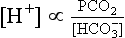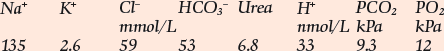Acid–base disorders
diagnosis and management
Specimens for blood gas analysis
If the [H+] and the PCO2 are known, the bicarbonate can be calculated. Indeed, blood gas analysers (Fig 24.1) are programmed to provide this on all samples, as the ‘standard bicarbonate’ i.e. under standard conditions. Other parameters usually included are the PO2, and the base excess, another way of assessing the metabolic component.
Interpreting results
The most important information available for the interpretation and classification of an acid-base disorder is provided by the patient’s clinical history. The predicted compensatory responses in [HCO3–] or PCO2 when [H+] changes as a result of primary acid–base disorders are shown in Table 24.1.
Table 24.1
Primary acid–base disorders and compensatory responses
| Primary disorder | Compensatory response |
| ↑PCO2 (Respiratory acidosis) | ↑HCO3– |
| ↓PCO2 (Respiratory alkalosis) | ↓HCO3– |
| ↓HCO3– (Metabolic acidosis) | ↓PCO2 |
| ↑HCO3– (Metabolic alkalosis) | ↑PCO2 |
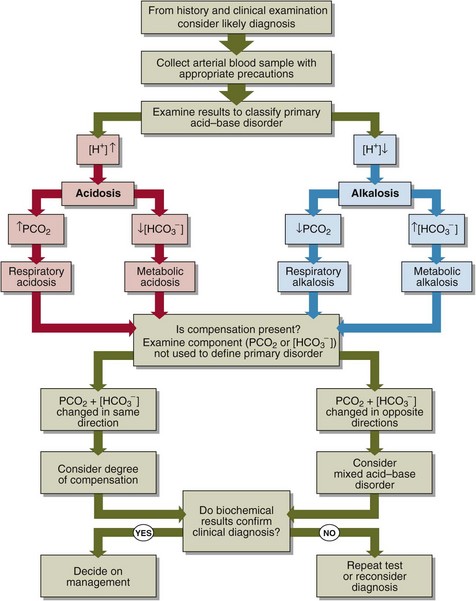
A practical approach to the interpretation of blood gas results is shown in Figure 24.2. The steps in classifying the acid–base disorder are:
 Look first at the [H+]. Decide if an acidosis or an alkalosis is present.
Look first at the [H+]. Decide if an acidosis or an alkalosis is present.
 If the [H+] is elevated, decide what is the primary cause of the acidosis. Look at the PCO2. If this is elevated, then there is a respiratory acidosis. Look at the bicarbonate. If this is decreased, there is a metabolic acidosis.
If the [H+] is elevated, decide what is the primary cause of the acidosis. Look at the PCO2. If this is elevated, then there is a respiratory acidosis. Look at the bicarbonate. If this is decreased, there is a metabolic acidosis.
 If the [H+] is decreased, decide what is the primary cause of the alkalosis. Look at the PCO2. If low, then there is a respiratory alkalosis. Look at the bicarbonate. If this is high, then there is a metabolic alkalosis.
If the [H+] is decreased, decide what is the primary cause of the alkalosis. Look at the PCO2. If low, then there is a respiratory alkalosis. Look at the bicarbonate. If this is high, then there is a metabolic alkalosis.
 Having decided on the primary acid–base disorder, look to see if there is compensation. If there is, there will be a change in the other component (the one which was not used to determine the primary disorder), in the direction which ‘compensates’ for the primary disorder, i.e. returns the ratio, and hence the [H+], towards normal. If there is not, the acid–base disorder may be uncompensated. If the change is in the opposite direction then a second acid–base disorder may be present. Even if there is compensation consider the possibility that there is a second acid–base problem that mimics the compensatory response.
Having decided on the primary acid–base disorder, look to see if there is compensation. If there is, there will be a change in the other component (the one which was not used to determine the primary disorder), in the direction which ‘compensates’ for the primary disorder, i.e. returns the ratio, and hence the [H+], towards normal. If there is not, the acid–base disorder may be uncompensated. If the change is in the opposite direction then a second acid–base disorder may be present. Even if there is compensation consider the possibility that there is a second acid–base problem that mimics the compensatory response.
 If there is compensation, decide if the disorder is fully compensated or partially compensated. If fully compensated, the resulting [H+] will be within the reference limits.
If there is compensation, decide if the disorder is fully compensated or partially compensated. If fully compensated, the resulting [H+] will be within the reference limits.
Clinical cases
The above practical advice is best illustrated by four case examples.
 A patient with chronic bronchitis. Blood gas results are: [H+] = 44 nmol/L; PCO2 = 9.5 kPa; [HCO3–] = 39 mmol/L. A compensated respiratory acidosis is present.
A patient with chronic bronchitis. Blood gas results are: [H+] = 44 nmol/L; PCO2 = 9.5 kPa; [HCO3–] = 39 mmol/L. A compensated respiratory acidosis is present.
 A patient who has had an acute asthmatic attack. Blood gas results are: [H+] = 24 nmol/L; PCO2 = 2.5 kPa; [HCO3–] = 20 mmol/L. An acute, and hence uncompensated, respiratory alkalosis is present.
A patient who has had an acute asthmatic attack. Blood gas results are: [H+] = 24 nmol/L; PCO2 = 2.5 kPa; [HCO3–] = 20 mmol/L. An acute, and hence uncompensated, respiratory alkalosis is present.
 A young man with a history of dyspepsia and excessive alcohol intake who gives a 24-hour history of vomiting. Blood gas results are [H+] = 28 nmol/L; PCO2 = 7.2 kPa; [HCO3–] = 48 mmol/L. This is a partially compensated metabolic alkalosis.
A young man with a history of dyspepsia and excessive alcohol intake who gives a 24-hour history of vomiting. Blood gas results are [H+] = 28 nmol/L; PCO2 = 7.2 kPa; [HCO3–] = 48 mmol/L. This is a partially compensated metabolic alkalosis.
 A 50-year-old man with a 2-week history of vomiting and diarrhoea. On examination he is dehydrated and his breathing is deep and noisy. Blood gas results are: [H+] = 64 nmol/L; PCO2 = 2.8 kPa; [HCO3–] = 8 mmol/L. These results show a partially compensated metabolic acidosis.
A 50-year-old man with a 2-week history of vomiting and diarrhoea. On examination he is dehydrated and his breathing is deep and noisy. Blood gas results are: [H+] = 64 nmol/L; PCO2 = 2.8 kPa; [HCO3–] = 8 mmol/L. These results show a partially compensated metabolic acidosis.