Achilles Tendon Repair and Rehabilitation
Jane Gruber, Eric Giza, James Zachazewski and Bert R. Mandelbaum
Achilles tendon injuries, whether acute or chronic, occur in many individuals. The severity of these injuries varies from mild, overuse-related inflammatory responses to acute, traumatic tendon rupture. Nonoperative treatment options are immobilization with a cast or functional bracing, while surgical options are operative repair with open or percutaneous procedures. Postoperative management varies with the length of immobilization and timing of early motion. This chapter describes current trends in surgical intervention, outlines rehabilitative guidelines and techniques, and details the rationales associated with treating Achilles tendon ruptures.
Surgical Indications And Considerations
Anatomy
The Achilles tendon complex is composed of contributions from the gastrocnemius, soleus, and plantaris (collectively known as the triceps surae) and inserts directly into the central third of the posterior calcaneal surface. The tendon is round in cross section to a level 4 cm proximal to calcaneus, where it flattens and rotates 90° so that its medial fibers insert posteriorly. This biomechanical “winding” of the fibers increases stored energy for higher shortening velocity and muscle power.1
During dorsiflexion, the tendon articulates with the superior third of the calcaneus. This articulation is cushioned by the retrocalcaneal bursa, which lies between the tendon and the superior third of the calcaneus.
The Achilles tendon does not possess a true synovial sheath. The peritendinous structures of the Achilles are composed of a triple-layered tissue.2 The superficial layer of tissue is the most durable and is analogous to the deep fascia. This layer comprises the posterior boundary of the superficial posterior compartment. The middle layer, the mesotendon, provides the major blood supply for the central portion of the Achilles tendon. The deepest layer of tissue is quite delicate and thin; however, it can always be isolated from the most superficial layer of the tendon, the epitenon.
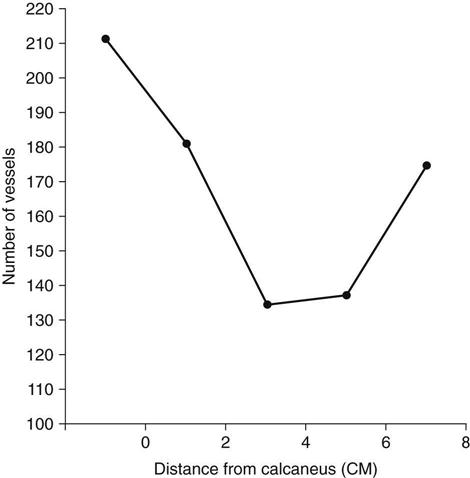
The Achilles tendon is supplied with blood and nutrients by three different sources.2 The most abundant supply is at the proximal and distal portions of the tendon, and the poorest is in the central portion of the tendon. As originally demonstrated by Lagerrgren and Lindholm3 and corroborated by others,4,5 a gradual decrease occurs in the number of blood vessels in the central part of the tendon 2 to 6 cm proximal to the calcaneal insertion (Fig. 31-1).
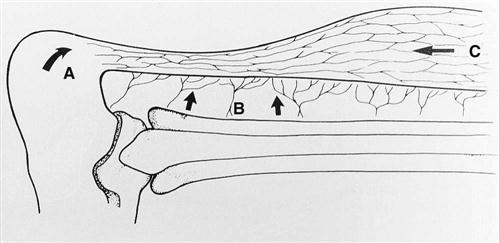
Nutrient branches emanate directly from the muscle to nourish the distal gastrocnemius aponeurosis and proximal portion of the tendon.6 The insertion of the Achilles tendon is supplied by anastomotic branches between the periosteal vessels and the tendon vessels. As already noted, the major blood supply comes from the mesotendon. Vessels enter the tendon itself via a network of fine connections with the deepest peritendinous layer. These vessels come off the deepest layer radially and enter the tendon perpendicular to its long axis. They then course proximally and distally. Because of the external forces that may be encountered by the posterior aspect of the tendon as a result of friction supplied by the skin, most of these fine vessels are found along the anterior aspect of the tendon, where they are afforded more protection (Fig. 31-2).
Pathogenesis
The theoretic explanation for tendon injuries suggests a continuum of events, including hypovascularity and repetitive microtrauma, that results in localized tendon degeneration and weakness and ultimately rupture with the application of an otherwise normal load that exceeds the tendon’s physiologic capacity. Based on clinical and histologic findings, the traditional description of Achilles tendonitis is not preferred,7 and Achilles tendon pathology is classified into three different categories: (1) paratendinitis, (2) paratendinitis with tendinosis, and (3) pure tendinosis.8–10
Paratendinitis involves inflammation only in the paratenon, regardless of whether it is lined by synovium. The paratenon thickens, and adhesions may form between the paratenon and the tendon.11 However, patients will rarely have isolated paratendinitis, and some authors believe that this process is the predecessor to tendinosis.7
Paratendinitis with tendinosis involves not only inflammation of the paratenon but also a degenerative change within the substance of the tendon. Paddu, Ippolito, and Postacchini10 and Kvist and Kvist11 have noted thickening, softening, and yellowing of the tendon, as well as cleavage planes and vascular budding during surgery for this condition. As in tendinitis, pain also is commonly noted because of the inflammatory process.
Pure tendinosis often appears as a nodule that is mobile with plantar flexion.7 It can present as a chronic nodule in the sedentary middle-aged person or as a contribution to the pathology of rupture in the tendons of persons older than 35 years who have suffered spontaneous rupture.12 Histopathologic changes such as hypoxic and mucoid degeneration, lipomatous infiltration, and calcifying tendinopathy have been noted at the time of surgical repair of acute ruptures.10,12
Hippocrates was the first to record an injury to the Achilles tendon, while Ambrose Pare was the first to describe Achilles tendon ruptures in 1575, and Gustave Paoaillon was the first to report operative repair of the tendon.1,2,13 Theories implicating the degenerative changes that take place in the Achilles tendon with the increased mechanical loads associated with various activities are the most common explanations of the pathogenesis of these ruptures.13,14 A combination of hypovascularity and repetitive microtrauma results in degenerative changes and inflammation, putting the tendon at risk for rupture. Healing and regeneration are hindered or halted because of poor blood supply to the tendon and recurrent microtrauma. Alfredson, Thorsen, and Lorentzon15 have shown that microtears in the tendon lead to areas of chronic damage that lack normal levels of prostaglandin E2, which are needed for healing. The combination of these factors may account for the fact that most ruptures occur 2 to 6 cm proximal to the tendon’s insertion into the calcaneus. Associated intrinsic factors include age (which leads to decreased vascularity and decreased deoxyribonucleic acid [DNA] synthesis),16–18 endocrine function, and nutrition.19 Extrinsic factors of compression and friction on the posterior aspect of the tendon (from the skin and inappropriately compressive footwear) further hinder vascularity, healing, and regeneration. Fluoroquinolone antibiotics are also associated with Achilles tendon rupture. Their use inhibits transcription of decorin, a protein important in collagen architecture, and the odds ratio for tendon rupture in patients taking the medication is 4.2.20 Acute Achilles ruptures are also often the result of corticosteroid injection for chronic inflammation of the tendon.7
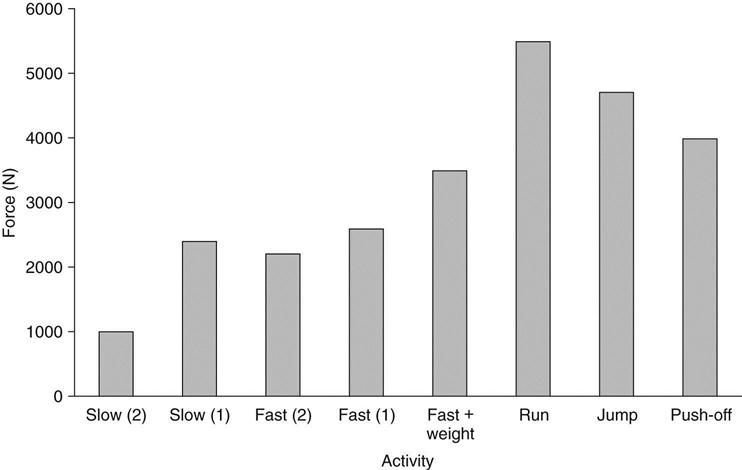
Normally, tendons are able to tolerate high forces associated with daily activities and athletics. However, if degenerative changes take place, then the mechanical load usually tolerated may exceed the tendon’s physiologic capacity. Mechanically, a tendon may be damaged or ruptured by a sudden application of force. This often involves a forceful lengthening or eccentric muscle contraction. Sudden maximal muscle activation results in a larger than normal force that is applied rapidly to the tendon, causing the rupture. The high load forces that cause such rupture are usually associated with vigorous activities such as sports. The forces to which the Achilles tendon is exposed during activities such as running and jumping have been calculated to be between 4000 and 5500 N21–23; they are summarized in Fig. 31-3.19 Arner and Lindholm24 describe three activities that can rupture a tendon:
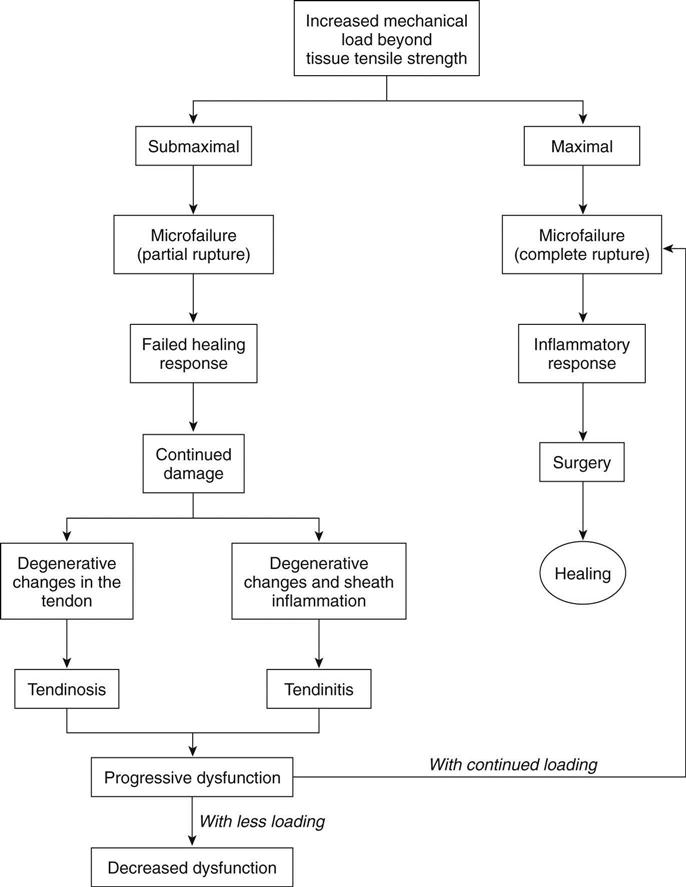
Curwin19 best summarized the possible progression of tendon injury (Fig. 31-4).
Epidemiology
Reports regarding the incidence, cause, and conservative, surgical, and postoperative management of Achilles tendon ruptures have increased during the past 50 to 60 years. The number of cases reported may be attributed not only to the observation and diligence of the health care community in publishing their research and thus improving patient care but also to the fact that the general population has increased its level of participation in recreational activity.
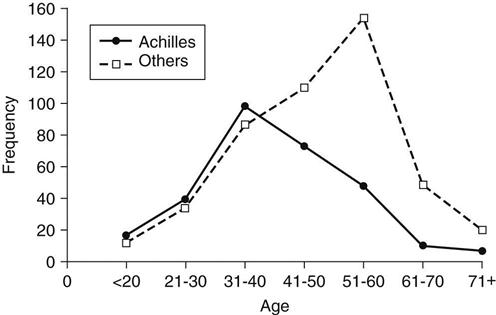
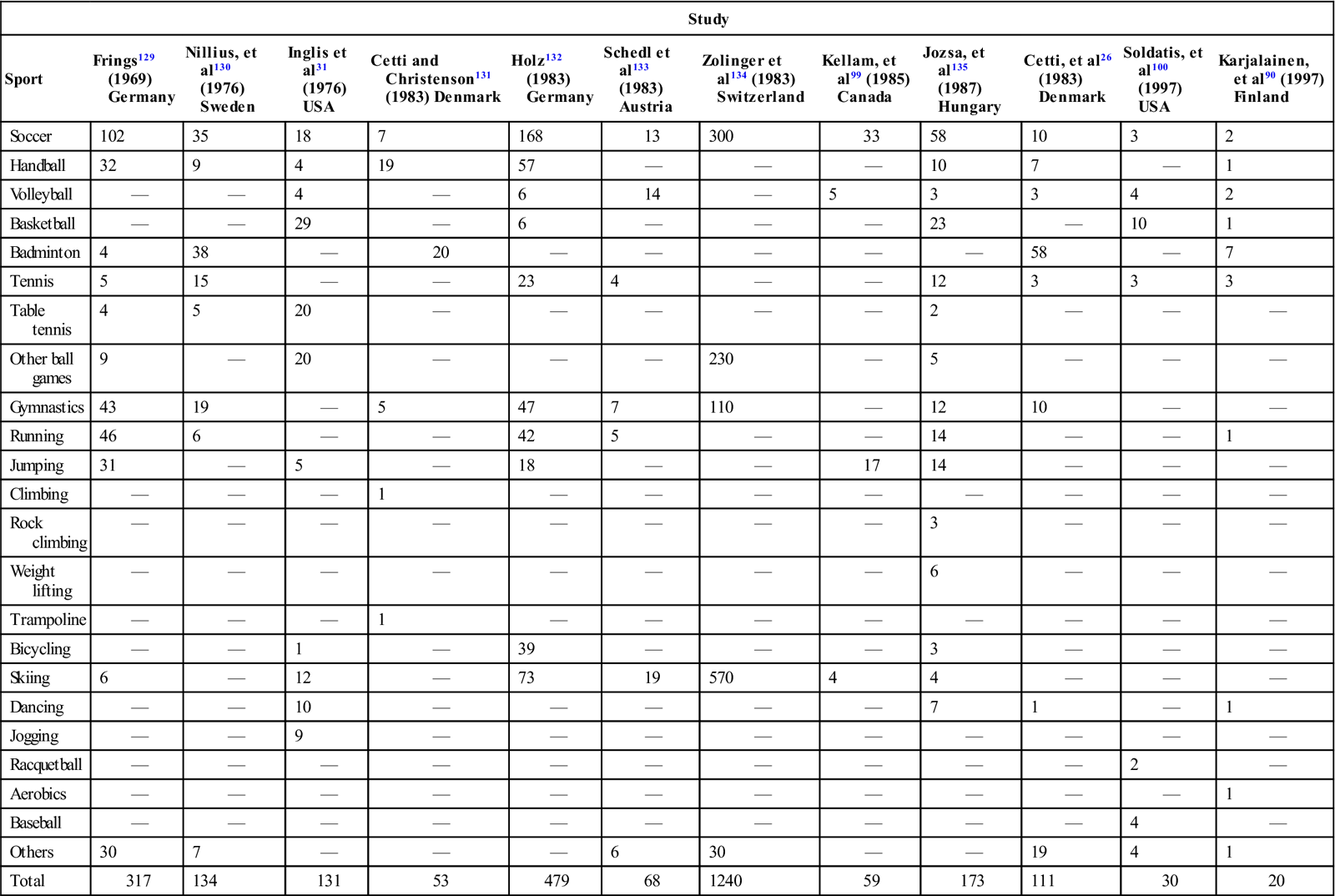
Although spontaneous tendon ruptures are rare, the Achilles tendon appears to be the one that is most frequently ruptured. Kannus and Jozsa12 report that 44.6% (397 out of 891) of tendon ruptures treated surgically between 1968 and 1989 involved the Achilles tendon, whereas the biceps brachii accounted for 33.9%. Achilles tendon ruptures are usually traumatic and occur between the ages of 30 and 40 years,25–27 which is younger than for other tendon ruptures (Fig. 31-5).
Most ruptures are suffered by recreational athletes rather than highly competitive, very active athletes. Of the 105 patients with Achilles ruptures described by Nistor,28 only 9 participated in competitive sports. Of the remaining patients, 35 exercised twice a week, 41 once a week, 20 took walks and occasionally exercised, and two were physically inactive. Of the 111 patients who ruptured their Achilles tendon while participating in a sports activity in Cetti and colleagues’ report,26 92 (83%) averaged only 3.6 hours of athletic activity per week. Of the 292 Achilles tendon ruptures documented by Jozsa and associates,27 59% occurred during recreational athletic activity—141 of these (83.2%) in men and 29 (16.8%) in women. No patients in this study by Jozsa and colleagues participated in competitive athletics. In their study, more than 625 of the Achilles tendon ruptures occurred in professional or white collar workers who tended to have generally sedentary lifestyles except when involved in sports. A review of numerous studies demonstrates that athletic activities that require sudden acceleration or deceleration are most likely to cause a rupture (Table 31-1). Ruptures not attributed to athletic activity are usually caused by falls or stumbles that also produce sudden acceleration and deceleration movements. Overall, Achilles tendon ruptures have been demonstrated to be more common in men than in women. Ratios of 2 : 1,29 4 : 1,30 1.6 : 1,31 8.7 : 1,28 10 : 1,25 and 12 : 110 have been reported.
TABLE 31-1
Distribution of Achilles Tendon Ruptures According to Sport
< ?comst?>
| Study | ||||||||||||
| Sport | Frings129 (1969) Germany | Nillius, et al130 (1976) Sweden | Inglis et al31 (1976) USA | Cetti and Christenson131 (1983) Denmark | Holz132 (1983) Germany | Schedl et al133 (1983) Austria | Zolinger et al134 (1983) Switzerland | Kellam, et al99 (1985) Canada | Jozsa, et al135 (1987) Hungary | Cetti, et al26 (1983) Denmark | Soldatis, et al100 (1997) USA | Karjalainen, et al90 (1997) Finland |
| Soccer | 102 | 35 | 18 | 7 | 168 | 13 | 300 | 33 | 58 | 10 | 3 | 2 |
| Handball | 32 | 9 | 4 | 19 | 57 | — | — | — | 10 | 7 | — | 1 |
| Volleyball | — | — | 4 | — | 6 | 14 | — | 5 | 3 | 3 | 4 | 2 |
| Basketball | — | — | 29 | — | 6 | — | — | — | 23 | — | 10 | 1 |
| Badminton | 4 | 38 | — | 20 | — | — | — | — | — | 58 | — | 7 |
| Tennis | 5 | 15 | — | — | 23 | 4 | — | — | 12 | 3 | 3 | 3 |
| Table tennis | 4 | 5 | 20 | — | — | — | — | — | 2 | — | — | — |
| Other ball games | 9 | — | 20 | — | — | — | 230 | — | 5 | — | — | — |
| Gymnastics | 43 | 19 | — | 5 | 47 | 7 | 110 | — | 12 | 10 | — | — |
| Running | 46 | 6 | — | — | 42 | 5 | — | — | 14 | — | — | 1 |
| Jumping | 31 | — | 5 | — | 18 | — | — | 17 | 14 | — | — | — |
| Climbing | — | — | — | 1 | — | — | — | — | — | — | — | — |
| Rock climbing | — | — | — | — | — | — | — | — | 3 | — | — | — |
| Weight lifting | — | — | — | — | — | — | — | — | 6 | — | — | — |
| Trampoline | — | — | — | 1 | — | — | — | — | — | — | — | — |
| Bicycling | — | — | 1 | — | 39 | — | — | — | 3 | — | — | — |
| Skiing | 6 | — | 12 | — | 73 | 19 | 570 | 4 | 4 | — | — | — |
| Dancing | — | — | 10 | — | — | — | — | — | 7 | 1 | — | 1 |
| Jogging | — | — | 9 | — | — | — | — | — | — | — | — | — |
| Racquetball | — | — | — | — | — | — | — | — | — | — | 2 | — |
| Aerobics | — | — | — | — | — | — | — | — | — | — | — | 1 |
| Baseball | — | — | — | — | — | — | — | — | — | — | 4 | — |
| Others | 30 | 7 | — | — | — | 6 | 30 | — | — | 19 | 4 | 1 |
| Total | 317 | 134 | 131 | 53 | 479 | 68 | 1240 | 59 | 173 | 111 | 30 | 20 |
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Adapted from Jozsa L, et al: The role of recreational sport activity in Achilles tendon rupture: A clinical, pathoanatomical, and sociological study of 292 cases. Am J Sports Med 17(3):338, 1989.
Diagnosis of Acute Achilles Tendon Rupture
In most situations the history is diagnostic. Patients describe hearing a pop as though someone had shot them in the back of the ankle. Prodromal symptoms of Achilles aching have been reported in 5% to 30% of cases.12,32 The rupture commonly occurs in the “watershed area,” between 2 and 6 cm proximal to the calcaneus.3 Avulsion fractures of the calcaneus are relatively uncommon.4 A careful history and physical examination are important because it has been shown that 22% of primary care physicians miss the diagnosis of acute Achilles rupture.26 The physical examination is characterized by palpation of the defect and documentation by Thompson test,33 which indicates discontinuity and loss of plantar flexion when the calf is squeezed. The Thompson test is performed by asking the patient to kneel on a chair with the feet hanging over the edge and the calf muscles relaxed. In this prone position, the examiner can usually denote a difference in resting angle from the contralateral side. Radiographic evaluation rules out the presence of a bony injury. Magnetic resonance imaging (MRI) can be helpful in demonstrating the presence, location, and severity of tears of the Achilles tendon. MRI also is helpful in assessing the status of an Achilles tendon repair.34 Ultrasonography has been used to define Achilles tendon discontinuity35 in countries where MRI is not routinely used. No one clinical test has been shown to be superior; however, a combination of a complete history and physical examination along with radiographic studies (if necessary) will lead to an accurate diagnosis.36
After an accurate diagnosis is made, a definitive physical therapy and rehabilitation program can be implemented by establishing the objectives for management. If any questions remain regarding the diagnosis or severity of the tear, then MRI and ultrasonography can be used to enhance diagnostic accuracy. The physical therapist (PT) should define the patient’s functional and athletic goals, personal needs, and temporal priorities before making therapeutic judgments.
Nonoperative Versus Operative Management
Treatment for Achilles tendon ruptures was nonoperative until the twentieth century. It included immobilization with strapping, wrapping, and braces for varying periods of time.37 In 1929 Quenu and Stoianovitch38 stated that a rupture of the Achilles tendon should be operated on without delay. Christensen30 (1953) and Arner, Lindholm, and Orell4 (1958) compared patients treated surgically and those managed nonoperatively; the surgical group had better results. As the field of sports medicine progressed with new surgical techniques, including rigid internal fixation combined with rehabilitation, the optimal treatment for Achilles tendon rupture became controversial. Some studies supported nonoperative management of Achilles ruptures,28,39 as shown in the following editorial statement made in 1973: “In view of the excellent results obtainable by conservative treatment, it is doubtful whether surgical repair in closed rupture of the Achilles tendon can be justified.40” In an updated meta-analysis of reports on Achilles tendon injury, Khan and associates41 found that open operative treatment of acute Achilles tendon ruptures significantly reduces the risk of rerupture compared with nonoperative treatment, but it produces a significantly higher risk of other complications, including wound infection. Significance varied among studies included. In total, reruptures in the operative groups were 3.5% vsersus 12.5% in the nonoperative groups. Rates for other complications (infection, adhesions, disruptions in sensation) in the operative groups were 34% versus 2.7% in the nonoperative groups.
In 2003, Weber and colleagues42 compared the results of acute ruptures in 23 patients treated nonoperatively to 24 patients treated operatively. Patients in the nonoperative group were allowed full weight bearing in 20° patellofemoral casts for 6 weeks and were verified with weekly ultrasonography and cast changes. Patients in the operative group were placed in casts and were non–weight bearing for 6 weeks with variable rehabilitation protocols. The results showed a decreased return to work and crutch time for nonoperative cases, with 4 of 23 reruptures at 7 to 12 weeks. Patients in the operative group had only 1 of 24 reruptures at 3 years. This study demonstrates that nonoperative treatment may be acceptable for some patient populations, but that operative treatment, even with traditional postoperative immobilization, yields a lower rerupture rate. The surgeon must explore the goals and expectations of the patient and decide which treatment is most appropriate. Indications for nonoperative treatment include concomitant illness in the patient, a sedentary lifestyle, or lower functional and athletic goals.
Early nonoperative options were well accepted and tolerated. But in the past 20 years, patient expectations and functional goals have increased so that surgical options have gained acceptance and preference.
Less invasive surgical procedures were first noted in the literature by Ma and Griffith.43 A lower overall rate of complications (particularly infection) in the percutaneously treated group has been noted.41,44,45 Studies comparing open versus percutaneous techniques have a relatively small number of subjects, limiting their reliability.
The patient selected for operative repair should be an individual who is extremely interested in optimal functional restoration.
In 2010 the American Academy of Orthopaedic Surgeons published guidelines on the treatment of acute Achilles tendon ruptures. A meta-analysis of all existing literature showed similar outcomes in operative and nonoperative treatment. The study demonstrated two moderate-strength recommendations that included suggestions for early postoperative protective weight bearing and for the use of protective devices that allow for postoperative mobilization in those patients who have surgical treatment.36
Functional postoperative treatment programs, regardless of surgical technique, avoid cast immobilization and are well tolerated, safe, and effective with well-motivated athletes and patients who especially desire the highest functional outcome.13,46–50
Acute Care of the Achilles Tendon Rupture
In the last century, the literature has proposed numerous approaches to the management of acute Achilles tendon ruptures. The most important design principles of the option selected should include the following:
1 The option and procedure are safe and effective.
2 The method allows the patient to accomplish realistic goals.
3 The surgeon can execute the method successfully.
4 The risks of the method are acceptable to the patient and surgeon.
The categorical options for the surgical treatment of acute Achilles tendon rupture include repair, repair with augmentation, and reconstruction.
Surgical Procedure
Repair
The rationale for any repair method is to restore continuity of the ruptured tendon end, facilitate healing, and restore muscle function. The technical difficulty is taking relative “mop ends” and opposing them in a stable fashion. Bunnell51 and Kessler52 were the first to popularize the end-to-end suture technique for ruptured tendons. Ma and Griffith43 described a percutaneous repair in 1977; however, a higher rerupture rate also occurred in their series. Beskin25 introduced the three-bundle suture, and Cetti46 demonstrated the suture weave in 1988, which was further modified by Mortensen and Saether53 in 1991 as a six-strand suture technique. Nada54 described the use of external fixation for Achilles tendon ruptures in 1985. Richardson, Reitman, and Wilson55 described good results using a pull-out wire, which has the advantage of minimal suture reaction but requires a second procedure for suture removal. More recently, a commercial device has become available that allows for the percutaneous passing of a suture to repair a rupture through a minimally invasive incision. Jung and colleagues evaluated 30 patients with limited open repair for Achilles tendon rupture using a commercial device with an average 18 months follow-up and found that 16 patients were very satisfied, 11 were satisfied, and 3 were dissatisfied.56
Each of these techniques has advantages and disadvantages, hence the wide spectrum of options. The surgeon’s selection of a method should be based on technical training, an accurate pathoanatomic diagnosis, and the patient’s goals and desires.
Repair With Augmentation
Historically, augmentation procedures evolved to supplement the repair construct of mop ends plus suture. Most augmentation procedures involved the local use of gastrocnemius fascia flaps or the plantaris tendon if available. Christensen30 described the use of the gastrocnemius aponeurosis flap to augment Achilles tendon repair in 1981. Silfverskiold57 described a central rotation gastrocnemius flap, and Lindholm58 devised a method of two turndown flaps. Lynn59 used the plantaris tendon, fanning it out to use the membrane to reinforce the repair. Kirschembaum and Kellman60 modified the technique in 1980 by placing the fascial flaps centrally rather than separating them. Chen and Wertheimer61 demonstrated the use of suture anchors distally at the calcaneus to repair the gastrocnemius turndown flap with semirigid fixation. Overall, these methods can facilitate the continuity and strength of the repair construct when doubt persists concerning the repair’s integrity. In practice these techniques are applied only in limited situations, but they should still be in the surgeon’s armamentarium.
Reconstruction
Acute ruptures are usually managed with the repair techniques already described with or without augmentation. Usually these are appropriate to promote continuity and healing of acute ruptures. Neglected, chronic ruptures, however, require reconstruction with endogenous or exogenous materials. Endogenous materials include fascia lata,62 peroneus brevis transfer,63 flexor digitorum longus,64 and flexor hallucis longus.65 Exogenous materials include carbon fiber,66 Marlex mesh,67 Dacron vascular grafts,49 polylactic acid implant, and a polypropylene braid.68 Once again, the surgeon must be familiar with these procedures and understand their advantages and disadvantages before applying them in appropriate scenarios.
Concepts of Achilles Tendon Repair
Prolonged cast immobilization has been used with both operative and nonoperative treatment of Achilles tendon ruptures. Although cast immobilization may promote healing, it also promotes one or more of the following manifestations of “cast disease69,70”:
Immobilization after tendon surgery was considered the single factor most responsible for postsurgical complications as long ago as 1954.24,30,43 Various clinical studies in the literature document residual isokinetic strength deficits between 10% and 16% after cast immobilization of Achilles tendon injuries, regardless of whether they were managed operatively or nonoperatively.9,28,71–73
The AO Group of Switzerland found that stable and rigid internal fixation of bone fractures allows early range of motion (ROM) and maximal rehabilitation, thus minimizing atrophy and the manifestations of cast disease. This principle indicates that early mobilization of tendon ruptures should be promoted as long as stable fixation is ensured. In support of this concept, it has been demonstrated that early motion limits atrophy,74 promotes fiber polymerization to collagen,75 and increases the organization of collagen at the repair site, leading to increased strength.76–79 Krackow, Thomas, and Jones80 initially described a suture technique that allows “rigid” internal fixation without tendon necrosis. This technique, coupled with complete repair of the peritenon, should result in progressive and successful healing of the Achilles tendon rupture without postoperative cast immobilization.
Mandelbaum, Myerson, and Forster13 reported on the successful use of the Krackow modified suture technique in a series of 29 athletes with acute Achilles tendon rupture. Postoperatively the patients were not rigidly immobilized and were started on early ROM and conditioning programs. No patients in this series experienced rerupture, persistent pain, frank infection, or skin necrosis as a complication. By 6 weeks after surgery, 90% of patients had full ROM. By 6 months, 92% had returned to sports participation; strength deficits were less than 3% on isokinetic testing. Rigid internal fixation of Achilles tendon tears allowed a more functional rehabilitation process in this series, including early motion and weight bearing. Maffulli and colleagues,32 who reported on the operative results of 42 patients who were randomized to a traditional protocol of postoperative immobilization or a full weight bearing protocol, strengthened these results. In the full weight-bearing group, patients were placed in a full weight-bearing cast for 2 weeks, then a full weight-bearing dorsiflexion splint for 4 weeks. In the traditional protocol, the patients were immobilized in a cast for 4 weeks, then a full weight bearing cast for 2 more weeks. They found that the full weight-bearing group had no crutch use at 1.5 weeks, a quicker return to work, less calf atrophy, greater patient satisfaction, less patient visits, and isometric strength equal to the traditional group.32 This method of accelerated but controlled postoperative management has proven to be safe and highly effective at returning the athlete and patient to activities of daily living (ADLs) and sports with the highest level of function.13,14,81
Preoperative Treatment
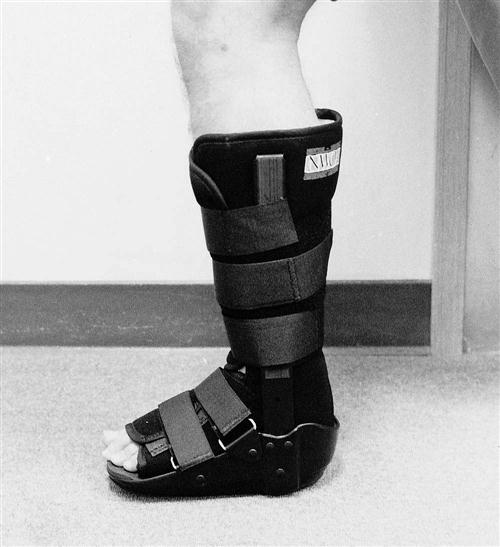
After the diagnosis is made, the patient should be placed in a compressive elastic or cohesive tape wrap to minimize swelling. They should be encouraged to use ice and elevate the extremity. If possible, the patient should be allowed to ambulate in a cam walker boot or with a cane to promote circulation and prevent venous thrombosis. Surgery should be performed in an outpatient setting 7 to 10 days after the injury. This delay allows consolidation of the tendon ends, making repair technically easier.
Surgical Technique
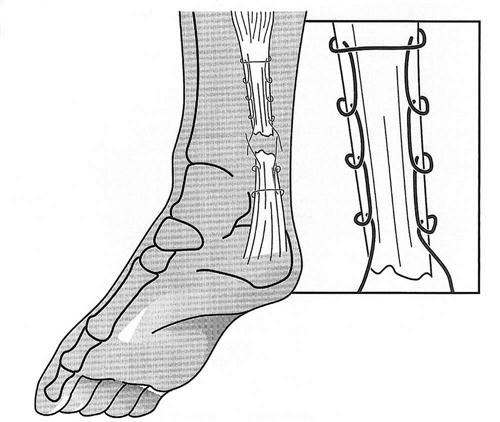
Surgery is performed with the patient in the prone position under general, regional, or local anesthesia. Care should be taken to note the resting tension of the opposite foot. To be most accurate, both feet can be prepped to allow accurate side-to-side comparison of tendon length. Either a straight midline or an anteromedial incision is made just medial to the gastrocnemius. A direct incision is made through the peritenon, which is split and tagged. Before repair of the tendon, any adhesions between the anterior surface of the muscle and tendon unit and the paratenon are removed. A relaxing incision in the anterior surface of the paratenon is made down to the muscle of the flexor hallucis longus, which will facilitate closure of the paratenon. Each end of the tear is sewn with a No. 2 nonabsorbable suture using the Krackow suture technique (Fig. 31-6). The recently introduced synthetic, polyethylene sutures such as Fiberwire* or Orthocord† are now commonly used for the repair and have been shown to have superior strength to traditional braided sutures.82 The sutures are tensioned appropriately to achieve the same resting angle as the contralateral extremity. If any doubt exists regarding the amount of tension, then the contralateral side may be used for comparison. The suture knots are passed to the anterior aspect of the tendon and secured. The peritenon is then closed anatomically with 4-0 absorbable suture. The ankle is taken through a ROM to evaluate the stability of the repair construct. The wound is closed with dermal mattress sutures, with the knots based on the medial side to protect the tendon; a posterior splint is applied. On the second day after surgery, the splint is removed and the ankle is taken gently through active range of motion (AROM). Gentle ROM exercises are begun. The sutures are removed by about day 14, and a progressive weight-bearing program is initiated using a walker boot.
Percutaneous Repair
Percutaneous repair of the Achilles tendon has become an acceptable alternative method to open repair. Proponents of the technique have cited a lower wound complication rate and similar rerupture rates.83–87 Halasi, Tallay, and Berkes46 performed an endoscopically assisted repair on 123 patients and had no wound problems, a return to sport in 4 to 6 months, and no wound complications. Although sural nerve irritation can be a complication of the technique, it has been postulated that endoscopic assistance of the procedure lowered the sural nerve complication rate.
Percutaneous Repair Technique
Buchgraber and Pässler83 described the following surgical technique. Patients are placed in a prone position and given a short-acting general anesthetic. The tendon defect is identified by palpation, and a transverse or longitudinal incision is made along the skin folds across its center. The associated hematoma is deliberately left in place. Using a 15.3-mm blade, stab incisions are made at the medial and lateral aspects of the tendon approximately 10 to 12 cm above the site of the tear. Another two stab incisions of the same length are made above the calcaneus, medial and lateral to the insertion of the Achilles tendon. To prevent sural nerve injury, a mosquito clamp is placed in the proximal lateral stab incision to retract the skin and the underlying fascia. With the help of a cutting needle,* a 1.2-mm polydioxanone suture (PDS) cord is pulled through proximally from the medial to the lateral stab incision. Then the cutting needle is introduced through the incision overlying the tear, passed through the tendon proximally, and advanced toward the lateral proximal stab incision. Using the cutting needle, the end of the PDS cord at this site is passed out through the central incision. Next the cutting needle is pushed into the tendon tissue from the lateral distal stab incision, brought out centrally to load it with the lateral end of the PDS cord, and pulled out distally. This end of the cord is grasped with the needle and introduced through the medial distal stab incision, brought out through the lateral distal stab incision, and pulled through medially. In the final step, the needle is passed through the proximal tendon end from the central incision and brought out at the medial proximal stab incision. The foot is put repeatedly through the full ROM to ensure that the cord will dig into the tendon tissue. The ends of the cord are tied with the foot in slight plantar flexion. Once the first knot is tied, the foot is dorsiflexed several times to check the tension on the cord. The procedure is concluded with another two knots added in a crisscross pattern.83
Potential Complications
The major complication with nonoperative treatment is a higher rerupture rate and an incomplete return of function and performance. Complications associated with the surgical technique include infection, anesthetic problems, rerupture, deep vein thrombosis, and an incomplete return of function. Infection can be a disastrous complication because soft tissue coverage is a major problem and can only be resolved with vascularized flaps and a reconstructive tendon procedure.88
Therapy Guidelines For Rehabilitation
Consideration Toward the Healing Tendon
The use of early motion during postoperative rehabilitation requires the PT to have a working knowledge of the process of tissue healing. With this knowledge, the therapist can apply appropriate amounts of stress at the correct times, progressing the rehabilitation program at an optimal pace and ensuring a good clinical outcome. Extensive summaries by Leadbetter,89 Curwin,19 and Curwin and Stanish22 fully describe tendon physiology and healing.
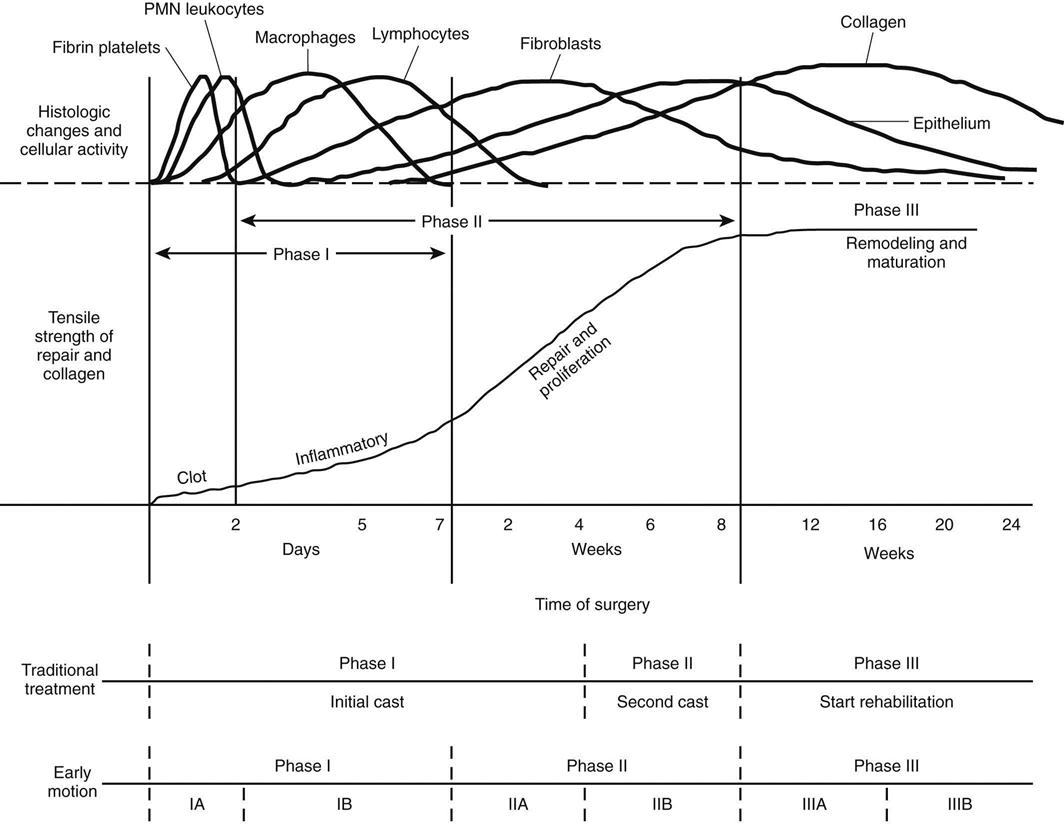
Fig. 31-7 summarizes the stages of the healing process and the implications they have for traditional postoperative management of Achilles tendon repairs and motion in the early postoperative period. Tendon healing occurs in four consecutive, related phases, with somewhat overlapping time frames.
Stages of Healing
Inflammatory Response
Minutes after injury, laceration, or the initiation of surgical repair, a coagulation response occurs, triggering the formation of a fibrin clot. This clot contains fibronectin, which is essential to reparative cell activity. Fibronectin eventually creates a scaffold for cell migration and supports fibroblastic activity. Soon after this clot forms, polymorphonuclear leukocytes and macrophages invade the area to clear cellular and tissue debris. The resulting arachidonic acid cascade is the primary chemical event during this stage. This stage is usually complete in less than 6 days unless infection and wound disturbance occur.
Repair and Proliferation
The repair and proliferation stage may begin as early as 48 hours after injury and may last for 6 to 8 weeks. Tissue macrophages are the key factors early in this stage. The macrophage is mobile and capable of releasing various growth factors, chemotactants, and proteolytic enzymes when necessary or appropriate for the activation of fibroblasts and tendon repair.89 Fibroblastic proliferation continues and produces increasing amounts of collagen. Type III collagen, which has poor cross-link definition, small fibril size, and poor strength, is initially deposited rapidly. As the repair process continues, collagen deposition shifts to type I collagen, which has greater cross-link definition, fibril size, and strength. The deposition of type I collagen accelerates and continues throughout this stage and well into the remodeling and maturation stage.
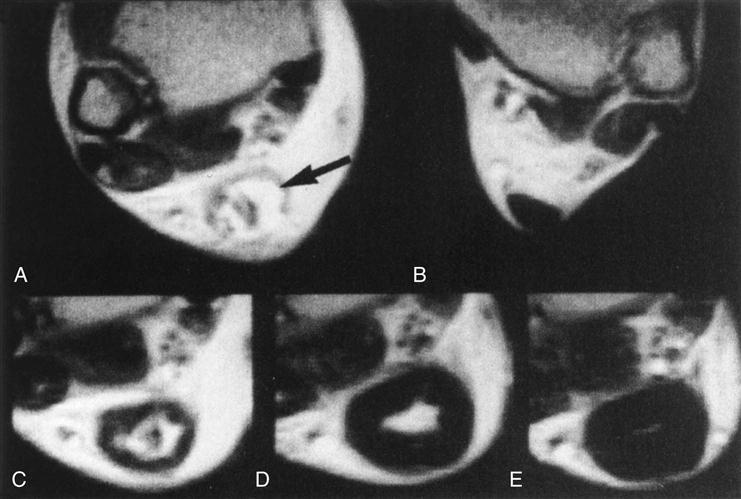
Changes in the postoperative internal structure of 21 surgically repaired Achilles tendons have been documented by Karjalainen and colleagues90 during the healing process using MRI. After surgery, patients were placed in casts in equinus position for 3 weeks without weight bearing and for another 3 weeks in a short walking cast in neutral position, with weight bearing as tolerated. After cast removal, patients began AROM and walking exercises. MRI was repeated at 3 weeks, 6 weeks, 3 months, and 6 months after repair to document changes (Fig. 31-8). During this stage the postoperative cross-sectional repaired area Achilles tendon increased dramatically, measuring 2.9 and 3.4 times the size of the uninjured contralateral tendon at 3 and 6 weeks, respectively. A diffuse, high-intensity heterogeneous signal was present at the repair site for all 21 tendons at 3 weeks and in 13 of 21 tendons at 6 weeks. In the other eight tendons, early formation of high-intensity intratendinous signal was present in the center of the tendon at the level of repair.
Remodeling and Maturation
Remodeling and maturation of collagen fibrils and cross-links characterize the third healing stage. Overall a trend toward decreased cellularity and synthetic activity, increased organization of the extracellular matrix, and a more normal biochemical profile is evident.91 Functional linear alignment of the collagen fibrils is usually present by 2 months.89 Although maturation appears to be complete a number of months after the injury, biochemical differences in collagen type and arrangement, water content, DNA content, and glycosaminoglycan content persist indefinitely. The material properties of these scars never become identical to those of intact tendon.8 Biomechanical properties can be reduced by as much as 30% despite the completion of all stages of healing and maturation.8,22,92,93 Karjalainen and associates61 found that the cross-sectional area of the tendon continues to increase. The area is 6.1 times the size of the unaffected tendon at 3 months and 5.6 times the size at 6 months. A variably sized, high-intensity signal demonstrating a central intratendinous lesion was detected in 19 of 21 repaired tendons at 3 months. The development of an intratendinous lesion appears to be a normal part of the healing process after surgical repair and casting.
Future Directions
Gene therapy and growth factors have an emerging role in the treatment of bone, cartilage, and tendon healing.94,95 Tendon callus can be influenced by cartilage derived morphogenic protein-2 (CDMP-2).95 Using an animal model of rabbit Achilles tendon tears, Forslund and Aspenberg96 showed that failure load and stiffness in a CDMP-2 group were greater at 14 days compared with controls. Future treatments may include addition of growth factors to the repair site.
Postoperative Management: Traditional Immobilization and Remobilization Versus Early Motion
Since surgical repair of Achilles tendon ruptures began almost 50 years ago,37 the traditional method of postoperative management has been to immobilize the repair in a plaster cast or other type of restrictive device until healing is considered complete, and then begin ROM and strengthening exercises.12,26,31,32,97–102 Based on an understanding of connective tissue physiology and the success achieved with other types of surgical repairs, such as anterior cruciate ligament reconstruction, several authors have begun using early postoperative motion to minimize the deleterious effects of immobilization on joints (e.g., stiffness and loss of muscle strength, endurance, and flexibility)74,93,103,104 and facilitate an earlier return to preoperative functional levels.* Numerous studies have compared immobilization to early postoperative motion12,32,101,108–110 and early weight bearing.12,32,101,102,109–111 In a meta-analysis, Khan found that functional bracing and early motion resulted in fewer adhesions, disturbed sensitivity, hypertrophic scarring, and infection with no difference in the rerupture rates between early motion and traditional immobilization groups.41 Early functional programs provide better patient satisfaction without an increase in rerupture rates compared with cast immobilization.109 However, no studies have compared different early motion rehabilitation regimens. Varying protocols make comparison between studies difficult. The guidelines presented are a compilation of various approaches considering appropriate tendon healing.
Outcomes Measurements
Several outcomes measurement instruments have been used in Achilles tendon rupture research. In addition to objective clinical measures such as ROM, calf circumference, and muscle testing, instruments for function and patient satisfaction help the clinician to set goals and determine success of treatment. Early research used nonvalidated scoring methods for pain, stiffness, activity limitations, footwear restrictions, patient satisfaction, strength, and ROM.111–114 The Victorian Institute of Sport Assessment was validated as an index of severity for patients with Achilles tendinopathy and has been used in Achilles tendon rupture research.32,115 Although found to be reliable and valid, the degree of responsiveness has not been established. Patients use a visual analogue scale to score eight items for morning stiffness, pain with walking, descending stairs, heel raises, single-leg hops, and ability to participate in sports and physical activities.116
The foot and ankle ability measure (FAAM) assesses activity limitations and vocational and avocational restrictions for individuals with general musculoskeletal foot and ankle disorders. The FAAM uses a Likert scale for a 21-item ADL/activities subscale and an 8-item sport subscale for a perfect score of 100 with a minimal clinically important difference (MCID) of 8 points for ADL and 9 points for sports.117,118 In 2007 an outcomes measurement specific to Achilles tendon ruptures was developed and found to be valid and reliable but has not been reported in a randomly controlled trial.110,119,120 The Achilles tendon total rupture score (ATRS ) uses 10 items rated on a 10-point scale for difficulty with strength, fatigue, stiffness, pain, ADLs, walking on level surfaces, ascending stairs or hills, running, jumping, and physical labor. We suggest that a change of 10 points in the score is clinically relevant.119
Guidelines for Traditional Immobilization and Remobilization
Immobilization might be in a conventional cast12,32,45,101,102 or a fixed-angle ankle foot orthosis.109 The time of immobilization has been reported from 4 weeks102,109 to 8 weeks.101 Cast changes can occur during the immobilization period for wound care or decreasing the degree of plantar flexion. Weight bearing has been reported as early as 2 weeks.102
Phases I and II
TIME: 1 to 8 weeks after surgery
GOALS: Minimize deconditioning, control edema and pain, encourage independent gait (non–weight bearing until cleared by the physician to progressive weight bearing, usually at 4 weeks) with assistive device as appropriate
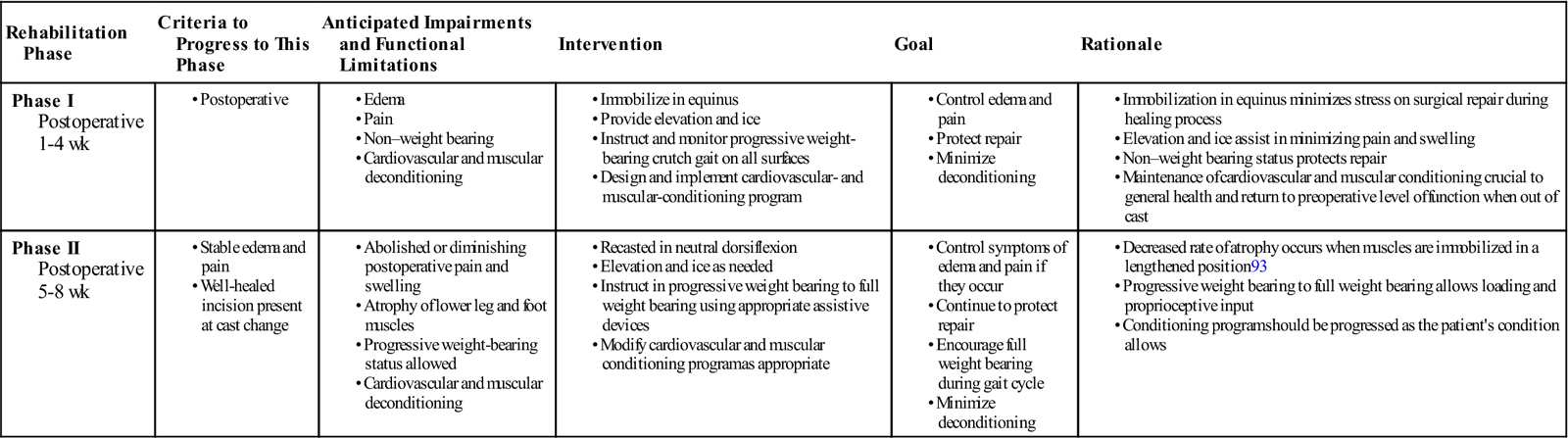
During traditional postoperative rehabilitation of surgically repaired Achilles tendon ruptures, a cast is usually in place throughout phases I and II of the healing process (Table 31-2). The PT can do little to influence healing and affect the outcome of the surgical repair. Casting incorporates varying degrees of plantar flexion to protect the repair from stress. Casts that were initially applied with the foot in plantar flexion are usually changed to neutral (0° dorsiflexion) for the final 3 to 4 weeks of immobilization. During this period, a conditioning program is designed to maintain the patient’s general strength and cardiovascular conditioning. After the cast is removed, the PT can further protect the tendon from full stress by using a heel lift of varying heights for as long as 8 weeks, if needed. Crutches with progressive weight bearing also may be used to control mechanical stress.
TABLE 31-2
Achilles Tendon Repair (Traditional Rehabilitation)
< ?comst?>
| Rehabilitation Phase | Criteria to Progress to This Phase | Anticipated Impairments and Functional Limitations | Intervention | Goal | Rationale |
| Phase I Postoperative 1-4 wk |
|
||||
| Phase II Postoperative 5-8 wk |
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Phase III
TIME: 9 to 16 weeks after surgery
GOALS: Normalize gait, increase ROM and strength, improve scar mobility
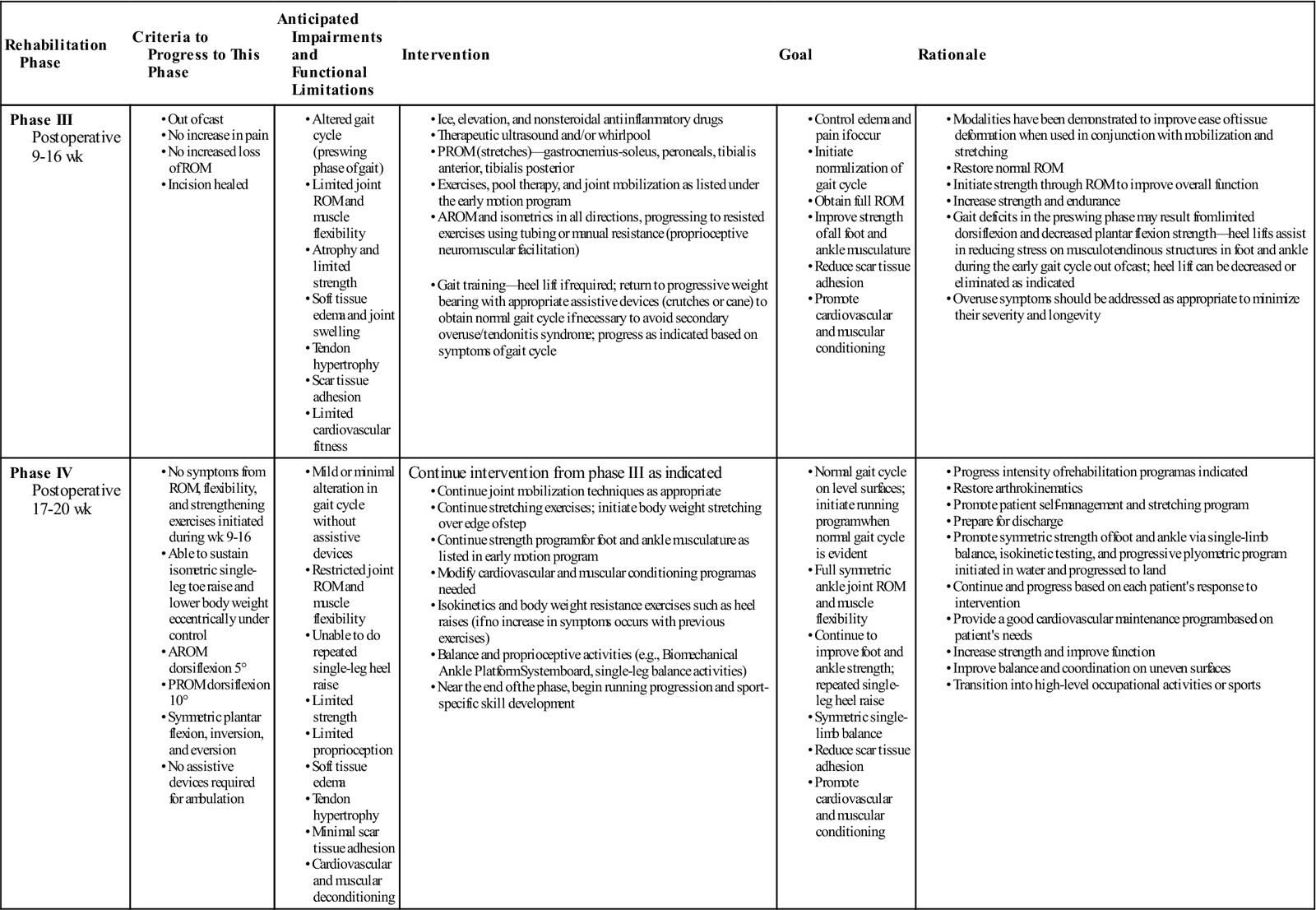
With the traditional approach, the rehabilitation program does not truly begin until phase III. The scar and tissue have begun to mature and therefore ROM, joint mobilization, stretching, strengthening, gait training, and return to function may progress as tolerated (Table 31-3). The sequence of treatment depends more on resolving the patient’s physical impairments and functional limitations than on the timeline of healing. Impairments are resolved to reestablish function, develop skills, and return the patient to full activity and sports participation.
TABLE 31-3
Achilles Tendon Repair (Traditional Rehabilitation)
< ?comst?>
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
AROM, Active range of motion; PROM, passive range of motion; ROM, range of motion.
The initial goal is to restore ROM. Restoration of mobility must occur before the patient can begin to work on strength. Active exercises in the sagittal (dorsiflexion and plantar flexion) and transverse (inversion and eversion) planes are initiated and progressed with the knee flexed and extended. The therapist should use joint mobilization techniques to assist in gaining joint ROM and stretching exercises to gain muscle flexibility and improve joint ROM (see figures in the Chapter 29 describing mobilization of the ankle).
![]() Any symptoms of pain and swelling that occur must be controlled during this phase through the use of appropriate modalities and adjustments in the intensity of the treatment plan and home program. For return to progressive weight bearing use appropriate assistive devices (crutches or cane) to obtain normal gait cycle if necessary to avoid secondary overuse/tendonitis syndrome. Progress as able based on symptoms of gait cycle. Soft tissue mobilization can be used to reduce scar adhesion. These adjuncts for restoring ROM, muscle and tendon flexibility, and strength are continued throughout the program as appropriate (see Table 31-3).
Any symptoms of pain and swelling that occur must be controlled during this phase through the use of appropriate modalities and adjustments in the intensity of the treatment plan and home program. For return to progressive weight bearing use appropriate assistive devices (crutches or cane) to obtain normal gait cycle if necessary to avoid secondary overuse/tendonitis syndrome. Progress as able based on symptoms of gait cycle. Soft tissue mobilization can be used to reduce scar adhesion. These adjuncts for restoring ROM, muscle and tendon flexibility, and strength are continued throughout the program as appropriate (see Table 31-3).
Phase IV
TIME: 17 to 20 weeks after surgery
GOALS: Demonstrate normal gait on level surfaces, initiate running program, have full ROM, increase strength, improve balance and coordination
The therapist can initiate strengthening as ROM progresses. Initially, isometric techniques are used for all motions. Strength training then progresses to the use of elastic tubing and manual resistive techniques, such as proprioceptive neuromuscular facilitation. Isokinetic exercise and body weight resistance can be added if symptoms do not arise from the other techniques. The patient should begin double-heel raises before single-heel raises to reduce tensile stress and the potential for symptoms such as pain and swelling. The heel raises should initially be performed on a level, flat surface, after which the patient can progress to doing them over the edge of a stair to use the full ROM available. Proprioceptive and balance activities should be initiated concurrently with strengthening. The PT can begin a running progression, sport-specific skill development, and functional activities after the patient has a normal gait and full ROM and can rapidly perform heel raises. Isokinetic measurement of strength, power, and endurance at 4 to 6 months may assist in the determination of return to sport activity, but it is not the sole determining factor.
The success of Achilles tendon repair using traditional immobilization and rehabilitation has been favorably measured, primarily by rerupture rates, strength, calf circumference, tendon width, and return to previous activity levels. In a meta-analysis comparing cast immobilization and functional bracing, Khan reported a pooled rerupture rate for traditional immobilization of 5% and other complications (adhesions, infection, and disturbed sensibility) at 35.7%.41 Early weight bearing in a cast was found to have no adverse effect as compared with the non–weight bearing group.102 The weight-bearing group discarded crutches approximately 3 weeks earlier than the non–weight bearing group, with no differences noted in tendon size measured by ultrasound and in isometric strength. Strength measurements include the ability to perform single-toe raises, manual muscle tests, and isokinetic strength measurements. Authors using peak torque measurements of isokinetic plantar flexion strength have reported that the gastrocnemius and soleus strength of the repaired Achilles tendon ranges from 83% to 101% of the uninvolved extremity.37 Some authors use combined isokinetic deficits at several speeds, ranking results poor to excellent, with good to excellent results in 71% to 79% of the subjects.112,114 Outcome measures in studies that have been reported in the literature are difficult to use in comparing various studies.37 These studies used different operational definitions, set forth different methodologies, and collected data at different times, making meaningful comparison difficult.
Guidelines for Early Motion
As early as 1984, in an effort to reduce the effects of immobilization, various authors began to present methods of repair that would allow early motion and rehabilitation.13,49,86,106,120–123 Orthoses or specialized splinting were developed to protect the repair after surgery.* Only one report, a case series, described an early motion protocol, no postoperative fixation, and no postoperative complications within 24 weeks. Protected AROM begins as early as day 1.120 In some cases AROM begins at 2 weeks.32,109,123 The functional orthosis is removed most frequently at 6 weeks, sometimes with use of a heel lift for several weeks. Weight bearing begins immediately32,101,110 or up to 2 weeks after surgery109 and is generally progressed as tolerated. ![]() Regardless of the type of repair, rehabilitation programs that emphasize early motion must consider the strength and integrity of the repaired Achilles tendon as it changes during the various stages of healing.
Regardless of the type of repair, rehabilitation programs that emphasize early motion must consider the strength and integrity of the repaired Achilles tendon as it changes during the various stages of healing.
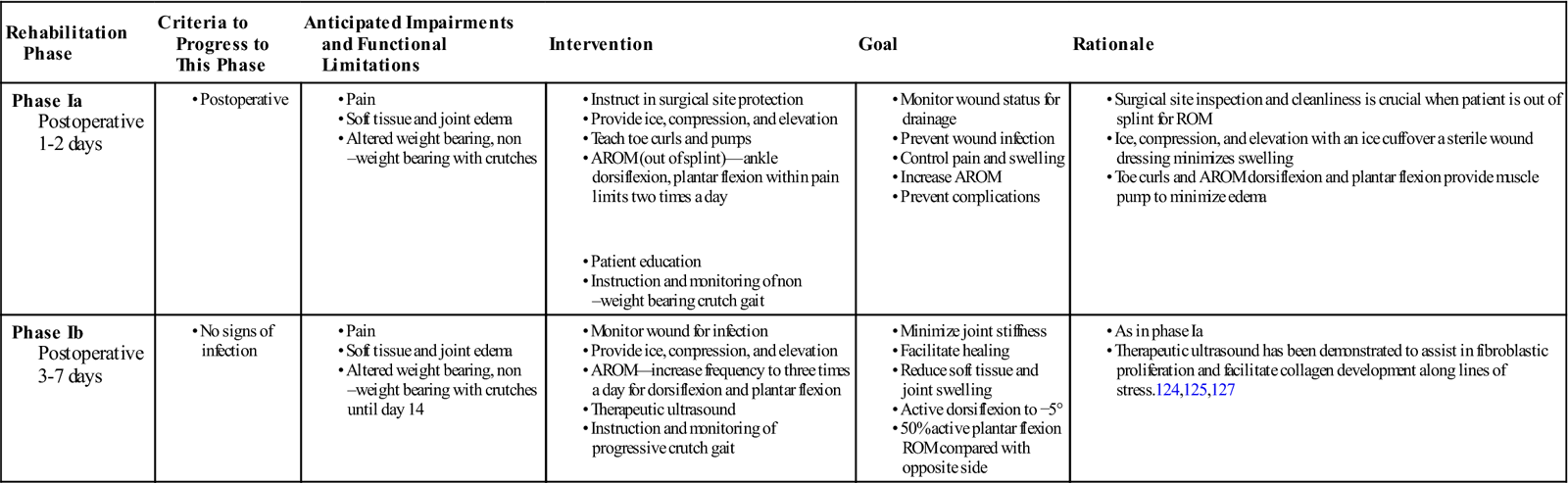
Phase Ia
TIME: 1 to 2 days after surgery
GOALS: Prevent infection, control edema and pain, increase AROM, prevent complications, promote independent gait using assistive device as appropriate
Phase Ib
TIME: 3 to 7 days after surgery
GOALS: Demonstrate AROM dorsiflexion to 5° and plantar flexion 50% of uninvolved side, control edema and pain
![]() When using an early motion program for postoperative rehabilitation, the therapist must keep in mind the phases of tissue healing and the degree to which tissues can tolerate tensile stress. During phases Ia and Ib of the early motion program, the primary concerns are evaluating wound status, decreasing swelling, and initiating ROM exercises (Table 31-4). However, ROM should not be pushed aggressively. The patient should work through PROM within the limits of pain and swelling intermittently throughout the day. The PT may prescribe ice, compression, and elevation to decrease swelling.
When using an early motion program for postoperative rehabilitation, the therapist must keep in mind the phases of tissue healing and the degree to which tissues can tolerate tensile stress. During phases Ia and Ib of the early motion program, the primary concerns are evaluating wound status, decreasing swelling, and initiating ROM exercises (Table 31-4). However, ROM should not be pushed aggressively. The patient should work through PROM within the limits of pain and swelling intermittently throughout the day. The PT may prescribe ice, compression, and elevation to decrease swelling.
TABLE 31-4
Achilles Tendon Repair (Early Motion)
< ?comst?>
| Rehabilitation Phase | Criteria to Progress to This Phase | Anticipated Impairments and Functional Limitations | Intervention | Goal | Rationale |
| Phase Ia Postoperative 1-2 days |
|||||
| Phase Ib Postoperative 3-7 days |
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
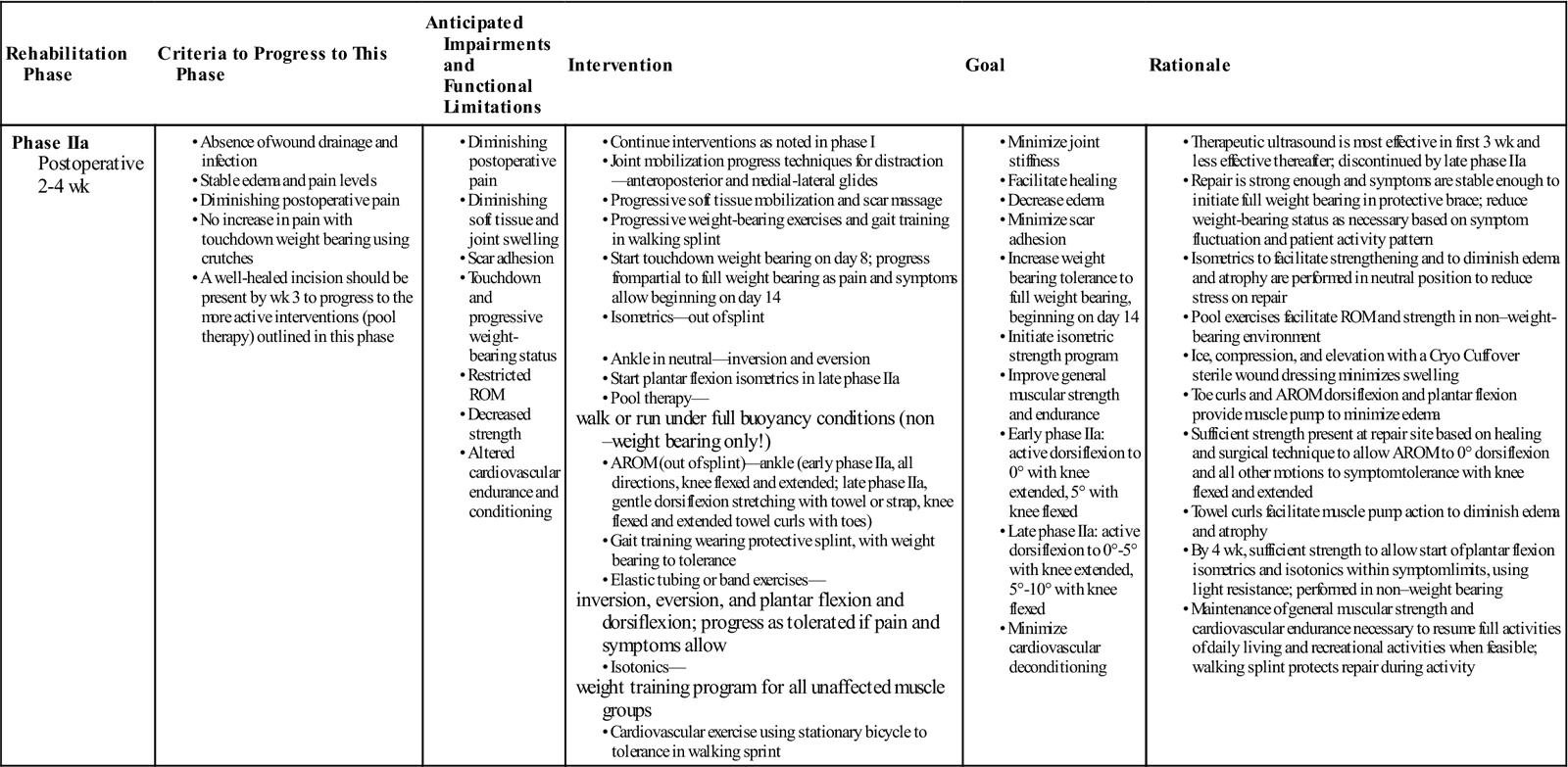
Phase IIa
TIME: 2 to 4 weeks after surgery
GOALS: Increase AROM and PROM, strength, and weight-bearing tolerance
During phase IIa (Table 31-5) of the early motion program, emphasis is placed on gaining active dorsiflexion past neutral, following a full weight-bearing program in a protective splint or boot with a fixed hinge (Fig. 31-9), and ![]() initiating a gentle strength-training program of all the muscle groups in a range that minimizes tensile stress on the repair.
initiating a gentle strength-training program of all the muscle groups in a range that minimizes tensile stress on the repair.
TABLE 31-5
Achilles Tendon Repair (Early Motion)
< ?comst?>
| Rehabilitation Phase | Criteria to Progress to This Phase | Anticipated Impairments and Functional Limitations | Intervention | Goal | Rationale |
| Phase IIa Postoperative 2-4 wk |
|
walk or run under full buoyancy conditions (non–weight bearing only!) inversion, eversion, and plantar flexion and dorsiflexion; progress as tolerated if pain and symptoms allow weight training program for all unaffected muscle groups |
|
|
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
During phases Ia and IIa, therapeutic ultrasound can be used to assist in the healing response. In some instances therapeutic ultrasound has been shown to increase collagen synthesis and tensile strength in experimentally repaired rabbit Achilles tendon.79,124–126 Enwemeka125 and Enwemeka, Rodriguez, and Mendosa124 reported favorable results that enhanced the healing response using 1 MHz at doses of 0.5 W/cm2 and 1 W/cm2 for 5 minutes during nine consecutive treatments. Jackson, Schwane, and Starcher126 noted changes in collagen synthesis and breaking strength as early as 5 days after injury when giving continuous therapeutic ultrasound at 1.5 W/cm2 for 4 minutes over 8 consecutive days and then every other day for up to 21 days. Freider and associates127 reported similar results in partially ruptured, nonsurgically repaired Achilles tendons of Marland rats that received continuous therapeutic ultrasound at 1.5 W/cm2 three times a week for either a 2- or a 3-week period. Ng reported no significant difference among treatment groups in the collagen fibril size in severed rat Achilles tendons after treatment with 1 Mhz continuous therapeutic ultrasound at 0.5, 1.2, and 2 W/cm2. There was a significant difference comparing treatment groups to control of sham ultrasound.128
Although the results of studies using animal models may not be directly applicable to the human response, the therapeutic use of therapeutic ultrasound in the inflammatory and proliferative stages could prove beneficial. Using therapeutic ultrasound during these stages is a common clinical intervention.
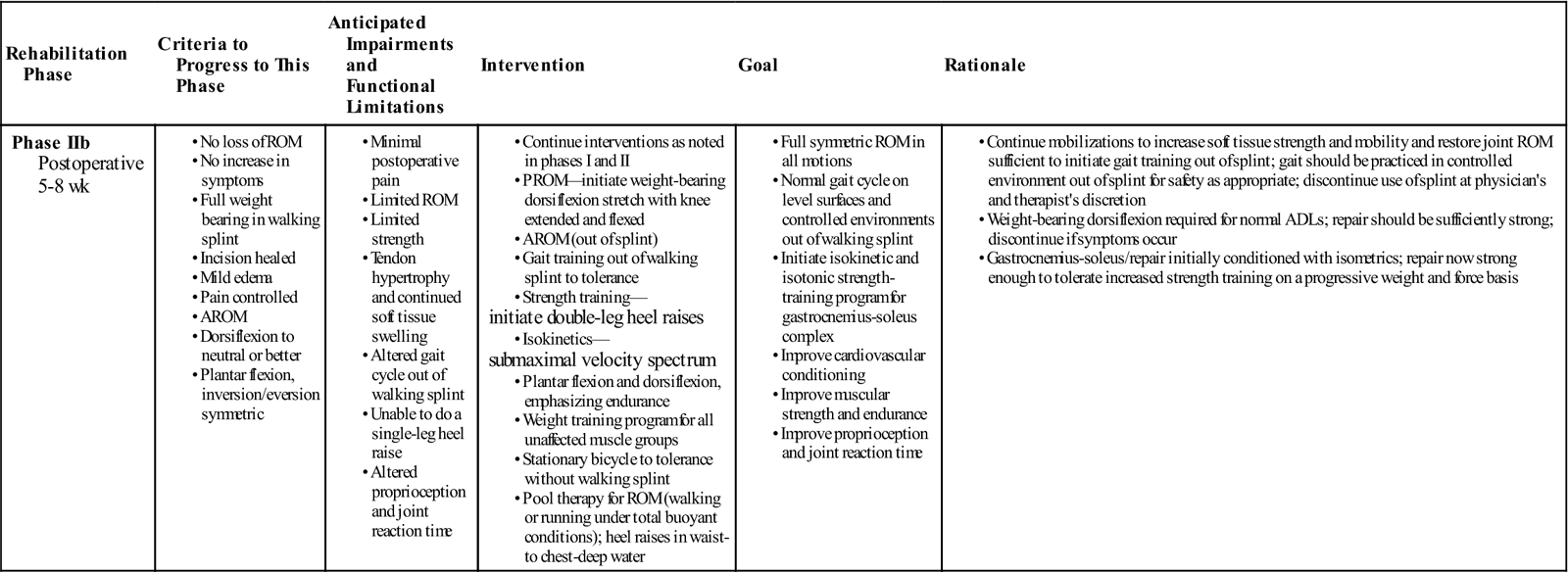
Phase IIb
TIME: 5 to 8 weeks after surgery
GOALS: Demonstrate normal gait on level surfaces, encourage full ROM (symmetric), increase strength and proprioception
During phase IIb (Table 31-6), the patient should strive to obtain full symmetric ROM, achieve a normal gait cycle on level surfaces and in controlled environments without the protective boot, progress the strength program, and initiate proprioceptive training.
TABLE 31-6
Achilles Tendon Repair (Early Motion)
< ?comst?>
| Rehabilitation Phase | Criteria to Progress to This Phase | Anticipated Impairments and Functional Limitations | Intervention | Goal | Rationale |
| Phase IIb Postoperative 5-8 wk |
initiate double-leg heel raises submaximal velocity spectrum |
|
|
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
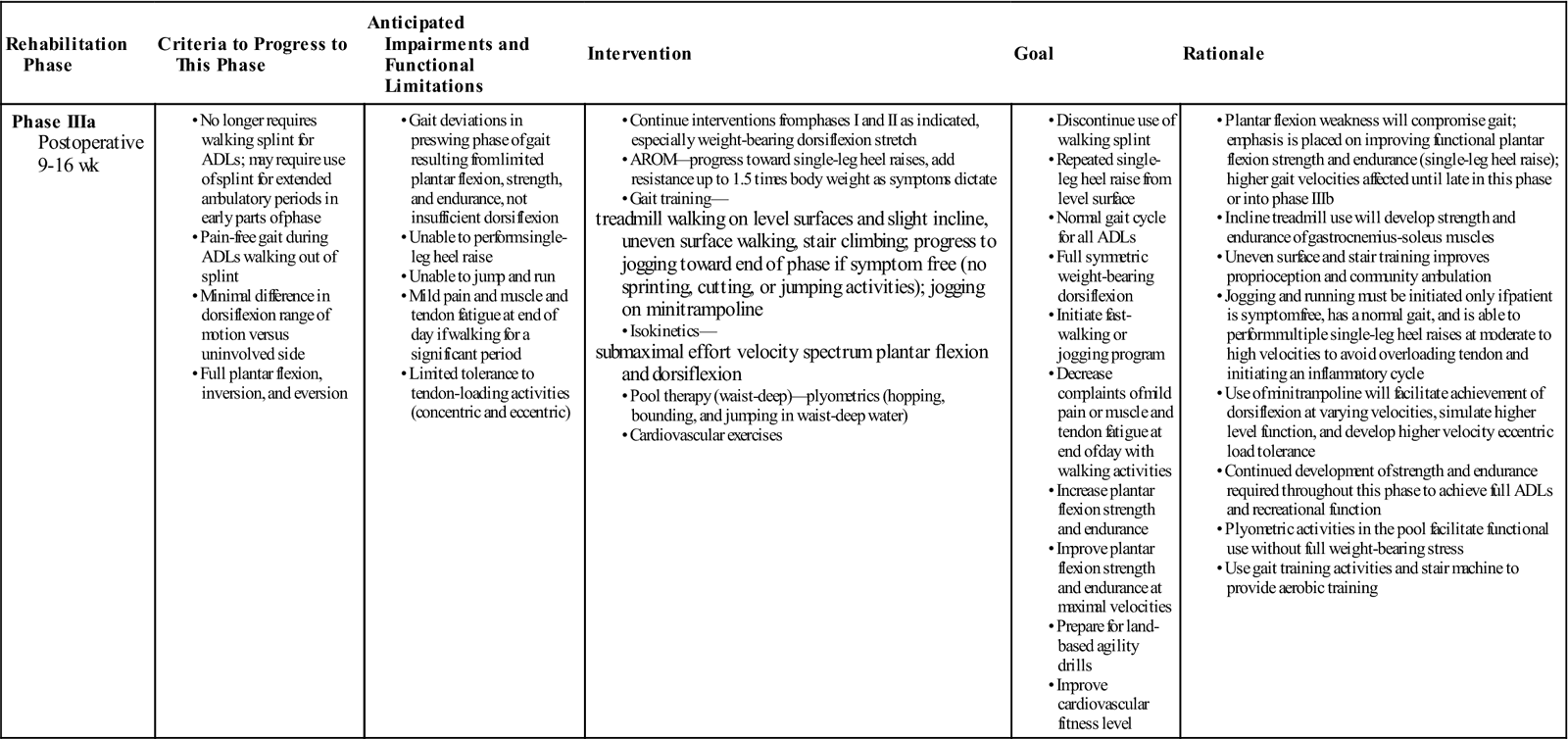
Phase IIIa
TIME: 9 to 16 weeks after surgery
GOALS: Demonstrate normal gait for all activities, have full weight bearing, increase strength and endurance; initiate walking with progression toward a jogging program (as appropriate), isokinetics, and pool therapy plyometrics as appropriate (toward end of phase)
Phase IIIb
TIME: 17 to 20 weeks after surgery
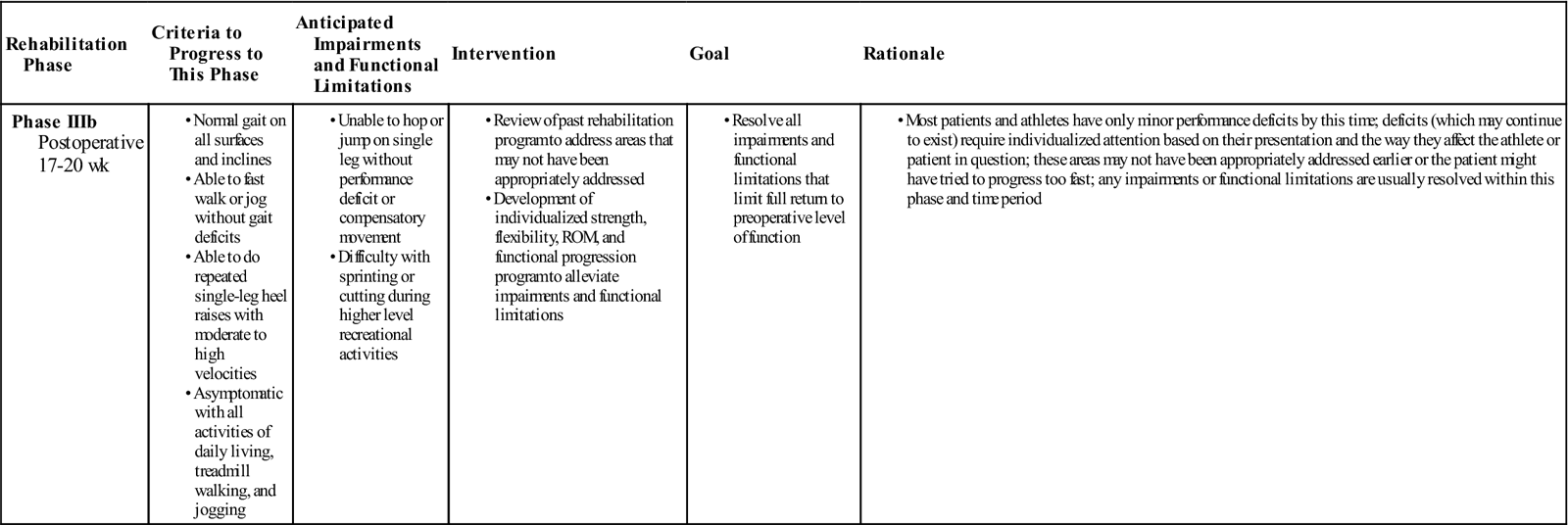
GOALS: Return to preoperative level of activity or sport
During phase IIIa (Table 31-7), emphasis is on increasing the velocity of activity, increasing the patient’s strength to perform a repeated single-leg heel raise, and improving the endurance of the gastrocnemius and soleus group to tolerate a functional progression. During phase IIIb (Table 31-8), a full functional progression is initiated to return the patient to the desired level of function.
TABLE 31-7
Achilles Tendon Repair (Early Motion)
< ?comst?>
| Rehabilitation Phase | Criteria to Progress to This Phase | Anticipated Impairments and Functional Limitations | Intervention | Goal | Rationale |
| Phase IIIa Postoperative 9-16 wk |
|
|
treadmill walking on level surfaces and slight incline, uneven surface walking, stair climbing; progress to jogging toward end of phase if symptom free (no sprinting, cutting, or jumping activities); jogging on minitrampoline submaximal effort velocity spectrum plantar flexion and dorsiflexion |
|
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
ADLs, Activities of daily living; AROM, active range of motion.
TABLE 31-8
Achilles Tendon Repair (Early Motion)
< ?comst?>
| Rehabilitation Phase |
Criteria to Progress to This Phase | Anticipated Impairments and Functional Limitations | Intervention | Goal | Rationale |
| Phase IIIb Postoperative 17-20 wk |
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
All authors using an early functional program, regardless of the type of augmentation used during surgery, have reported excellent results. Success was measured by the same methods as for traditional rehabilitation. Limitation of ROM for dorsiflexion and plantar flexion are rare; patients are able to walk heel-toe and do single-leg raises at the time of follow-up examination. In addition, isokinetic peak torque levels are within 5% of the uninvolved side.13,105
![]() Reruptures are rare and are usually attributed to lack of retraining before a return to sports123 or return to vigorous activity against advice.101 However, these outcomes are also difficult to compare for the same reasons as cited for traditional rehabilitation programs.
Reruptures are rare and are usually attributed to lack of retraining before a return to sports123 or return to vigorous activity against advice.101 However, these outcomes are also difficult to compare for the same reasons as cited for traditional rehabilitation programs.
Summary
The superiority of early motion for Achilles tendon repairs compared with traditional postoperative casting cannot yet be fully determined. Although studies reported to date state that the results are excellent, the different rehabilitation philosophies cannot be compared critically because of differences in operational definitions, criteria monitored, postoperative time when the data are collected, and surgical technique. Although the ultimate level of function achieved appears to be the same regardless of the rehabilitative program, the authors of this chapter believe that using early motion after Achilles tendon repair enables the patient to obtain a more independent and active quality of life sooner than that seen with traditional postoperative casting. However, patient compliance and ongoing oversight of the rehabilitation program are crucial factors that must always be considered.
Clinical Case Review
1 What are the three stages of healing?
(a) Inflammatory response—Minutes after injury, laceration, or the initiation of surgical repair. (b) Repair and proliferation—The repair and proliferation stage may begin as early as 48 hours after injury and may last for 6 to 8 weeks. (c) Remodeling and maturation—can last months.
2 Scott is 35 years old and is undergoing his initial evaluation 7 days after surgery. What part of his history will help the therapist avoid being too aggressive and putting the repair at risk?
His general health, family, and past medical history are keys to determining if any factors may delay the healing process. Diabetes, peripheral vascular disease, medications (corticosteroids/NSAIDs), anticoagulants, smoking, past responses to healing (surgical, nonsurgical, or both), pain tolerance, nutrition, and high body mass index all influence soft tissue healing response.
3 Nancy is following an early motion program and began therapy at 1 week postoperation with an ATRS score of 10. At 6 weeks, she has made clinically significant progress as demonstrated by an ATRS score of 44. How do you use this data to plan her continued therapy?
Review the detailed scores to find that Nancy is limited more so in her activity scores than in pain, stiffness, and strength which correlates with your objective findings. Evaluate Nancy’s ADLs, vocational, and recreational activity goals to begin activity-specific training.
4 Kerry had an Achilles tendon repair 7 weeks ago. She is partial weight bearing and uses a fixed protective splint. At 9 weeks she will initiate non–weight bearing stretching exercises for all ankle motions. During phases I and II (weeks 1 through 8) of the healing process, fibroblastic proliferation continues, producing a rapid increase in the amount of collagen. Even though collagen amounts are increased, the repaired tissue is weak and requires protection (e.g., restricted weight bearing and nonaggressive ROM stretches). Why do the healing tissues remain weak despite the increase in collagen fibers?
Type III collagen is deposited; it has poor cross-link definition, small fibril size, and poor strength. As the repair process continues, the collagen deposition shifts to type I collagen, which has better cross-link definition, fibril size, and strength.
5 Kim is 40 years old and ruptured her Achilles tendon playing tennis. She had surgery 7 weeks ago and is eager to resume her prior activity level. What exercises can she safely perform, laying the foundation for her return to the court?
At this stage of Kim’s rehabilitation, she can safely perform deep-water running (in a floatation vest without her feet touching the bottom), core training exercises that avoid straining the repair (sitting or supine using a medicine ball), and upper extremity exercises (scapula stabilization and rotator cuff program).
6 Doug is 43 years old and underwent an Achilles tendon repair 9 weeks ago. The physician has chosen the traditional immobilization approach for rehabilitation. He presented today 1 week after cast removal and is still quite painful with ambulation (stance phase). What can the therapist do to help control his symptoms?
The PT can assist in protecting the tendon from full stress by using a heel lift of varying heights, and wean the height as able. In addition, by using crutches, progressive weight bearing may be used to control mechanical stress. Modalities can also be used to help manage any residual pain and inflammation.
7 Brian is 3 months postoperation and is concerned that his repaired Achilles tendon looks much larger than his unaffected side. Is this normal?
The area of repair can be 6.1 times the size of the unaffected tendon at 3 months and 5.6 times the size at 6 months. A variably sized, high-intensity signal demonstrating a central intratendinous lesion was detected in 19 of 21 repaired tendons at 3 months. The development of an intratendinous lesion appears to be a normal part of the healing process after surgical repair and casting.
8 Tom is 3 weeks postoperation in the early motion guidelines. What can be done to assist his healing response.
Once the incision is healed and dry, therapeutic ultrasound can be used to help assist in the healing response. In some instances therapeutic ultrasound has been shown to increase collagen synthesis and tensile strength.
9 Dan is 22 weeks postoperation and demonstrates good ROM and strength. He has a normal gait on level surfaces, and he initiated the Return to Running program. He appears to be holding back his effort on some agility drills and he mentions that he “just doesn’t feel that he can trust it.” What can you do as a clinician to alleviate his concerns?
Reruptures are rare and are usually attributed to lack of retraining before a return to sports. The demands of his sport should be broken down into phases and accomplished one phase at a time. This will allow him to be successful in smaller demand activities and gain confidence that he can “trust” his repair. Refer to the chapters on Return to Running and Return to Jumping for a complete program.