CHAPTER 289 Acquired Abnormalities of the Craniocervical Junction
The craniocervical junction is the most complex portion of the axial skeleton. The geometry of the articular surfaces provides mobility at the cost of stability. The latter is provided by the ligamentous structures as well as the cervical musculature.1 The synovial lining of the craniocervical joints is affected early in rheumatoid disease and plays a significant role in the subsequent destructive changes that occur in this region.2,3 Other nonrheumatoid entities that affect the craniocervical junction are inflammatory (e.g., ankylosing spondylitis, juvenile rheumatoid arthritis, psoriasis, regional ileitis, Reiter’s syndrome), degenerative (e.g., osteoarthritis, calcium pyrophosphate pseudogout), and infective (e.g., pyogenic, Grisel’s syndrome).4
Normal Anatomy
The complex relationships between the occipital bone and the first and second cervical vertebrae constitute the occipitocervical junction. The foramen magnum is composed of three parts: the squamosal portion dorsally, the basal portion ventrally, and the condylar portions laterally. The occipital condyles are prominences that articulate with the lateral masses of C1. C1 is unique among the vertebrae in that it is ring-shaped, without a vertebral body. Instead, the dens of C2 occupies this position within the ring of C1. The lateral masses are located at the anterolateral portions of the C1 ring. The superior articular processes of C1 join with the occipital condyles, and the inferior articular processes join with C2, allowing a significant degree of flexion and extension movement at the occipitoatlantoaxial complex. Each of these joints, along with the articulation between the dens and the anterior arch of C1, is a true synovial joint.5,6
The complex ligaments of the occipitocervical junction provide some stability to the region while also allowing a significant range of motion. The cruciate ligament is the most important of these structures. This ligament is composed of two portions: the transverse portion, which runs between the lateral aspects of the C1 ring dorsal to the dens, and the cervical portion, which runs from the basion to the C2 vertebral body, also dorsal to the dens. In addition to the cruciate ligament, there is an apical ligament connecting the tip of the dens to the basion, two alar ligaments running from the anterolateral portions of the foramen magnum to the tip of the dens, and the paired accessory atlantoaxial ligaments securing the lateral masses of C1 to the body of C2. In addition to these ligamentous structures, the tectorial membrane provides support to this region. This membrane is the rostral extension of the posterior longitudinal ligament and runs from C3 to the clivus.6
The craniovertebral junction receives its blood supply from the vertebral and occipital arteries. These vessels anastomose to form the apical arcade over the superior aspect of the dens. These feeding vessels, the apical arcade, and branches from the carotid arteries provide most of the blood supply to this region. The lymphatic ducts of this region drain primarily into the retropharyngeal space and subsequently to the deep cervical chain. This pattern of lymphatic drainage explains the development of Grisel’s syndrome, whereby inflammation of the craniocervical synovial joints develops following infection of the nasopharynx or retropharyngeal space.6
Rheumatoid Arthritis
Rheumatoid arthritis (RA) is one of the most common disabling diseases today. The overall prevalence in Europe of proven RA is 0.8% of those older than 15 years of age.7 The prevalence in Sudbury, Massachusetts, is similar, at 0.9%,8 and a national sample of the white population in the United States yielded a prevalence of 1%.9,10 Thus, the number of patients with RA in the United States is about 2 to 2.4 million, and in the United Kingdom it is 0.63 to 0.65 million. Based on the various studies available, a significant number will develop cervical spine involvement requiring attention.
RA of the cervical spine was first described as a clinical entity by Garrod11 in 1890. In that series of 500 patients, 178 had involvement of the cervical spine. According to Conlon and coworkers,12 if lateral cervical radiographs were obtained among the general population, cervical spine involvement compatible with RA would be detected in about 6%. However, the clinical involvement is a fraction of this. Based on their initial studies, Conlon and coworkers believed that involvement of the cervical spine did not cause neurological deficits. Unfortunately, this led to the common but erroneous belief that treatment of rheumatoid involvement of the craniocervical junction should be conservative. However, in 1974, Mathews13 pointed out that progressive atlantoaxial subluxation occurred in up to 25% of RA patients, and rheumatoid cranial settling occurred in 6% to 18% of individuals.
RA is a chronic relapsing inflammatory arthritis that usually affects multiple diarthrodial joints with varying degrees of systemic involvement.14–16 RA occurs worldwide and in all racial groups; it affects females two to four times more frequently than males. It causes substantial morbidity and an attendant economic burden; approximately 50% of patients are unable to work within 10 years of onset, and the lifetime cost of the disease rivals that of coronary artery disease or stroke.16 Joints, articular tissues, serosae, and the eyes are commonly affected, but the spectrum of organ damage may be vast, especially when vasculitis develops during the course of the disease.17
Immunologic Features
Rheumatoid factors are antibodies with specificity for the Fe fragment of immunoglobulin G (IgG).17,18 The latex agglutination test is positive in 80% of patients meeting the American Rheumatism Association criteria (Table 289-1).19 High titers are associated with the presence of subcutaneous nodules, extra-articular manifestations, and vasculitis. A negative rheumatoid factor test by routine laboratory procedures does not exclude the diagnosis of RA because 20% of patients who meet the American Rheumatism Association criteria test negative for RA. These patients may have IgG or IgM rheumatoid factor that is not detectable by routine techniques.20
TABLE 289-1 American Rheumatism Association Criteria for Classic or Definite Rheumatoid Arthritis
Antinuclear antibodies have been found in 14% to 28% of patients with RA. Tests that use monoclonal rheumatoid factors in the CLq binding assay are more frequently positive in patients with RA than all the various assays for the detection of immune complexes.15 Although the assays correlate poorly with indices of disease activity, a positive test is usually associated with an increased incidence of extra-articular manifestations, particularly vasculitis.
More recently, anti–cyclic citrullinated peptide (anti-CCP) antibodies have been identified in patients with RA. These antibodies react to a variety of citrullinated proteins, including filaggrin and keratin. These antibodies have been identified in the serum of patients before the onset of RA symptoms, suggesting that they play a role in the pathogenesis of the disease. Anti-CCP antibodies have a greater specificity for RA than does rheumatoid factor. Taken together, positivity for rheumatoid factor and anti-CCP antibodies is 98% specific for RA.21
The cause of RA is unknown. However, it is postulated that the disease is triggered by an infectious agent. Macrophages present this antigen on their surface, inciting the release of cytokines such as tumor necrosis factor (TNF) and initiating the inflammatory cascade. This macrophage activation is dependent on human leukocyte antigen (HLA) molecules, explaining the predisposition for RA in patients with particular HLA haplotypes (HLA-DR4 and HLA-DR1). In addition to macrophages, T cells (predominantly Th1 cells), B cells, plasma cells, natural killer cells, dendritic cells, and mast cells infiltrate the synovium, transforming it into the rheumatoid pannus. T cells interact with B cells, which subsequently secrete autoantibodies that further promote the inflammatory response through the recruitment and activation of complement and neutrophils. In addition, synovial fibroblasts respond, developing into RA synovial fibroblasts, which play an active role in joint destruction. RA synovial fibroblasts, macrophages, and T cells secrete a number of chemokines, including interleukin-1β, interleukin-17, and TNF, which promote the production of matrix metalloproteinases. Other molecules such as cathepsins, collagenases, and stromelysin are secreted and participate in the destructive processes. In addition, the cytokine milieu results in the activation of osteoclasts.22–24
Rheumatoid pannus forms in the inflamed joints from proliferating fibroblasts and inflammatory cells, leading to granulation tissue.25 The pannus itself produces collagenase and proteolytic enzymes capable of destroying adjacent cartilage, tendons, and bones.26 In addition, some patients develop lymphoid germinal centers within the synovium consisting of follicular aggregates of T cells, B cells, and dendritic cells.21 The end result is loss of cartilage, ligamentous laxity, rupture of tendons, and bone erosion. In general, the joints ultimately undergo severe destruction and become symptomatic within the first year of disease onset.
Clinical manifestations of RA include constitutional symptoms; arthritis involving large, medium, and small joints, and extra-articular manifestations that include subcutaneous nodules, nail bed thrombi, pleurisy, pulmonary fibrosis, pericarditis, nerve entrapment syndromes, scleritis, and vasculitis. The most common vascular complications are peripheral cutaneous ulcers and neuropathy.27–31
The earliest changes in the cervical spine most likely take place at the superficial joints and the lateral margins of the disks. Neurocentral synovitis results in granulation tissue with fibroblasts and capillaries that erode and replace the disk annulus and neighboring disk-bone border. This is not accompanied by active bone formation.32 Therefore, osteophyte formation around disks and apophyseal joints is inconspicuous, enhancing the possibility of dislocation.13,33,34
Fibrosis and ankylosis of apophyseal joints are not uncommon in untreated cases, and there is a natural tendency for the disease to terminate in segmental immobilization, with loss of disk and stepwise subluxation in the subaxial cervical region. The active inflammatory lesions with fibrinoid changes are similar to those in the tendons of the hand and are thus seen in the apical ligaments, the transverse ligament of the craniocervical junction, and biopsy specimens of the interspinous ligaments.35 The transverse ligament becomes insufficient because of inflammatory erosion of the posterior surface of the odontoid process by granulation tissue arising in the synovial joints between the transverse ligament and the posterior surface of the odontoid process. Osteoporosis is a frequent finding in the rheumatoid cervical spine and may contribute to weakening of the bone beneath the ligamentous attachments.35,36
Rheumatoid Involvement of the Spine
The cervical spine is the most common site affected because of the large number of synovial joints. There is a predilection for involvement of the craniocervical junction for this reason. The most common lesion found is atlantoaxial dislocation, followed by cranial settling and then rheumatoid granulation tissue. Subaxial subluxation occurs in 12% to 22% of individuals and is seen predominantly at the C4 and C5 levels.37 Winfield and colleague38 evaluated 100 rheumatoid patients diagnosed within 1 year of the onset of disease. At 5-year follow-up they found that 12 patients had atlantoaxial subluxation of more than 7 mm, and subaxial subluxation had occurred in 20. In 3 individuals, vertical migration of the odontoid process had occurred. Zikou and coworkers39 reported a series of 165 consecutive patients with RA, 66 of whom reported symptoms of cervical spine involvement, including neck pain, neck stiffness, and limited cervical range of motion. Interestingly, 146 of 165 patients had radiographic evidence of rheumatoid disease of the cervical spine, including 80 patients who had no symptoms referable to the cervical spine. The radiographic findings included atlantoaxial subluxation greater than 2.5 mm (20.6%), subaxial subluxation greater than 1 mm (43.6%), and odontoid erosions (2.4%). This same group reported on a series of 51 patients with RA who underwent magnetic resonance imaging (MRI) investigation of the cervical spine. Thirty of these patients had clinical evidence of cervical spine disease, and 40 had other radiographic evidence of cervical spine involvement. On MRI, 88% of the patients demonstrated some degree of pannus formation at the dens, 23.5% had dens erosion, 13.7% had atlantoaxial subluxation, and 10% had subaxial subluxation.40 In longitudinal studies, it appears that atlantoaxial subluxation begins with horizontal subluxation, progresses to a combination of vertical and horizontal subluxation, and ultimately develops into vertical subluxation alone in a subset of patients. It is rare for patients to develop vertical subluxation alone without first passing through a stage of horizontal subluxation. More severe atlantoaxial subluxation was observed in patients with more severe systemic disease.41
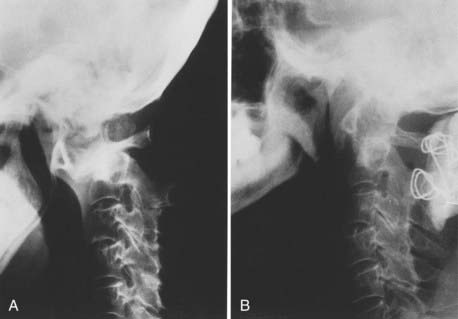
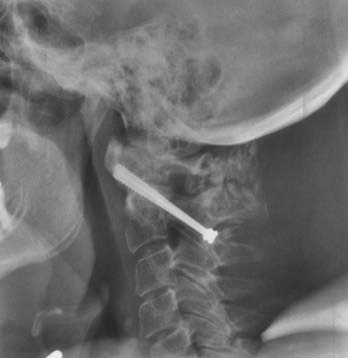
Atlantoaxial subluxation is initiated by a loss of tensile strength and stretching of the transverse ligament. Similar changes occur in the synovial joints around the odontoid process as in the joints of the lateral atlantoaxial and occipitoatlantal regions. Thus, there are erosive changes in the adjacent bone, formation of granulation tissue in the synovial joints, loss of bone volume, osteoporosis, angulation of the softened bone, and occasionally fracture. The laxity of the ligaments, together with the bone changes, leads to horizontal or anteroposterior translation as well as rotary luxation of the atlas and the axis vertebrae (Fig. 289-1).42–49
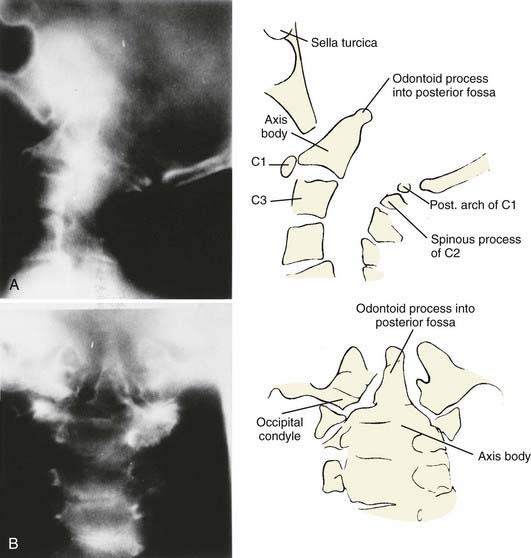
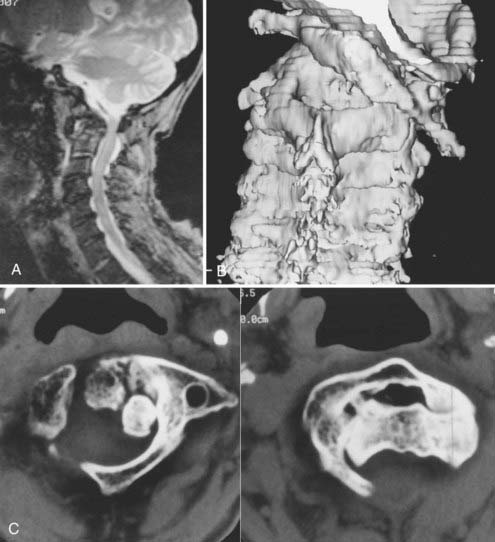
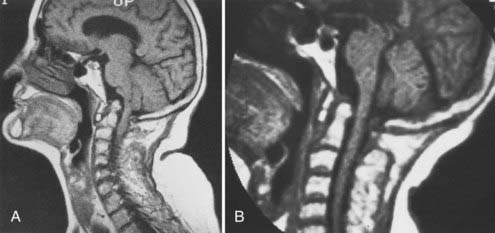
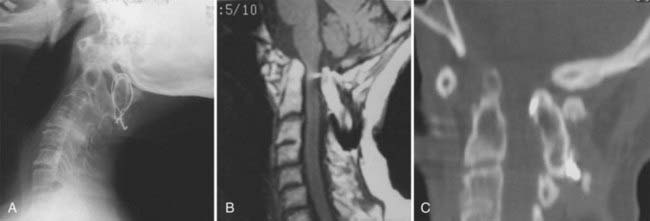
Vertical penetration of the odontoid process into the foramen magnum has been variably termed rheumatoid basilar invagination, odontoid vertical migration, rheumatoid translation, and cranial settling.9,13 It is caused by the loss of bone and the lateral mass of the atlas vertebra, with rostral migration of the axis. The lateral atlantal masses may fracture in severe cases with lateral displacement.25 The odontoid then penetrates further through the foramen magnum. The destructive changes may be severe enough that the occipital condyles rest on the lateral masses of the axis vertebrae, separating and displacing the anterior and posterior arches of the atlas and their respective directions (Fig. 289-2).50 The anterior arch of the atlas settles downward, telescoping onto the axis body, and there is an upward invagination of the odontoid process. This is not basilar invagination or basilar impression but cranial settling.9,25,28,51–53 Casey and colleagues54 reported on a prospective review of 256 patients with RA. Of the 186 patients with cervical myelopathy, 62% had cranial settling (vertical translocation). The patients with cranial settling had more severe neurological dysfunction than those with horizontal subluxation. In addition, patients with cranial settling experienced pseudostabilization, with a decrease in the atlantodental interval and a resulting decrease in the size of the pannus.
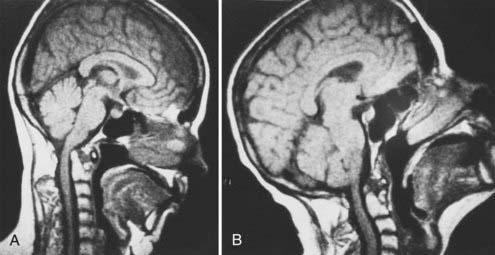
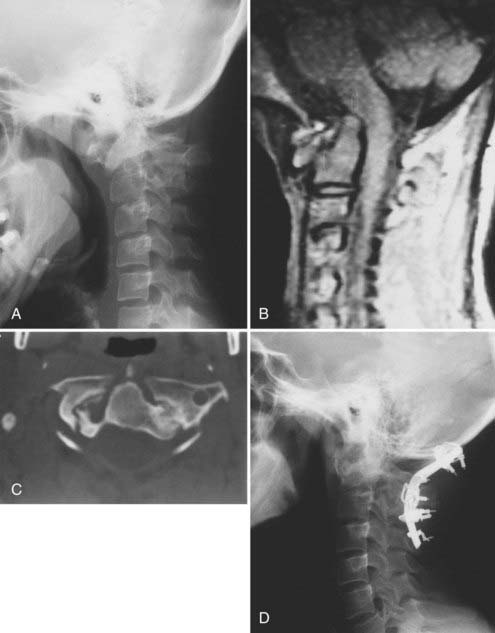
Excessive proliferation of granulation tissue may lead to complete destruction of the odontoid process, with the granulation mass and pannus emanating out of the rest of the synovial joints.31 This causes ventral and lateral cervicomedullary compression with gross dislocation in all directions (Fig. 289-3). At times the bony spicule of the odontoid process may be left as a ghost and can penetrate the tectorial membrane, subsequently embed in the ventral aspect of the pons and the medulla, and attach itself to the vertebrobasilar arterial tree. Occasionally, rheumatoid dural nodules and pachymeningitis occur secondary to the rheumatoid process. The rheumatoid pannus may be associated with granulation tissue and become tough and fibrotic.31 This does not usually recede with immobilization. In contrast, active pannus that resembles the active disease in other joints recedes with cervical immobilization.53,55–57
Subluxation below the second cervical vertebra occurs in 17% to 29% of patients with RA, most commonly at the C3-4 and C4-5 levels. Serial cervical subluxations producing a staircase appearance are common.12,58
The natural history of rheumatoid disease of the cervical spine is grim. Fujiwara and associates41 reported on a series of 173 patients with RA followed from 1992 to 1997. Initially, 29% of the patients had atlantoaxial subluxation. Over the 5-year period, 63% of these patients experienced progression of their atlantoaxial disease. In addition, 39% of the patients who initially had no atlantoaxial subluxation developed upper cervical spine disease over the course of the study. At the beginning of the study period, no patients had neurological deficits; by the end of the study, 10 patients were myelopathic. Pellicci and coworkers59 studied RA patients over a 5-year period and found a mortality rate of 17%, as opposed to a 9% mortality rate for the same age group without rheumatoid disease. Subluxation worsened in 80%, and new subluxation occurred in 27%. Mikulowski and associates60 reported on a postmortem study of 104 patients with RA and found the cause of death in 11 patients to be atlantoaxial dislocation with cervicomedullary compression. This had not been suspected antemortem. Two patients had myelomalacia, and three others had cervical vertebral artery vascular complications related to dislocation. In 1981 Marks and Sharp33 reported on 31 patients with RA and upper cervical spine involvement with dislocation. Fifteen deaths occurred within 6 months of presentation. All those who were untreated died, as did 50% of those treated with a soft cervical collar alone. Only fusion provided a reasonable chance of survival. Delamarter and Bohlman3 performed a postmortem analysis of patients with paralysis secondary to RA of the cervical spine and found cervical cord compression to be the main cause of death in 10. Thus, once cervical myelopathy sets in, the natural history is grave unless surgical intervention takes place.31,61–63
Autopsy analyses of spinal cords from rheumatoid patients suffering from paralysis secondary to atlantoaxial dislocation or cranial settling have shown abnormal histology within the spinal cord at the site of compression.3 Common to all patients were gliosis and axonal degeneration, with nuclear damage within the ganglion cells frequently appearing in different stages of progression. No parenchymal hemorrhage was seen. Delamarter and Bohlman3 identified three histologic types of spinal cord compression. In the first type, four patients with severe, chronic mechanical cord compression exhibited distortion with flattening and destruction of the cord and secondary wallerian degeneration of the ascending and descending tracts, without anoxic or ischemic neural changes, suggesting that the damage was due to chronic mechanical compression. In the intermediate stage of compression (type II), there was vascular compression showing ischemic damage to the cord, with necrosis of the lateral columns and the ischemic watershed regions supplied by the anterior and posterior spinal arteries. In the patients with mild mechanical compression (type III), there was only focal gliosis at the site of compression, without ascending or descending tract injury. Nakano and coworkers45 analyzed two autopsy cases and found that maximal changes occurred in the central gray matter and adjacent posterior and lateral columns. This was thought to be due to direct intermittent compression and narrowing of the transverse branches of the anterior spinal artery. This was previously observed by Bland2 and confirmed more recently by Casey and colleagues.62 Thus, the available space for the spinal cord at the craniocervical junction is of great prognostic significance.4,62,63
In addition to the cervical involvement described earlier, patients with RA are at higher risk for the development of compression fractures in other areas of the spine. Orstavik and associates64 showed that patients with RA had a significantly higher risk of multiple thoracic and lumbar compression fractures than matched controls (odds ratio 2.60). In this study, presence of RA, bone mineral density, and corticosteroid use were independent predictors of vertebral deformities. In addition, there are reports of spontaneous (nontraumatic) odontoid fractures in patients with RA. Although rare, this condition should be considered in RA patients with neck pain whose work-up is otherwise unrevealing.65,66
Extra-articular Manifestations
Extra-articular manifestations are myriad in patients with severe RA and high titers of rheumatoid factor. Pericarditis, myocarditis, and coronary vasculitis are not infrequent.20,29,30,67 RA can present in the lungs as pleural effusions and pleuritis, intraparenchymal rheumatoid nodules, and Caplan’s syndrome (rheumatoid nodules with pneumoconiosis, diffuse pulmonary fibrosis, bronchiolitis, and pulmonary hypertension out of proportion to coexisting lung disease).18,29,68–70 Pleuritis is the most common thoracic manifestation (5% to 15%), and rheumatoid effusions occur in 50% of patients in the first 5 years after the onset of arthritis.71 Pulmonary fibrosis was seen in 10 of 18 patients with RA who underwent thin-section computed tomography (CT) scanning of the chest, but the plain radiographs were reported as normal.72 Thus, it is important that pulmonary function tests be performed in RA patients before any operative procedure and general anesthesia.
Medical Treatment
Current recommendations for the medical management of RA include the use of nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, and disease-modifying antirheumatic drugs (DMARDs). NSAIDs and cyclooxygenase-2 (COX-2) inhibitors provide symptomatic relief but do not alter the course of the disease. A substantial number of patients are placed on corticosteroids, although the use of this class of medications is limited by side effects. The current mainstay of RA management is DMARDs. Synthetic DMARDs include methotrexate, leflunomide, sulfasalazine, minocycline, intramuscular gold, penicillamine, chloroquine, and hydroxychloroquine. Biologic DMARDs are newer agents that target the immune response. Currently, there are four such medications, three of which inhibit TNF-α (infliximab, etanercept, adalimumab) and one that inhibits interleukin-1 (anakinra). Previously, single DMARD therapy was preferred, but more recent studies have shown a substantial advantage to using combination DMARD therapy.73 A recent study showed that combination DMARD therapy reduces the incidence of atlantoaxial subluxation in patients with RA compared with DMARD monotherapy.74
Clinical Presentation
Atlantoaxial subluxation may present within 2 years of the disease, but it is unusual for myelopathy to develop early.50,59,75 The most frequent symptom of rheumatoid abnormalities of the craniocervical junction is occipital headaches.31 This is typically described as occipital pain radiating to the skull vertex and aggravated by an upright posture. It was present in 60% of patients with atlantoaxial subluxation and 90% of individuals with cranial settling. Between 1977 and 1994, 780 symptomatic patients with RA affecting the craniocervical region were evaluated by Menezes.31 Three main categories of abnormalities were recognized from a radiographic standpoint: atlantoaxial instability, cranial settling, and primary granulation tissue masses.
Cervical myelopathy is insidious, and disability may be mistaken for progression of rheumatoid disease, rheumatoid joint dysfunction, hypothyroidism, or poor nutritional habits.13,25,61,76,77 The clearest indication of cervical myelopathy is progressive physical disability and the inability to carry out daily tasks.45,61 It is difficult to detect abnormal neurological signs of myelopathy in rheumatoid patients with deforming painful arthritis and associated neuropathy. The peripheral neuropathy itself can cause areflexia. Thus, the presence of normal reflexes in a patient with advanced RA should raise the suspicion of myelopathy. We have found neurophysiologic testing with somatosensory evoked potentials to be of no use in this situation, despite some reports to the contrary.9
Limb paresthesia, numbness, weakness, and sphincter disturbance may herald myelopathy. Dizziness, vertigo, and syncope may be associated with vertebrobasilar ischemia. Nystagmus may be evident in this situation. Transient blackout spells were reported by 55% of individuals with cranial settling.25 Gradual, progressive difficulty with ambulation was a chief complaint in 76% of individuals with cervical myelopathy. In 15% of individuals, an acute onset of quadriparesis was seen.31
Abnormal neurological signs in Menezes’31 series included brainstem evidence of internuclear ophthalmoplegia, facial diplegia, nystagmus, spastic quadriparesis, and sleep apnea, especially in patients with cranial settling. These signs, either singly or in combination, were present in 20% of individuals. An additional 20% had loss of pain and touch sensation in the distribution of the trigeminal nerve, and each patient had invagination of the odontoid process in excess of 10 mm above the foramen magnum. The cranial nerves most affected by cranial settling were the glossopharyngeal, vagus, and hypoglossal. With cranial settling, 20% of individuals had evidence of dysfunction of one or more cranial nerves.78 Nine individuals with cranial settling had undergone previous tracheostomy for the diagnosis of rheumatoid pharyngeal dysfunction.
Radiologic Features of Cervical Spine Involvement
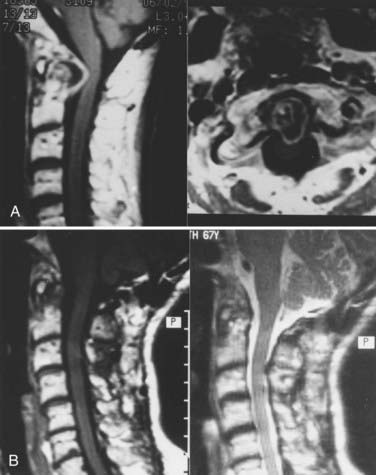
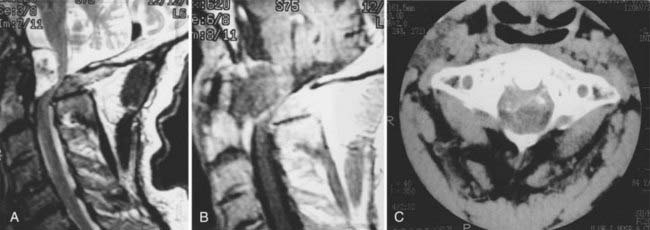
Radiographic changes reflect the pathologic processes described. Plain lateral cervical spine radiographs as well as anteroposterior and open-mouth views are usually obtained. At times, dynamic flexion-extension views are obtained, with the extended and flexed positions assumed by the patient without forced extremes of head position. Neurological deficits, predental space of 7 to 8 mm, abnormal radiographic pathology such as cranial settling, atrophy of the odontoid process, and subaxial subluxation justify MRI. This identifies the relationship of the cervical spine to the contained cervicomedullary junction and cervical spinal cord. It is important that MRI scans be obtained in the sagittal and axial planes using both T1- and T2-weighted modes. Dynamic views in the flexed and extended positions identify the instability as well as the manner of compression (Fig. 289-4).56,58,79,80 Active synovitis with ligamentous involvement is best identified with the spin echo technique. Contrast-enhanced T1-weighted spin echo MRI can discriminate between joint effusion and various forms of pannus in patients with RA of the craniocervical junction. With joint effusions, Stiskal and coworkers57 found a low signal intensity on unenhanced T1-weighted images and a high signal intensity on T1-weighted enhanced images, with a rim-like zone at the periphery in all lesions; this was redistributed with delayed images (Fig. 289-5). In hypervascular pannus, T2-weighted graded images showed high intensity, as did contrast-enhanced images. However, in fibrous pannus, there was reduced signal intensity in both T2-weighted GRE images and pre- and postcontrast T1-weighted spin echo images.80 This is important from a surgical standpoint. In the case of fibrous hypovascular pannus, craniocervical immobilization with fusion does not result in disappearance of the ventral mass (see Fig. 289-3). However, active pannus with hypervascular tissue, like active synovitis, resolves with dorsal stabilization.
Thin-section CT and three-dimensional CT of the affected area provide additional information regarding the osseous integrity, as well as the relationships and extent of osteoporosis and osteopenia (Fig. 289-6).31
Overall, MRI is the preferred method for evaluating patients with suspected rheumatoid involvement of the cervical spine. In one study, plain radiographs identified craniocervical junction disease in 32.6% of patients, CT showed disease in 41.3% of patients, and MRI identified craniocervical junction disease in 61% of patients with RA. Therefore, the false-negative rate for both plain radiography and CT was high.81
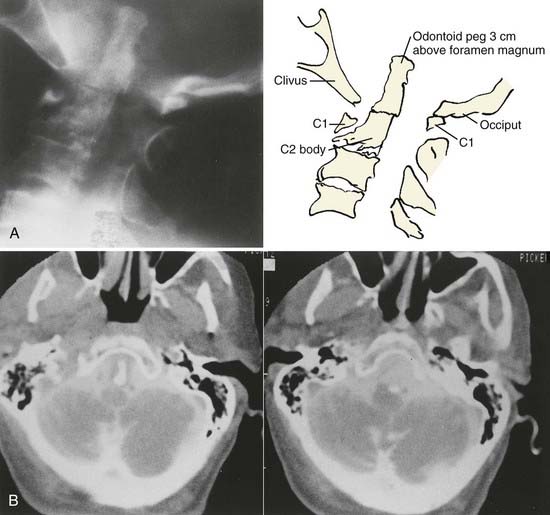
Cranial settling is reducible in 77% to 80% of individuals by means of positioning or cervical traction of up to 9 to 10 pounds over 4 to 5 days (Fig. 289-7). Cranial settling is irreducible in individuals who exhibit odontoid penetration through the foramen magnum greater than 15 mm, large pannus, fracture of the odontoid process, or cranial settling complicated by lateral or rotational dislocation in addition to the primary phenomenon (Fig. 289-8).31,78 Complex cranial settling with posterior dislocation is considered potentially lethal at any time owing to the distraction of the vertebral artery complex. In such individuals, a fusion procedure should be done immediately.
Operative Indications
There is wide variation among natural history studies, including the results. Failure to identify and appreciate cranial settling25 makes it difficult to draw conclusions about the efficacy of surgical intervention. Likewise, the identification of abnormal pathology without attempted reduction in potentially reducible lesions has led to an increased incidence of ventral decompression and dorsal occipitocervical fusion. However, there is a consensus that surgical intervention must be advocated in patients with progressive neurological dysfunction, cranial settling, and intractable pain.4,35,36,45,49,50,75,82 Cervical myelopathy and instability after decompressive laminectomy are urgent indications for operative intervention.
Atlantoaxial dislocation greater than 8 to 9 mm must be carefully examined with MRI. Boden,63 in a 1994 publication, showed that a posterior atlantodental interval greater than 14 mm (on plain films) was generally safe. This does not include the presence of pannus or other tissues that reduce the effective diameter of the subarachnoid space. If the posterior atlantodental interval was greater than 14 mm, there was a 94% chance the patient would have no paralysis. Unfortunately, a patient with a posterior atlantodental interval of 13 mm could have a much smaller available space for the spinal cord, depending on the thickness of pannus.
Casey and colleagues,62 reporting on Crockard’s series, recognized high rates of postoperative morbidity and mortality in patients classified as Ranawat class IIIB (Table 289-2).83 They cited a detailed investigation of a cohort of 55 such patients. The early postoperative mortality was 12.7%, and one quarter of the patients were dead within 6 months. When the spinal cord area was less than 44 mm2, a poor outcome was more likely; this also correlated with patients who had an increased degree of vertical translocation. Weissman and associates,84 in an earlier publication before the MRI era, actually proposed the same idea without a true statistical analysis. Boden63 likewise recognized that when the posterior atlantodental interval was less than 10 mm preoperatively, the prognosis for return of motor function was poor. In contrast, significant motor recovery could be appreciated after surgery in patients who had preoperative intervals of 14 mm or more.
| CLASS | DESCRIPTION |
|---|---|
| I | Pain, no neurological deficit |
| II | Subjective weakness, hyperreflexia, dysesthesias |
| III | Objective weakness, long-tract signs |
| IIIA | Ambulatory |
| IIIB | Nonambulatory |
Surgical Treatment
The primary treatment goals for patients with RA at the craniocervical junction are relief of neuraxial compression and elimination of instability.25 Achievement of these goals is dependent on the precise identification of the pathology and motion mechanics. For treatment purposes, these lesions are divided into reducible and irreducible lesions.4 A reducible lesion is defined as one in which relief of compression on the cervicomedullary neural structures can be obtained by restoring the craniovertebral junction to a more anatomic relationship. This may be accomplished by positioning or cervical traction. A stabilization procedure then becomes paramount. In irreducible lesions, the basic tenet is decompression in the manner in which encroachment occurred before dorsal fixation.
All patients who require surgical attention must first be evaluated for articular and extra-articular involvement by the rheumatoid process. Nutritional status is best evaluated with a total lymphocyte count and liver function tests.31 A lymphocyte count less than 1000 is indicative of protein deficiency. All salicylate and NSAIDs must be discontinued for 1 week before any surgical intervention. During this time it is imperative to maintain drugs such as methotrexate and steroids for the symptomatic relief of pain. Cardiac status and pulmonary function are always evaluated in the senior author’s unit.
The senior author strongly believes that before patients undergo any other treatment for rheumatoid involvement of the craniocervical junction, an attempt should be made to align the osseous anatomy to relieve the neural compression. Traction is contraindicated only when there is a complex rotary luxation or posterior occipitoatlantoaxial dislocation.31 These individuals actually require an immediate stabilization procedure. All patients undergoing cervical traction should be observed in a monitored nursing setting with pulse oximetry and the capacity to monitor respiratory function. Traction is applied via a crown halo ring. This is started at 5 to 7 pounds and gradually increased to a maximum of 10 to 11 pounds, achieved at 36 hours. Periodic radiographic evaluation is essential to identify the degree of reduction and to adjust the vector of force for distraction. Mild extension to a neutral position is necessary in most individuals. If reduction is not achieved in 4 to 5 days, this should be considered an irreducible state, especially in the conditions previously described. Dorsal fusion in the face of ventral irreducible pathology can have adverse results (Fig. 289-9).
Irreducible pathology causing ventral cervicomedullary neural compression (e.g., cranial settling with odontoid invagination into the medulla) requires ventral removal of the offending pathology first.31,78 The transoral transpharyngeal route provides a rapid, safe, and effective approach to the ventral craniovertebral junction. This procedure must be accompanied by dorsal fixation. Cranial settling mandates occipitocervical arthrodesis.25
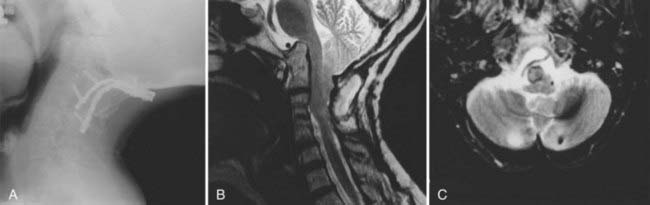
Reducible atlantoaxial dislocation is best managed with transarticular screw fixation if the lateral atlantal masses are intact and the quality of bone is satisfactory, as in a younger individual (Fig. 289-10).9,27,31,85–87 However, all fusions must be supplemented with bone; in the senior author’s practice, this is done with dorsal interlaminar fixation using rib graft.78 The reported fusion rate is 98.2%.87 The Halifax clamp for atlantoaxial dorsal fixation is associated with failure in 17% to 20% of individuals.78 Placement of a large rectangle of iliac crest between the posterior arch of C1 and C2 with cable retention also has a high failure rate (Fig. 289-11). Internal fixation obviates the need for postoperative halo immobilization and similar measures. Dorsal occipitocervical fusion is necessary in all individuals with rheumatoid cranial settling and in those who have had resection of the rheumatoid pannus. In a few individuals with active pannus, dorsal fixation allows stabilization and reduction of the ventral soft tissue mass because the active process abates immediately with stabilization (as with active effusion in other joints). Dorsal occipitocervical stabilization necessitates the placement of bone to anchor the cervical spine to the occiput as far laterally as possible; this prevents lateral rotation as well as flexion and extension and provides for axial loading.78,88–90 In most rheumatoid individuals undergoing dorsal occipitocervical fusion, instrumentation is essential. This is handled with a custom-contoured threaded titanium loop fixed to the skull and the upper cervical spine.31,90 This allows bone supplementation for long-term osseous integration. Use of calvarial bone in a disabled rheumatoid patient does not add to morbidity or mortality, as questioned by several authors.9,50 The success rate of occipital bone grafts has been well documented.91–93 In the senior author’s experience, a modified custom-built occipitocervical shell brace has proved satisfactory for occipitocervical stabilization. This custom-molded orthosis is similar to a modified Minerva brace and has a high degree of patient acceptance and compliance.
Transarticular screw fixation between C2 and C1 requires satisfactory width of the pars interarticularis at C2 and integrity of the lateral mass of C1—that is, no compromise by atrophy, compression, or significant osteoporosis.27 Unfortunately, in about 25% to 30% of individuals who fulfill these criteria for transarticular screw fixation, the vertebral artery groove may encroach on the pars, risking injury even in the most experienced hands.86 This can be readily identified as a potential problem by preoperative CT scans through the area of screw placement.
Following occipitocervical or cervical arthrodesis, careful follow-up is required. Clarke and coworkers94 found that 39% of RA patients undergoing surgery for atlantoaxial subluxation subsequently developed subaxial subluxation; 54% of these patients required fusion for this new condition. Ronkainen and associates95 reported long-term follow-up in a series of patients who underwent surgery for RA involvement of the cervical spine. Perioperative mortality was within the range reported by other authors (5.6%). However, long-term mortality over the median follow-up time of 7.5 years was 37%. In this study, age and postoperative complications were associated with higher mortality. In addition, patients with horizontal atlantoaxial subluxation fared better than those with other forms of atlantoaxial subluxation.
Seronegative Spondyloarthritides
The term seronegative spondyloarthritides refers to conditions that cause inflammatory states of the spine and sacroiliac joints in the absence of rheumatoid factor.96,97 These conditions include ankylosing spondylitis, Reiter’s syndrome, some forms of psoriatic arthritis, infectious arthritis, and arthritis associated with inflammatory bowel disease. In these conditions the anatomic site of involvement in the ligament is the tendinous insertion into bone—the enthesis.93,96–107 This metabolically active transition in the region between two collagen surfaces is particularly susceptible to torsion-related injury and can be the site of chronic inflammation. The conditions that result from inflammation of the enthesis are referred to as enthesopathies, and they overlap with the seronegative spondyloarthritides. In contrast, the central structure involved in rheumatic conditions is the synovial membrane. Although synovial involvement can occur in the seronegative spondyloarthritides, it is generally less severe and is believed to be secondary to the primary area of injury. Atlantoaxial instability, although common in RA, is uncommon in the seronegative spondyloarthritides.
Ankylosing spondylitis, or bamboo spine, usually affects the axial skeleton, sparing the atlantoaxial region.103 The fusion that takes place in the subaxial spine leads to an excessive dynamic load at the atlantoaxial level, with subsequent dislocation and further progression that may lead to secondary basilar invagination.106 The fused mid and lower cervical spine, together with the thoracic spine, acts as a single segment, transmitting load to the craniocervical region (Fig. 289-12). Suarez-Almazor and Russell108 observed a substantial increase in atlantoaxial instability in patients with ankylosing spondylitis who had associated psoriasis, Reiter’s syndrome, or inflammatory bowel disease. Of the 39 patients, one third had peripheral disease involving psoriasis or inflammatory bowel. Lee and colleagues109 reported on a series of 61 patients with ankylosing spondylitis. Of these patients, 60.6% had some degree of atlantodental ossification, and 18% had atlantoaxial subluxation.
Juvenile RA occurs in children and adolescents and is not a single disease but a group of disorders classified by mode of onset.20,110 They include Still’s disease, polyarticular onset, pauciarticular onset, and RA. The causes are multiple and include factors such as infection, autoimmunity, and trauma. Viruses have been considered because they are associated with arthritis in children, and the rubella virus has been isolated from phytohemagglutinin-stimulated lymphoblasts.31,98 Illness that simulates juvenile RA commonly occurs in subjects with IgA deficiency, agammaglobulinemia, or C2 complement deficiency. Seventy percent of patients experience a spontaneous and prominent remission by adulthood; however, children with positive latex fixation tests have the worst prognosis.31,44 The major complications are impaired growth and irreversible developmental retardation owing to early apophyseal closure. Ten percent of children may develop amyloidosis. Laiho and associates111 reviewed a series of 159 patients with juvenile chronic arthritis (including juvenile RA, ankylosing spondylitis, inflammatory bowel disease, and psoriatic arthritis) who had cervical spine radiographs available as adults. They found that 62% of these patients had inflammatory changes of the cervical spine, including 17% with atlantoaxial subluxation and 25% with atlantoaxial impaction.
Psoriatic arthropathy occurs in 7% of patients with psoriasis.105,112–114 Skin changes generally precede the onset of arthritis; spinal involvement develops in 20% of patients with psoriatic arthritis. This affects the craniocervical junction in much the same way as ankylosing spondylitis.114–116 Blau and Kaufman104 reviewed the clinical histories of 28 patients with both psoriasis and inflammatory arthritis between 1971 and 1984. Cervical spine involvement was found in 75% of the group; 13 of 21 had ankylosing characteristics and 8 of 21 had inflammatory characteristics. Three patients developed cervical myelopathy. More recently, Quiero and colleagues117 reviewed a series of 70 patients with psoriatic spondyloarthropathy. They found that 41% of these patients manifested involvement of the cervical spine, but only one patient had atlantoaxial subluxation. Laiho and Kauppi118 reviewed the cervical spine radiographs of 65 patients with psoriatic arthritis and identified 12 patients with cervical spine involvement. Of these 12 patients, 5 had atlantoaxial subluxation and 3 demonstrated atlantoaxial impaction.
The relationship between inflammatory bowel disease and craniocervical disease has not often been addressed in the literature. In 1986, Jordan and coworkers99 claimed the first description of a case in which inflammatory bowel disease was associated with atlantoaxial instability. The patient presented with C1-2 instability and ultimately was diagnosed as having Crohn’s disease. The triad of arthritis, urethritis, and ocular disease—referred to as Reiter’s syndrome—is usually not seen simultaneously. The arthropathy may be acute and is sometimes associated with reactive arthritis; as a result of the synovitis, atlantoaxial subluxation may occur. The treatment of these conditions is as previously outlined for RA.
Calcium pyrophosphate dihydrate (CPPD) deposition disease is considered one of the most common forms of crystal-induced arthritis.119 The disease has not been recognized is those younger than 70 years and is more common in individuals older than 85.31,120 CPPD deposition can act as a retro-odontoid mass, causing ventral cervicomedullary compression. The diagnostic features are small areas of calcification within the mass on CT; mostly isointense mass on T1-weighted MRI; mixed density, with the signal changing from hypo- to hyperintense, on T2-weighted MRI; and peripheral enhancement of the mass on postgadolinium MRI (Fig. 289-13). Retro-odontoid CPPD deposition–induced masses can be diagnosed preoperatively with a high degree of certainty based on these findings. They can be approached via the transoral route to halt or reverse the progression to neurological deterioration. The surgical pathologist needs to be alerted to the possibility of this diagnosis so that the surgical specimen can be handled appropriately to reveal the diagnostic birefringent rhomboid CPPD crystals. In a recent review, 21 surgically treated patients with an average age of 70.3 years were identified with pathologically confirmed CPPD deposition at the craniovertebral junction.121 Eighty-five percent of patients presented with occipital and neck pain, and 61% presented with numbness or paresthesias. Lower cranial nerve palsies were less frequent (29%). Gross total resection was achieved in all cases, primarily via a transoral-transpalatopharyngeal approach. Seventy-six percent of the patients also underwent dorsal occipitocervical fusion. With a mean follow-up of 15 months, 86% of the patients demonstrated improvement or resolution of symptoms.121
Arnett FC, Edworthy SM, Bloch DA. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315-324.
Boden SD, Dodge LD, Bohlman HH, et al. Rheumatoid arthritis of the cervical spine. A long-term analysis with predictors of paralysis and recovery. J Bone Joint Surg Am. 1993;75:1282-1297.
Casey ATH, Crockard HA, Geddes JF, et al. Vertical translocation: The enigma of the disappearing interval in patients with myelopathy and rheumatoid arthritis. Part I. Clinical, radiological, and neuropathological features. J Neurosurg. 1997;87:856-862.
Conlon PW, Isdale IC, Rose BS. Rheumatoid arthritis of the cervical spine. An analysis of 333 cases. Ann Rheum Dis. 1966;25:120-126.
Fenoy AJ, Menezes AH, Donovan KA, et al. Calcium pyrophosphate dihydrate crystal deposition in the craniovertebral junction. J Neurosurg Spine. 2008;8:22-29.
Koch AE. The pathogenesis of rheumatoid arthritis. Am J Orthop. 2007;36(7 suppl):5-8.
Menezes AH, Traynelis VC. Anatomy and biomechanics of normal craniovertebral junction (a) and biomechanics of stabilization (b). Childs Nerv Syst. 2008;24:1091-1100.
Menezes AH, VanGilder JC, Clark CR, et al. Odontoid upward migration in rheumatoid arthritis. An analysis of 45 patients with “cranial settling.”. J Neurosurg. 1985;63:500-509.
Muller-Ladner U, Pap T, Gay RE, et al. Mechanisms of disease: The molecular and cellular basis of joint destruction in rheumatoid arthritis. Nat Clin Pract Rheumatol. 2005;1:102-110.
Neva MH, Kauppi MJ, Kautiainen H, et al. Combination drug therapy retards the development of rheumatoid atlantoaxial subluxations. Arthritis Rheum. 2000;43:2397-2401.
O’Dell JR. Therapeutic strategies for rheumatoid arthritis. N Engl J Med. 2004;350:2591-3025.
O’Leary P, Ranawat CS, Pellicci PM. The cervical spine in rheumatoid arthritis. Contemp Surg. 1975;7:13-17.
Pellicci PM, Ranawat CS, Tsairis P, et al. A prospective study of the progression of rheumatoid arthritis of the cervical spine. J Bone Joint Surg Am. 1981;63:342-350.
Ranawat CS, O’Leary P, Pellicci PM, et al. Cervical spine fusion in rheumatoid arthritis. J Bone Joint Surg Am. 1979;61:1003-1010.
Ronkainen A, Niskanen M, Auvinen A, et al. Cervical spine surgery in patients with rheumatoid arthritis: long-term mortality and its determinants. J Rheumatol. 2006;33:517-522.
Ryken TC, Menezes AH. Inflammatory bowel disease and the craniocervical junction. Neurosurg Focus. 1999;6(6):e10.
Scrivo R, Di Franco M, Spadaro A, et al. The immunology of rheumatoid arthritis. Ann N Y Acad Sci. 2007;1108:312-322.
1 Goel VK, Clark CR, Gallaes K, et al. Movement-rotation relationships of the ligamentous occipito-atlanto-axial complex. J Biomech. 1988;21:678-680.
2 Bland JH. Rheumatoid arthritis of the cervical spine. Review. J Rheumatol. 1974;1:319-342.
3 Delamarter RB, Bohlman HH. Postmortem osseous and neuropathologic analysis of the rheumatoid cervical spine. Spine. 1994;19:2267-2274.
4 Menezes AH, VanGilder JC. Anomalies of the craniovertebral junction, 3rd ed. Youmans J, editor. Neurological Surgery, Vol 2. Philadelphia: WB Saunders. 1990:1359-1420.
5 Menezes AH, Traynelis VC. Anatomy and biomechanics of normal craniovertebral junction (a) and biomechanics of stabilization (b). Childs Nerv Syst. 2008;24:1091-1100.
6 Schweitzer ME, Hodler J, Cervilla V, et al. Craniovertebral junction: Normal anatomy with MR correlation. AJR Am J Roentgenol. 1992;158:1087-1090.
7 Engel A. Rheumatoid arthritis in US adults 1960-1962. In: Bennett PH, Wood PHN, editors. Population Studies of the Rheumatic Diseases. Amsterdam: Excerpta Medica, 1968.
8 Cathcart E, O’Sullivan JB. Rheumatoid arthritis in a New England town. N Engl J Med. 1970;282:421-442.
9 Casey ATH, Crockard HA. Rheumatoid arthritis. In: Dickman CA, Spetzler RF, Sonntag VKH, editors. Surgery of the Craniovertebral Junction. New York: Thieme; 1998:151-174.
10 Kauppi M, Hakala M. Prevalence of cervical spine subluxations and dislocations in a community-based rheumatoid arthritis population. Scand J Rheumatol. 1994;23:133-136.
11 Garrod AE. A Treatise on Rheumatism and Rheumatoid Arthritis. London: Charles Griffin; 1890.
12 Conlon PW, Isdale IC, Rose BS. Rheumatoid arthritis of the cervical spine. An analysis of 333 cases. Ann Rheum Dis. 1966;25:120-126.
13 Mathews JA. Atlanto-axial subluxation in rheumatoid arthritis. A 5-year followup study. Ann Rheum Dis. 1974;33:526-531.
14 Vasey FB. Psoriatic arthritis. In Schumacher HR, editor: Primer in the Rheumatic Diseases, 9th ed, Atlanta: Arthritis Foundation, 1988.
15 Silman AJ. Rheumatoid arthritis and infection: A population approach. Ann Rheum Dis. 1989;48:707-710.
16 Koopman WJ. Prospects for autoimmune disease. Research advances in rheumatoid arthritis. JAMA. 2001;285:648-650.
17 Zvaifler NJ. Rheumatoid arthritis: Epidemiology, etiology, rheumatoid factor, pathology, pathogenesis. In Schumacher HR, editor: Primer on the Rheumatic Diseases, 9th ed, Atlanta: Arthritis Foundation, 1988.
18 Young ID, Ford SE, Ford PM. The association of pulmonary hypertension with rheumatoid arthritis. J Rheumatol. 1989;16:1266-1269.
19 Arnett FC, Edworthy SM, Bloch DA. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315-324.
20 Condemi JJ. The autoimmune diseases. JAMA. 1992;268:2882-2892.
21 Scrivo R, Di Franco M, Spadaro A, et al. The immunology of rheumatoid arthritis. Ann N Y Acad Sci. 2007;1108:312-322.
22 Koch AE. The pathogenesis of rheumatoid arthritis. Am J Orthop. 2007;36(7 suppl):5-8.
23 Firestein GS. Immunologic mechanisms in the pathogenesis of rheumatoid arthritis. J Clin Rheumatol. 2005;11:S39-S44.
24 Muller-Ladner U, Pap T, Gay RE, et al. Mechanisms of disease: The molecular and cellular basis of joint destruction in rheumatoid arthritis. Nat Clin Pract Rheumatol. 2005;1:102-110.
25 Menezes AH, VanGilder JC, Clark CR, et al. Odontoid upward migration in rheumatoid arthritis. An analysis of 45 patients with “cranial settling.”. J Neurosurg. 1985;63:500-509.
26 Cush JJ, Lipsky PE. The immunopathogenesis of rheumatoid arthritis: The role of cytokines in chronic inflammation. Clin Aspects Autoimmun. 1987;1:2-13.
27 Clark CR, Menezes AH. Rheumatoid arthritis: Surgical considerations, 4th ed. Herkowitz HN, Garfin SR, Balderston RA, et al, editors. The Spine, Vol 2. Philadelphia: WB Saunders. 1999:1281-1301.
28 Delamarter RB, Bolesta MJ, Bohlman HH. Rheumatoid arthritis. In: Frymoyer J, editor. The Adult Spine: Principles and Practice, Vol 1. New York: Raven Press; 1991:745-762.
29 Helmers R, Galvin J, Hunninghake GH. Pulmonary manifestations associated with rheumatoid arthritis. Chest. 1991;100:235-238.
30 Hurd ER. Extra-articular manifestations of rheumatoid arthritis. Semin Arthritis Rheum. 1979;8:151-176.
31 Menezes AH. Rheumatological disorders. In: Menezes AH, Sonntag VKH, editors. Principles of Spinal Surgery, Vol 1. New York: McGraw-Hill; 1996:705-722.
32 Sharp J, Purser DW, Lawrence JS. Rheumatoid arthritis of the cervical spine in the adult. Ann Rheum Dis. 1958;17:303-313.
33 Marks JS, Sharp J. Rheumatoid cervical myelopathy. QJM. 1981;50:307-319.
34 Ball J, Sharp J. Rheumatoid arthritis of the cervical spine. In: Hill AGS, editor. Modern Trends in Rheumatology. 2nd ed. London: Butterworth; 1971:117-138.
35 Lorber A, Pearson CM, Rene RM. Osteolytic vertebral lesions as a manifestation of rheumatoid arthritis and related disorders. Arthritis Rheum. 1961;4:514-532.
36 The upper cervical spine. Regional anatomy, pathology and traumatology. A systematic radiological atlas and textbook. In: Verlat GT, editor. A Systemic Radiological Atlas and Textbook. New York: Grune & Stratton; 1972:1-99.
37 Hernandez LA, Buchanan WW, Sturrock RD. C4 and C5 body vertebral erosions in early rheumatoid disease. Clin Rheumatol. 1988;7:331-334.
38 Winfield J, Cooke D, Brook AS, et al. A prospective study of the radiological changes in early rheumatoid disease. Ann Rheum Dis. 1981;40:109-114.
39 Zikou AK, Alamanos Y, Argyropoulou MI, et al. Radiological cervical spine involvement in patients with rheumatoid arthritis: A cross sectional study. J Rheumatol. 2005;32:801-806.
40 Zikou AK, Argyropoulou MI, Alamanos Y, et al. Magnetic resonance imaging findings of the cervical spine in patients with rheumatoid arthritis. A cross-sectional study. Clin Exp Rheumatol. 2005;23:665-670.
41 Fujiwara K, Owaki H, Fujimoto, et al. A long-term follow-up study of cervical lesions in rheumatoid arthritis. J Spinal Disord. 2000;13(6):519-526.
42 Mikulowski P, Wollheim FA, Rotmil P, et al. Sudden death in rheumatoid arthritis with atlanto-axial dislocation. Acta Med Scand. 1975;198:445-451.
43 Dodge LD, Bohlman HH, Rechtine GR. Paralysis secondary to rheumatoid arthritis—pathogenesis and results of treatment. Orthop Trans. 1987;11:473.
44 Martel W. The occipito-atlanto-axial joints in rheumatoid arthritis and ankylosing spondylitis. AJR Am J Roentgenol. 1961;86:223-240.
45 Nakano KK, Schoene WC, Baker RA, et al. The cervical myelopathy associated with rheumatoid arthritis: analysis of 32 patients with two postmortem cases. Ann Neurol. 1978;3:144-151.
46 O’Leary P, Ranawat CS, Pellicci PM. The cervical spine in rheumatoid arthritis. Contemp Surg. 1975;7:13-17.
47 Redlund-Johnell I, Pettersson H. Radiographic measurements of the craniovertebral region. Acta Radiol Diagn. 1984;25:23-28.
48 Sambrook PN, Eisman JA, Champion GD, et al. Determinants of axial bone loss in rheumatoid arthritis. Arthritis Rheum. 1987;30:721-728.
49 Santavirta S, Slatis P, Kankaanpea U, et al. Treatment of the cervical spine in rheumatoid arthritis. J Bone Joint Surg Am. 1988;70:658-667.
50 Crockard HA. Spine update. Surgical management of cervical rheumatoid problems. Spine. 1995;20:2584-2590.
51 Macedo TF, Gow PJ, Heap SW, et al. Bilateral hypoglossal nerve palsy due to vertical subluxation of the odontoid process in rheumatoid arthritis. Br J Rheumatol. 1988;27:317-320.
52 Sherk HH. Atlantoaxial instability and acquired basilar invagination in rheumatoid arthritis. Orthop Clin North Am. 1978;9:1053-1063.
53 Zygmunt S, Saveland H, Brattstrom H, et al. Reduction of rheumatoid periodontoid pannus following posterior occipitocervical fusion visualized by magnetic resonance imaging. Br J Neurosurg. 1988;2:315-320.
54 Casey ATH, Crockard HA, Geddes JF, et al. Vertical translocation: the enigma of the disappearing interval in patients with myelopathy and rheumatoid arthritis. Part I. Clinical, radiological, and neuropathological features. J Neurosurg. 1997;87:856-862.
55 Zoma A, Sturrock R, Fisher WD, et al. Surgical stabilization of the rheumatoid cervical spine. A review of indications and results. J Bone Joint Surg Br. 1987;69:8-12.
56 Larsson EM, Holtas S, Zygmunt S. Pre- and postoperative MR imaging of the craniocervical junction in rheumatoid arthritis. AJNR Am J Neuroradiol. 1989;10:89-94.
57 Stiskal MA, Neuhold A, Szolar DH, et al. Rheumatoid arthritis of the craniocervical region by MR imaging: detection and characterization. AJR Am J Roentgenol. 1995;165:585-592.
58 Bundschuh C, Modic MT, Kearney F, et al. Rheumatoid arthritis of the cervical spine: surface-coil MR imaging. AJR Am J Roentgenol. 1988;151:181-187.
59 Pellicci PM, Ranawat CS, Tsairis P, et al. A prospective study of the progression of rheumatoid arthritis of the cervical spine. J Bone Joint Surg Am. 1981;63:342-350.
60 Mikulowski P, Wollheim FA, Rotmil P, et al. Sudden death in rheumatoid arthritis with atlanto-axial dislocation. Acta Med Scand. 1981;198:445-451.
61 Crockard HA, Essigman WK, Stevens JM, et al. Surgical treatment of cervical cord compression in rheumatoid arthritis. Ann Rheum Dis. 1985;44:809-816.
62 Casey ATH, Crockard HA, Bland JM, et al. Predictors of outcome in the quadriparetic nonambulatory myelopathic patient with rheumatoid arthritis: a prospective study of 55 surgically treated Ranawat class IIIb patients. J Neurosurg. 1996;85:574-581.
63 Boden SD. Rheumatoid arthritis of the cervical spine. Surgical decision making based on predictors of paralysis and recovery. Spine. 1994;19:2275-2280.
64 Orstavik RE, Haugeberg G, Mowinckel P, et al. Vertebral deformities in rheumatoid arthritis: a comparison with population-based controls. Arch Intern Med. 2004;164:420-425.
65 Al Khayer A, Sawant N, Emberton P, et al. Spontaneous odontoid process fracture in rheumatoid arthritis: diagnostic difficulties, pathology and treatment. Injury. 2006;37:659-662.
66 Lewandrowski KU, Park PP, Baron JM, et al. Atraumatic odontoid fractures in patients with rheumatoid arthritis. Spine J. 2006;6:529-533.
67 Scully RE. Case records of the Massachusetts General Hospital (Case 37-1992). N Engl J Med. 1992;327:873-880.
68 Eaton AM, Serota H, Kernodle GHJr, et al. Pulmonary hypertension secondary to serum hyperviscosity in a patient with rheumatoid arthritis. Am J Med. 1987;82:1039-1045.
69 Price TML, Skelton MO. Rheumatoid arthritis with lung lesions. Thorax. 1956;11:234-240.
70 Wiedemann HP, Matthay RA. Pulmonary manifestations of the collagen vascular diseases. Clin Chest Med. 1989;10:677-722.
71 Epler GR, McLoud TC, Gaensler EA, et al. Normal chest roentgenograms in chronic diffuse infiltrative lung disease. N Engl J Med. 1978;298:934-939.
72 Fewins HE, McGowan I, Whitehouse GH, et al. High definition computed tomography in rheumatoid arthritis associated pulmonary disease. Br J Rheumatol. 1991;30:214-216.
73 O’Dell JR. Therapeutic strategies for rheumatoid arthritis. N Engl J Med. 2004;350:2591-3025.
74 Neva MH, Kauppi MJ, Kautiainen H, et al. Combination drug therapy retards the development of rheumatoid atlantoaxial subluxations. Arthritis Rheum. 2000;43:2397-2401.
75 Boden SD, Dodge LD, Bohlman HH, et al. Rheumatoid arthritis of the cervical spine. A long-term analysis with predictors of paralysis and recovery. J Bone Joint Surg Am. 1993;75:1282-1297.
76 Christophidis N, Huskinsson EC. Misleading symptoms and signs of cervical spine subluxation in rheumatoid arthritis. BMJ. 1982;285:364-365.
77 Saway PA, Blackburn WD, Halla JT, et al. Clinical characteristics affecting survival in patients with rheumatoid arthritis undergoing cervical spine surgery: a controlled study. J Rheumatol. 1989;16:890-896.
78 Menezes AH. Surgical approaches to the craniocervical junction. In: Frymoyer J, editor. The Adult Spine: Principles and Practice, Vol 2. New York: Raven Press; 1991:967-986.
79 Semble EL, Elster AD, Loeser RF, et al. Magnetic resonance imaging of the craniovertebral junction in rheumatoid arthritis. J Rheumatol. 1988;15:1367-1375.
80 Winalski CS, Aliabadi P, Wright RJ, et al. Enhancement of joint fluid with intravenously administered gadopentetate dimeglumine: Technique, rationale, and implications. Radiology. 1993;187:179-185.
81 Zoli A, Priolo F, Galossi A, et al. Craniocervical junction involvement in rheumatoid arthritis: a clinical and radiological study. J Rheumatol. 2000;27:1178-1182.
82 Grob D, Wursch R, Grauer W, et al. Atlantoaxial fusion and retrodental pannus in rheumatoid arthritis. Spine. 1997;22:1580-1584.
83 Ranawat CS, O’Leary P, Pellicci PM, et al. Cervical spine fusion in rheumatoid arthritis. J Bone Joint Surg Am. 1979;61:1003-1010.
84 Weissman BNW, Aliabadi P, Weinfeld MS, et al. Prognostic features of atlanto-axial subluxation in rheumatoid arthritis patients. Radiology. 1982;144:745-751.
85 Dickman CA, Crawford NR, Paramore CG. Biomechanical characteristics of C1-2 cable fixations. J Neurosurg. 1996;85:316-322.
86 Paramore CG, Dickman CA, Sonntag VKH. The anatomical suitability of the C1-2 complex for transarticular screw fixation. J Neurosurg. 1996;85:221-224.
87 Sawin PD, Traynelis VC, Menezes AH. A comparative analysis of fusion rates and donor site morbidity for autogenic rib and iliac crest bone grafts in posterior cervical fusions. J Neurosurg. 1998;88:255-265.
88 Newman P, Sweetnam R. Occipito-cervical fusion. An operative technique and its indications. J Bone Joint Surg Br. 1969;51:423-431.
89 Sakou T, Kawaida H, Morizono Y, et al. Occipito-atlantoaxial fusion utilizing a rectangular rod. Clin Orthop. 1989;239:136-144.
90 MacKenzie AI, Uttley D, Marsh HT, et al. Craniocervical stabilization using Luque/Hartshill rectangles. Neurosurgery. 1990;26:32-36.
91 Robertson SC, Menezes AH. Occipital calvarial bone graft in posterior occipitocerivcal fusion. Spine. 1998;23:249-255.
92 Walters BC. Cranial bone grafts for use in posterior fixation of the cervical spine. J Neurosurg. 1993;79:286-288.
93 Sagher O, Malik JM, Lee JH, et al. Fusion with occipital bone for atlantoaxial instability: technical note. Neurosurgery. 1993;33:926-929.
94 Clarke MJ, Cohen-Gadol AA, Ebersold MJ, et al. Long-term incidence of subaxial cervical spine instability following cervical arthrodesis surgery in patients with rheumatoid arthritis. Surg Neurol. 2006;66:136-140.
95 Ronkainen A, Niskanen M, Auvinen A, et al. Cervical spine surgery in patients with rheumatoid arthritis: long-term mortality and its determinants. J Rheumatol. 2006;33:517-522.
96 Fries JF. The reactive enthesiopathies. Dis Mon. 1985;31:1-46.
97 Ryken TC, Menezes AH. Inflammatory bowel disease and the craniocervical junction. Neurosurg Focus. 1999;6(6):e10.
98 Helliwell PS, Hickling P, Wright V. Do the radiological changes of classic ankylosing spondylitis differ from the changes found in the spondylitis associated with inflammatory bowel disease, psoriasis, and reactive arthritis? Ann Rheum Dis. 1998;57:135-140.
99 Jordan JM, Obeid LM, Allen NB. Isolated atlantoaxial subluxation as the presenting manifestation of inflammatory bowel disease. Am J Med. 1986;80:517-520.
100 Kerr R, Resnick D. Radiology of the seronegative spondyloarthropathies. Clin Rheum Dis. 1985;11:113-146.
101 Lee ST, Lui TN. Psoriatic arthritis with C1-C2 subluxation as a neurosurgical complication. Surg Neurol. 1986;26:428-430.
102 Yulish BS, Lieberman JM, Newman AJ, et al. Juvenile rheumatoid arthritis: Assessment with MR imaging. Radiology. 1987;165:149-152.
103 Sharp J, Purser DW. Spontaneous atlanto-axial dislocation in ankylosing spondylitis and rheumatoid arthritis. Ann Rheum Dis. 1961;20:47-77.
104 Blau RH, Kaufman RL. Erosive and subluxing cervical spine disease in patients with psoriatic arthritis. J Rheumatol. 1987;14:111-117.
105 Fam AG, Cruickshank B. Subaxial cervical subluxation and cord compression in psoriatic spondylitis. Arthritis Rheum. 1982;25:101-106.
106 McEwen C, DiTata D, Lengg C. Ankylosing spondylitis and spondylitis accompanying ulcerative colitis, regional enteritis, psoriasis and Reiter’s disease. A comparative study. Arthritis Rheum. 1971;14:291-318.
107 Kransdorf MJ, Wehrle PA, Moser RPJr. Atlantoaxial subluxation in Reiter’s syndrome. A report of three cases and review of the literature. Spine. 1988;13:12-14.
108 Suarez-Almazor ME, Russell AS. Anterior atlantoaxial subluxation in patients with spondyloarthropathies: association with peripheral disease. J Rheumatol. 1988;15:973-975.
109 Lee JY, Kim JI, Park JY, et al. Cervical spine involvement in longstanding ankylosing spondylitis. Clin Exp Rheumatol. 2005;23:331-336.
110 Ball J. Enthesopathy of rheumatoid and ankylosing spondylitis. Ann Rheum Dis. 1971;30:213-223.
111 Laiho K, Savolainen A, Kautiainen H, et al. The cervical spine in juvenile chronic arthritis. Spine J. 2002;2:89-94.
112 Dzioba RB, Benjamin J. Spontaneous atlantoaxial fusion in psoriatic arthritis. Spine. 1985;10:102-103.
113 Hanly JG, Rusell MI, Gladman DD. Psoriatic spondyloarthropathy: A long-term prospective study. Ann Rheum Dis. 1988;47:386-393.
114 Killebrew K, Gold RH, Sholkoff SD. Psoriatic spondylitis. Radiology. 1973;108:9-16.
115 Paimela L, Laasonen L, Kankaanpaa E, et al. Progression of cervical spine changes in patients with early rheumatoid arthritis. J Rheumatol. 1997;24:1280-1284.
116 Reynolds MD, Rankin TJ. Diagnosis of “rheumatoid variants” ankylosing spondylitis, the arthritides of gastrointestinal diseases and psoriasis, and Reiter’s syndrome. West J Med. 1974;120:441-447.
117 Quiero R, Belzungui J, Gonzalez C, et al. Clinical asymptomatic axial disease in psoriatic spondyloarthropathy. A retrospective study. Clin Rheumatol. 2002;21:10-13.
118 Laiho K, Kauppi M. The cervical spine in patients with psoriatic arthritis. Ann Rheum Dis. 2002;61:650-652.
119 Chuzin Y, Panush RS. CPPDDD: What it is and why it is under-recognized. Bull Rheum Dis. 1995;44:3-5.
120 Zunkeler B, Schelper R, Menezes AH. Periodontoid calcium pyrophosphate dihydrate deposition disease: “pseudogout” mass lesions of the craniocervical junction. J Neurosurg. 1996;85:803-809.
121 Fenoy AJ, Menezes AH, Donovan KA, et al. Calcium pyrophosphate dihydrate crystal deposition in the craniovertebral junction. J Neurosurg Spine. 2008;8:22-29.