CHAPTER 65 Abdominal oesophagus and stomach
ABDOMINAL OESOPHAGUS
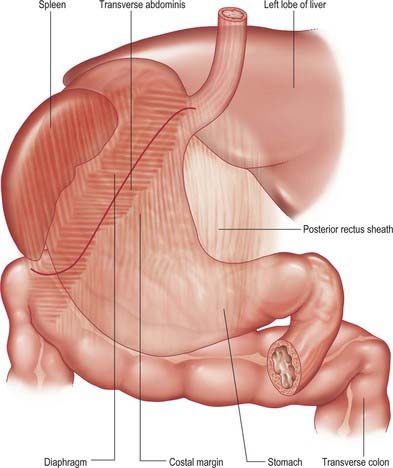
The abdominal oesophagus is effectively tethered to the diaphragm by connective tissue (Fig. 65.1); the phreno-oesophageal ligament. This is formed of two thickened bands of elastin-rich connective tissue; the inferior phreno-oesophageal ligament is effectively an extension of the transversalis fascia extending beneath the parietal peritoneum as it is reflected from the diaphragm onto the abdominal oesophagus. The fibres are only loosely attached to the adventitial tissues and a variable amount of fat often lies beneath it, between the oesophageal wall and the crural sling. This oesophageal fat pad tends to act to tether the oesophagus to the fibres of the crura but tends to regress with age. On the thoracic side of the diaphragm the superior phreno-oesophgeal ligament is similarly formed from an extension of the subpleural endothoracic fascia. It is denser than its inferior counterpart with more elastin present and is tethered much more firmly through the muscle fibres of the oesophageal wall into the submucosal tissues. It may well act to restore lower oesophageal position after the movement engendered by the peristalsis of swallowing (Kwok et al 1999). Anteriorly, the subperitoneal connective tissue is particularly dense and blends with both the outer layer of the oesophageal wall and the apex of the crural fibres of the diaphragm. On the posterior aspect the peritoneal reflection is extremely short since the crura lie steeply angled, and the posterior oesophageal wall has a much shorter ‘effective length’ than the anterior. This short reflection of peritoneum is sometimes referred to as the gastrophrenic ligament and, via the peritoneum over the oesophagus continues directly onto the posterior surface of the stomach. It covers the oesophageal branches of the left gastric vessels and the coeliac branches of the posterior vagus and can thus be said to form an extremely short, wide mesentery to the abdominal oesophagus. In all but the thinnest individuals a pad of adipose tissue is found beneath the peritoneum covering the anterior surface of the lower abdominal oesophagus and the adjacent gastric wall. It is a useful surgical marker for the external location of the gastro-oesophageal junction.
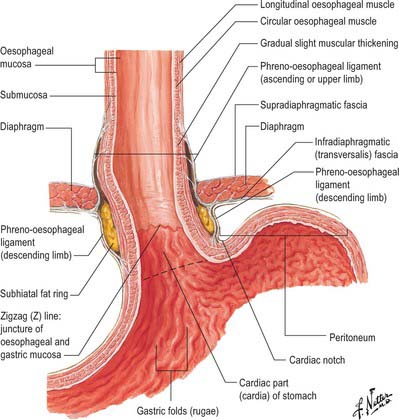
Fig. 65.1 The anatomical structures around the abdominal oesophagus.
(Reprinted from Netter Anatomy Illustration Collection, © Elsevier Inc. All Rights Reserved.)
VASCULAR SUPPLY AND LYMPHATIC DRAINAGE
STOMACH
PARTS OF THE STOMACH
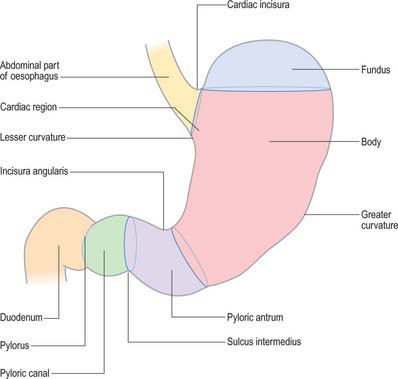
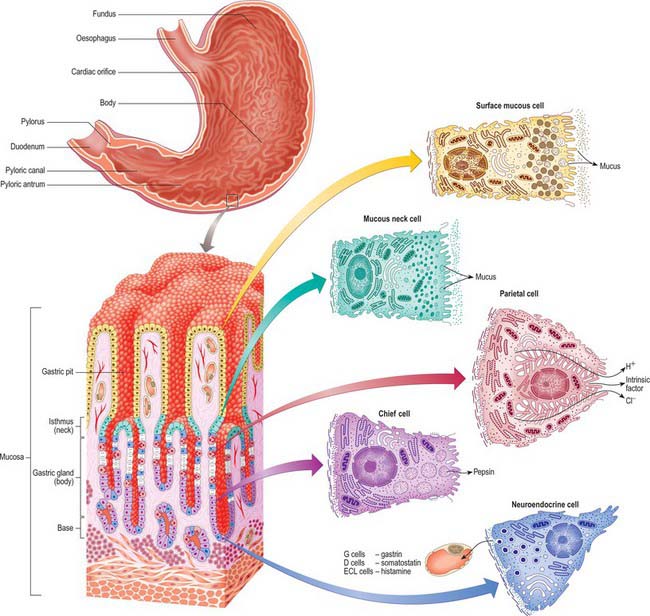
For descriptive purposes, the stomach is divided by arbitrary lines drawn on its external surface into a fundus, body, pyloric antrum and pylorus (Fig. 65.2). The internal appearance and microstructure of these regions varies to some degree. The fundus is dome shaped and projects above and to the left of the cardiac orifice to lie in contact with the left dome of the diaphragm. It lies above a line drawn horizontally from the incisura cardiaca to the greater curvature. The body extends from the fundus to the incisura angularis, which is a constant external notch at the lower end of the lesser curvature. A line drawn from the incisura angularis to an indentation on the greater curvature defines the lower boundary of the body. The pyloric antrum extends from this line to the sulcus intermedius, where the stomach narrows to become the pyloric canal (1–2 cm long), which terminates at the pyloric orifice.
GASTRIC RELATIONS
Gastric curvatures
Lesser curvature
The lesser curvature extends between the cardiac and pyloric orifices and forms the medial (posterior and superior) border of the stomach. It descends from the medial side of the oesophagus in front of the decussating fibres of the right crus of the diaphragm, curves downwards and to the right and lies anterior to the superior border of the pancreas (Fig. 65.3). It ends at the pylorus just to the right of the midline. In the most dependent part there is typically a notch, the incisura angularis, whose position and appearance vary with gastric distension. The lesser omentum is attached to the lesser curvature and contains the right and left gastric vessels.
Gastric surfaces
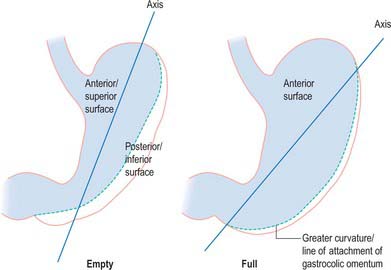
When the stomach is empty and contracted, the two surfaces tend to lie facing almost superiorly and inferiorly, but with increasing degrees of distension they come to face progressively more anteriorly and posteriorly (Fig. 65.4).
Anterior (superior) surface
The lateral part of the anterior surface is posterior to the left costal margin and in contact with the diaphragm, which separates it from the left pleura, the base of the left lung, the pericardium and the left sixth to ninth ribs (Fig. 65.5). It lies posterior to the costal attachments of the upper fibres of transversus abdominis, which separate it from the seventh to ninth costal cartilages. The upper and left part of this surface curves posterolaterally and is in contact with the gastric surface of the spleen. The right half of the anterior surface is related to the left and quadrate lobes of the liver and the anterior abdominal wall. When the stomach is empty, the transverse colon may lie adjacent to the anterior surface. The entire anterior (superior) surface is covered by peritoneum.
Posterior (inferior) surface
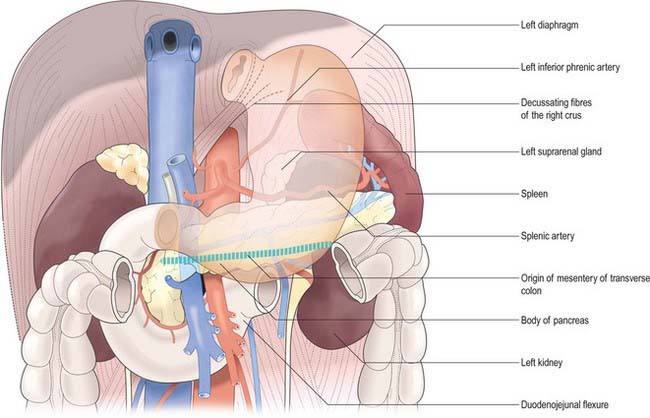
The posterior surface lies anterior to the left crus and lower fibres of the diaphragm, the left inferior phrenic vessels, the left suprarenal gland, the superior pole of the left kidney, the splenic artery, the anterior pancreatic surface, the splenic flexure of the colon and the upper layer of the transverse mesocolon (Fig. 65.3). Together these form the shallow stomach bed: they are separated from the stomach by the lesser sac (over which the stomach slides as it distends). The upper left part of the surface curves anterolaterally and lies in contact with the gastric surface of the spleen. The greater omentum and the transverse mesocolon separate the stomach from the duodenojejunal flexure and ileum. The posterior surface is covered by peritoneum, except near the cardiac orifice, where a small, triangular area contacts the left diaphragmatic crus and sometimes the left suprarenal gland. The left gastric vessels reach the lesser curvature at the right extremity of this bare area in the left gastropancreatic fold. The gastrophrenic ligament passes from the lateral aspect of this bare area to the inferior surface of the diaphragm.
GASTRIC ORIFICES
Cardiac orifice and gastro-oesophageal junction
The opening from the oesophagus into the stomach is the cardiac orifice (Fig. 65.1). It is typically situated to the left of the midline behind the seventh costal cartilage at the level of the eleventh thoracic vertebra. It is typically 10 cm from the anterior abdominal wall and 40 cm from the incisor teeth. The abdominal oesophagus is continuous with the cardiac orifice; the right side is continuous with the lesser curvature, the left side with the greater curvature. There is no specific anatomically discernible cardiac sphincter related to the orifice.
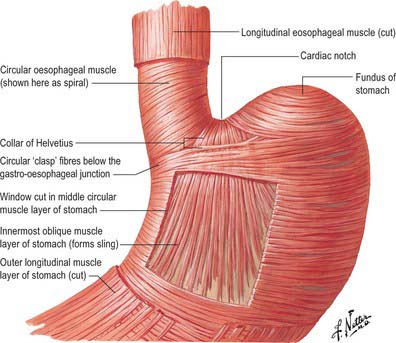
Internally, the transition between oesophagus and stomach is difficult to define because mucosa of gastric fundal pattern extends a variable distance up into the abdominal oesophagus. It usually forms a ‘zig-zag’ squamo-columnar epithelial junction with the oesophageal epithelium above this Z line: for histological and endoscopic purposes, this is often referred to as the gastro-oesophageal junction. A sling of longitudinal and oblique gastric muscle fibres forms a loop on the superior, left, side of the gastro-oesophageal junction between the oesophagus and the lesser curvature, and this is taken as the external boundary of this junction. Bands of thickened circular smooth muscle in the upper wall of the greater curvature and the distal oesophagus are sometimes confusingly referred to as ‘clasp’ or ‘sphincter’ fibres.
Lower oesophageal sphincter and gastro-oesophageal reflux
At rest there is a gastro-oeosophageal pressure gradient due to the presence of negative intra-thoracic pressure (transferred to the thoracic oesophagus) and positive intra-abdominal pressure (transferred to the stomach and augmented by any contraction of the stomach wall itself). Several anatomical and physiological factors normally prevent gastro-oesophageal reflux. Minor factors include the folds of gastric mucosa present in the gastro-oesophageal junction, the mucosal rosette, which contribute to the formation of a fluid- and gas-tight seal and also help to ensure that even low levels of tone within the lower oesophageal wall muscles may occlude the lumen of the junction against low pressures of gastric gas; the angle of the cardiac orifice, which is formed, in part, by the pull of the long oblique fibres of the inner layer of the gastric smooth muscle and may help to form a type of ‘flap valve’; and the length of the abdominal oesophagus, which is buttressed externally by pads of adipose connective tissue at and below the level of the diaphragmatic hiatus. However, the major anti-reflux mechanisms are the tonic contractions of the specialized smooth muscle of the wall lower oesophageal and the encircling fibres of the right diaphragmatic crus, which, together, exert a radial pressure that can be measured by electromyography or manometric testing (Paterson 2001, Mittal 2006), and form an effective high pressure zone (HPZ). At and just below the level of the entry of the abdominal oesophagus into the stomach, the circular fibres of the intermediate layer of the muscularis externa lying over the upper lesser curvature are particularly pronounced and sometimes referred to as ‘clasp’ fibres and exert fairly constant myogenic tone (Fig. 65.6). Since the oesophagus passes obliquely into the stomach, with increasing gastric distension the tone in the clasp fibres rises and they may act to draw the anterior and posterior surfaces together, increasing the tone at the gastro-oesophageal junction, contributing to the HPZ. These anatomical and physiological features are together referred to as the lower oesophageal sphincter (LOS). If reflux is to be prevented, the pressure within the HPZ must exceed the difference between the pressures on either side of the junction. The oesophageal part of the LOS is controlled by the intramural plexuses of the enteric nervous system via the neural release of nitric oxide which relaxes the smooth muscle of the LOS. Tone is reduced in advance of the oesophageal peristaltic wave during swallowing and raised again after the food bolus has passed. During inspiration, the greater negative intrathoracic pressure which increases the gastro-oesophageal pressure gradient is offset by increased pressure in the HPZ due to contraction of the peri-oesophageal fibres of the right diaphragmatic crus. Activation of the crural diaphragm slightly before the costal fibres ensures that this increase precedes the increase in the gradient.
GASTRIC FORM AND INTERNAL APPEARANCES
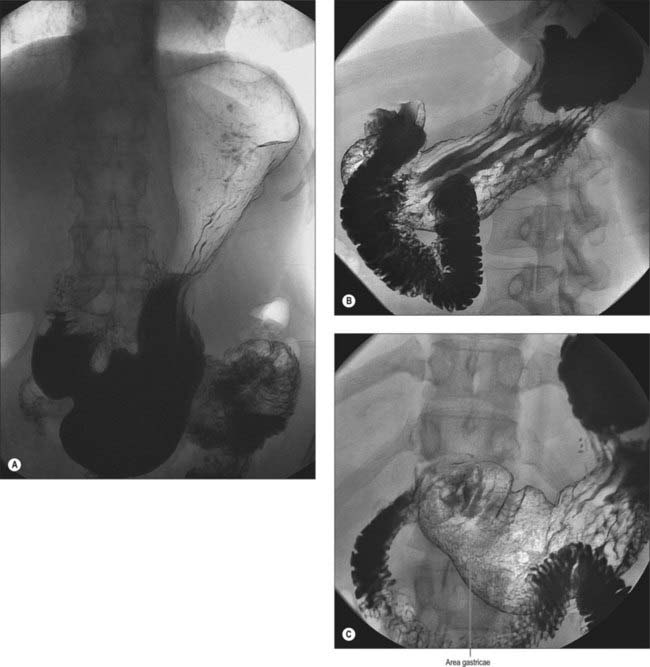
Numerous factors influence both the form and position of the stomach, including the posture and build of the individual, the extent of filling of the stomach, the position of the surrounding viscera, and the tone of the abdominal wall and gastric musculature. The empty stomach is most commonly J-shaped, the fundus usually contains gas, and, in the erect posture, the pylorus descends to the level of the second or the third lumbar vertebra. The lowest part of the antrum often lies below the level of the umbilicus, and the overall axis of the organ is slightly inclined from the vertical (Fig. 65.7). In short, obese individuals, the axis of the stomach lies more towards the horizontal as a ‘steer-horn’ shape.
Internal appearances
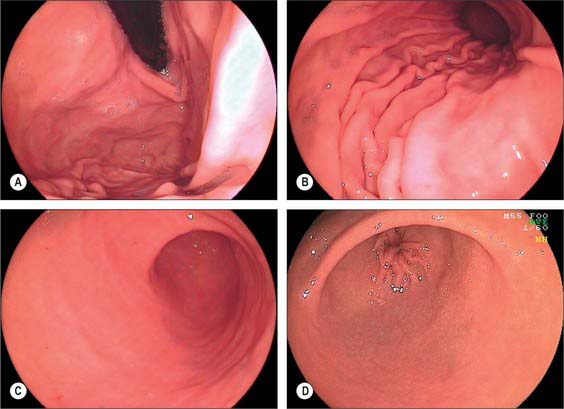
During endoscopic examination (Fig. 65.8), the stomach is typically at least partially distended by air. The cardiac orifice and the lowest portion of the abdominal oesophagus viewed from above are typically closed at rest by tonic contraction of the lower oesophageal musculature. The gastric mucosa lining the orifice is puckered into ridges and is present for a short but variable distance up the abdominal oesophagus; the transition between columnar and squamous epithelium is usually clearly visible. The presence of abnormal columnar epithelium within the oesophagus is referred to as Barrett’s oesophagus but the precise definition of this condition is difficult (see above). From within the distended stomach, the cardiac orifice appears in the medial wall of the fundus and is asymmetrical. The medial edge of the cardiac orifice is continuous with the medial wall of the body of the stomach. The mucosa is slightly thickened at this point with a raised profile, forming part of the ‘mucosal rosette’ that lines the orifice. The ‘rosette’ aids closure of the cardiac orifice and helps prevent reflux of stomach contents into the oesophagus. The medial edge of the orifice is more clearly visible than the lateral edge because it forms a more acute angle with the mucosal lining of the abdominal oesophagus.
In the partly distended stomach, the mucosa of the fundus is thrown into gentle folds with no particular pattern (rugae). As the stomach fills towards capacity, these folds rapidly become less pronounced, and the wall is nearly smooth when the stomach is over-inflated. The body of the stomach has the most pronounced mucosal folds. Even in moderate distension, they appear as long, broad mucosal ridges running in sinuous strips from fundus to pyloric antrum (Fig. 65.7). They are seen on all mucosal surfaces of the body but are most obvious on the anterolateral, lateral and posterolateral parts (which correspond to the inner surface of the anterior and posterior external surfaces and to the greater curvature). Here they are occasionally called the magenstrasse, a reference to their possible role in directing liquid entering the stomach immediately down into the pyloric antrum. These folds are least prominent on the medial surface (corresponding to the inner surface of the lesser curvature), which is much smoother, particularly when the stomach distends.
The areae gastricae within the antrum are small elevations or puckerings of the mucosal surface that are readily seen on double contrast barium meal (Fig. 65.7). The few folds present in the antrum when the stomach is relaxed disappear with distension. The antrum adjacent to the pyloric canal, the prepyloric antrum, has a smooth mucosal surface that culminates in a slight puckering of the mucosa at the pyloric orifice caused by the contraction of the pyloric sphincter.
VASCULAR SUPPLY AND LYMPHATIC DRAINAGE
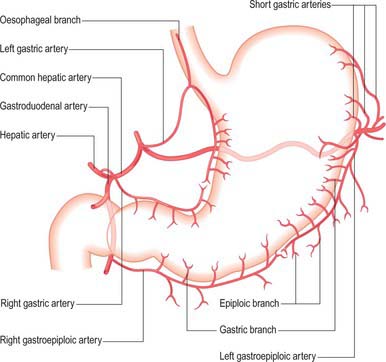
Arteries
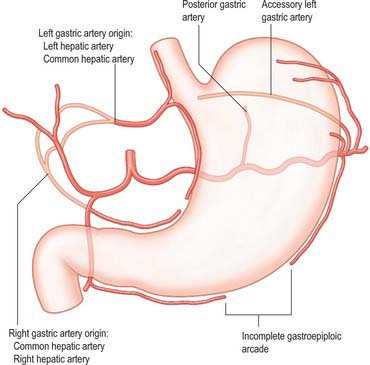
The arterial supply to the stomach comes predominantly from the coeliac axis although intramural anastomoses exist with vessels of other origins at the two ends of the stomach (Figs 65.9, 65.10, 65.11). The left gastric artery arises directly from the coeliac axis. The splenic artery gives origin to the short gastric arteries as well as the left gastroepiploic artery and may occasionally give origin to a posterior gastric artery. The hepatic artery gives origin to the right gastric artery and to the gastroduodenal artery, which in turn gives origin to the right gastroepiploic artery.
Short gastric arteries
There is no universally accepted definition of what constitutes the short gastric arteries but a practical definition is ‘vessels which supply the fundus of the stomach above the level of the splenic artery and which arise to the left of the greater curvature of the stomach’. The short gastric arteries are variable in number, commonly between five and seven. They arise from the splenic artery or its divisions, or from the proximal left gastroepiploic artery, and pass between layers of the gastrosplenic ligament to supply the cardiac orifice and gastric fundus. They anastomose with branches of the left gastric and left gastroepiploic arteries. An accessory left gastric artery may arise with these vessels from the distal splenic artery.
Posterior gastric artery
A distinct posterior gastric artery may occur. When present, it arises from the splenic artery in its middle section posterior to the body of the stomach (Fig. 65.11). It ascends behind the peritoneum of the lesser sac towards the fundus and reaches the posterior surface of the stomach in the gastrophrenic fold.
Gastroduodenal artery
The gastroduodenal artery arises from the common hepatic artery posterior and superior to the first part of the duodenum. It gives origin to the right gastroepiploic and superior pancreaticoduodenal arteries at the lower border of the first part of the duodenum. (For details of the gastroduodenal artery and its relevance to clinical situations, see Chapter 66.)
Arterial anastomoses of the stomach
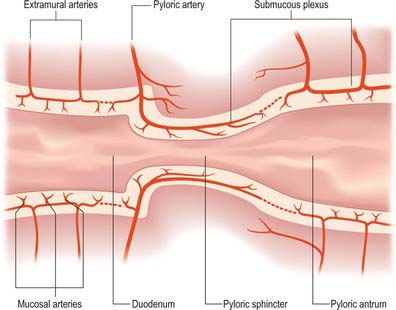
The pyloric arteries are rami of the right gastric and right gastroepiploic arteries. They pierce the duodenum distal to the sphincter around its entire circumference and pass through the muscular layer to the submucosa where they divide into two or three rami. The latter turn back into the pyloric canal beneath the mucosa and run to the end of the pyloric antrum: they supply the entire mucosa of the pyloric canal (Fig. 65.12). Branches of the pyloric submucosal arteries may anastomose close to their origin with the duodenal submucosal arteries, and their terminal rami also anastomose with gastric arteries from the prepyloric antrum. The pyloric sphincter is supplied by the gastric and pyloric arteries via rami that leave their parent vessels in the subserosal and submucosal levels to penetrate the sphincter.
Veins
Oesophageal and gastric varices
Varices are progressive dilatations which occur predominantly in the submucosal plexus of the distal abdominal oesophagus and occasionally on the gastric side of the gastric orifice at portal venous pressures above 15 mmHg. Portal venous hypertension, of any cause, predisposes to the opening up of pre-existing embryonic venous channels between venous tributaries of the portal system and the systemic venous circulation (Paquet 2000), such as the venous plexuses which exist in the lower abdominal oesophagus and gastric orifice. In portal hypertension, the valves within the perforating vessels become incompetent, permitting retrograde flow, and this causes variceal dilatation of the deep and superficial veins. Varices in the distal oesophagus are often easily visible at endoscopy, because they are situated superficially in the lamina propria and protrude into the oesophageal lumen; they are a frequent cause of substantial upper gastrointestinal bleeding. Spontaneous cessation of the bleeding is less common than at other sites, in part because the pressure and the flow rate within the veins is high, but also because there is a relative lack of submucosal supporting connective tissue to allow for venous contraction and thrombosis. Gastric varices are often present on the inferior aspect of the gastric orifice. They present less commonly in clinical practice than oesophageal varices, are less easy to diagnose because of their location, and occasionally exist without the presence of oesophageal varices. In these circumstances, it may be that the embryonal ‘point of meeting’ between portal and systemic venous systems is lower than usual and occurs in the upper stomach rather than the lower oesophagus.
Lymphatic drainage
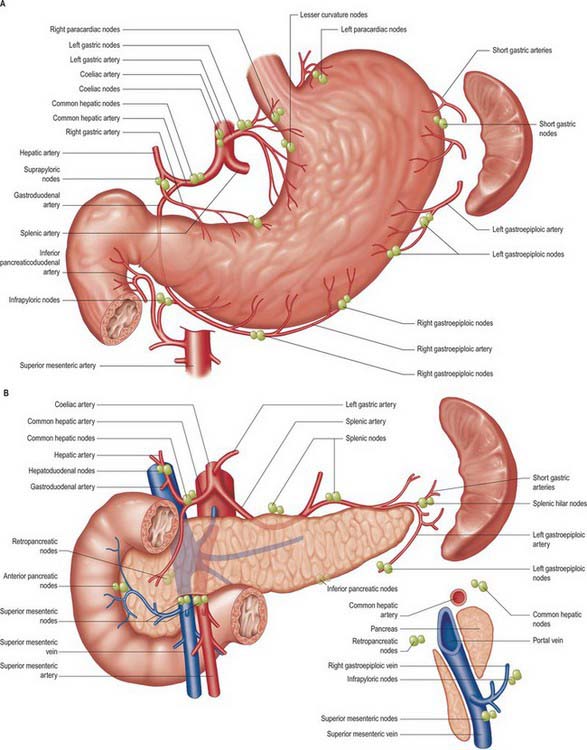
The stomach has a rich network of lymphatics that connect with lymphatics draining the other visceral organs of the upper abdomen. At the gastro-oesophageal junction the lymphatics are continuous with those draining the lower oesophagus, and in the region of the pylorus they are continuous with those draining the duodenum. In general, they follow the course of the arteries supplying the stomach. However, many separate groups of nodes are now recognized (Fig. 65.13), and their relationship to the regions of the stomach and the vascular territories supplied, is of great importance during resection of the stomach, particularly for malignancy. Pancreatic and hepatic lymphatics play a significant role in draining areas of the stomach during disease.
Gastrectomy and gastric lymphadenectomy
Surgery for gastro-oesophageal malignancy involves varying degrees of radical resection of the associated lymph nodes that potentially drain the tumour area. The wide ramification of the gastro-oesophageal lymph node drainage and the multiple possible nodes which may be involved in a tumour inevitably mean that the extent of lymphadenectomy undertaken may be wide. The extent of possible/actual nodal involvement by the tumour is classified as N1 (loco-regional nodes specific to the tumour site), N2 (regional and major named vessel nodes draining the tumour) and N3 (wider draining nodes including para-aortic nodes). Gastrectomies can be classified according to the node groups excised for the tumour: D1 (e.g. removal of the portion of the stomach involved and the local lymph nodes directly related to the arteries ligated), D2 (e.g. total gastrectomy including all gastric lymph node groups often including the spleen) and D3 (e.g. total gastrectomy plus extensive lymphadenectomy that includes the associated upper abdominal lymph nodes, namely pancreatic, superior mesenteric, coeliac, hepatic and transverse colic). (For details of the lymph node stations relevant to surgical gastric resection, see Japanese Gastric Cancer Association 1998 and UICC 1997.)
INNERVATION
The stomach is innervated by sympathetic and parasympathetic fibres.
Parasympathetic innervation
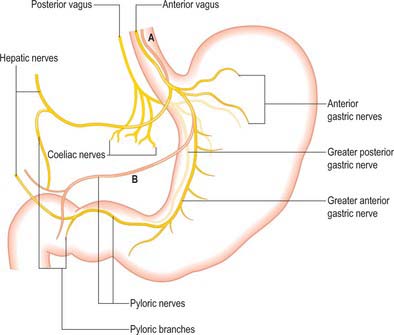
The parasympathetic supply to the stomach is from the anterior and posterior vagus nerves (Fig. 65.14). The anterior vagus (formed mainly from fibres from the left vagus originating from the oesophageal plexuses) is often double or even triple and supplies filaments to the cardiac orifice. The nerve lies very closely applied to the outer muscle of the abdominal oesophagus and divides near the oesophageal end of the lesser curvature into gastric and pyloric/hepatic branches. The upper anterior gastric branches radiate on the anterior surface of the upper body and fundus. The main gastric branch is termed the greater anterior gastric nerve and lies in the lesser omentum near the lesser curvature; branches from this nerve pass to the body and antrum with the gastric arterial supply branches. Hepatic/pyloric branches (generally one main and possibly one accessory) originate below the cardiac orifice. The main hepatic/pyloric nerve runs between the peritoneal layers of the lesser omentum almost horizontally towards its free edge to reach the hilum of the liver where hepatic branches ramify and pyloric branches turn down on the left side of the hepatic artery to reach the pylorus. The second nerve usually arises from the greater anterior gastric nerve during its course and runs inferomedially to the pyloric antrum where pyloric (and antral) branches form and hepatic branches (one or two) run superiorly to contribute to the hepatic plexus. Variations in the anterior nerve include accessory pyloric branches, long gastric branches with a high lying nerve trunk and a low origin of the pyloric branch with a low lying nerve trunk.
MICROSTRUCTURE
The gastric wall consists of the major layers found elsewhere in the gut, i.e. mucosa, submucosa, muscularis externa and serosa, together with gastric vessels and nerves (Figs 65.15, 65.16). The microstructure reflects the functions of the stomach as an expandable muscular sac lined by secretory epithelium, although there are local structural and functional variations in this pattern.
Mucosa
Epithelium
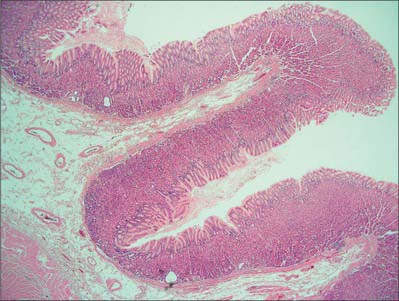
When viewed microscopically at low magnification, the internal surface of the stomach wall appears honeycombed by small, irregular gastric pits approximately 0.2 mm in diameter (Figs 65.15, 65.16). The base of each gastric pit receives several long, tubular gastric glands that extend deep into the lamina propria as far as the muscularis mucosae. Simple columnar mucus-secreting epithelium covers the entire luminal surface including the gastric pits, and is composed of a continuous layer of surface mucous cells which release gastric mucus from their apical surfaces to form a thick protective, lubricant layer over the gastric lining. This epithelium commences abruptly at the cardiac orifice, where there is a sudden transition from oesophageal stratified squamous epithelium.
Gastric glands
Principal gastric glands
Chief (peptic) cells (Fig. 65.15) are the source of the digestive enzymes pepsin and lipase. They are usually basal in position and cuboidal in shape, and their nuclei are rounded and euchromatic. They contain secretory zymogen granules and their abundant cytoplasmic RNA renders them strongly basophilic. Parietal (oxyntic) cells are the source of gastric acid and of intrinsic factor, a glycoprotein necessary for the absorption of vitamin B12. They are large, oval and strongly eosinophilic, and have centrally placed nuclei. Parietal cells occur intermittently along the walls of the more apical half of the gland, but can reach as far as the isthmus; they bulge laterally into the surrounding connective tissue. They have a unique ultrastructure related to their ability to secrete hydrochloric acid. The luminal side of the cell is deeply invaginated to form a series of blind-ended channels (canaliculi) that bear numerous irregular microvilli covered by a plasma membrane rich in H+/K+ ATPase antiporter channels. The latter actively secrete hydrogen ions into the lumen; chloride ions follow along the electrochemical gradient. The mitochondria-rich cytoplasm facing these channels contains a tubulo-vesicular system of abundant fine membranous tubules directed towards the canalicular surface. The precise structure of the cell varies with its secretory phase: when stimulated, the number and surface area of the microvilli increases up to five-fold, probably as a result of the rapid fusion of the tubulo-vesicular system with the plasma membrane. This process is reversed at the end of stimulated secretion, when the excess membrane retreats back into the tubulo-alveolar system and microvilli are lost.
Stem cells are relatively undifferentiated mitotic cells from which the other types of gland cell are derived. They are relatively few in number, and are situated in the isthmus of the gland and the bases of the gastric pits. Stem cells are columnar and possess a few short apical microvilli. They periodically undergo mitosis; their progeny migrate either apically, to differentiate into new surface mucous cells, or basally, to form mucous neck, parietal, chief or neuroendocrine cells. All of these cells have a limited lifespan, especially the mucus-secreting types, and so they are constantly replaced. The typical replacement period for surface mucous cells is 3 days, and for mucous neck cells is 1 week. Other cell types appear to live much longer.
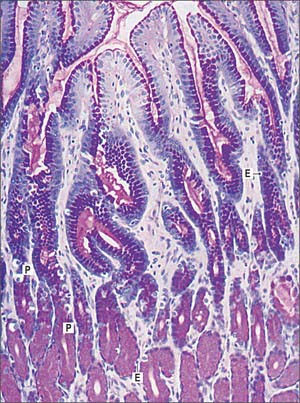
Pyloric glands
Pyloric glands empty via groups of two or three short convoluted tubes into the bases of the deep gastric pits of the pyloric antrum: the pits occupy about two-thirds of the mucosal depth (Fig. 65.17). The glands are populated mainly by mucus-secreting cells, but they also contain neuroendocrine cells, especially G cells, which secrete gastrin when activated by appropriate mechanical stimulation (causing increased gastric motility and secretion of gastric juices). Although parietal and chief cells are scarce, parietal cells are always present in both fetal and postnatal pyloric glands, and may also appear in the duodenal mucosa, proximally near the pylorus, in adult tissue.
DiDio LJ, Anderson MC. The ‘Sphincters’ of the Digestive System. Baltimore: Williams and Wilkins, 1968.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma. Gastric Cancer. 1998;1:10-24. 2nd English edition.
Japanese Research Society for Gastric Cancer. Japanese Classification of Gastric Carcinoma. Tokyo: Kanehara & Co Ltd, 1998. and Gastric Cancer 1: 10–24, 25–30
Kwok H, Marriz Y, Al-Ali S, Windsor JA. Phrenoesophageal ligament revisited. Clin Anat. 1999;12:164-170.
Mittal RK, Goyal RK 2006 Sphincter mechanisms at the lower end of the esophagus. GI Motility online.
Paquet KJ. Causes and mechanisms of oesophageal varices development. Med Sci Monit. 2000;6:915-928.
Paterson WG. The normal anti-reflux mechanism. Chest Surg Clin N Am. 2001;11:473-483.
Silverstein FE, Tytgat GNJ. Atlas of Gastrointestinal Endoscopy, 2nd edition. New York: Gower Medical Publishing, 1991.
UICC, Sobin LH, Wittekind CH. TNM classification of malignant tumours, 5th edition, Berlin: Springer-Verlag, 1997.