Chapter 1 ABNORMAL PAP SMEAR (ABNORMAL CERVICAL CYTOLOGIC FINDINGS)
Cervical cytology screening has significantly decreased rates of mortality from cervical cancer; however, 400 women die each year in the United States from cervical cancer, mostly as a result of inadequate screening.
Cervical cytology results are classified according to the Bethesda 2001 system (Box 1-1), which describes the categories of epithelial cell abnormalities. Histologic diagnoses of abnormalities are reported as cervical intraepithelial neoplasia (CIN) grades 1-3.
Box 1-1. The 2001 Bethesda System Categorizing of Epithelial Cell Abnormalities
Squamous Cell
Modified from Solomon D, Davey D, Kurman R, et al: The 2001 Bethesda System: terminology for reporting results of cervical cytology. Forum Group Members; Bethesda 2001 Workshop. JAMA 2002;287:2114-2119. Copyright © 2002, American Medical Association. All rights reserved.
Other risk factors for cervical cancer include cigarette smoking, immunocompromised status (e.g., human immunodeficiency virus [HIV] infection), early age at onset of sexual activity, multiple sexual partners, and sexual activity with male partners at high risk for sexually transmitted diseases.
Causes of Abnormal Cervical Cytologic Findings
• Causes of ASC-US include atrophy, infection, inflammation, cervical dysplasia, and cervical cancer.
• Invasive cervical cancer is present in 0.1% to 0.2% of women with ASC-US and in 1% to 2% of women with high-grade squamous intraepithelial lesions (HSIL). About 5% to 10% of women with AGC have adenocarcinoma in situ or adenocarcinoma.
• About 5% of women with ASC-US, 24% to 94% of women with atypical squamous cells with high-grade intraepithelial lesion (ASC-H), 15% to 30% of women with low-grade squamous intraepithelial lesions (LSIL), 75% of women with HSIL, and up to 50% of women with AGC have moderate to severe cervical dysplasia.
Key Historical Features
Key Physical Findings
✓ External genital examination to evaluate for erythema (sign of infection with Trichomonas or Candida organisms) or ulcerative lesions, which may indicate herpes infection
✓ Speculum examination to evaluate for discharge, bleeding, or vaginal lesions (the vagina may appear erythematous with Trichomonas or Candida infection) and to evaluate the cervix for masses, erosions, ulcers, friability, or bleeding
✓ Bimanual examination to evaluate for any uterine or adnexal masses or tenderness
✓ Rectovaginal examination to evaluate for any masses or tenderness
Suggested Work-Up
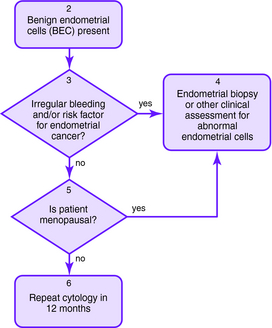
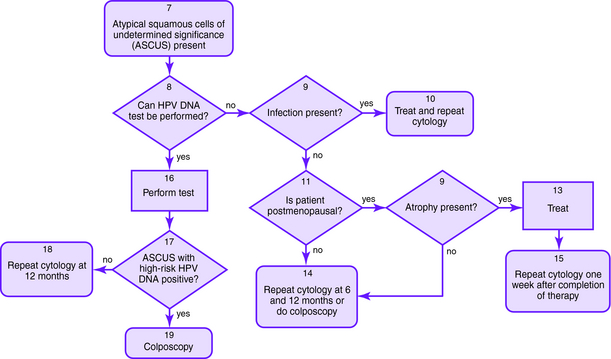
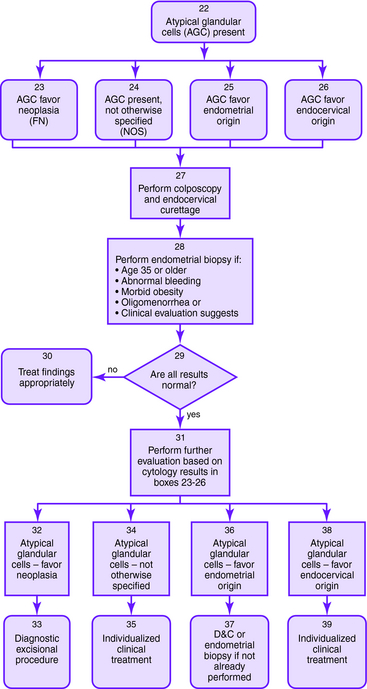
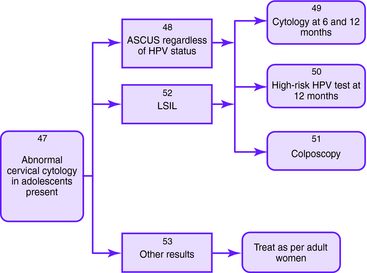
The Bethesda 2001 classification system was used to create the American Society for Colposcopy and Cervical Pathology Consensus Guidelines in 2001 to distinguish women at risk for significant cervical disease from those with mild or no disease. The American College of Obstetrics and Gynecology also published guidelines for the management of abnormal Pap smears/cervical cytologic findings (Figs. 1-1 to 1-5). The guidelines involve substantial use of HPV DNA testing and colposcopy. Other testing used includes endocervical sampling, biopsy, and excisional procedures.
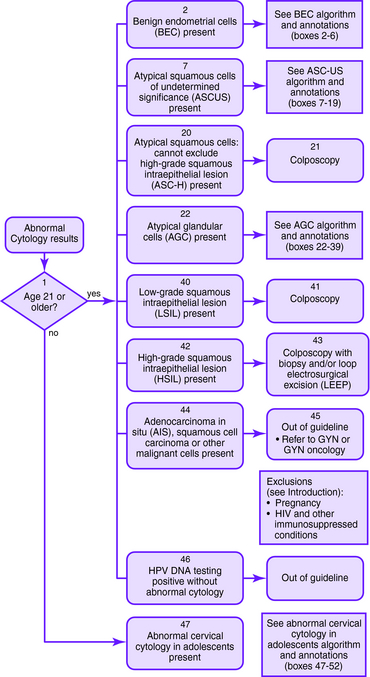
Figure 1-1. Initial management of abnormal cervical cytology (Pap smear) and human papillomavirus testing. Algorithm for initial abnormal cytologic result. Box numbers refer to algorithm boxes in Figure 1-1 to 1-5.
The following are recommendations for evaluation that are based upon cervical cytologic results. Algorithms are provided in Figures 1-1 to 1-5.
| ASC-US | If HPV testing is positive, colposcopy should be performed with consideration of endocervical sampling with a brush or curette; if finding is negative, then the Pap smear should be repeated in 1 year |
| If HPV test result is negative, then a Pap smear should be repeated in 1 year; if HPV testing is not performed, other options include immediate colposcopy or repeat Pap smear at 6 and 12 months | |
| If the patient is immunocompromised, then colposcopy should be performed immediately | |
| In adolescents with ASC-US who are HPV positive, Pap smears may be repeated at 6 and 12 months or HPV testing may be undertaken at 12 months instead of immediate colposcopy, since clearance rate of HPV is high | |
| ASC-H | Colposcopy should be performed with consideration of endocervical sampling |
| LSIL | Colposcopy should be performed with consideration of endocervical sampling |
| In adolescents with LSIL, Pap smears may be repeated at 6 and 12 months or HPV testing may be repeated at 12 months instead of immediate colposcopy, since the clearance rate of HPV is high | |
| HSIL | Colposcopy with endocervical sampling and biopsy should be performed. If the colposcopic finding is negative or inconclusive, then an excision should be performed |
| AGC | Colposcopy and endocervical sampling should be performed |
| Endometrial sampling should be performed if the patient is older than 35 years or is at risk for endometrial cancer (abnormal bleeding, obesity, or oligomenorrhea) | |
| Figure 1-1 to 1-4 outlines the management of AGC based on initial cytology results. | |
| AGC, favor neoplasia, or adenocarcinoma in situ | If the previously described work-up for AGC does not show invasive disease, then a diagnostic excisional procedure should be performed (cold-knife conization is preferred) |
Additional Work-Up
| Test for HIV infection | In patients at risk |
| Chlamydia and gonorrhea cultures or a nucleic acid amplification test | In patients at risk |
| Urine pregnancy test | Should be performed before an invasive procedure if the patient may be pregnant |
| Wet mount evaluation | To evaluate for Candida infection, bacterial vaginosis, or Trichomonas infection if any of these conditions is suspected |
American College of Obstetrics and Gynecology. ACOG Practice Bulletin number 66, September 2005. Management of abnormal cervical cytology and histology. Obstet Gynecol. 2005;106:645-664.
Apgar BS, Brotzman G. Management of cervical cytologic abnormalities. Am Fam Physician. 2004;70:1905-1916.
Apgar BS, Zoschnick L, Wright TCJr. The 2001 Bethesda system terminology. Am Fam Physician. 2003;68:1992-1998.
Buechler EJ. Pap tests and HPV infection. Postgrad Med. 2005;118(2):37-46.
Bundrick JB, Cook DA, Gostout BS. Screening for cervical cancer and initial treatment of patients with abnormal results from Papanicolaou testing. Mayo Clin Proc. 2005;80:1063-1108.
Institute for Clinical Systems Improvement. In Initial Management of Abnormal Cervical Cytology (Pap Smear) and HPV Testing. Bloomington, MN: Institute for Clinical Systems Improvement; 2006.
Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. Forum Group Members; Bethesda 2001 Workshop. JAMA. 2002;287:2114-2119.
U.S. Preventive Services Task Force: Screening for Cervical Cancer. Available at http://www.ahrq.gov. Accessed on Web 8/8/07