcomes after repair of pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries: experience with 85 patients. Circulation. 2000;101(15):1826-1832.
162 Pawade A, Karl T. Management strategy in neonates presenting with pulmonary atresia with intact ventricular septum. Curr Opin Pediatr. 1994;6(5):600-605.
163 Matsui H, Gardiner H. Fetal intervention for cardiac disease: the cutting edge of perinatal care. Semin Fetal Neonatal Med. 2007;12(6):482-489.
164 Veldtman GR, et al. Radiofrequency applications in congenital heart disease. Expert Rev Cardiovasc Ther. 2004;2(1):117-126.
165 Pawade A, et al. Pulmonary atresia with intact ventricular septum: surgical management based on right ventricular infundibulum. J Card Surg. 1993;8(3):371-383.
166 Miyaji K, et al. Pulmonary atresia with intact ventricular septum: long-term results of “one and a half ventricular repair”. Ann Thorac Surg. 1995;60(6):1762-1764.
167 Freedom RM, Anderson RH, Perrin D. The significance of ventriculo-coronary arterial connections in the setting of pulmonary atresia with an intact ventricular septum. Cardiol Young. 2005;15(5):447-468.
168 Martins P, Castela E. Transposition of the great arteries. Orphanet J Rare Dis. 2008;3:27.
169 Warnes CA. Transposition of the great arteries. Circulation. 2006;114(24):2699-2709.
170 Hovels-Gurich HH, et al. Long-term results of cardiac and general health status in children after neonatal arterial switch operation. Ann Thorac Surg. 2003;75(3):935-943.
171 Sarkar D, et al. Comparison of long-term outcomes of atrial repair of simple transposition with implications for a late arterial switch strategy. Circulation. 1999;100(19 Suppl):II176-II181.
172 Foran JP, et al. Primary arterial switch operation for transposition of the great arteries with intact ventricular septum in infants older than 21 days. J Am Coll Cardiol. 1998;31(4):883-889.
173 Legendre A, et al. Coronary events after arterial switch operation for transposition of the great arteries. Circulation. 2003;108(Suppl 1):II186-II190.
174 Boutin C, et al. Rapid two-stage arterial switch operation. Evaluation of left ventricular systolic mechanics late after an acute pressure overload stimulus in infancy. Circulation. 1994;90(3):1294-1303.
175 Jonas RA. Fontan or septation: when I abandon septation in complex lesions with two ventricles. Semin Thorac Cardiovasc Surg. Pediatric Cardiac Surgery Annual. 2009:94-98.
176 Schwartz SM, et al. Single-ventricle physiology. Crit Care Clin. 2003;19(3):393-411.
177 de Leval MR. The Fontan circulation: What have we learned? What to expect? Pediatr Cardiol. 1998;19(4):316-320.
178 Stamm C, et al. Long-term results of the lateral tunnel Fontan operation. J Thorac Cardiovasc Surg. 2001;121(1):28-41.
179 Petrossian E, Thompson LD, Hanley FL. Extracardiac conduit variation of the Fontan procedure. Adv Card Surg. 2000;12:175-198.
180 Amin Z, et al. Hemidiaphragmatic paralysis increases postoperative morbidity after a modified Fontan operation. J Thorac Cardiovasc Surg. 2001;122(5):856-862.
181 Momma K. ACE inhibitors in pediatric patients with heart failure. Paediatr Drugs. 2006;8(1):55-69.
182 Thompson LD, et al. Perioperative administration of angiotensin converting enzyme inhibitors decreases the severity and duration of pleural effusions following bidirectional cavopulmonary anastomosis. Cardiol Young. 2001;11(2):195-200.
183 Cheung YF, Penny DJ, Redington AN. Serial assessment of left ventricular diastolic function after Fontan procedure. Heart. 2000;83(4):420-424.
184 Tchervenkov CI, et al. The nomenclature, definition and classification of hypoplastic left heart syndrome. Cardiol Young. 2006;16(4):339-368.
185 Ohye RG, Bove EL. Advances in congenital heart surgery. Curr Opin Pediatr. 2001;13(5):473-481.
186 Akinturk H, et al. Hybrid transcatheter-surgical palliation: basis for univentricular or biventricular repair: the Giessen experience. Pediatr Cardiol. 2007;28(2):79-87.
187 Rupp S, et al. Implantation of stents to ensure an adequate interatrial communication in patients with hypoplastic left heart syndrome. Cardiol Young. 2007;17(5):535-540.
188 Ohye RG, Sleeper LA, Mahoney L, Newburger JW, Pearson GD, Lu M, et al. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362:1980-1992.
189 Tworetzky W, et al. Improved surgical outcome after fetal diagnosis of hypoplastic left heart syndrome. Circulation. 2001;103(9):1269-1273.
190 Tabbutt S, et al. Neurodevelopmental outcomes after staged palliation for hypoplastic left heart syndrome. Pediatrics. 2008;121(3):476-483.
191 Kitagawa T, et al. Techniques and results in the management of multiple ventricular septal defects.[comment]. J Thorac Cardiovasc Surg. 1998;115(4):848-856.
192 Tibby SM, Durward A. Interpretation of the echocardiographic pressure gradient across a pulmonary artery band in the setting of a univentricular heart. Intensive Care Med. 2008;34(1):203-207.
193 Bonnard A, et al. Vascular ring abnormalities: a retrospective study of 62 cases. J Pediatr Surg. 2003;38(4):539-543.
194 Dodge-Khatami A, et al. Vascular rings and pulmonary arterial sling: from respiratory collapse to surgical cure, with emphasis on judicious imaging in the hi-tech era. Cardiol Young. 2002;12(2):96-104.
195 Backer CL, Mavroudis C. Surgical approach to vascular rings. Adv Card Surg. 1997;9:29-64.
196 Takeuchi S, et al. New surgical method for repair of anomalous left coronary artery from pulmonary artery. J Thorac Cardiovasc Surg. 1979;78(1):7-11.
197 Azakie A, et al. Anatomic repair of anomalous left coronary artery from the pulmonary artery by aortic reimplantation: early survival, patterns of ventricular recovery and late outcome. Ann Thorac Surg. 2003;75(5):1535-1541.
198 McElhinney DB, et al. Management and outcomes of delayed sternal closure after cardiac surgery in neonates and infants.[comment]. Crit Care Med. 2000;28(4):1180-1184.
199 Iyer RS, et al. Outcomes after delayed sternal closure in pediatric heart operations: a 10-year experience.[comment]. Ann Thorac Surg. 1997;63(2):489-491.
200 Horvath R, et al. Cerebral and somatic oxygen saturation decrease after delayed sternal closure in children after cardiac surgery. J Thorac Cardiovasc Surg. 2010;139(4):894-900.
201 Ferrieri P, et al. Unique features of infective endocarditis in childhood. Pediatrics. 2002;109(5):931-943.
202 Nishimura RA, et al. ACC/AHA 2008 guideline update on valvular heart disease: focused update on infective endocarditis: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;118(8):887-896.
203 O’Grady NP, et al. Guidelines for the prevention of intravascular catheter-related infections. The Hospital Infection Control Practices Advisory Committee, Centers for Disease Control and Prevention, U.S. Pediatrics. 2002;110:e51.