CHAPTER 53 ABDOMINAL VASCULAR INJURIES
Abdominal vascular injuries remain among the most lethal of injuries that the trauma surgeon will encounter. The successful management of these injuries requires a well-organized trauma system capable of swiftly transporting the patient to the appropriate facility, a trauma center capable of rapidly mobilizing an appropriate surgical team, and a trauma surgeon capable of expeditiously resuscitating the patient, localizing the injury, and controlling the source of hemorrhage.
EPIDEMIOLOGY
Penetrating abdominal injuries are the most common cause of abdominal vascular injuries, accounting for 67%–91%.1 Abdominal vascular injuries are treated much more commonly in today’s urban trauma centers than in recent military conflicts.2 Indeed, in DeBakey and Simeone’s3 classic review of 2471 arterial injuries from World War II, only 49 (2%) were abdominal arterial injuries. Similarly, Hughes4 reported on 304 arterial injuries from the Korean conflict and found 7 (2.3%) occurred in the iliac arteries. Finally, Rich and colleagues5 reviewed 1000 arterial injuries from the Vietnam conflict and found that only 29 (2.9%) involved abdominal vessels. In contrast, a 30-year review of 5760 cardiovascular injuries from Ben Taub General Hospital in Houston found 1947 (33.8%) abdominal vascular injuries.6 The Emory University trauma service at Grady Memorial Hospital consistently treats over 30 patients per year with abdominal vascular injuries. The marked difference between the two settings is believed to reflect the increased wounding power of military firearms, delayed transport to appropriate surgical facilities and, more recently, the protection of torso body armor.
Patients undergoing laparotomy after sustaining abdominal gunshot wounds will be found to have an injury to a major vessel 20%–25% of the time,7 while penetrating stab wounds will produce a major abdominal vascular injury in only 10% of patients.8 In contrast, only 5% of patients with blunt abdominal trauma are found to have an injury to a major abdominal vessel at laparotomy.9 Blunt trauma to the abdominal vasculature typically arises from either rapid deceleration or anterior crush injuries. Deceleration injuries result either in the avulsion of small branches from major vessels (such as avulsion of intestinal branches from the superior mesenteric artery) or in a proximal intimal tear with secondary thrombosis (i.e., renal artery thrombosis).10 Anterior crush injury also results in two different types of vascular trauma including an intimal flap resulting in secondary thrombosis (superior mesenteric artery, infrarenal abdominal aorta and iliac artery) or a direct blow that completely disrupts an exposed vessel (left renal vein over the aorta).11
Abdominal vascular injuries rarely occur in isolation because of a predominantly posterior-central location of a majority of the vasculature. Approximately two to four associated intra-abdominal injuries occur with abdominal vascular injuries, and patients with an injured abdominal vessel have an approximately 50% chance of having injured multiple vessels.6,12
INITIAL RESUSCITATION
Physical findings in patients with abdominal vascular trauma depend on the degree of containment of the injury. Patients with contained hematomas in the retroperitoneum, base of the mesentery, or hepatoduodenal ligament frequently present with transient hypotension that responds to an initial bolus of a crystalloid solution. These patients may remain hemodynamically stable with minimal physical findings until the hematoma is opened in the operating room. Conversely, patients presenting with free intraperitoneal hemorrhage have marked hypotension that does not respond to crystalloid boluses and may have a rigid abdomen on physical examination. Comparing patients with abdominal vascular injuries who had hypotension (a lowest emergency department systolic blood pressure of <100 mm Hg) with those without hypotension, it was noted in one study that patients with active bleeding had worse physiologic parameters (mean base deficit of −14.7 compared with −7.2), required more transfusions (15.1 units compared with 8.6 units of blood in the operating room), and had a worse survival rate (43% vs. 96%).13
Initial resuscitation for patients with suspected blunt or penetrating injuries to abdominal vessels includes basic airway maneuvers and optimizing pulmonary function. Peripheral intravenous lines should be inserted to start resuscitation with crystalloid solutions. There is no consistent evidence to definitively support either the prehospital administration of crystalloid solutions14 or the “delayed resuscitation” practice of withholding fluid.15
Agonal patients presenting to the trauma bay with a rigid abdomen secondary to penetrating trauma may require an emergency department thoracotomy with cross-clamping of the descending thoracic aorta in order to maintain cerebral and coronary arterial blood flow.16 This maneuver will clearly complicate the patient’s intraoperative course, and the need for performing this maneuver is predictive of a less than 5% survival rate.17
When transferred to the operating room, standard maneuvers need to be used to prevent hypothermia, including warming the room to more than 85° F (24.9° C), covering the head and exposed upper and lower extremities with a heating unit, using a heating cascade on the anesthetic circuit, and irrigating the body cavities with warm saline.18 If the patient’s systolic pressure is less than 70 mm Hg, some centers use a preliminary operating room thoracotomy with cross-clamping of the descending thoracic aorta before beginning a celiotomy. Even though this maneuver can assist with maintaining cerebral and cardiac blood flow, it has little effect on intra-abdominal vascular injuries because of the significant back flow. Persistent shock (systolic blood pressure <90 mm Hg) after placing a cross-clamp on the descending thoracic aorta portends a universally fatal prognosis.19
CLASSIFICATION OF INJURIES
Major abdominal vascular injuries are best classified as occurring in one of four zones as follows:
INJURIES IN SUPRAMESOCOLIC REGION OF ZONE 1
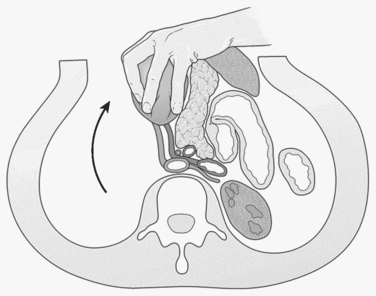
An abdominal hematoma or hemorrhage in the supramesocolic region of zone 1 typically results from an injury to the suprarenal aorta, celiac axis, proximal superior mesenteric artery, or proximal renal artery. Suprarenal aortic injuries carry reported survival rates ranging from 10%–50%.20 Proximal vascular control of the aorta should be obtained at the aortic hiatus of the diaphragm. This can be achieved either by manual compression with an aortic compressor (which is correctly positioned by dividing the lesser omentum and retracting the stomach and esophagus to the left; formal cross-clamping at this level will mandate separating the crura from the supraceliac aorta with the electrocautery) or by performing a formal left-sided medial visceral rotation (reflecting all left-sided intraabdominal viscera, including the colon, kidney, spleen, tail of the pancreas, and fundus of the stomach) (Figure 1). The advantage of the latter is that it provides exposure of the entire abdominal aorta from the hiatus to the aortic bifurcation. This is achieved at a cost of 5 minutes of exposure time and a significant risk of injury to the spleen, left kidney, or left renal artery.21,22 Supraceliac exposure can be improved by dividing the left crus of the diaphragm at the 2 o’clock position and exposing the distal descending thoracic aorta.23 An aortic cross-clamp can be applied quickly at this level with minimal dissection. Prolonged cross-clamping of the supraceliac aorta can lead to hepatic hypoperfusion, which may induce a primary fibrinolytic state24 and severe ischemia of the lower extremities. The lower extremities should be examined at the end of the procedure and compartment pressures measured below the knee; bilateral below-knee, two-skin incision, four-compartment fasciotomies should be considered for pressures over 30–35 mm Hg.
Asensio and colleagues25 recently reviewed 13 celiac axis injuries with an overall survival rate of 38% (5 of 13). Four of the five survivors were managed with ligation of the celiac axis. This is well-tolerated because of extensive collateral circulation, especially in the splenic and left gastric arterial beds. Injuries to the common hepatic artery proximal to the gastroduodenal artery are also amenable to ligation. Ligation of the celiac axis may result in necrosis of the gallbladder, however, and a cholecystectomy should be strongly considered.26 Graham et al.27 reported a series of patients with injuries to the celiac axis and noted a 100% survival in those with isolated injuries, 33% survival in those with one associated vascular injury, and 100% mortality in those with three associated vascular injuries.
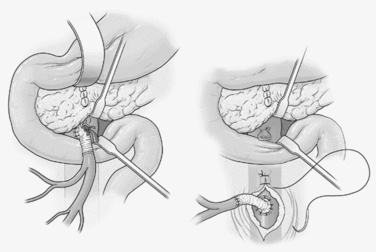
Mesenteric arterial injuries occur in only 12% of penetrating vascular injuries and are associated with 33%–57% mortality.28 Fullen and colleagues29 described four anatomic zones of the superior mesenteric artery which dictate options for repair. Fullen zone I injuries lie beneath the pancreas. Exposure requires either transection of the neck of the pancreas between Glassman clamps or a left medial visceral rotation in order to control bleeding. Fullen zone II injuries lie between the pancreaticoduodenal and the middle colic branches of the artery and lie between the pancreas and the base of the transverse mesocolon. Repairs at this level will lie adjacent to the pancreas and are susceptible to pancreatic leaks. Therefore, these lesions and those in zone I in hemodynamically unstable patients are best temporized with the insertion of a temporary intraluminal shunt until the patient is stabilized. Definitive repair can then be accomplished in a more stable patient by performing a saphenous vein or PTFE bypass from the distal infrarenal aorta through the posterior aspect of the small bowel mesentery to the distal superior mesenteric artery in an end-to-side fashion30 (Figure 2). The aortic suture line must then be protected with retroperitoneal fat or omentum in order to avoid the formation of an aorto-enteric fistula. Fullen zone III injuries lay beyond the middle colic branch, and Fullen zone IV injuries lay at the level of the enteric branches. These injures must be primarily repaired to preserve adequate blood flow to the affected regions of small bowel.
The superior mesenteric vein lies to the right of the superior mesenteric artery, and proximal injuries are difficult to manage. Injuries behind the pancreas will also require pancreatic transection to expose and repair. The perforation may also lie inferior to the lower border of the pancreas. These injuries can be digitally controlled by the surgeon and repaired by an assistant with a continuous row of 5-0 polypropylene sutures. In a damage control setting, the superior mesenteric vein can be ligated with reported survival rates ranging up to 85%.31 If ligation is required, Stone et al.32 recognized the ongoing need for vigorous postoperative fluid resuscitation lasting up to 3 days. Because these patients often develop massive edema of the small bowel, the abdomen should be left open under a temporary silo.
INJURIES IN INFRAMESOCOLIC REGION OF ZONE 1
Injuries to the infrarenal abdominal aorta and the inferior vena cava are the most common vascular injuries in the inframesocolic area of the central abdomen. Exposure at this level is similar to that required for repair of an abdominal aortic aneurysm. With the transverse mesocolon elevated and the small bowel eviscerated to the right, the retroperitoneum is opened at the ligament of Treitz and the opening is extended superiorly to expose the left renal vein. When the aortic injury is underneath a large central retroperitoneal hematoma inferior to the transverse mesocolon, one option is to manually split the hematoma at its highest point and advance the fingers down to identify and compress the point of injury. The injury is always found under the highest point of the hematoma (“Mt. Everest phenomenon”). After obtaining proximal and distal control, the aorta can be repaired primarily with 3-0 polypropylene sutures, with a PTFE patch angioplasty or with an interposition tube graft. The aorta in young trauma patients will usually accommodate a 12–16 mm graft. The gastrocolic omentum should be mobilized and used to cover the graft to prevent a postoperative aortoduodenal fistula.33
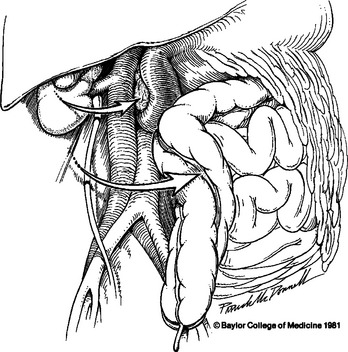
Injuries to the inferior vena cava present with an inframesocolic hematoma that is more extensive on the right and may have active hemorrhage coming through the base of the mesentery of the ascending colon or at the hepatic flexure. Exposure of the entire abdominal vena cava (from the confluence of the iliac veins to the inferior hepatic margin) is best provided by performing a right medial visceral rotation. This involves mobilization of the right colon and C-loop of the duodenum, leaving the right kidney in situ (Figure 3). The site of hemorrhage can be identified only after stripping the loose retroperitoneal tissue around the inferior vena cava. The injury in this vessel may be harder to identify than one in the adjacent aorta, and sponge sticks may be used to occlude the vessel proximal and distal to the site of hemorrhage until all the fatty tissue is dissected free and the injury is identified. An anterior injury can be controlled using Allis clamps to pull the wound margins into a Satinsky clamp. An extensive injury may require the placement of proximal and distal DeBakey aortic clamps. Another useful adjunct can be the placement of a 5-ml or 30-ml balloon catheter into the caval lumen at the site of laceration and inflating the balloon until better exposure can be obtained or the repair completed.34 Repair can then be accomplished with a continuous 4-0 or 5-0 polypropylene suture with meticulous bites used to avoid an “hourglass” effect on the vessel at the site of repair.
The two most difficult locations to obtain vascular control of the infrahepatic vena cava are (1) at the junction of the renal veins, and (2) at the confluence of the common iliac veins. Perforations at the junction of the renal veins require proximal and distal compression of the vena cava while vessel loops are passed around the right and left renal veins. If this dissection is not possible, the right kidney can be mobilized from lateral to medial allowing for visualization and placement of a side-biting clamp at the junction of the right renal vein and inferior vena cava. Exposure of perforations at the confluence of the iliac veins is limited by the overlying aortic bifurcation. If necessary, the right common iliac artery can be temporarily divided between vascular clamps, allowing the surgeon to mobilize the aortic bifurcation to the left, primarily repair the venous injury, and then reanastomose the right common iliac artery.35 Alternatively, the ipsilateral internal iliac artery may be ligated, which allows greater mobilization of the right iliac artery away from the vein.
INJURIES IN ZONE 2
Blunt injuries to the kidney identified on CT rarely require exploration. Stable patients who have a penetrating injury to the flank are evaluated by CT and, if found to have a minor renal injury, can also be observed.36 In addition, patients undergoing abdominal exploration for other blunt injuries do not need to have a stable hematoma in zone 2 opened, especially if a preoperative CT scan has visualized a reasonably intact kidney. Conversely, in patients who have not undergone a preoperative CT and who are undergoing celiotomy for penetrating abdominal injury, any hemorrhage or hematoma in the lateral upper retroperitoneal area suggests injury to the renal artery, renal vein, or the kidney itself and should be explored. Preliminary vascular control of the renal hilum has not been shown to have an impact on the rate of nephrectomy, transfusion requirements, or blood loss; its use is, therefore, quite variable among trauma centers.37 Exposure of the traumatized kidney is best obtained by dividing the retroperitoneum lateral to the affected kidney, manually elevating the kidney into the wound, and placing a large vascular clamp proximal to the hilum.
Injuries to the renal artery are difficult to manage because of the small size of the vessel and its location deep in the retroperitoneum. In an unstable patient with two kidneys, multiple intra-abdominal injuries, or a long preoperative period of ischemia, a nephrectomy is clearly warranted. In stable patients, renal arterial injuries can be managed with a lateral arteriorrhaphy or with resection and end-to-end anastomosis. Interposition grafts and borrowed arterial repairs (splenic or right hepatic) have been reported, but are rarely indicated.38 Survival for patients with isolated injuries to the renal arteries ranges from 56%–74%.
INJURIES IN ZONE 3
The iliac artery and iliac veins lie in the lateral pelvis and can be injured by blunt or penetrating forces. Blunt injuries to branches of the iliac artery, with or without associated pelvic fractures, are typically evaluated and embolized with arteriography. Penetrating injury to the pelvic vessels requires urgent laparotomy. Initial control of hemorrhage can be accomplished with digital or sponge-stick compression until formal proximal and distal control can be achieved by opening the retroperitoneum over the aortic bifurcation and passing vascular tapes around the common, external, and internal iliac vessels. Injuries to the common or external iliac artery should be repaired. In patients with multiple injuries or severe shock, a temporary Argyle shunt may be placed to allow for damage control. Once the patient is stabilized, options for primary repair include lateral arteriorrhaphy, end-to-end anastomosis, insertion of a saphenous vein or PTFE interposition graft, mobilization and rotation of the internal iliac artery as a replacement, and transposition of one iliac artery to the side of the contralateral iliac for wounds close to the bifurcation.39 Primary arterial repair in the pelvis (especially with synthetic grafts) is contraindicated in the presence of significant enteric or fecal contamination. In this situation, the proximal and distal artery around the site of injury should be ligated with a double running row of 4-0 polypropylene suture and buried in the retroperitoneum or in an omental pedicle. If the patient is unstable, a four-compartment fasciotomy should be performed on the affected side, and resuscitation continued. When the patient is stable, an extra-anatomic femoro-femoral crossover graft should be performed with an externally ringed, 8-mm PTFE graft. The overall survival for injuries to the common or external iliac artery depends on whether associated vascular injuries or free bleeding into the peritoneal cavity is present. In recent series, survival rates after injury to the external iliac artery has been approximately 65%.
Injuries to the common and external iliac veins can be repaired with 4-0 or 5-0 polypropylene suture or ligated. If repaired and narrowed, postoperative anticoagulation should be administered to avoid thrombotic complications. If ligated, the extremity should be managed as described after ligation of the inferior vena cava.40 In recent series, survival rates after injuries to the common or external iliac veins were 60% and 72%, respectively.
INJURY GRADING
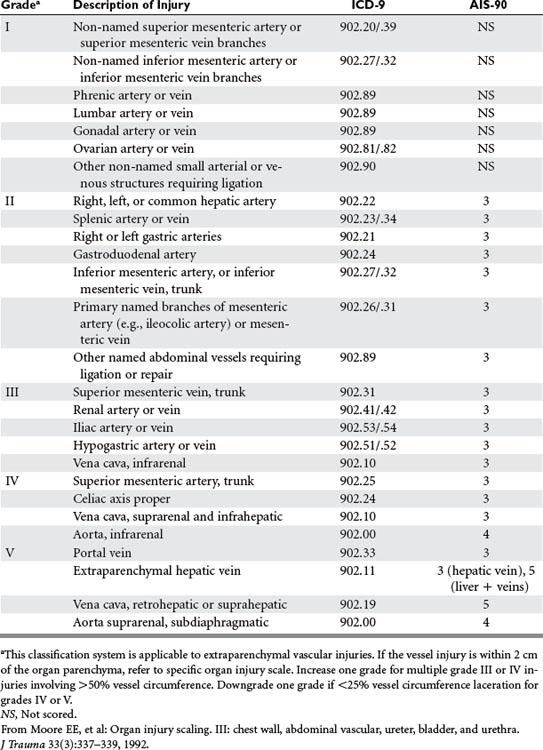
The American Association for the Surgery of Trauma (AAST) has advanced the Abdominal Vascular Organ Injury Score (AAST-OIS) in order to better describe and define abdominal vascular injuries located more than 2 cm from an organ parenchyma (Table 1).41 Overall mortality has been shown by Asensio and colleagues1 to correlate well with the grade of injury: grade II 25%, grade III 32%, grade IV 65%, and grade V 88%.
Asensio JA, Chahwan S, Hanpeter D, Demetriades D, et al. Operative management and outcomes of 302 abdominal vascular injuries. Am J Surg. 2000;180:528-534.
Asensio JA, Forno W, Roldan G, et al. Abdominal vascular injuries. Surg Clin North Am. 2001;81(6):1395-1416.
Davis TP, Feliciano DV, Rozycki GS, et al. Results with abdominal vascular trauma in the modern era. Am Surg. 2001;67:565-571.
Feliciano DV, Burch JM, Spjut-Patrinely V, et al. Abdominal gunshot wounds: an urban trauma center’s experience with 300 consecutive patients. Ann Surg. 1988;208:362-370.
Feliciano DV, Rozycki GS. The management of penetrating abdominal trauma. Adv Surg. 1995;28:1-39.
Feliciano DV. Vascular exposure: aorta and vena cava, renal artery and vein, iliac artery and vein, hepatic artery, and portal vein. In: Zietlow SP, Feliciano DV, editors. Operative Techniques in General Surgery. Philadelphia: WB Saunders; 2000:253-264.
Feliciano DV. Abdominal vascular injury. In: Moore EE, Feliciano DV, Mattox KL, editors. Trauma. 5th ed. New York: McGraw-Hill; 2004:755-777.
Feliciano DV. Injuries to the great vessels of the abdomen. In: Souba WW, Fink MP, Jurkovich GJ, et al, editors. ACS Surgery: Principles and Practice. WebMD; 2006:1250-1261.
Mattox KL, Feliciano DV, Burch J, Beall ACJr, Jordan GLJr, Debakey ME. Five thousand seven hundred sixty cardiovascular injuries in 4459 patients. Epidemiologic evolution 1958 to 1987. Ann Surg. 1989;209:698-707.
Stone HH, Fabian TC, Turkleson ML. Wounds of the portal venous system. World J Surg. 1982;6:335-341.
1 Asensio JA, Chahwan S, Hanpeter D, et al. Operative management and outcome of 302 abdominal vascular injuries. Am J Surg. 2000;180:528-534.
2 Asensio JA, Forno W, Roldan G, et al. Abdominal vascular injuries. Surg Clin North Am. 2001;81(6):1395-1416.
3 DeBakey ME, Simeone FA. Battle injuries of the arteries in World War II: an analysis of 2471 cases. Ann Surg. 1946;123:534-579.
4 Hughes CW. Arterial repair during the Korean War. Ann Surg. 1958;147:555-561.
5 Rich NM, Baugh JH, Hughes CW. Acute arterial injuries in Vietnam: 1,000 cases. J Trauma. 1970;10:359-369.
6 Mattox KL, Feliciano DV, Burch J, et al. Five thousand seven hundred sixty cardiovascular injuries in 4459 patients. Epidemiologic evolution 1958 to 1987. Ann Surg. 1989;209:698-707.
7 Feliciano DV, Burch JM, Spjut-Patrinely V, et al. Abdominal gunshot wounds: an urban trauma center’s experience with 300 consecutive patients. Ann Surg. 1988;208:362.
8 V Spjut-Patrinely & DV Feliciano: Trauma Data from Ben Taub General Hospital, Houston, Texas, July 1985 to June 1988
9 Cox CE. Blunt abdominal trauma. A 5-year analysis of 870 patients requiring celiotomy. Ann Surg. 1984;199:467-474.
10 Haas CA, Dinchman KH, Nasrallah PF, et al. Traumatic renal artery occlusion: a 15-year review. J Trauma. 1998;45:557-561.
11 Feliciano DV. Abdominal vascular injuries. Surg Clin North Am. 1988;68:741-755.
12 Feliciano DV. Abdominal vessels. In: Ivatury R, Cayten CG, editors. The Textbook of Penetrating Trauma. Baltimore: Williams and Wilkins; 1996:702-716.
13 WI Ingram, DV Feliciano & BM Renz, et al: Blood pressure in the emergency department in patients with abdominal vascular injuries: effect on management and prognostic value. Presented at the 55th Meeting of American Association for the Surgery of Trauma, Halifax, Nova Scotia, Canada, September 27–30, 1995
14 Kaweski SM, Sise MJ, Virgilio RW. The effect of prehospital fluids on survival in trauma. J Trauma. 1990;30:1215-1218.
15 Bickell WH, Wall MJJr, Pepe PE, et al. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331:1105-1109.
16 Feliciano DV, Bitondo CG, Cruse PA, et al. Liberal use of emergency center thoracotomy. Am J Surg. 1986;152:654-659.
17 Asensio JA, Wall M, Minei J, et al. Practice management guidelines for emergency department thoracotomy. J Am Coll Surg. 2001;193:303-309.
18 Feliciano DV, Rozycki GS. The management of penetrating abdominal trauma. Adv Surg. 1995;28:1-39. Cameron JL, et al., editors:
19 Wiencek RGJr, Wilson RF. Injuries to the abdominal vascular system: how much does aggressive resuscitation and prelaparotomy thoracotomy really help? Surgery. 1987;102:731-736.
20 Feliciano DV. Abdominal vascular injury. In: Moore EE, Feliciano DV, Mattox KL, editors. Trauma. 5th ed. New York: McGraw-Hill; 2004:755-775.
21 Mattox KL, McCollum WB, Jordan GLJr, et al. Management of upper abdominal vascular trauma. Am J Surg. 1974;128:823-828.
22 Fry WR, Fry RE, Fry WJ. Operative exposure of the abdominal arteries for trauma. Arch Surg. 1991;126:289-291.
23 Feliciano DV. Injuries to great vessels of the abdomen. In: Souba WW, Fink MP, Jurkovich GJ, et al, editors. ACS Surgery: Principles and Practice. Web MD; 2006:1250-1261.
24 Illig KA, Green RM, Ouriel K, et al. Primary fibrinolysis during supraceliac aortic clamping. J Vasc Surg. 1997;25:244-251.
25 Asensio JA, Petrone P, Kimbrell B, Kuncir E. Lessons learned in the management of thirteen celiac axis injuries. South Med J. 2005;98(4):462-466.
26 Kavic SM, Atweh N, Ivy ME, et al. Celiac axis ligation after gunshot wound to the abdomen: case report and literature review. J Trauma. 2001;50:738-739.
27 Graham LM, Mattox KL, Beall ACJr, et al. Injuries to the visceral arteries. Surgery. 1978;84:835-839.
28 Feliciano DV, Mattox KL. Thoracic and abdominal vascular trauma. In: Hobson RWII, Wilson SE, Veith FJ, editors. Vascular Surgery. Principles and Practice. New York: Marcell Dekker Inc.; 2004:1049-1070.
29 Fullen WD, Hunt J, Alexander WA. The clinical spectrum of penetrating injury to the superior mesenteric arterial circulation. J Trauma. 1972;12:656-664.
30 Accola KD, Feliciano DV, Mattox KL, et al. Management of injuries to the superior mesenteric artery. J Trauma. 1986;26:313-319.
31 Donahue TK, Strauch GO. Ligation as definitive management of injury to the superior mesenteric vein. J Trauma. 1988;28:541-543.
32 Stone HH, Fabian TC, Turkleson ML. Wounds of the portal venous system. World J Surg. 1982;6:335-341.
33 Nothmann A, Tung TC, Simon B. Aortoduodenal fistula in the acute trauma setting: case report. J Trauma. 2002;53:106-108.
34 Feliciano DV, Burch JM, Mattox KL, et al. Balloon catheter tamponade in cardiovascular wounds. Am J Surg. 1990;160:583-587.
35 Salam AA, Stewart MT. New approach to wounds of the aortic bifurcation and inferior vena cava. Surgery. 1985;98:105-108.
36 McAninch JW, Carrol PR. Renal trauma: kidney preservation through improved vascular control. J Trauma. 1985;22:285-290.
37 Gonzalez RP, Falimirski M, Holevar MR, et al. Surgical management of renal trauma: is vascular control necessary? J Trauma. 1999;47:1039-1042.
38 Barone GW, Kahn MB, Cook M, et al. Traumatic left renal artery stenosis managed with splenorenal bypass: case report. J Trauma. 1990;30:1594-1596.
39 Landreneau RJ, Mitchum P, Fry WJ. Iliac artery transposition. Arch Surg. 1989;124:978-981.
40 Mullins RJ, Lucas CE, Ledgerwood AM. The natural history following venous ligation for civilian injuries. J Trauma. 1980;20:737-743.
41 Moore EE, et al. Organ injury scaling. III: chest wall, abdominal vascular, ureter, bladder, and urethra. J Trauma. 1992;33(3):337-339.