Acquired Abnormalities (Stone Disease and Infection)
Renal Infection
Acute Bacterial Pyelonephritis
Overview: The term pyelonephritis encompasses infections of the renal parenchyma and pelvocaliceal system. Bacterial infections of the kidneys are further categorized as acute or chronic, unilateral or bilateral, and focal, multifocal, or diffuse. Potential complications of pyelonephritis include renal or perinephric abscess. A bacterial infection in which purulent material fills a dilated collecting system is termed pyonephrosis.1
Pathophysiology and Clinical Presentation: The most common cause of acute bacterial pyelonephritis is ascending infection from the lower urinary tract. Parenchymal renal infection occasionally occurs as a result of hematogenous inoculation; this mechanism is more common in infants than in older children. Although children with vesicoureteral reflux are at elevated risk for pyelonephritis, reflux is not a prerequisite for renal infection. Potential clinical manifestations of acute bacterial pyelonephritis include flank pain, abdominal pain, fever, pyuria, nausea, and vomiting. Young infants with pyelonephritis often have nonspecific findings such as irritability and poor feeding; fever is sometimes lacking.2–4
Imaging: Renal cortical scintigraphy with technetium-99m dimercaptosuccinic acid (DMSA) or technetium-99m glucoheptonate is highly sensitive (at least 90%) for the detection of acute bacterial pyelonephritis. Infected regions of the kidneys have diminished or absent accumulation of the radiopharmaceutical agent, often with a spherical or flarelike pattern (Fig. 115-1). A renal abscess produces a scintigraphic defect that usually is indistinguishable from that of uncomplicated parenchymal infection.5,6
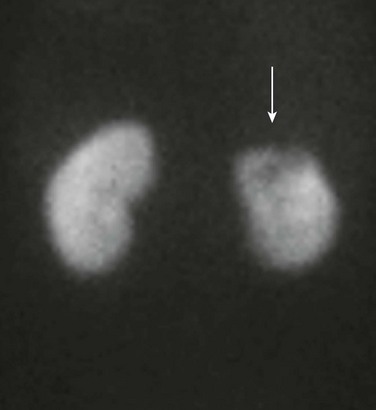
Figure 115-1 Acute bacterial pyelonephritis.
A posterior technetium-99m dimercaptosuccinic acid image of a febrile 16-year-old shows absent uptake in the upper pole of the left kidney (arrow).
Contrast-enhanced computed tomography (CT) provides sensitivity that is similar to renal cortical scintigraphy for the diagnosis of acute bacterial pyelonephritis. Infected renal parenchyma has diminished contrast enhancement on images obtained immediately after injection of contrast material (Fig. 115-2). Potential patterns of infected parenchyma include radially oriented linear streaks of diminished attenuation, round or irregular hypoattenuating foci, wedge-shaped defects, and heterogeneous diminished enhancement throughout an enlarged kidney. The nephrogram intensity usually is diminished relative to the contralateral normal kidney. Delayed CT images of the infected kidney show retention of contrast in obstructed tubules. A parenchymal abscess appears as a hypoattenuating focus, sometimes with a prominently enhancing rim. A perinephric abscess also is hypoattenuating.7
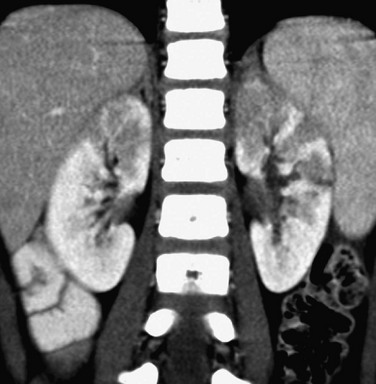
Figure 115-2 Multifocal bacterial pyelonephritis.
Multiple areas of deficient contrast enhancement of the kidneys are present on this coronal computed tomography image of a 6-year-old girl.
Renal parenchymal edema resulting from infection leads to diminished signal intensity on T1-weighted magnetic resonance imaging (MRI) and increased signal intensity on T2-weighted images (Fig. 115-3). Nephromegaly or localized parenchymal expansion may be present. Corticomedullary differentiation is sometimes deficient. The parenchyma may have a striated appearance. Edema in the perinephric space is a common MRI finding. As with CT, contrast enhancement is deficient in the involved portions of parenchyma. Urothelial thickening is sometimes appreciable.8,9
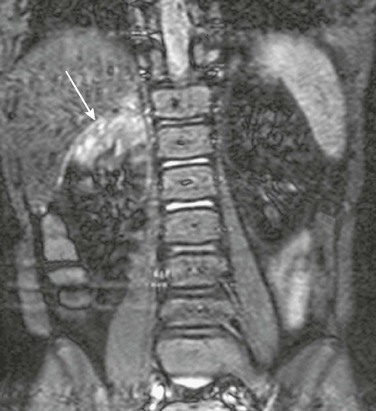
Figure 115-3 Acute bacterial pyelonephritis.
A coronal contrast-enhanced short tau inversion recovery magnetic resonance image shows abnormal increased signal in the upper pole of the right kidney (arrow). Normal renal parenchyma is hypointense. (Courtesy Damien Grattan-Smith, MD, Atlanta, GA.)
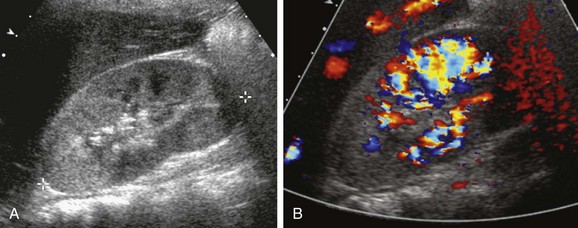
Reported sensitivities of sonography for the detection of acute bacterial pyelonephritis range from 25% to 50%. The findings include nephromegaly, abnormal parenchymal echogenicity, loss of corticomedullary differentiation, renal sinus hyperechogenicity, and urothelial thickening. Color Doppler or power Doppler imaging demonstrates diminished perfusion of the infected regions of parenchyma, sometimes with a wedge shape (Fig. 115-4). A parenchymal abscess usually appears as a spherical hypoechoic focus with acoustic enhancement. Occasionally, pus within the cavity results in an isoechoic or hypoechoic appearance. A perinephric abscess appears as a hypoechoic fluid collection immediately peripheral to the capsule.10–13
Treatment and Follow-up: Longer and more intensive antibiotic therapy is required for upper urinary tract infections than for those confined to the bladder. Antibiotic prophylaxis against reinfection is indicated for selected patients, particularly for those with predisposing factors. The utility of follow-up diagnostic imaging studies for children with acute bacterial pyelonephritis is a topic of ongoing investigation and debate.14,15
Pyonephrosis
Overview: Pyonephrosis is a bacterial infection of the kidney in which purulent material fills a dilated collecting system. Most often, a preexisting chronic obstruction is present, such as congenital ureteropelvic junction obstruction. Acute obstruction because of a calculus is an occasional cause in children. The clinical presentation of pyonephrosis is similar to that of other bacterial urinary tract infections. Fever, flank pain, pyuria, and hematuria are common.16
Imaging: Sonography shows echogenic material within a dilated pelvocaliceal system (Fig. 115-5). The purulent material often layers in the dependent portions of the collecting system and may shift with changes in patient position. Thickening of the wall of the dilated renal pelvis usually is present. Uncommon additional potential findings include a fluid-debris level in the collecting system, echogenic foci due to gas-forming organisms, and complete filling of the dilated collecting system with echogenic material.17,18
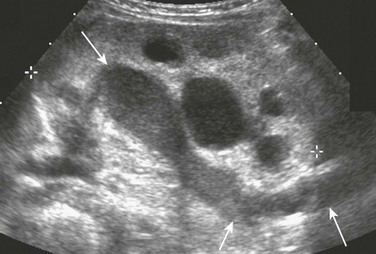
Figure 115-5 Pyonephrosis.
A longitudinal sonogram shows a hydronephrotic duplex collecting system. Echogenic debris is present within the upper pole collecting system and ureter (arrows). Lesser debris is present within the lower pole collecting system.
Imaging of pyonephrosis with CT, MRI, or scintigraphy shows diminished function of the involved kidney. Deficient excretion of intravenously administered contrast or radiopharmaceutical material is noted. Contrast enhancement of the renal parenchyma of the infected kidney is heterogeneous and delayed on CT. Excreted contrast may outline filling defects in the dilated collecting system on delayed images. Debris usually is visible in the dilated renal collecting system on MRI. The renal parenchyma has abnormal heterogeneous signal intensity. The pus-filled collecting system is markedly hyperintense on diffusion-weighted images. Contrast-enhanced MRI confirms reduced function of the kidney.19
Treatment and Follow-up: The treatment of pyonephrosis includes prompt initiation of aggressive antimicrobial therapy. Drainage of the obstructed collecting system often is required, usually by percutaneous nephrostomy. Surgical correction of the underlying obstruction typically is performed after resolution of the acute infection.
Xanthogranulomatous Pyelonephritis
Overview: Xanthogranulomatous pyelonephritis (XGP) is an uncommon form of severe chronic renal parenchymal infection. The pathogenesis typically involves chronic infection of an obstructed kidney. Histologic examination demonstrates inflammatory cells interspersed within fibrogranulomatous tissue, nodules of lipid laden macrophages, and areas of necrotic parenchyma. Diffuse involvement of the kidney is most common; segmental or focal forms also can occur, sometimes in association with obstruction of a duplicated system or infundibulum. Common clinical findings include fever, flank pain, malaise, pyuria, weight loss, and anemia.20–22
Imaging: In the early stages of XGP, the involved portion of the kidney typically has an irregular hyperechoic character on sonography. With the diffuse form, the kidney may be massively enlarged but usually maintains a reniform shape. Echogenic foci with shadowing indicate the presence of calcifications. With progression, necrotic tissue and fluid are usually hypoechoic. Echogenic debris is sometimes visible within abscesses or the dilated collecting system.23
CT of diffuse XGP typically demonstrates an enlarged nonfunctioning kidney that has multiple low-attenuation parenchymal foci (Fig. 115-6). A staghorn calculus often is present in a contracted renal pelvis. Irregular contrast enhancement of the inflamed renal parenchyma occurs, often accompanied by inflammatory enhancement of the perinephric fat. Abscesses are moderately hypoattenuating and do not enhance with contrast. The pus-filled collecting system also is hypoattenuating. Little or no contrast excretion occurs. Regional retroperitoneal lymphadenopathy is common. With the focal form of XGP, CT typically demonstrates an expansile renal mass. Peripheral granulation tissue or compressed renal parenchyma may result in an enhancing peripheral rim. The involved renal parenchyma typically has low or intermediate signal intensity on T1-weighted MRI and high intensity on T2-weighted images.24
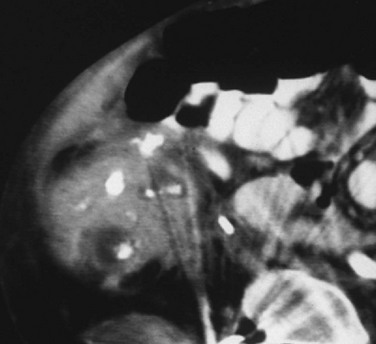
Figure 115-6 Xanthogranulomatous pyelonephritis.
Contrast-enhanced computed tomography shows a right kidney that contains multiple stones surrounded by low-attenuation regions of abscess or necrosis. The process extends into the perirenal tissues, which are edematous and poorly defined, and into the soft tissues beneath the right abdominal wall, which are thickened. Metal-induced artifacts are produced by spinal fixation hardware in this child with dysraphism.
Treatment and Follow-up: The usual treatment for XGP is antibiotic therapy followed by nephrectomy or heminephrectomy. A percutaneous biopsy occasionally is useful to allow differentiation from a neoplasm and to provide material for culture. With acute disease, percutaneous abscess drainage can serve as a temporizing measure.
Candidiasis
Overview: Candidiasis, typically due to Candida albicans, accounts for about 80% of renal fungal infections. Premature neonates are particularly susceptible. The clinical presentation in neonates usually is nonspecific: hypertension, oliguria, or anuria. Older children with renal candidiasis usually have immunocompromise. The clinical findings typically are indistinguishable from those of a bacterial infection: fever, chills, dysuria, and flank pain.25
Imaging: Potential sonographic findings of renal candidiasis include parenchymal hyperechogenicity, nephromegaly, one or more small abscesses, and debris within the collecting system. A fungus ball (mycetoma) in the pelvocaliceal system appears as an echogenic object, with or without acoustic shadowing (Fig. 115-7). Dilation of the collecting system proximal to a fungus ball is common. Findings on contrast-enhanced CT include nephromegaly, diffuse or multifocal edema, renal abscess, and hydronephrosis. Infected parenchyma lacks normal contrast enhancement. Disseminated candidiasis sometimes results in tiny bilateral renal lesions, which often are associated with splenic and hepatic disease. CT may demonstrate organ enlargement and a heterogeneous (salt and pepper) pattern of contrast enhancement.26,27
Treatment and Follow-up: The typical treatment for renal candidiasis is systemic antifungal medication. Percutaneous nephrostomy is an option for children with an obstructing fungus ball. This treatment allows decompression of the collecting system and provides a pathway for installation of antifungal medication.
Tuberculosis
Overview: The urinary system is the most common site of extrapulmonary tuberculosis. Initial infection of the kidney consists of small caseous foci in the renal parenchyma that release the organism into the collecting tubules. Eventually, a larger mass may develop in the renal cortex, resulting in additional discharge of bacteria. Antegrade seeding frequently allows spread of infection to the ureters, prostate, or epididymis. Involvement of the collecting system can progress to fibrosis and calcification, sometimes leading to obstruction. Potential symptoms of urinary tuberculosis include dysuria, flank pain, and gross hematuria.28
Imaging: In the acute phase of tuberculous infection, imaging of the renal parenchyma may be normal or show manifestations of edema and vasoconstriction. Involved areas lack normal contrast enhancement on CT and MRI and sometimes have altered echogenicity on sonography. Inflamed urothelium may appear thickened on cross-sectional imaging studies and undergo prominent contrast enhancement. Parenchymal edema and large tubercles may cause distortion of the calyceal system. Later in the course of the disease, compromised renal function results in diminished contrast excretion. Contrast sometimes pools within parenchymal cavitations. Multiple small filling defects may be present in the collecting system or ureter. Fibrotic strictures of the collecting system that are common late in the course of the disease result in focal dilation or hydronephrosis. Dystrophic calcifications develop in persons with necrotic parenchyma.29,30
Treatment and Follow-up: The mainstay treatment of urinary system tuberculosis is systemic antituberculous medication. Imaging studies serve to monitor treatment effectiveness and to detect complications. Apparent obstructive strictures of the ureter sometimes are due to edema that can regress during treatment. A persistent stricture may require surgical intervention.
Stone Disease
Nephrocalcinosis
Overview: In more than 90% of children with nephrocalcinosis, the calcification predominantly is in the medullary region. The most common causes of medullary renal calcification in children are metabolic conditions such as renal tubular acidosis, diuretic use, and metabolic conditions that produce hypercalcemia and hypercalciuria (Box 115-1).31–33
Etiologies, Pathophysiology, and Clinical Presentation: Type 1 renal tubular acidosis (RTA) is the most common metabolic condition associated with nephrocalcinosis in children. Nephrocalcinosis occurs in about three quarters of patients with type 1 RTA. Progression to urolithiasis can occur.
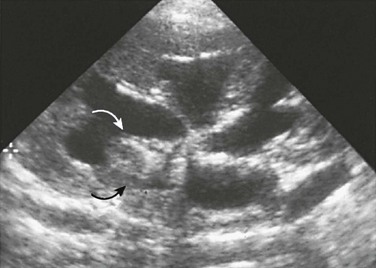
Imaging: The major sonographic feature of medullary nephrocalcinosis is hyperechogenicity of one or more renal pyramids (Fig. 115-8). With macroscopic calcification, acoustic shadowing is present. The earliest sonographic sign of medullary nephrocalcinosis is loss of normal papillary hypoechogenicity. In some instances, hyperechogenicity occurs only at the tips of the pyramids.34,35
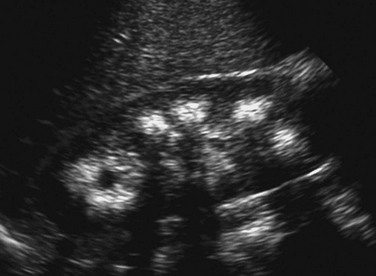
Figure 115-8 Medullary nephrocalcinosis.
A longitudinal sonographic image of a child with type 1 renal tubular acidosis and medullary nephrocalcinosis shows markedly echogenic renal pyramids, some of which produce acoustic shadowing.
With extensive nephrocalcinosis, medullary calcifications are visible on standard radiographs. Most common are diffuse or uniform calcifications within the medullary pyramids, resulting in a triangular pattern. Renal calcification in patients with type 1 RTA typically is quite dense and involves all the medullary pyramids in a uniform fashion. Calcifications associated with medullary sponge kidney tend to be asymmetric.36
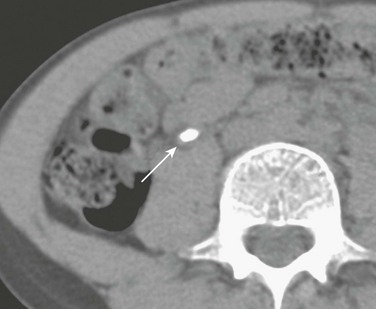
CT allows definitive localization of calcifications to the medullary or cortical regions (Fig. 115-9). With medullary sponge kidney, medullary calculi typically occur in clusters within the renal pyramids. Stagnation of contrast material is evident within the dilated collecting tubules on enhanced CT. With mild disease, stagnation of contrast material appears as linear papillary opacities; small cystic components may be visible with more advanced disease. With medullary sponge kidney, contrast material within the collecting tubules surrounds calculi. In patients with type 1 RTA, the contrast does not uniformly surround calcific deposits because the calcifications are within the medullary interstitium and tubular lumina.37
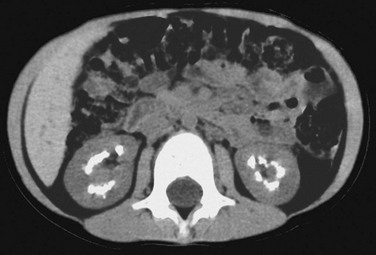
Figure 115-9 Medullary nephrocalcinosis.
Unenhanced computed tomography shows dense renal pyramid calcifications in a child with type 1 renal tubular acidosis.
The Anderson-Carr-Randall progression theory of urolithiasis suggests that microscopic calcifications within a renal pyramid can coalesce to form a plaque that migrates toward the calyx and thereby form a stone nidus. High-resolution ultrasonography of young children may reveal papillary hyperechogenicity caused by these microscopic calcifications, despite normal findings on other imaging studies. Hyperechoic pyramids are present in about 50% of children with diseases that predispose to nephrocalcinosis. Subepithelial calcium phosphate plaques sometimes are visible on radiographs or CT as slivers of calcification adjacent to the papillary tip.38
Treatment and Follow-up: Adequate hydration is an important preventive measure in patients with known nephrocalcinosis or a metabolic condition that can cause nephrocalcinosis. Various medical therapies are designed to reduce renal calcium excretion and increase the solubility of urinary calcium. These techniques occasionally serve to lessen the severity of nephrocalcinosis over time. However, nephrocalcinosis is irreversible for most patients. Imaging studies provide early detection of renal calcifications, monitor response to therapy, and detect complications such as obstructive urolithiasis.
Cortical Nephrocalcinosis
Overview and Imaging: Cortical nephrocalcinosis involves the periphery of the kidney and the central septa of Bertin. In the pure forms of cortical nephrocalcinosis, the medullary pyramids are spared. The most common causes of cortical nephrocalcinosis are chronic glomerulonephritis, acute cortical necrosis, and oxalosis (Box 115-2). Standard abdominal radiographs may show thin linear peripheral calcifications, diffuse homogeneous renal calcification, or diffuse punctate calcifications. On sonography, the involved cortex is echogenic, but acoustic shadowing does not occur unless conglomerate calcifications are present. CT accurately demonstrates the cortical location of calcification.33,39
Urolithiasis
Overview: The prevalence of urolithiasis varies according to geographic area, age, sex, and race. In the United States, urolithiasis affects approximately 1 in 1000 children. Urolithiasis is slightly more common in Europe than in North America and is considerably more common in Asia. In American children, the prevalence of urolithiasis is greatest in southern California and in the southeastern states. Urolithiasis is more common in white persons than in African Americans.31,40–42
Pathophysiology: About 70% of patients with urolithiasis have a known predisposing condition such as hypercalciuria, urinary stasis, or chronic infection (Table 115-1). A genitourinary anomaly is present in at least one third of children with nephrocalcinosis and in nearly all patients with renal calculi related to infection. Urolithiasis is common in patients with a urinary tract infection because of a urea-splitting gram-negative enteric organism. These “infection stones” usually are a mixture of magnesium ammonium phosphate (struvite) and calcium phosphate (apatite), that is, a “triple phosphate stone.” Struvite is the predominant composition of most staghorn calculi. Nonstruvite stones that occur in association with urinary tract infections are termed “infection-associated stones.” Patients with a neurogenic bladder, a congenital urinary tract obstruction, and other forms of urinary stasis have a propensity for calcium stones and infection stones.43–45
Hypercalciuria is a common cause of calcium stones. Hyperoxaluria is an additional potential cause of urinary stones that contain calcium. Uric acid stones due to hyperuricosuria account for only 5% of stones in North American children, but they are common in some areas of the world (e.g., Israel). Cystinuria and xanthinuria are rare causes of urolithiasis. Matrix stones are rare stones that consist of nonradiopaque inspissated mucoproteins, usually in conjunction with laminated or scattered calcific components. About 30% of pediatric urolithiasis is idiopathic. The typical composition of idiopathic stones is calcium phosphate or calcium oxalate. Idiopathic calcium stones sometimes have a nucleus of urate.46
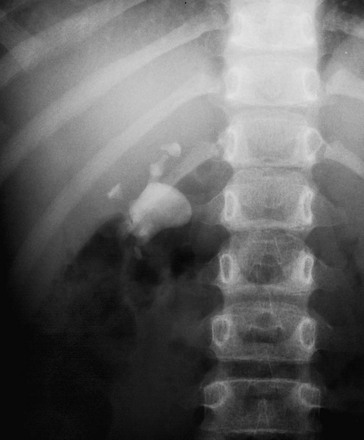
Bladder stones can either originate from the upper urinary tract or develop within the bladder (Fig. 115-10). Bladder stones often are laminated and sometimes reach a very large size. Bladder stones also can form in a bladder diverticulum, surgical pouch, or urachal remnant.
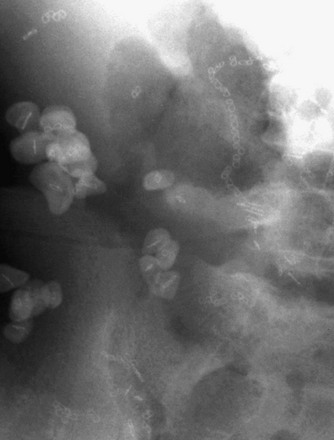
Figure 115-10 Bladder calculi.
A radiograph of the right lower quadrant shows multiple calculi within a bladder augmentation. A surgical clip serves as the nidus for each of the stones.
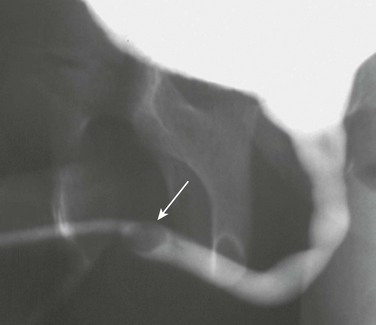
Urethral calculi are rare and only occur in males (Fig. 115-11). They can originate from a more proximal portion of the urinary tract or arise locally. Primary urethral calculi sometimes develop in association with prolonged obstruction or within a urethral diverticulum or the prostatic utricle.
Clinical Presentation: Pain is present in 50% to 75% of children with urolithiasis at the time of diagnosis. Pain can localize to the abdomen or flank and may or may not radiate. Additional potential clinical manifestations include hematuria (which is common), urgency, dysuria, frequency, fever, pyuria, and bacteriuria. Urinary retention can occur in patients with stones of the bladder or urethra.
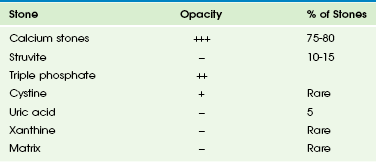
Imaging: About 90% of urinary tract calculi are sufficiently radiopaque for visualization on standard radiographs (Table 115-2). Classification of a stone as radiopaque versus nonopaque (or radiolucent) is according to its appearance on standard radiographs (Fig. 115-12); most nonopaque stones are hyperattenuating on CT. In general, calcium stones are radiopaque. Stones composed of pure uric acid, xanthine, or struvite usually are nonopaque. Cystine stones are moderately radiopaque.47,48
Both radiopaque and nonopaque urinary tract stones appear echogenic on sonography and produce acoustic shadowing (Fig. 115-13). Sonography is sensitive for the detection of nephrolithiasis but is of limited utility for visualizing calculi in the ureters. An obstructing ureteral calculus usually is associated with some degree of ureteropelvocalyectasis, however. The sonographic appearance of acoustic shadowing aids in the differentiation of a bladder calculus from blood clot, debris, or tumor.
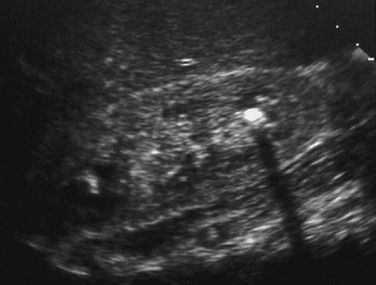
Figure 115-13 Nephrolithiasis.
A longitudinal sonographic image of an infant with hypercalcemia and Williams syndrome reveals an echogenic calculus in a right lower pole calyx.
Multi-detector CT is the most sensitive imaging technique for the detection of urolithiasis (Fig. 115-14). Regardless of composition, nearly all urinary tract stones are hyperattenuating on CT. Calcium oxalate stones generally have attenuation values in the 800 to 1000 HU range, infection stones are in the 300 to 900 HU range, and uric acid stones usually measure between 150 to 500 HU. Secondary CT signs of an obstructing urinary tract stone include hydronephrosis, hydroureter, nephromegaly, delayed nephrogram, periureteral edema, and perinephric stranding or fluid. Usually, the portion of the ureteral wall that surrounds a calculus thickens (the “rim sign”); this sign typically is lacking with a phlebolith or other mimicking calcification. A linear soft tissue density (the involved pelvic vein) often extends from a phlebolith; this feature is the “comet tail sign.”47,49,50
Craig, WD, Wagner, BJ, Travis, MD. Pyelonephritis: radiologic-pathologic review. Radiographics. 2008;28(1):255–277. quiz 327-328
Lavocat, MP, Granjon, D, Allard, D, et al. Imaging of pyelonephritis. Pediatr Radiol. 1997;27(2):159–165.
Hoppe, B, Kemper, MJ. Diagnostic examination of the child with urolithiasis or nephrocalcinosis. Pediatr Nephrol. 2010;25(3):403–413.
Kraus, SJ, Lebowitz, RL, Royal, SA. Renal calculi in children: imaging eatures that lead to diagnoses: a pictorial essay. Pediatr Radiol. 1999;29(8):624–630.
References
1. Raszka, WV, Jr., Khan, O. Pyelonephritis. Pediatr Rev. 2005;26(10):364–370.
2. Smith, EA. Pyelonephritis, renal scarring, and reflux nephropathy: a pediatric urologist’s perspective. Pediatr Radiol. 2008;38(suppl 1):S76–S82.
3. Rollino, C, Boero, R, Ferro, M, et al. Acute pyelonephritis: analysis of 52 cases. Ren Fail. 2002;24(5):601–608.
4. Chang, SL, Shortliffe, LD. Pediatric urinary tract infections. Pediatr Clin North Am. 2006;53(3):379–400. [vi].
5. Majd, M, Nussbaum Blask, AR, Markle, BM, et al. Acute pyelonephritis: comparison of diagnosis with 99mTc-DMSA, SPECT, spiral CT, MR imaging, and power Doppler US in an experimental pig model. Radiology. 2001;218(1):101–108.
6. Lavocat, MP, Granjon, D, Allard, D, et al. Imaging of pyelonephritis. Pediatr Radiol. 1997;27(2):159–165.
7. Dacher, JN, Boillot, B, Eurin, D, et al. Rational use of CT in acute pyelonephritis: findings and relationships with reflux. Pediatr Radiol. 1993;23(4):281–285.
8. Poustchi-Amin, M, Leonidas, JC, Palestro, C, et al. Magnetic resonance imaging in acute pyelonephritis. Pediatr Nephrol. 1998;12(7):579–580.
9. Lonergan, GJ, Pennington, DJ, Morrison, JC, et al. Childhood pyelonephritis: comparison of gadolinium-enhanced MR imaging and renal cortical scintigraphy for diagnosis. Radiology. 1998;207(2):377–384.
10. Dacher, JN, Pfister, C, Monroc, M, et al. Power Doppler sonographic pattern of acute pyelonephritis in children: comparison with CT. AJR Am J Roentgenol. 1996;166(6):1451–1455.
11. Eggli, KD, Eggli, D. Color Doppler sonography in pyelonephritis. Pediatr Radiol. 1992;22(6):422–425.
12. Dacher, JN, Avni, F, Francois, A, et al. Renal sinus hyperechogenicity in acute pyelonephritis: description and pathological correlation. Pediatr Radiol. 1999;29(3):179–182.
13. Sorantin, E, Fotter, R, Aigner, R, et al. The sonographically thickened wall of the upper urinary tract system: correlation with other imaging methods. Pediatr Radiol. 1997;27(8):667–671.
14. Roberts, JA. Management of pyelonephritis and upper urinary tract infections. Urol Clin North Am. 1999;26(4):753–763.
15. Pecile, P, Miorin, E, Romanello, C, et al. Age-related renal parenchymal lesions in children with first febrile urinary tract infections. Pediatrics. 2009;124(1):23–29.
16. Yoder, IC, Lindfors, KK, Pfister, RC. Diagnosis and treatment of pyonephrosis. Radiol Clin North Am. 1984;22(2):407–414.
17. Schneider, K, Helmig, FJ, Eife, R, et al. Pyonephrosis in childhood—is ultrasound sufficient for diagnosis? Pediatr Radiol. 1989;19(5):302–307.
18. Jeffrey, RB, Laing, FC, Wing, VW, et al. Sensitivity of sonography in pyonephrosis: a reevaluation. AJR Am J Roentgenol. 1985;144(1):71–73.
19. Chan, JH, Tsui, EY, Luk, SH, et al. MR diffusion-weighted imaging of kidney: differentiation between hydronephrosis and pyonephrosis. Clin Imaging. 2001;25(2):110–113.
20. Smith, EA, Styn, N, Wan, J, et al. Xanthogranulomatous pyelonephritis: an uncommon pediatric renal mass. Pediatr Radiol. 2010;40(8):1421–1425.
21. Korkes, F, Favoretto, RL, Broglio, M, et al. Xanthogranulomatous pyelonephritis: clinical experience with 41 cases. Urology. 2008;71(2):178–180.
22. Zugor, V, Schott, GE, Labanaris, AP. Xanthogranulomatous pyelonephritis in childhood: a critical analysis of 10 cases and of the literature. Urology. 2007;70(1):157–160.
23. Kim, J. Ultrasonographic features of focal xanthogranulomatous pyelonephritis. J Ultrasound Med. 2004;23(3):409–416.
24. Kim, JC. US and CT findings of xanthogranulomatous pyelonephritis. Clin Imaging. 2001;25(2):118–121.
25. Bryant, K, Maxfield, C, Rabalais, G. Renal candidiasis in neonates with candiduria. Pediatr Infect Dis J. 1999;18(11):959–963.
26. Erden, A, Fitoz, S, Karagulle, T, et al. Radiological findings in the diagnosis of genitourinary candidiasis. Pediatr Radiol. 2000;30(12):875–877.
27. Kale, H, Narlawar, RS, Rathod, K. Renal fungal ball: an unusual sonographic finding. J Clin Ultrasound. 2002;30(3):178–180.
28. Eastwood, JB, Corbishley, CM, Grange, JM. Tuberculosis and the kidney. J Am Soc Nephrol. 2001;12(6):1307–1314.
29. Rui, X, Li, XD, Cai, S, et al. Ultrasonographic diagnosis and typing of renal tuberculosis. Int J Urol. 2008;15(2):135–139.
30. Mapukata, A, Andronikou, S, Fasulakis, S, et al. Modern imaging of renal tuberculosis in children. Australas Radiol. 2007;51(6):538–542.
31. Hoppe, B, Kemper, MJ. Diagnostic examination of the child with urolithiasis or nephrocalcinosis. Pediatr Nephrol. 2010;25(3):403–413.
32. Ammenti, A, Pelizzoni, A, Cecconi, M, et al. Nephrocalcinosis in children: a retrospective multi-centre study. Acta Paediatr. 2009;98(10):1628–1631.
33. Cremin, B, Wiggelinkhuizen, J, Bonnici, F. Nephrocalcinosis in children. Br J Radiol. 1982;55(654):413–418.
34. Weber, G, Cazzuffi, MA, Frisone, F, et al. Nephrocalcinosis in children and adolescents: sonographic evaluation during long-term treatment with 1,25-dihydroxycholecalciferol. Child Nephrol Urol. 1988;9(5):273–276.
35. Glazer, GM, Callen, PW, Filly, RA. Medullary nephrocalcinosis: sonographic evaluation. AJR Am J Roentgenol. 1982;138(1):55–57.
36. Manz, F, Jaschke, W, van Kaick, G, et al. Nephrocalcinosis in radiographs, computed tomography, sonography and histology. Pediatr Radiol. 1980;9(1):19–26.
37. Afschrift, M, Nachtegaele, P, Van Rattinghe, R, et al. Nephrocalcinosis demonstrated by ultrasound and CT. Pediatr Radiol. 1983;13(1):42–43.
38. Patriquin, H, Robitaille, P. Renal calcium deposition in children: sonographic demonstration of the Anderson-Carr progression. AJR Am J Roentgenol. 1986;146(6):1253–1256.
39. Alon, US. Nephrocalcinosis. Curr Opin Pediatr. 1997;9(2):160–165.
40. Knoll, T, Schubert, AB, Fahlenkamp, D, et al. Urolithiasis through the ages: data on more than 200,000 urinary stone analyses. J Urol. 2011;185(4):1304–1311.
41. Routh, JC, Graham, DA, Nelson, CP. Trends in imaging and surgical management of pediatric urolithiasis at American pediatric hospitals. J Urol. 2010;184(4 suppl):1816–1822.
42. Mandeville, JA, Nelson, CP. Pediatric urolithiasis. Curr Opin Urol. 2009;19(4):419–423.
43. Abrahams, HM, Stoller, ML. Infection and urinary stones. Curr Opin Urol. 2003;13(1):63–67.
44. Cameron, MA, Sakhaee, K, Moe, OW. Nephrolithiasis in children. Pediatr Nephrol. 2005;20(11):1587–1592.
45. Gillespie, RS, Stapleton, FB. Nephrolithiasis in children. Pediatr Rev. 2004;25(4):131–139.
46. DeFoor, WR, Jackson, E, Minevich, E, et al. The risk of recurrent urolithiasis in children is dependent on urinary calcium and citrate. Urology. 2010;76(1):242–245.
47. Palmer, JS, Donaher, ER, O’Riordan, MA, et al. Diagnosis of pediatric urolithiasis: role of ultrasound and computerized tomography. J Urol. 2005;174(4 pt 1):1413–1416.
48. Kraus, SJ, Lebowitz, RL, Royal, SA. Renal calculi in children: imaging features that lead to diagnoses: a pictorial essay. Pediatr Radiol. 1999;29(8):624–630.
49. Strouse, PJ, Bates, DG, Bloom, DA, et al. Non-contrast thin-section helical CT of urinary tract calculi in children. Pediatr Radiol. 2002;32(5):326–332.
50. Bellin, MF, Renard-Penna, R, Conort, P, et al. Helical CT evaluation of the chemical composition of urinary tract calculi with a discriminant analysis of CT-attenuation values and density. Eur Radiol. 2004;14(11):2134–2140.