Chapter 18 Achalasia
Etiology
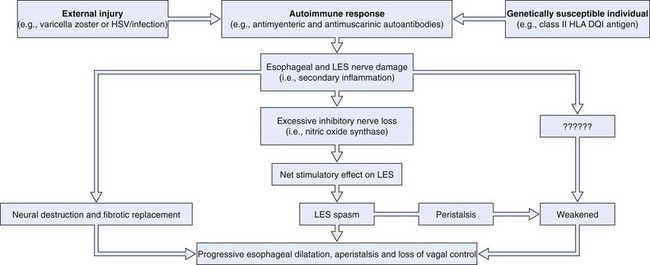
Fig. 18.1 illustrates the putative events in the development of achalasia. Extensive experimental data support this hypothesis. First, the neurotropic virus, varicella zoster, has been detected by in situ hybridization in cardiomyotomy specimens from patients with achalasia.1 More recent data have also implicated herpes simplex virus type 1 as a cause of achalasia by showing a population of cells and cytokines in LES tissue typically found in herpes simplex virus infection.2 In addition, Chagas’ disease, a disease caused by Trypanosoma cruzi, a protozoan that is endemic in Central and South America, causes esophageal disease that may be indistinguishable from achalasia on manometric and radiographic grounds as a consequence of denervation of Auerbach’s plexus.3 Second, some investigators have shown an association between white patients with achalasia and the class II human leukocyte antigen (HLA) DQ1.4 This genetic relationship has been characterized more specifically as an association with the DQB1*0602 and DRB1*15 HLA alleles.5 Third, antimyenteric6 and antimuscarinic cholinergic receptor autoantibodies7 have been detected in patients with achalasia. Fourth, studies of surgical specimens from patients with achalasia have shown a chronic inflammatory infiltrate leading to ganglionic destruction and fibrosis.8–12 Fifth, vagal dysfunction has been shown in natural and experimental animal models of achalasia.13,14 Sixth, a selective deficiency of nitric oxide synthase, the predominant inhibitory neurotransmitter of the LES, has been shown in animal models and patients with achalasia.15,16 Seventh, patients with less advanced forms of achalasia (from both a clinical and a radiographic point of view) have more inflammation and less fibrosis in the ganglia of the LES than patients with more advanced forms of the disease,11,17 suggesting that LES basal and residual tone may increase and esophageal body function deteriorates as the disease progresses.18
Much more data are needed to prove this hypothesis. The data referred to were generated from a relatively small number of patients, given the rarity of the disease. The abnormalities described are rarely identified in all patients with achalasia. Only 3 of 9 patients had detectable varicella zoster in the myenteric plexus,1 and only 7 of 18 patients had antimyenteric neuronal antibodies.5 These preliminary findings are encouraging, however, particularly because the general theory proposed here may apply to other gastrointestinal (GI) disease states in addition to achalasia (e.g., chronic idiopathic pseudoobstruction).
Diagnosis
Symptoms
The hallmark symptoms of achalasia are dysphagia, chest pain, and regurgitation (Table 18.1). Various series of patients with achalasia have reported dysphagia in 82% to 100% of cases, chest pain in 17% to 95%, and regurgitation in 59% to 81%.19–24 Dysphagia occurs typically for both solids and liquids, but solid food dysphagia often precedes liquid dysphagia. Some patients with early disease describe the need to “wash down their food with water,” which possibly affords relief by raising a sufficient head of pressure above to overcome the LES spasm below. In others, there may be superimposed acute episodes of solid food dysphagia in which patients present with symptoms suggestive of food impaction that resolve spontaneously or are severe enough to warrant endoscopic removal.
| Symptoms | Frequency (%)* |
|---|---|
| MAJOR | |
| Dysphagia | 82–100 |
| Chest pain | 17–95 |
| Regurgitation | 59–81 |
| Weight loss | 32 |
| MINOR | |
| Slow eating | |
| Stereotypic maneuvers during eating | |
| Halitosis | |
| Heartburn | |
| Accumulation of oral debris at night | |
| Staining of pillow during sleep | |
| Nocturnal coughing or choking | |
| Acute airway obstruction | |
| Inability to belch | |
| Postprandial syncope | |
| Dental caries | |
| Asthma | |
| Pneumonia |
Chest pain often occurs during ingestion and may signify food impaction. A spasmlike chest pain that may mimic gastroesophageal reflux disease (GERD) (i.e., heartburn) or cardiac pain (i.e., angina) may also occur spontaneously. The origin of the chest pain is generally unclear, and it may be independent of the mechanisms that cause dysphagia and regurgitation; this is suggested by data showing that chest pain commonly persists after achalasia treatment that is effective for the other two major symptoms.24
Regurgitation may occur minutes to hours after ingestion of a meal. Patients may even identify food that they ingested many days before in the regurgitant. Patients with severe symptoms may be unable to drink a glass of water without regurgitating. The nature of the regurgitant may be useful to differentiate achalasia from GERD. In achalasia, the regurgitant is typically described as nonsour or nonbitter, and patients may mention that it “tastes just like it did when it was initially swallowed.” Because of the difficulty with eating, weight loss is also a common feature of achalasia; this was seen in 32% of patients in one more recent study.25
In our experience, a careful history emphasizing and dissecting the major symptoms of dysphagia, chest pain, regurgitation, and weight loss can be quite specific for the diagnosis of achalasia, although we are constantly surprised by the varied patterns of presentation of this unusual disease. As with many chronic disease states in which life-threatening consequences are rare and take months to years to develop, achalasia is a disease that lends itself to self-accommodation on the part of the patient. Behavioral adaptation may lead to the emergence of a whole host of subtle compensatory symptoms accompanied by the patient denying or downplaying the more classic symptoms described previously (see Table 18.1).
One more recent study25 elicited numerous compensatory symptoms, including slow eating and other stereotypic maneuvers to aid swallowing such as walking, standing, sitting straight up, or arching of the neck during swallowing. Potential patients should also be interviewed about other subtle symptoms, including halitosis, heartburn, accumulation of oral debris or staining of the pillow during sleep, nocturnal coughing or choking, acute airway obstruction,26,27 an inability to belch because of upper esophageal sphincter dysfunction,28,29 and postprandial syncope.30 Heartburn, the cardinal symptom of gastroesophageal reflux, is particularly important because its presence is often erroneously believed to be a strong negative indicator of achalasia. Although unproved, the sensation may be a consequence of bacterial fermentation of retained ingested foodstuff leading to lowering of the esophageal luminal pH mimicking GERD. Some studies have shown that heartburn and specialist referral of patients on proton pump inhibitors can be quite common in the early stages of achalasia.25,31 Other important symptoms often erroneously ascribed to GERD include the presence of dental caries (from food bathing the teeth at night), asthma, and a history of pneumonia (presumably from microaspiration). Accurately differentiating between GERD and achalasia by history alone may be surprisingly difficult, given that the two diseases represent completely opposite ends of the pathophysiologic spectrum.
Radiography
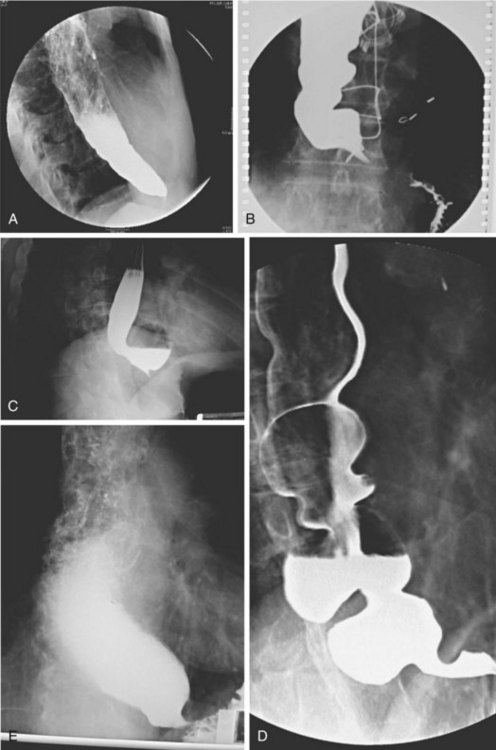
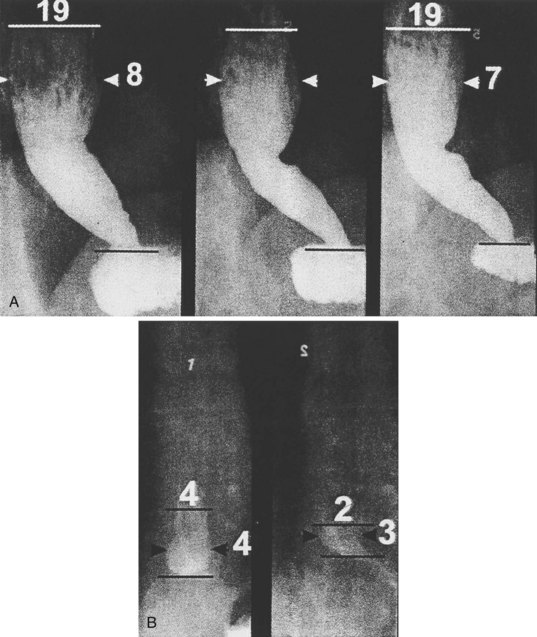
Achalasia may be suspected on the basis of a routine chest x-ray film (particularly a lateral view) by the presence of an air-fluid level or a dilated esophagus or both. However, barium swallow is the most reliable radiographic method for making the diagnosis (Fig. 18.2). The pathognomonic findings include a smooth, tapered narrowing of the gastroesophageal junction (the so-called bird’s beak appearance) with a proximally dilated esophagus that may be filled with fluid or food debris. Features of candidal infection, a consequence of long-standing esophageal stasis, may also be present. However, this basic template can include remarkable heterogeneity. The degree of esophageal dilation may vary from minimal to massive, and end-stage disease may reveal sigmoidization of the esophagus. Similarly, the diameter of the gastroesophageal junction may range from less than 1 mm to greater than 8 mm. Radiographs may also show single or multiple distal esophageal diverticula of varying sizes or a corkscrew or “spastic” appearance with several simultaneous lumen-obliterating contractions (this has been termed vigorous achalasia).
To assess and characterize these esophageal radiographic findings, various scoring systems have been devised.19,25 Although these scoring systems help to standardize interpretation of radiographic appearance, which may be useful from a research point of view, there is generally very poor correlation between symptom severity and radiographic score.25 This limitation is discussed further in the treatment section. Finally, there has been an old tenet in esophagology that a hiatal hernia is unusual in achalasia.32 More recent data have questioned this dictum, showing the presence of a hiatal hernia in 10 of 71 radiographs reviewed from patients with achalasia (14.5%) compared with 9 of 35 patients (25.7%) 51 years old or older, a frequency that is similar to the normal population.33 This finding makes intuitive sense because achalasia may develop at any age, sometimes in the presence of long-standing GERD with a hiatal hernia that preceded the development of the achalasia. Perhaps older studies had a bias of either underdiagnosis of hiatal hernia or overenrollment of younger patients.
Esophageal Manometry
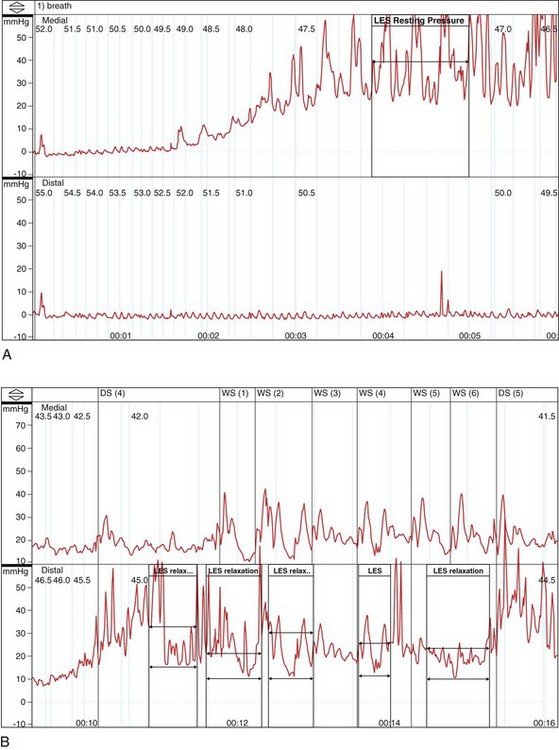
In the absence of a true “gold standard,” manometry is the best test for the diagnosis of achalasia (Fig. 18.3). However, manometric diagnosis of achalasia has undergone great revision in recent years. Classic teaching required the presence of a hypertensive LES with incomplete relaxation (i.e., an increased residual pressure) and esophageal body aperistalsis, whereas it is now clear that these classic criteria do not occur in all patients. Part of the reason for this reevaluation has been prolonged combined ambulatory pH and manometric recordings in which peristaltic contractions, complete LES relaxation, and acid reflux may be shown intermittently in patients who otherwise fulfill the classic criteria on stationary manometry.34 As a result, it now seems conceivable that “normal” manometric findings might occur on stationary manometry as well in patients with achalasia, depending on the timing of the study.
Hirano and coworkers35 in a study of 58 patients with achalasia proposed a manometric heterogeneity in patients with achalasia, including features such as a normal basal LES pressure, normal residual pressures, high-amplitude contractions, varying lengths of aperistalsis, and the presence of transient LES relaxations (the hallmark of GERD). In interpreting this important study, one might wonder what the “gold standard” for the diagnosis of achalasia was in these “atypical” patients. This study shows the importance of making the diagnosis of achalasia based on a combination of compatible clinical, radiographic, and manometric findings in a specific patient rather than routinely relying on classic criteria. Similarly, Vantrappen and coworkers36 in a landmark article proposed that diffuse esophageal spasm is in itself one end of the spectrum of achalasia. Several case reports have now shown spasm-type manometric (and perhaps radiographic) findings either as a component of established achalasia or as an early manifestation of dysmotility that may evolve into achalasia in due course.37,38
Currently, many esophagologists consider diffuse esophageal spasm a true variant of achalasia. Attention has also focused on the concept of vigorous achalasia, which is distinguished from classic achalasia by the presence of high-amplitude nonperistaltic esophageal body contractions. Vigorous achalasia may also represent an early manometric manifestation of the disease, before the development of complete aperistalsis. Although pathologic studies have shown progression of histologic injury within the myenteric plexus during the transition from vigorous to classic achalasia,10 clinical studies comparing radiographic appearance and response to therapy in vigorous and classic achalasia have not borne out these differences.39
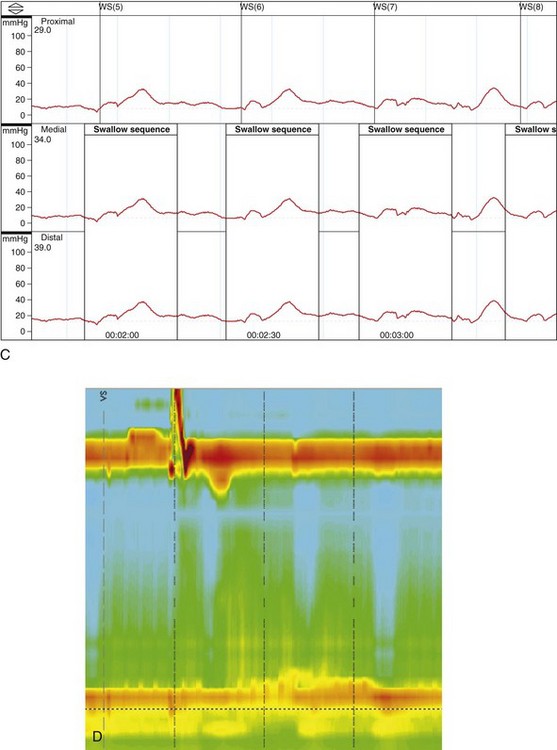
Significant advances have been made in motility testing of the esophagus in recent years with the advent of high-resolution manometry.40–42 This technique expands on prior techniques by permitting simultaneous manometry measurements at centimeter intervals along the entire length of the esophagus. Three-dimensional color plots of change in pressure measurements with time (pressure topography) allow more accurate recording of the upper esophageal sphincter and LES pressures and the proximal (striated) and distal (smooth) muscular contractions of the esophageal body. These sophisticated tracings have permitted a new manometric classification of achalasia as proposed by Kahrilas and colleagues.40 This new classification divides achalasia into three manometric types: type I, achalasia with minimal esophageal pressurization, previously referred to as classic achalasia (with aperistalsis and an elevated LES pressure that fails to react; see Fig. 18.3D), occurring in roughly 21% of cases; type II, achalasia with esophageal compression, previously referred to as vigorous achalasia (with swallow-induced pressurization), occurring in roughly 49% of cases; and type III, achalasia with spasm, a new variant characterized by high pressures in the distal esophageal body, occurring in roughly 29% of cases. The utility of this new classification has yet to be definitively shown, although proponents have suggested that it can be used prognostically (type II being more likely to respond to standard therapy than type I or III).
Endoscopy
Several studies have also examined the role of endoscopic ultrasound in diagnosing achalasia.43,44 Although some studies suggest that patients with achalasia may have increased LES and esophageal body wall thickness, these findings are not present in all patients. Currently, endoscopic ultrasound should be considered a research tool for patients with primary achalasia. Its role in secondary achalasia is discussed later.
Treatment
Many important therapeutic principles must be kept in mind before prescribing treatment options for patients with achalasia (Box 18.1). The first is the need to individualize the definition of a treatment threshold because no defined criteria exist. To date, investigators have been unable to define specific criteria for therapy or to evaluate treatment response. This controversy arises from data showing extremely poor correlation between symptoms and radiographic appearance before and after treatment. One more recent study25 comparing the number and the severity of achalasia symptoms with radiographic scores (based on predefined radiographic parameters, such as the degree of esophageal dilation, the LES diameter, and the presence or absence of sigmoidization or retained food debris) found no correlation at all between symptoms and x-ray appearance either before or after treatment. Other studies have had similar results.45,46
Box 18.1 Therapeutic Principles in Achalasia
In addition, one must keep in mind age when evaluating symptoms and treatment response. Studies have shown that older patients with achalasia (>45 to 60 years old depending on the study) tend to have better symptomatic responses to treatment. These improved responses have been shown with botulinum toxin injection47 and with pneumatic dilation.48 Whether the improvement is objective or subjective is unclear because older patients tend to have esophagi that are less sensitive to stimulation49; this population may be less reliable in conveying objective improvement. No outcome trials have been designed as yet to show whether or not early therapeutic intervention leads to an improved clinical outcome.
The second issue is whether one should standardize the criteria used for symptom assessment or radiographic evaluation. There is a wide variation in the way individual physicians take histories from patients with achalasia. Specific symptom scoring methods have been proposed to address this problem to allow standardized evaluation of treatment responses, but they have not been found to be very useful clinically.25,50,51 Similarly, there is great variability in the way a barium swallow is performed in different radiology departments. Difference exists in terms of the amount and consistency of oral contrast medium given, the performance of video techniques versus static images, the use of double-contrast versus single-contrast imaging, and the number and rate of swallows administered.
Some investigators have studied the use of a standardized “timed” barium swallow.45,52,53 The proposed technique requires ingestion of low-density barium sulfate suspension within 30 to 45 seconds (the maximum amount tolerated is measured) with spot films taken at 1 minute, 2 minutes, and 5 minutes. The height and width of the barium column is measured at each time point, and the rate of esophageal emptying from 1 to 5 minutes is recorded (Fig. 18.4).51 In one of the studies using this technique,52 32 patients with near-complete symptom relief after therapy were followed. Initially, there was a significant association between patient symptoms and improvement in barium column height, although esophageal barium emptying was less predictive of the symptomatic response during longer term follow-up. Despite this limitation, patients whose symptom response correlated best with emptying were far less likely to have symptomatic relapse during follow-up.
Fourth, when evaluating the results of therapeutic studies, one must bear in mind that most studies deal with a heterogeneous population of patients with varying degrees of disease severity. Because of significant variability among patients in terms of specific symptoms,25 extrapolating symptom response rates from clinical studies to individual patients may not always be valid. Because of the rarity of this disease, most achalasia studies group patients with all stages of severity together for analysis. These data include therapeutic responses ranging from patients with minimal esophageal dilation and some LES opening to patients with frank sigmoidization, complete aperistalsis, and severe LES spasm. Although the proportion of patients with end-stage disease may be similar among some studies, patients with end-stage disease are often excluded in other studies. In one study evaluating the efficacy of Heller myotomy for achalasia, symptomatic success was related to preoperative disease stage.54 The relatively small numbers of achalasia patients in general also predisposes to type II errors in study design. Finally, the precise details of how therapeutic procedures (botulinum toxin injection, pneumatic dilation, and surgical myotomy) are performed often vary greatly among operators.
Botulinum Toxin Injection
From the innovative work of Pashricha and coworkers,55 botulinum toxin injection into the LES has become a cornerstone of treatment for achalasia. Gastroenterologists have embraced this approach to treatment for three reasons: (1) It is easy to administer, (2) it is safe, and (3) it works. Because of its extremely favorable risk-to-benefit ratio, we commonly use botulinum toxin injection as a temporizing measure in patients presenting with severe disease who are concerned about undergoing more invasive procedures or in patients at poor risk for more invasive procedures.
The precise injection technique is unspecified. In Pashricha’s original work, “the lower esophageal sphincter was visualized endoscopically by identification of the sphincteric rosette typically seen at the squamocolumnar junction. Botulinum toxin was injected through a 5-mm sclerotherapy needle into the region of the lower esophageal sphincter.”55 In a more recent report by Kolbasnik and colleagues,56 botulinum toxin was administered “approximately 5 mm above the Z line.” In a study by Annese and coworkers,57 the toxin was injected into “the LES region identified at endoscopy.” The fact that all these studies show similar efficacy for botulinum toxin injection suggests that the precise site of injection may not be critical to the success of the technique. The LES is generally longer in patients with achalasia (approximately 5 cm) compared with control subjects and patients with GERD. It is unknown whether clinical results vary with botulinum toxin injection into the proximal, mid, or distal LES region. In addition, there are no clear data to support an advantage to administering the injection using endoscopic ultrasound guidance or from a retroflexed position in the gastric cardia.
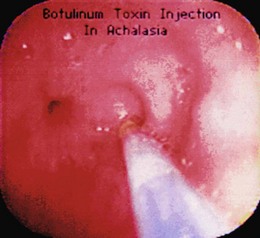
Additional important aspects of botulinum toxin administration include not preparing the solution until just before its use (because it is extremely expensive), using nonbacteriostatic saline without preservatives (preservatives may destroy the toxin), admixing the toxin with the saline solution by slowly injecting the saline into the vial followed by gently rolling the vial back and forth (to prevent denaturing of the toxin), and ensuring that the sclerotherapy catheter is fully flushed before injection (to prevent submucosal injection of air). Fig. 18.5 illustrates performance of an injection using this technique.
That botulinum toxin works well for patients with achalasia has been clear from the first published trial.58 Numerous subsequent trials59–61 have confirmed this fact. The symptom response after one injection ranges from 64% to 100%,57–61 and a more recent meta-analysis showed a 78.7% efficacy of botulinum toxin injection in 315 patients described in nine articles.62 Manometric and radiologic parameters in these studies also improve significantly. There is a 33% to 49% reduction in basal LES pressure and a 35% to 47% reduction in esophageal retention. The duration of response ranged from 7.1 to 11 months with overall efficacy of 68.3% at 12 months in the aforementioned meta-analysis.62 Prolonged responses (>2 years) have been documented in several patients. In addition, patients who respond well to their initial injection also seem to respond well to a second injection. Studies vary, however, on the symptomatic response to a second injection in initial nonresponders (from 0% to 33%).
We believe a second injection is worthwhile in patients who initially fail to respond, particularly if the patient is elderly or a poor operative risk. Data on the efficacy of a third or fourth injection are scant, but anecdotal and published data suggest it may still work. As discussed, age may be an important predictor of response to botulinum toxin injection. In the initial study by Pashricha and coworkers,55 53 patients older and younger than 50 years had an 82% (>50 years old) and 43% (<50 years old) chance of responding symptomatically. Other, but not all, studies show similar trends.59 Another predictor of success is the presence of vigorous achalasia, although the number of patients available for analysis is very small.58 This smaller patient population is not unexpected because vigorous achalasia may represent an earlier form of the disease process in which esophageal peristalsis is less impaired.
The most important predictor of initial and long-term symptomatic response is a documented reduction in LES pressure (this holds true in general for all achalasia treatment modalities). One study59 showed that a decrease in basal LES pressure to less than 20 mm Hg predicted a 100% symptomatic response. One study60 suggested only that a low basal LES pressure before treatment was also predictive of a better outcome (see figures presented by Pashricha and coworkers55). Side effects of botulinum toxin injection are infrequent. Studies have reported transient chest pain, heartburn-type symptoms, and rash. In one case report,63 a 10-year-old girl with achalasia developed a sinus tract between the esophagus and fundus, but this occurred after six botulinum toxin injections. One other potential side effect of this type of therapy is its potential impact on future surgical therapy. Specifically, several surgical studies have shown more difficult surgical dissection and perhaps poorer surgical outcome.64 Whether a poorer surgical outcome is uniformly accepted is unclear, but one should not use botulinum toxin without considering the possibilities of its effect on subsequent myotomy.
Pneumatic Dilation
In evaluating studies of pneumatic dilation for achalasia, evaluation of the precise method used and the age of the study are paramount for two reasons. First, as discussed previously, there is marked variation in the precise details of the technique used. Methods vary from brief 3- to 5-second dilations at 12 to 15 pounds per square inch (PSI),65 to maintaining an average dilation pressure of 7 PSI for less than 30 seconds, to maintaining a pressure of 6 to 12 PSI for up to 2 minutes. Whether such variations in technique influence outcome has not been well studied. Studies specifically examining the effect of the dilator size or the duration or number of inflations have not shown any clear differences,66,67 although one early study showed an increased benefit if a larger balloon size was used (35 mm vs. 30 mm),67 and another showed a benefit to inflating the balloon to a pressure of greater than 7 PSI.65
Second, there has been a progressive change in the types of dilators used over time. Earlier studies described the use of the bulky, cumbersome Brown-McCarty or Mosher bags. However, these have been replaced over the past decade by Rigiflex dilators. Although the Rigiflex dilator is considered easier to use, few data suggest that it actually yields superior results.68 There is no agreed-on method for balloon dilation, and many methodologic variations exist based mainly on anecdotal, personal experience. The following points regarding performance of balloon dilation for achalasia should be emphasized. We always start with a 30-mm balloon Rigiflex dilator. Although data suggest that there may be an incremental benefit to using a larger diameter balloon,66 we prefer to start off with the smaller 30-mm balloon in the hopes of limiting the likelihood of perforation. We are willing to repeat the procedure with a 35-mm balloon at a later setting if the initial dilation is unsuccessful, but we are very averse to using the 40-mm balloon given its high rate of perforation and the fact that alternative therapies (especially laparoscopic myotomy) are so successful.
We first inflate the balloon to about 2 to 3 PSI to confirm appropriate positioning of the balloon looking for a “waist” in its center. This commonly is just below, not at, the diaphragm. The catheter is fixed in place by grasping it firmly against the bite-block. If there is too much give on the catheter, it is squeezed inferiorly by the hypertensive LES during inflation. The balloon is inflated slowly up to a limit of 10 PSI. During the process, we attempt first to identify the waist and then notice its obliteration to confirm that the balloon has reached its full diameter. We prefer to deflate the balloon as soon as the waist is obliterated rather than maintaining full inflation for any set amount of time. We do not exceed a balloon pressure of 10 PSI based on data suggesting an increased chance of perforation above this limit.69 The dilator and guidewire are removed and examined for blood to confirm that the LES region has been torn. We generally do not reintubate the esophagus endoscopically, but we always follow therapeutic dilation with a Gastrografin oral solution swallow immediately after the patient has awakened from the intervention. Gastrografin oral solution is primarily administered to exclude a procedural perforation rather than as an attempt to document efficacy; postdilation spasm may hide a perforation that manifests later as the spasm resolves.
There is general consensus among most authorities that a single, successful balloon dilation has a duration of efficacy of a few years. Specifically, 5-year follow-up data show that the effect of a single dilation remains effective in 30% to 50% of patients.67,69–71 Typically, patients require two to three dilations over a 5-year period to stay in remission. In the classic study by Parkman and associates,72 three dilations maintained remission in 90% of patients after 5 years of follow-up (Fig. 18.6). Only 15% of patients required myotomy during this period (although two of these were a consequence of procedurally induced perforation). Longer term follow-up studies have also been reported. In one of these,73 pneumatic dilation was effective in 61 of 72 patients followed for a mean length of 6.5 years, and a second dilation was required in only 4 patients. One patient responded for 25 years with a single procedure. Another series67 followed patients for more than 15 years, at which time the therapeutic response to balloon dilation was classified as excellent in 12%, good in 28%, moderate in 20%, and poor in 40% with a requirement of a median four dilations.
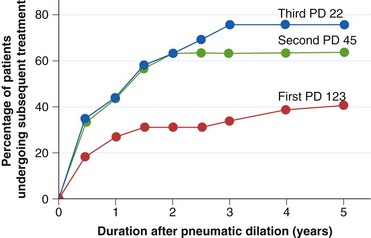
Fig. 18.6 Efficacy of repeated balloon dilations in patients with achalasia. PD, Pneumatic dilation.
(From Parkman HP, Reynolds JC, Ouyang A, et al: Pneumatic dilatation or esophagomyotomy treatment for idiopathic achalasia: Clinical outcomes and cost analysis. Dig Dis Sci 38:75–85, 1993.)
Two caveats must be kept in mind when evaluating these long-term follow-up studies. First, a significant number of patients were lost to follow-up, which hampers the quality of the report.74 Second, one of the reports75 describes the personal experience of one of the most experienced esophagologists in the world, and it is unclear whether or not physicians with less experience can expect similar outcomes. Finally, some authors have advocated the use of endoscopically guided pneumatic dilation without fluoroscopy.76 In this technique, a colored mark is placed on the center of the balloon for visualization; whether or not this is an advantageous approach is unclear.
As with botulinum toxin injection, pneumatic dilation works well for patients with achalasia. An effective symptom response occurs in most patients, and, in contrast to botulinum toxin injection, the effect may be quite durable. Also, objective data show that the technique is effective in terms of producing a reduction in LES pressure and esophageal diameter and improved esophageal emptying by scintigraphy.66,67 Patients commonly require repeated procedures, and some patients have little, if any, response. One study67 showed little or no improvement in 12 of 54 patients (23%) 4 weeks after dilation. There is a wide range of potential outcomes from no response at all to long-term resolution of symptoms.
Several factors have been proposed as useful predictors of a response to balloon dilation (Box 18.2). First (and intuitively), the more frequent the dilations are needed to induce remission, the less likely they are to provide long-term responses. This has been shown in 5-year studies when multiple dilations were required for symptom control.67 Second, early relapse (i.e., within 2 months) predicts a poor long-term outcome.65,67 Third, age is an important predictor of success. In various studies, older age may be defined as older than 40 years,65,67 older than 45 years,69 or older than 60 years.71 In one of these studies,65,67 four of five patients younger than 18 years had symptomatic relapse within 2 months. In another study of 132 patients,71 patients younger than 60 years were much more likely to need myotomy (14 patients) than patients older than 60 years (1 patient). Fourth, advanced stage achalasia is also less likely to respond to dilation. Many studies evaluating balloon dilation specifically exclude patients with sigmoidization of the esophagus because such patients are believed to have end-stage disease that is unlikely to respond to any therapeutic modality other than esophagectomy.
Box 18.2 Predictors of Response to Balloon Dilation
LES, Lower esophageal sphincter.
(From Kostic SV, Rice TW, Baker ME, et al: Timed barium esophagogram: A simple physiologic assessment for achalasia. J Thorac Cardiovasc Surg 120:935–946, 2000.)
From an objective point of view, neither radiographic findings nor the degree of esophageal emptying after dilation can predict long-term clinical course.70 However, absolute LES pressure does seem to correlate with long-term clinical benefit (Fig. 18.7), and a reassessment of LES pressure after the procedure may be helpful in some cases.70 As discussed previously, newer balloons and balloons with a greater diameter have not been shown to influence outcome, although one study suggested that higher balloon inflation pressures and radiographic visualization of a fully expanded balloon diameter predicted greater success.70
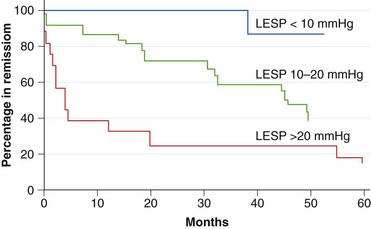
Fig. 18.7 Correlation between postdilation lower esophageal sphincter pressure (LESP) and clinical outcome.
(From Eckardt VF, Aignherr C, Bernhard G: Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology 103:1732–1738, 1992.)
Pneumatic balloon dilation of the LES for achalasia is by no means a risk-free procedure. The most concerning complication is esophageal perforation. Most studies quote a perforation rate of 0% to 6%.66,71,77–80 This complication is particularly important because esophageal perforation may have a poor outcome even with rapid careful management. The quoted mortality rate from traumatic esophageal perforation is 10%.81,82 Factors associated with an increased likelihood of esophageal perforation after balloon dilation include (1) a first dilation, (2) high-amplitude esophageal contractions, and (3) malnutrition.65,83,84
Perforation rates may be lower in the Rigiflex era,83 possibly as a result of the use of lower inflation pressures and shorter inflation times compared with prior reports. A more recent meta-analysis of 15 articles including 1065 patients who underwent pneumatic dilation quoted only a 1.6% perforation rate.85 Patients still must be cautioned very carefully before dilation. They especially need to be made aware that the operative management of a traumatic esophageal perforation generally requires an open approach through either the chest or the abdomen, whereas a primary Heller myotomy is easily accomplished laparoscopically in most cases. For this reason, many esophagologists are moving away from recommending pneumatic dilation as the primary modality for therapy, unless patients have a particular aversion or contraindication to surgery. Other worrisome complications of balloon dilation include hematomas (which rarely may cause esophageal obstruction68) and chest pain. As expected, chest pain is quite common, and sometimes it may be severe enough to warrant hospitalization even if contrast studies fail to show a perforation. Patients with achalasia who present initially with chest pain are more likely to develop severe chest pain after balloon dilation.65
Long-term complications may also develop after dilation. Gastroesophageal reflux is common when measured by ambulatory pH monitoring,86 but it usually is not severe, and it responds readily to proton pump inhibitor therapy. Nevertheless, severe reflux complications such as esophageal stricture formation and Barrett’s esophagus have been reported.65 The development of a diverticulum in the distal esophagus after balloon dilation has also been described,65 but whether this represents true diverticular formation or an esophageal ballooning from an effective dilation-induced myotomy87 is unclear.
Surgical Myotomy
Surgical cardiomyotomy (the Heller procedure) is an important and effective therapy for achalasia. It has stood the test of time (many long-term series have been published), it is relatively safe, and it has been successfully adapted to a minimally invasive laparoscopic approach.88–113 Patient satisfaction remains high 5 to 10 years after surgery with good to excellent results persisting in 85% to 90% of patients. Laparoscopic myotomy, a newer technique for which the available duration of follow-up is shorter by necessity, seems to be associated with similar outcomes to traditional open myotomy. Compared with pneumatic dilation, myotomy (whether performed in an open or laparoscopic fashion) outperforms dilation.77,88–91 However, there are two important limiting factors that keep pneumatic dilation a viable option. First is the ever-present, albeit small, risk of perioperative complications. Second is the steep learning curve involved in learning to perform laparoscopic myotomy reliably (at least 20, but probably more, cases). Several series note that most, if not all, serious complications occur within the first 20 to 30 patients.102,114 Similar to many other foregut surgical procedures, the results of laparoscopic myotomy are best if the procedure is performed by an expert, experienced surgeon.
Prior surgical controversies regarding the performance of a Heller myotomy have largely been resolved in recent years. Most esophagologists currently believe that a laparoscopic approach is superior to a thoracoscopic approach, and most believe a loose antireflux procedure should be performed at the same time, although debate still rages regarding what type of antireflux surgery is best. Esophagologists who argue against the need for an antireflux procedure point out that a carefully performed thoracoscopic or laparoscopic myotomy permits excellent visualization of the gastroesophageal junction allowing preservation of the vagus nerve and oblique sling fibers of the cardia, resulting in only minor traumatic mobilization of the lower esophagus through the diaphragmatic crural sphincter. As a consequence, reflux is unlikely to occur,114 as is borne out by some studies that show no differences in the development of reflux symptoms whether or not an antireflux operation is performed.96
Numerous other series have identified a high incidence postoperatively of reflux symptoms, abnormal ambulatory pHmetry scores, and subsequent development of peptic strictures and Barrett’s esophagus.109 In particular, over long-term follow-up, patients studied for greater than 6 years even with fundoplication have a greater than 40% chance of developing reflux manifest by esophagitis or abnormal pH monitoring or both.115,116 Surgeons continue to debate whether to perform a loose, but complete, fundoplication (i.e., a Nissen operation) or a partial fundoplication (e.g., a Dorr or Toupet procedure), and no clear recommendations have emerged to date. There is a fine line between not extending the myotomy too far down the cardia (leading to reflux) and paying the price with an incomplete myotomy (leading to failure and requiring a repeat operation). Consequently, we believe that most patients, if not all, should undergo an antireflux procedure with an extended (at least 8 cm above the LES and 2 cm below it) laparoscopic myotomy specifically. We do not favor a thoracoscopic approach, given the ease, lower complication rate, shorter hospital stay, and less postoperative pain associated with the laparoscopic approach.
Occasionally, patients with end-stage achalasia require esophagectomy. Numerous studies have shown that a favorable surgical outcome is closely related to the preoperative stage of disease.54 Patients with marked sigmoidization and dilation of the esophagus may not respond well to sphincter-directed therapy alone, although some investigators still advocate an attempt at surgical myotomy first.117 The few data available regarding these end-stage patients suggest that esophagectomy (preferably with a gastric pull-up rather than a colonic or jejunal interposition) enables most of these patients to regain their weight and eat within the expected limitations of any patient undergoing a total esophagectomy.118,119 Finally, successful robot-assisted laparoscopic Heller myotomy has been described.120 This technique provides further potential advances over a standard laparoscopic approach by providing a three-dimensional view of the gastroesophageal junction, restoring depth perception to the operator, and by improving precision by scaling down the surgeon’s movements so that large movements of the robotic console are translated into smaller movements in the patient. Robotic surgery also eliminates hand tremor. Whether or not these theoretical advantages result in better long-term outcomes remains to be shown.
Miscellaneous and Emerging Treatments
Pharmacologic therapy for achalasia has been used in the past and is presently undergoing further investigation. Sublingual nifedipine has been reported to improve symptoms, esophageal retention, and LES pressures.121,122 This approach may be useful in isolated, rare instances, but potential side effects and lack of published long-term efficacy limit its use. Similarly, sildenafil, by augmenting nitric oxide effects, lowers the LES pressure and improves some manometric measurements in patients with achalasia.123 Symptoms are not improved significantly, however, so there are no indications for its use at this time. Finally, expandable metal stents have been attempted in six poor surgical candidates with achalasia.124 There was no sustained symptom relief, and the 1-month mortality was extremely high, suggesting no role for this approach at the present time.
One of the newest and potentially useful methods of therapy for achalasia may be the use of peroral endoscopic myotomy (POEM). This method of myotomy is performed completely endoscopically without the need for breaching the abdomen. The seeds of success for this operation were first developed with the ability to perform submucosal endoscopy with mucosal flap safety valve (SEMF), at first used for access to the abdominal cavity.125 Following this breakthrough technique, the first successful POEM was performed in pigs.126 Most recently, this technique was adapted successfully to a series of 17 patients with achalasia.127 In this study, a submucosal myotomy, on average 8.0 cm in length including a 2-cm extension onto the cardia, leg to significant improvement of dysphagia score and reduction in LES pressure without serious complications. The follow-up for this group of patients was only 5 months, however. Whether this will become an effective long-term treatment comparable to laparoscopic myotomy has yet to be determined. It is also important to keep in mind that a fundoplication is not performed with this technique.
Treatment Summary
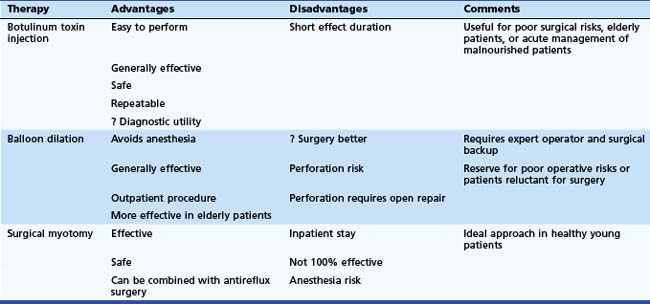
Trying to reach consensus on the appropriate initial treatment strategy for achalasia is one of the great debates in esophagology. Even decision models cannot agree, with one recent study recommending pneumatic dilation128 and another recommending laparoscopic cardiomyotomy.129 The latter publication indicated a preference for botulinum toxin injection over balloon dilation.130 Each of the three major approaches has its own advantages and disadvantages (Table 18.2). Botulinum toxin injection is easy to perform, generally works well, has only a small chance of significant complications, and can be given multiple times. One placebo-controlled trial showed similar outcomes for symptom control and objective measurements of changes in LES pressure and esophageal retention in patients randomly assigned to pneumatic dilation or botulinum toxin injection,57 although the number of patients studied was small (16), and the trend was in favor of dilation. The duration of effect with botulinum toxin is much shorter than with either balloon dilation or surgical myotomy requiring either repeated injections or ultimately a more definitive intervention such as balloon dilation or surgical myotomy.
In comparing balloon dilation with surgical myotomy, the available data favor a surgical approach, especially when one factors in the advantages afforded by using the laparoscopic approach. Although anecdotal evidence130 has suggested that dilation-induced esophageal perforation may be repaired laparoscopically with little lost compared with an elective procedure, this is by no means generally accepted. The disadvantages of balloon dilation over surgical myotomy include a poorer efficacy and a potentially missed opportunity to undergo a minimally invasive procedure. Some medical centers have all but abandoned pneumatic dilation in favor of laparoscopic myotomy. We have not yet embraced this approach completely, but the number of dilations we are performing currently has decreased markedly.
Our general approach for the management of patients with achalasia is as follows. Botulinum toxin injection is our preferred initial management strategy for patients who are elderly, who are poor operative risks, or who have an unclear or atypical form of achalasia (i.e., as a diagnostic approach131). We also favor this approach initially for patients who present in extremis to permit stabilization of their condition before definitive therapy is undertaken later or for patients who are reluctant to undergo definitive therapy until we can show the likely benefit of lowering LES pressure by another means later on. For young, healthy patients (arbitrarily, one might say, <25 years old), laparoscopic myotomy is indicated initially. We favor combining cardiomyotomy with a loose fundoplication at the same time. For older patients (arbitrarily >60 years old) who are otherwise in good health, pneumatic dilation may be indicated initially.
Achalasia and Cancer
The relationship of achalasia with cancer warrants special mention (Box 18.3). The disease has two important associations with malignancy. First is the development of an achalasialike syndrome as a consequence of malignancy (i.e., secondary achalasia), and second is the development of malignancy in patients with long-standing achalasia (i.e., secondary malignancy).
Secondary Achalasia
Secondary achalasia accounts for approximately 2.4% to 4% of all cases of achalasia.131,132 Malignancy may cause an achalasialike syndrome through two unrelated mechanisms. The first is through direct anatomic compression or infiltration of the LES133 mandating the performance of a careful upper GI endoscopy with careful retroflexion in all patients. The second is through an antibody-mediated paraneoplastic effect on esophageal function. In a study of 13 patients with tumor-induced achalasia,134 11 cases had direct involvement of the LES with penetration into the muscularis propria. Three of these cases showed extensive neoplastic involvement of the myenteric plexus of the LES. Ganglion cells appeared normal suggesting that the effect is purely due to an obstruction to esophageal outflow. That some of these malignancies induced achalasia through extrinsic compression alone is supported by case reports of benign processes, such as mediastinal fibrosis, surgical procedures, or large pancreatic pseudocysts, also causing achalasialike syndromes.135,136 In one of the patients in the aforementioned series, anti-Hu antibodies (antineuronal nuclear antibody type 1) were detected. Histologic analysis of the LES in this patient revealed marked lymphocytic myenteric plexus inflammation and marked depletion of ganglion cells identical to that seen in primary achalasia.
Several types of malignancy cause secondary achalasia. Their distribution depends partly on the mechanism involved. For malignancies that cause achalasia by direct involvement of the distal esophagus, adenocarcinoma of the cardia or gastroesophageal junction (with or without Barrett’s esophagus) predominates,129 but many other types of cancer have also been described, including cancer of the breast, liver, prostate, lung, pleura, pancreas, cervix, and uterus.134,137–141 For paraneoplastic achalasia, small cell carcinoma of the lung predominates, but other cancer types including lymphoma have also been described.134,142
The diagnosis of secondary achalasia requires a high index of suspicion. Although these patients tend to be older, have a shorter duration of symptoms, and commonly exhibit weight loss, studies have shown that even with these “red flags” primary achalasia is still statistically much more common.143 The onset of achalasia symptoms may precede signs of cancer by months or years144 in patients with paraneoplastic achalasia. One clue may be the presence of other neurologic symptoms or the presence of a more global GI motility syndrome (e.g., colonic pseudoobstruction) together with the presence of achalasia symptoms. In addition, although patients with paraneoplastic achalasia may appear radiologically and endoscopically identical to patients with primary disease, patients with secondary achalasia from direct tumor involvement tend to have longer areas of distal esophageal narrowing and less esophageal dilation than patients with primary achalasia.145 Endoscopically, as opposed to the characteristic “pop” that one feels on traversing the LES with primary achalasia, there is moderate to severe resistance during passage of the endoscope.
Treatment for secondary achalasia is usually directed at the tumor. The one exception might be patients with paraneoplastic disease, especially patients with antibody-confirmed disease but no obvious primary tumor in whom a botulinum toxin injection may be effective144 (anecdotal personal observation).
Secondary Malignancy
The second association of achalasia with malignancy is the increased risk of squamous cell carcinoma and perhaps adenocarcinoma of the esophagus in patients with long-standing primary disease. The estimated relative risk of developing squamous cell carcinoma of the esophagus in achalasia is 8-fold to 140-fold higher than in the normal population.146–149 In a long-term follow-up study of 249 patients with achalasia (a mean of 12 years), 6 (2.4%) developed esophageal cancer.149 A more recent study similarly showed the occurrence of squamous cell carcinoma in 5 of 228 consecutive patients with achalasia studied over a median of 18.3 years.150
Esophageal cancer generally develops at a younger age in patients with achalasia. Symptoms of achalasia generally precede the development of squamous cell carcinoma of the esophagus by 17 to 20 years.146–149 Consequently, there has been a long debate regarding the role, if any, of endoscopic surveillance in patients with achalasia, which remains unresolved. One more recent report described the potential role of flow cytometric analysis of esophageal biopsy specimens as a means of surveying these patients, but this recommendation was based on a single case report.151 No data are available to show whether effective treatment of achalasia reduces the development of esophageal cancer.150
1 Robertson CS, Martin BA, Atkinson M. Varicella-zoster virus DNA in the esophageal myenteric plexus in achalasia. Gut. 1993;34:299-302.
2 Facco M, Brun P, Baesso I, et al. T cells in the myenteric plexus of achalasia patients show a skewed TCR repertoire and react to HSV-1 antigens. Am J Gastroenterol. 2008;103:1598-1609.
3 Wong RK, Maydonovitch CL, Metz SJ, et al. Significant DQw association with achalasia. Dig Dis Sci. 1989;34:349-352.
4 Verne GN, Hahn AB, Pineau BC, et al. Association of LSA-DR and -DQ alleles with idiopathic achalasia. Gastroenterology. 1999;117:26-31.
5 Ruiz-de-Leon A, Mendoza J, Sevilla-Mantilla C, et al. Myenteric antiplexus antibodies and class II HLA in achalasia. Dig Dis Sci. 2002;47:15-19.
6 Verne GN, Sallusto JE, Baker EY. Anti-myenteric neuronal antibodies in patients with achalasia: A prospective study. Dig Dis Sci. 1997;42:307-313.
7 Goin JC, Sterin-Borda L, Bilder CR, et al. Functional implications of circulating muscarinic cholinergic receptor autoantibodies in chagasic patients with achalasia. Gastroenterology. 1999;117:798-805.
8 Cassella RR, Brown AL, Sayre GP, et al. Achalasia of the esophagus: Pathologic and etiologic considerations. Ann Surg. 1964;160:474-479.
9 Goldblum JR, Whyte RI, Orringer MB, et al. Achalasia: a morphologic study of 42 resected specimens. Am J Surg Pathol. 1994;18:327-337.
10 Goldblum JR, Rice TW, Richter JE. Histopathologic features in esophagomyotomy specimens from patients with achalasia. Gastroenterology. 1996;111:648-654.
11 Clark SB, Rice TW, Tubbs RR, et al. The nature of the myenteric infiltrate in achalasia: An immunohistochemical analysis. Am J Surg Pathol. 2000;24:1153-1158.
12 Tottrup A, Fredens K, Funch-Jensen P, et al. Eosinophil infiltration in primary esophageal achalasia: A possible pathogenic role. Dig Dis Sci. 1989;34:297-303.
13 Higgs B, Kerr FW, Ellis FHJr. The experimental production of esophageal achalasia by electrolytic lesions in the medulla. J Thorac Cardiovasc Surg. 1965;50:613-625.
14 Holland CT, Satchell PM, Farrow BR. Selective vagal afferent dysfunction in dogs with congenital idiopathic megaesophagus. Auton Neurosci. 2002;99:18-23.
15 Mearin F, Mourelle M, Guarner F, et al. Patients with achalasia lack nitric oxide synthase in the gastro-esophageal junction. Eur J Clin Invest. 1993;23:724-728.
16 Sivarao DV, Mashimo HL, Thatte HS, et al. Lower esophageal sphincter is achalasic in nNOS(−/−) and hypotensive in W/W(v) mutant mice. Gastroenterology. 2001;121:34-42.
17 Whyte RI, Orringer MB, Appelman HD. Achalasia: A morphologic study of 42 resected specimens. Am J Surg Pathol. 1994;18:327-337.
18 Mearin F, Vasconez C, Zarate N, et al. Esophageal tone in patients with total aperistalsis: Gastroesophageal reflux disease versus achalasia. Am J Physiol. 2000;279:G374-G379.
19 Vantrappen G, Hellemans J, Deloof W, et al. Treatment of achalasia with pneumatic dilatations. Gut. 1971;12:268-275.
20 Okike N, Payne WS, Neufeld DM, et al. Esophagomyotomy versus forceful dilatation for achalasia of the esophagus: Results in 899 patients. Ann Thorac Surg. 1979;28:119-123.
21 Wong RK, Johnson LF. Achalasia. In: Castell DO, Johnson LF, editors. Esophageal function in health and disease. New York: Elsevier Biomedical; 1983:99-123.
22 Howard PJ, Maher L, Pryde A, et al. Five year prospective study of the incidence, clinical features, and diagnosis of achalasia in Edinburgh. Gut. 1992;33:1011-1015.
23 Eckardt VF, Kohne U, Westermeier T. Risk factors for diagnostic delay in achalasia. Dig Dis Sci. 1997;42:580-585.
24 Eckardt VF, Stauf B, Bernhard G. Chest pain in achalasia: Patient characteristics and clinical course. Gastroenterology. 1999;116:1300-1304.
25 Blam ME, Delfyett W, Levine MS, et al. Achalasia: A disease of varied and subtle symptoms that do not correlate with radiographic findings. Am J Gastroenterol. 2002;97:1916-1923.
26 Becker DJ, Castell DO. Acute airway obstruction in achalasia: Possible role of defective belch reflex. Gastroenterology. 1989;97:1323-1326.
27 Arcos E, Medine C, Mearin F, et al. Achalasia presenting as acute airway obstruction. Dig Dis Sci. 2000;45:2079-2083.
28 Dudnick RS, Castell JA, Castell DO. Abnormal upper esophageal sphincter function in achalasia. Am J Gastroenterol. 1992;87:1712-1715.
29 Massey B, Hogan WJ, Dodds WJ, et al. Alteration of the upper esophageal sphincter belch reflex in patients with achalasia. Gastroenterology. 1992;103:1574-1579.
30 Schima W, Sterz F, Pokieser P. Syncope after eating. N Engl J Med. 1993;328:1572.
31 Fisichella PM, Raz D, Palazzo F, et al. Clinical, radiological, and manometric profile in 145 patients with untreated achalasia. World J Surg. 2008;32:1974-1979.
32 Binder HJ, Clemett AR, Thayer WR, et al. Rarity of hiatus hernia in achalasia. N Engl J Med. 1965;272:680-682.
33 Goldenberg SP, Vos C, Burrell M, et al. Achalasia and hiatal hernia. Dig Dis Sci. 1992;37:528-531.
34 Van Herwaarden MA, Sansom M, Smout AJ. Prolonged manometric recordings of esophagus and lower esophageal sphincter in achalasia patients. Gut. 2001;49:813-821.
35 Hirano I, Tatum RP, Shi G, et al. Manometric heterogeneity in patients with idiopathic achalasia. Gastroenterology. 2001;120:789-798.
36 Vantrappen G, Janssens J, Hellemans J, et al. Achalasia, diffuse esophageal spasm, and related motility disorders. Gastroenterology. 1979;76:450-457.
37 Anggiansah A, Bright NF, McCullagh M, et al. Transition from nutcracker esophagus to achalasia. Dig Dis Sci. 1990;35:1162-1166.
38 Golioto M, McGrath K, Smith J, et al. Achalasia with high-amplitude esophageal body contractions. Dig Dis Sci. 2001;46:1960-1962.
39 Goldenberg SP, Burrell M, Fette GG, et al. Classic and vigorous achalasia: A comparison of manometric, radiographic, and clinical findings. Gastroenterology. 1991;101:743-748.
40 Pandolfino JE, Kwiatek MA, Nealis T, et al. Achalasia: A new clinically relevant classification by high-resolution manometry. Gastroenterology. 2008;135:1526-1533.
41 Kahrilas PJ, Sifrim D. High-resolution manometry and impedance-pH/manometry: Valuable tools in clinical and investigational esophagology. Gastroenterology. 2008;135:756-769.
42 Pandolfino JE, Ghosh SK, Rice J, et al. Classifying esophageal motility by pressure topography characteristics: A study of 400 patients and 75 controls. Am J Gastroenterol. 2008;103:27-37.
43 Miller LS, Liu JB, Barbarevich CA, et al. High-resolution endoluminal sonography in achalasia. Gastrointest Endosc. 1995;42:545-549.
44 Van Dam J, Falk GW, Sivak MV, et al. Endosonographic evaluation of the patient with achalasia: Appearance of the esophagus using the echoendoscope. Endoscopy. 1995;27:185-190.
45 Kostic SV, Rice TW, Baker ME, et al. Timed barium esophagogram: A simple physiologic assessment for achalasia. J Thorac Cardiovasc Surg. 2000;120:935-946.
46 Eckardt VF, Aignherr C, Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology. 1992;103:1732-1738.
47 Neubrand M, Scheurlen C, Schepki M, et al. Long-term results and prognostic factors in the treatment of achalasia with botulinum toxin. Endoscopy. 2002;34:519-523.
48 Robertson CS, Fellows IW, Mayberry JF, et al. Choice of therapy for achalasia in relation to age. Digestion. 1988;40:244-250.
49 Lasch H, Castell DO, Castell JA. Evidence for diminished visceral pain with aging: Studies using graded intraesophageal balloon distension. Am J Physiol. 1997;272(1 Pt 1):G1-G3.
50 Ellis FH, Olsen AM. Achalasia of the esophagus. In: Ellis FH, Olsen AM, editors. Major problems in clinical surgery, vol 9. Philadelphia: WB Saunders; 1969:205.
51 Vantrappen G, Hellemans J. Treatment of achalasia and related motor disorders. Gastroenterology. 1980;79:144-154.
52 Vaezi MF, Baker ME, Achkar E, et al. Timed barium esophagram: Better predictor of long term success after pneumatic dilation in achalasia than symptom assessment. Gut. 2002;50:765-770.
53 De Oliveira JM, Birgisson S, Doinoff C, et al. Timed barium swallow: A simple technique for evaluating esophageal emptying in patients with achalasia. AJR Am J Roentgenol. 1997;169:473-479.
54 Pechlivanides G, Chrysos E, Athanasakis E, et al. Laparoscopic Heller cardiomyotomy and Dor fundoplication for esophageal achalasia. Arch Surg. 2001;16:1240-1243.
55 Pashricha PJ, Rai R, Ravich WJ, et al. Botulinum toxin for achalasia: Long-term outcome and predictors of response. Gastroenterology. 1996;110:1410-1415.
56 Kolbasnik J, Waterfall WE, Fachnie B, et al. Long-term efficacy of botulinum toxin in classical achalasia: A prospective study. Am J Gastroenterol. 1999;94:3434-3439.
57 Annese V, Basciani M, Perri F, et al. Controlled trial of botulinum toxin injection versus placebo and pneumatic dilation in achalasia. Gastroenterology. 1996;111:1418-1424.
58 Pasricha PJ, Ravich WJ, Hendrix TR, et al. Intrasphincteric botulinum toxin for the treatment of achalasia. N Engl J Med. 1995;332:774-778.
59 Cuilliere C, Ducrotte P, Zerbib R, et al. Achalasia: Outcome of patients treated with intrasphincteric injection of botulinum toxin. Gut. 1997;41:87-92.
60 Neubrand M, Scheurlen C, Schepki M, et al. Long-term results and prognostic factors in the treatment of achalasia with botulinum toxin. Endoscopy. 2002;34:519-523.
61 Kolbasnik J, Waterfall WE, Fachnie B, et al. Long-term efficacy of Botulinum toxin in classical achalasia: A prospective study. Am J Gastroenterol. 1999;94:3434-3439.
62 Campos GM, Vittinghoff E, Rabl C, et al. Endoscopic and surgical treatment for achalasia. Ann Surg. 2009;249:45-57.
63 Sukerek H, Tolia V. Clinical quiz. J Pediatr Gastroenterol Nutr. 2002;35:38-39.
64 Sweet MP, Nipomnick I, Gasper WJ, et al. The outcome of laparoscopic Heller myotomy for achalasia is not influenced by the degree of esophageal dilatation. J Gastrointest Surg. 2008;12:159-165.
65 Csendes A, Velasco N, Braghetto I, et al. A prospective randomized study comparing forceful dilatation and esophagomyotomy in patients with achalasia of the esophagus. Gastroenterology. 1981;80:789-795.
66 Gelfand MD, Kozarek RA. An experience with polyethylene balloons for pneumatic dilation in achalasia. Am J Gastroenterol. 1989;84:924-927.
67 Eckardt VF, Kanzler G, Westermeier T. Complications and their impact after pneumatic dilation for achalasia: Prospective long-term follow-up study. Gastrointest Endosc. 1997;45:349-353.
68 Kim CH, Cameron AJ, Hsu JJ, et al. Achalasia: Prospective evaluation of relationship between lower esophageal sphincter pressure, esophageal transit, and esophageal diameter and symptoms in response to pneumatic dilation. Mayo Clin Proc. 1993;68:1067-1073.
69 West RL, Hirsch DP, Bartelsman JF, et al. Long term results of pneumatic dilation in achalasia followed for more than 5 years. Am J Gastroenterol. 2002;97:1346-1351.
70 Eckardt VF, Aignherr C, Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology. 1992;103:1732-1738.
71 Nair LA, Reynolds JC, Parkman HP, et al. Complications during pneumatic dilation for achalasia or diffuse esophageal spasm: Analysis of risk factors, early clinical characteristics, and outcome. Dig Dis Sci. 1993;38:1893-1904.
72 Parkman HP, Reynolds JC, Ouyang A, et al. Pneumatic dilatation or esophagomyotomy treatment for idiopathic achalasia: Clinical outcomes and cost analysis. Dig Dis Sci. 1993;38:75-85.
73 Robertson CS, Fellows IW, Mayberry JF, et al. Choice of therapy for achalasia in relation to age. Digestion. 1988;40:244-250.
74 Penagini R, Cantu P, Mangano M, et al. Long-term effects of pneumatic dilatation on symptoms and lower esophageal sphincter pressure in achalasia. Scand J Gastroenterol. 2002;37:380-384.
75 Katz PO, Gilbert J, Castell DO. Pneumatic dilatation is effective long-term treatment for achalasia. Dig Dis Sci. 1998;43:1973-1977.
76 Chuah S-K, Hu T-H, Wu K-L, et al. Endoscope-guided pneumatic dilatation of esophageal achalasia without fluoroscopy is another safe and effective treatment option: A report of Taiwan. Surg Laparosc Endosc Percutan Tech. 2008;18:8-12.
77 Arvanitakis C. Achalasia of the esophagus: A reappraisal of esophagomyotomy vs. forceful pneumatic dilation. Dig Dis Sci. 1975;20:841-846.
78 Sanderseon DR, Ellis FH, Olsen AM. Achalasia of the esophagus: Results of therapy by dilation. Chest. 1970;48:116-121.
79 Vantrappen G, Hellemans J, Deloof W, et al. Treatment of achalasia with pneumatic dilations. Gut. 1971;12:268-275.
80 Fellows IW, Oglivie AL, Atkinson M. Pneumatic dilatation in achalasia. Gut. 1983;12:268-275.
81 Bladergroen MR, Lowe JE, Postlethwait RW. Diagnosis and recommended management of esophageal perforation and rupture. Ann Thorac Surg. 1986;42:235-239.
82 Wesdorp IC, Bartelsman JF, Huibregtse K, et al. Treatment of instrumental esophageal perforation. Gut. 1984;25:398-404.
83 Borotto E, Gaudric M, Danel B, et al. Risk factors of esophageal perforation during pneumatic dilatation for achalasia. Gut. 1996;39:9-12.
84 Fennerty B. Esophageal perforation during pneumatic dilatation for achalasia: A possible association with malnutrition. Dysphagia. 1990;5:227-228.
85 Campos GM, Vittinghoff E, Rabl C, et al. Endoscopic and surgical treatment for achalasia. Ann Surg. 2009;249:45-57.
86 Shoenut JP, Duerksen D, Yaffe CS. A prospective assessment of gastroesophageal reflux before and after treatment of achalasia patients: Pneumatic dilation versus transthoracic limited myotomy. Am J Gastroenterol. 1997;92:1109-1112.
87 Rubesin SE, Kennedy M, Levine MS, et al. Distal esophageal ballooning following Heller myotomy. Radiology. 1988;167:345-347.
88 Okike N, Payne WS, Meufeld DM, et al. Esophagomyotomy versus forceful dilation for achalasia of the esophagus: Results in 899 patients. Ann Thorac Surg. 1979;28:119-125.
89 Csendes A, Braghetto I, Henriquez A, et al. Late results of a prospective randomised study comparing forceful dilatation and esophagomyotomy in patients with achalasia. Gut. 1989;30:299-304.
90 Suarez J, Mearin F, Boque R, et al. Laparoscopic myotomy vs. endoscopic dilation in the treatment of achalasia. Surg Endosc. 2002;16:75-77.
91 Yon J, Christensen J. An uncontrolled comparison of treatments for achalasia. Ann Surg. 1975;182:672-676.
92 Paricio PP, Martinez de Haro L, Ortiz A, et al. Achalasia of the cardia: Long-term results of esophagomyotomy and posterior partial fundoplication. Br J Surg. 1990;77:1371-1374.
93 Sariyannis C, Mullard KS. Esophagomyotomy for achalasia of the cardia. Thorax. 1975;30:539-542.
94 Hirashima T, Sato H, Hara T, et al. Results of esophagocardioplasty with gastric patch treatment of esophageal achalasia. Ann Surg. 1978;188:38-42.
95 Ellis FH, Crozier RE, Watkins E. Operation for esophageal achalasia: Results of esophagomyotomy without an antireflux operation. J Thorac Cardiovasc Surg. 1984;88:344-351.
96 Jordan PHJr. Longterm results of esophageal myotomy for achalasia. J Am Coll Surg. 2001;193:137-145.
97 Pai G, Ellison R, Rubin JW, et al. Two decades of experience with modified Heller’s myotomy for achalasia. Ann Thorac Surg. 1984;38:201-206.
98 Black J, Vorbach AN, Leigh Collis J. Results of Heller’s operation for achalasia of the esophagus: The importance of hiatal hernia repair. Br J Surg. 1976;63:949-953.
99 Wingfield HV, Karwowski A. The treatment of achalasia by cardiomyotomy. Br J Surg. 1972;59:281-284.
100 Ben-Meir A, Urbach DR, Khajanchee YS, et al. Quality of life before and after laparoscopic Heller myotomy for achalasia. Am J Surg. 2001;181:471-474.
101 Patti MG, Molena D, Fisichella PM, et al. Laparoscopic Heller myotomy and Dor fundoplication for achalasia: Analysis of successes and failures. Arch Surg. 2001;136:870-877.
102 Finley RJ, Clifton JC, Stewart KC, et al. Laparoscopic Heller myotomy improves esophageal emptying and the symptoms of achalasia. Arch Surg. 2001;136:892-896.
103 Maher JW, Conklin J, Heitshusen DS. Thoracoscopic esophagomyotomy for achalasia. Surgery. 2001;130:570-577.
104 Yamamura MS, Gilstger JC, Myers BS, et al. Laparoscopic Heller myotomy and anterior fundoplication for achalasia results in a high degree of patient satisfaction. Arch Surg. 2000;135:902-906.
105 Luketich JD, Fernando HC, Christie NA, et al. Outcomes after minimally invasive esophagomyotomy. Ann Thorac Surg. 2001;72:1909-1913.
106 Ackroyd R, Watson EI, Devitt PG, et al. Laparoscopic cardiomyotomy and anterior partial fundoplication for achalasia. Surg Endosc. 2001;15:683-686.
107 Diener U, Patti MG, Molena D, et al. Laparoscopic Heller myotomy relieves dysphagia in patients with achalasia and low LES pressure following pneumatic dilatation. Surg Endosc. 2001;15:687-690.
108 Finley RJ, Clifton JC, Stewart KC, et al. Laparoscopic Heller myotomy improves esophageal emptying and the symptoms of achalasia. Arch Surg. 2001;136:892-896.
109 DiSimone MP, Felice V, D’Errico A, et al. Onset timing of delayed complications and criteria of follow-up after operation for esophageal achalasia. Ann Thorac Surg. 1996;61:1106-1111.
110 Holzman MD, Sharp KW, Lapido JK, et al. Laparoscopic surgical treatment of achalasia. Am J Surg. 1997;173:308-311.
111 Ancona E, Anselmino M, Zanitto G, et al. Esophageal achalasia: Laparoscopic versus conventional open Heller-Dor operation. Am J Surg. 1995;170:265-270.
112 Raiser F, Perdikis G, Hinder RA, et al. Heller myotomy via minimal-access surgery: An evaluation of antireflux procedures. Arch Surg. 1996;131:593-598.
113 Watson EI, Devitt PG, Jamieson GG. Laparoscopic cardiomyotomy and anterior partial fundoplication for achalasia. Surg Endosc. 2001;15:683-686.
114 Robertson GS, Lloyd DM, Wicks ACB, et al. Laparoscopic Heller’s cardiomyotomy without an antireflux procedure. Br J Surg. 1995;82:957-959.
115 Csendes A, Braguetto I, Burdiles P, et al. Very late results of esopohagomyotomy for patients with achalasia. Ann Surg. 2006;243:196-203.
116 Ortiz A, Martinez de Haro LF, Parrilla P, et al. Very long-term objective evaluation of Heller myotomy plus posterior partial fundoplication in patients with achalasia of the cardia. Ann Surg. 2008;247:258-264.
117 Sweet MP, Nipomnick I, Gasper WJ, et al. The outcome of laparoscopic Heller myotomy for achalasia is not influenced by the degree of esophageal dilatation. J Gastrointest Surg. 2008;12:159-165.
118 Peters JH, Kauer WK, Crookes PF, et al. Esophageal resection with colon interposition for end-stage achalasia. Arch Surg. 1995;130:632-637.
119 Gupta NM, Goenka MK, Behera A, et al. Transhiatal esophagectomy for benign obstructive conditions of the esophagus. Br J Surg. 1997;84:262-264.
120 Shah J, Rockall R, Carzi A. Robot-assisted laparoscopic Heller’s cardiomyotomy. Surg Laparosc Endosc Percutan Tech. 2002;12:30-32.
121 Berger K, McCallum RW. Nifedipine in the treatment of achalasia. Ann Intern Med. 1982;96:61-62.
122 Coccia G, Bortolotti M, Michetti P, et al. Return of esophageal peristalsis after nifedipine therapy in patients with idiopathic achalasia. Am J Gastroenterol. 1992;87:1705-1708.
123 Bortolotti M, Mari C, Lopilato C, et al. Effects of sildenafil on esophageal motility of patients with idiopathic achalasia. Gastroenterology. 2000;118:253-257.
124 Mukherjee S, Kaplan DS, Parasher G, Sipple MS. Expandable metal stents in achalasia—is there a role? Am J Gastroenterol. 2000;95:2185-2188.
125 Sumiyama K, Tajiri H, Gostout CJ. Submucosal endoscopy with mucosal flap safety valve (SEMF) technique: A safe access method into the peritoneal cavity and mediastinum. Minimally Invasive Therapy. 2008;17:365-369.
126 Pasricha PJ, Hawari R, Ahmed L, et al. Submucosal endoscopic esophageal myotomy: A novel experimental approach for the treatment of achalasia. Endoscopy. 2007;39:761-764.
127 Inoue H, Minami H, Kobayashi Y, et al. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42:265-271.
128 O’Connor JB, Singer ME, Imperiale TF, et al. The cost-effectiveness of treatment strategies for achalasia. Dig Dis Sci. 2002;47:1516-1525.
129 Urbach DR, Hansen PD, Khajanchee YS, et al. A decision analysis of the optimal initial approach to achalasia: Laparoscopic Heller myotomy with partial fundoplication, thoracoscopic Heller myotomy, pneumatic dilation, or botulinum toxin injection. J Gastrointest Surg. 2001;5:192-205.
130 Katzka DA, Castell DO. Use of botulinum toxin as a diagnostic/therapeutic trial to help clarify an indication for definitive therapy in patients with achalasia. Am J Gastroenterol. 1999;94:637-642.
131 Sandler RS, Bozymksi EM, Orlando RC. Failure of clinical criteria to distinguish between primary achalasia and achalasia secondary to tumor. Dig Dis Sci. 1982;27:209-213.
132 Moonka R, Patti MG, Feo CV, et al. Clinical presentation and evaluation of malignant pseudoachalasia. J Gastrointest Surg. 1999;3:456-461.
133 Raymond L, Lach B, Shamji FM. Inflammatory etiology of primary esophageal achalasia: An immunohistochemical and ultrastructural study of Auerbach’s plexus. Histopathology. 1999;35:445-453.
134 Liu W, Fackler W, Rice TW, et al. The pathogenesis of pseudoachalasia: A clinicopathologic study of 13 cases of a rare entity. Am J Surg Pathol. 2002;26:784-788.
135 Awad ZT, Selima MA, Filipi CJ. Pseudoachalasia as a late complication of gastric wrap performed for morbid obesity: Report of a case. Surg Today. 2002;32:906-909.
136 Colarian JH, Sekkarie M, Rao R. Pancreatic pseudocyst mimicking idiopathic achalasia. Am J Gastroenterol. 1998;93:103-105.
137 Tanigawa H, Kida Y, Kuwao S, et al. Hepatoid adenocarcinoma in Barrett’s esophagus associated with achalasia: First case report. Pathol Int. 2002;52:141-146.
138 Bholat OS, Haluck RS. Pseudoachalasia as a result of metastatic cervical cancer. J Soc Laparoendosc Surg. 2001;5:57-62.
139 Song CW, Chun HJ, Kim CD, et al. Association of pseudoachalasia with advancing cancer of the gastric cardia. Gastrointest Endosc. 1999;50:486-491.
140 Lopez-Liuchi JV, Kraytem A, Uldry PY. Oesophageal achalasia secondary to pleural mesothelioma. J R Soc Med. 1999;92:24-25.
141 Moonka R, Patti MG, Feo CV, et al. Clinical presentation and evaluation of malignant pseudoachalasia. J Gastrointest Surg. 1999;3:456-461.
142 Lucchinetti CF, Kimmel DW, Lennon VA. Paraneoplastic and oncologic profiles of patients seropositive for type 1 antineuronal nuclear autoantibodies. Neurology. 1998;50:652-657.
143 Sandler RS, Bozymksi EM, Orlando RC. Failure of clinical criteria to distinguish between primary achalasia and achalasia secondary to tumor. Dig Dis Sci. 1982;27:209-213.
144 Valera RA, Brazer SR. Botulinum toxin for suspected pseudoachalasia. Am J Gastroenterol. 1995;90:1319-1321.
145 Woodfield CA, Levine MS, Rubesin SE, et al. I. Diagnosis of primary versus secondary achalasia: Reassessment of clinical and radiographic criteria. AJR Am J Roentgenol. 2000;175:727-731.
146 Brossard E, Ollyo JB, Fontolliet C, et al. Achalasia and squamous cell carcinoma of the esophagus: Is an endoscopic surveillance justified? [abstract]. Gastroenterology. 1992;102:A4.
147 Meijssen MA, Tilanus HW, van Blankenstein M, et al. Achalasia complicated by esophageal squamous cell carcinoma: A prospective study of 195 patients. Gut. 1992;33:155-158.
148 Just-Viera JO, Haight C. Achalasia and carcinoma of the esophagus. Surg Gynecol Obstet. 1969;128:1081-1095.
149 Brucher BL, Stein HJ, Bartels H, et al. Achalasia and esophageal cancer: Incidence, prevalence, and prognosis. World J Surg. 2001;25:745-749.
150 Zaninotto G, Rizzetto C, Zambon P, et al. Long-term outcome and risk of esophageal cancer after surgery for achalasia. Br J Surg. 2008;95:1488-1494.