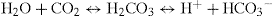1.4 Acid–base balance
The basics
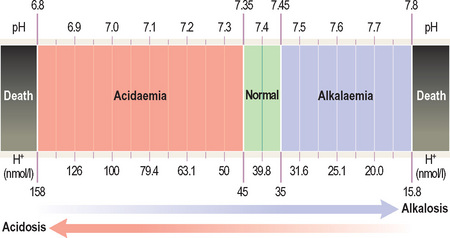
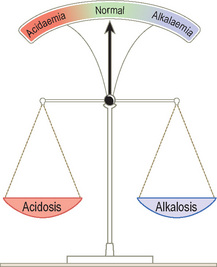
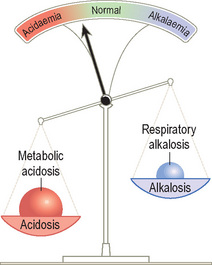
An acidosis is any process that lowers blood pH whereas an alkalosis is any process that raises blood pH.
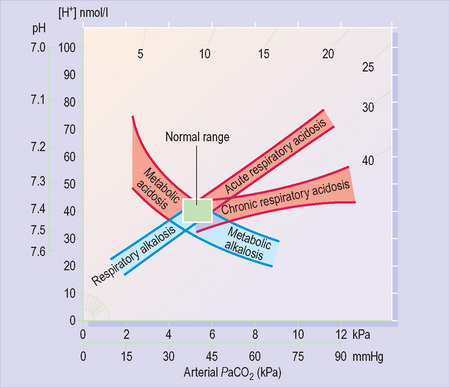
It is a negative logarithmic scale (Figure 10). The ‘negative’ means that pH values get lower as the H+ concentration increases (so a pH of 7.1 is more acidic than 7.2). The ‘logarithmic’ means that a shift in pH by one number represents a 10-fold change in H+ concentration (so 7 is 10 times more acidic than 8).
Maintaining acid–base balance
What generates H+ ions in our bodies?
Metabolism of protein produces hydrochloric, sulphuric and other so-called ‘metabolic acids’.
H+ ions must, therefore, be removed to maintain normal blood pH.
What removes H+ ions from our bodies?
Renal (metabolic) mechanisms
The kidneys are responsible for excreting metabolic acids. They secrete H+ ions into urine and reabsorb HCO3 from urine. HCO3 is a base (and therefore accepts H+ ions), so it reduces the concentration of H+ ions in blood. The kidneys can adjust urinary H+ and HCO3 excretion in response to changes in metabolic acid production.
Maintaining acid–base balance
This one equation is crucial to understanding acid–base balance:
Firstly it shows that CO2, when dissolved in blood, becomes an acid.
Secondly, it predicts that blood pH depends not on the absolute amounts of CO2 or HCO3 present but on the ratio of CO2 to HCO3. Thus, a change in CO2 will not lead to a change in pH if it is balanced by a change in HCO3 that preserves the ratio (and vice versa). Since CO2 is controlled by respiration and HCO3 by renal excretion, this explains how compensation can prevent changes in blood pH.
Disturbances of acid–base balance
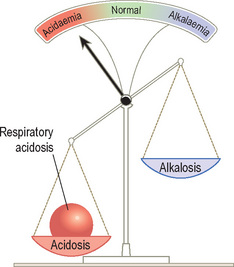
PaCO2 raised = Respiratory acidosis
PaCO2 low = Respiratory alkalosis
Acid–base disturbances can be considered as a set of scales.
Compensated acid–base disturbance
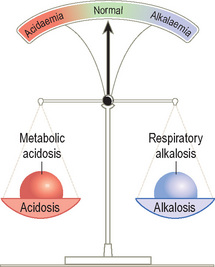
Figures 13 and 14 represent two scenarios in which the lungs have responded to a primary metabolic acidosis by increasing alveolar ventilation to eliminate more CO2 (compensatory respiratory alkalosis). In Figure 13 an acidaemia persists despite compensation (partial compensation); in Figure 14, blood pH has returned to the normal range (full compensation).
The second rule is that the patient is more important than the ABG. When considering an ABG, one must always take account of the clinical context. For example, if the patient in Figure 14 were diabetic, with high levels of ketones in the urine, it would be obvious that the metabolic acidosis was a primary process (diabetic ketoacidosis).
Mixed acid–base disturbance
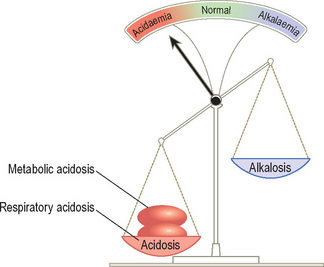
If these two processes oppose each other, the pattern will be similar to a compensated acid–base disturbance (Figure 14) and the resulting pH derangement will be minimised. A good example is salicylate poisoning, where primary hyperventilation (respiratory alkalosis) and metabolic acidosis (salicylate is acidic) occur independently.
On the other hand, if the two processes cause pH to move in the same direction (metabolic acidosis and respiratory acidosis or metabolic alkalosis and respiratory alkalosis), a profound acidaemia or alkalaemia may result (Figure 15).