Chapter 8 Acids and bases
The Involvement of Water in Acid–Base Reactions
As you have already seen, water is a very active molecule, forming and breaking hydrogen bonds between its molecules (see Figure 3.12, p. 19). This constant juggling of hydrogen bonds gives the water molecule the capability to break apart or disassociate.
The environment of living tissues is largely water and when certain compounds enter this environment they can be encouraged to disassociate. The degree to which they dissociate depends on the number of hydrogen ions present. It is this balance of hydrogen ions that forms the basis for acid–base mechanisms.
Acids and Bases
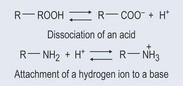
Acids disassociate in water to form a hydrogen ion and the ion of the acid (Figure 8.1). In other words, in the right conditions, acids tend to give away their hydrogen ions, donating them to the aqueous solution.
Bases accept hydrogen ions from aqueous solutions in the right conditions (Figure 8.1). A strong base is one that is very keen to grasp free hydrogen ions, whereas a weak base is not good at grasping free hydrogen ions. The result, regardless of strength, is a compound that has a positively charge ion.
What is pH?
So, the higher the number, the stronger the base; the lower the number, the stronger the acid.
What is pK?
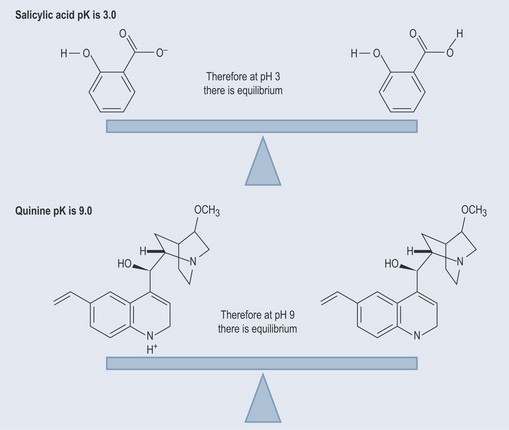
The degree to which compounds are prepared to dissociate (break apart to form a hydrogen ion and a charged compound) can be measured and something called a pK value is used. This dissociation constant will tell you at exactly what pH a compound is half ionized and half not ionized. In other words, the pH at which the compound is in equilibrium.
How Are the pH and pK Values Relevant to Absorption?
The pK of salicylic acid is 3.0. At pH 3, 50% of the salicylic acid will be un-ionized and 50% will be in the form of salicylate ions (negatively charged), having lost its hydrogen (Figure 8.2).
What Happens to Acids and Bases in a low pH?
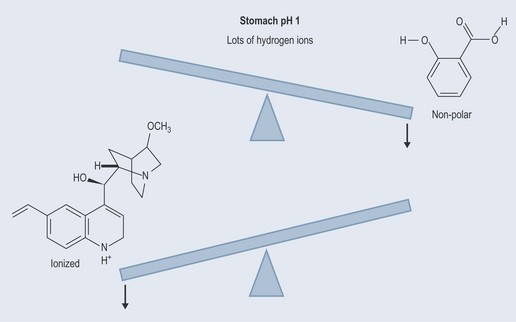
The stomach has a pH of 1 (a low pH, a high concentration of hydrogen ions). With this many hydrogen ions, an acid with a pK value of 3 is not going to be able to give away its hydrogen ions (donate hydrogen ions) because there are too many in solution. Rather like a housing market flooded with properties makes it difficult for a vendor to sell their properties. However, a base with a pK of 9 will be only too happy to soak up the spare hydrogen ions. This is the same as a buyer having a great deal of choice when a house market is flooded with properties (a buyer’s market).
Therefore, in the acidic environment of the stomach, the salicylic acid stays in its configuration but quinine becomes positively charge due to the extra hydrogen ions (Figure 8.3). The salicylic acid is therefore uncharged (un-ionized, non-polar) and the quinine is charged (ionized, polar).
What Happens to Acids and Bases in a High pH?
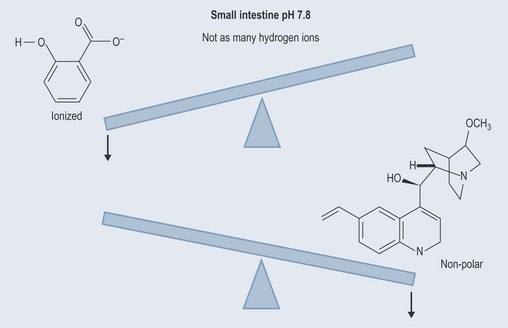
By losing a hydrogen ion the salicylic acid therefore becomes charged (ionized, polar; Figure 8.4) and the quinine, by being unable to attach to a hydrogen ion is uncharged (un-ionized, non-polar).
How the Degree of Charge on a Compound will Affect its Absorption Rate into the Body
The environmental pH therefore has an effect on acids and bases, as different pHs can make them charged (ionized or polar) or uncharged (un-ionized or non-polar). This in turn affects the absorption of an acidic or basic compound through a membrane that is largely non-polar due to its composition of non-polar lipids.
How the Degree of Charge on a Compound will Affect its Removal from the Body
The kidneys are a very active area when it comes to pH (see Figure 18.3, p. 135). The excretion of certain drugs can be altered depending on the pH of the urine.
The Importance of pH and pK
Under the right conditions, substances such as organic acids that have carboxyl groups (see Figure 5.1, p. 30) can ionize and form negatively charged COO− ions and positively charged H+ ions (or protons). This capacity of a molecule to lose or gain a proton is very important in maintaining stability of pH in the bloodstream and how easily a chemical is absorbed or removed from the body (see Chapter 18 ‘Drug excretion’, p. 135). pH is kept very tightly controlled in the plasma.
Conjugate Acids and Bases
You might come across the terms ‘conjugate acid’ and ‘conjugate base’ in the literature. It is an interesting paradox that occurs in chemistry and one that you have already seen in action above. Figure 8.5 will enable you to follow the discussion more easily.
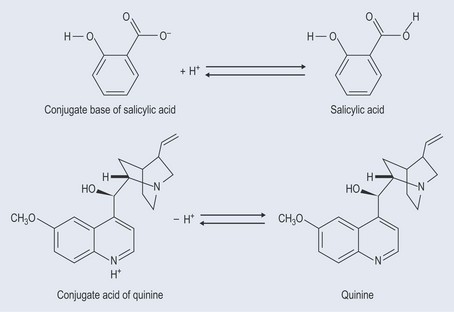
Figure 8.5 The principle of a conjugate base and a conjugate acid. Note that the reaction is reversible.
A conjugate acid is formed when a base gains a hydrogen ion. The compound is now an acid because it has gained a hydrogen ion and is willing to donate it, just like an acid.
How is pH – or Hydrogen ion Concentration – Controlled in the Body?
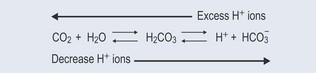
Bicarbonate Buffer System
When there are excess hydrogen ions (low pH; Figure 8.6, top arrow, right to left) the equilibrium is shifted to the left. To restore the correct hydrogen ion concentration (pH), the excess of hydrogen ions will react with the bicarbonate ions (HCO3−) eventually producing carbon dioxide and water.
When there is a decrease in hydrogen ions (high pH; Figure 8.6, bottom arrow, left to right), carbon dioxide will combine with water, eventually producing more hydrogen ions and bicarbonate ions. The respiratory centre will then increase respiration to remove excess carbon dioxide from the blood.
Control of pH by the Kidneys
There is a large flow of blood through the kidneys and – potentially – a large capability to remove fluids and solutes from the body (see Chapter 18 ‘Drug excretion’, p. 135 and Chapter 26 ‘Cardiovascular disorders’, p. 192). The kidneys are therefore a convenient way to control pH.