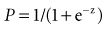201 Abdominal Compartment Syndrome
 Definitions
Definitions
To date, the most common way to measure intraabdominal pressure (IAP) is the intravesical technique via a urinary catheter (often referred to as urinary bladder pressure).1–3 The mean value of IAP in hospitalized nontrauma patients is 6.5 mm Hg (range, 0.2-16.2 mm Hg).4 In critically ill ICU patients or trauma patients with shock and subsequent resuscitation, IAP is typically higher (12-16 mm Hg).5
Secondary ACS refers to conditions that do not originate from the abdominopelvic region.
 Damage Control
Damage Control
Patients undergoing laparotomy for major abdominal bleeding or sepsis are at risk for entering a “vicious circle” of acidosis, hypothermia, and coagulopathy; selected patients benefit from an abbreviated laparotomy (“damage-control” strategy).6,7 The goals are to quickly control bleeding and prevent further contamination or spillage from hollow viscus perforations. The abdomen is temporarily closed without fascial approximation, and the patient is triaged to the intensive care unit (ICU), where resuscitation can be optimized and the vicious-circle physiology corrected. Damage control has saved the lives of severely injured and septic patients who otherwise would have died. Nevertheless, use of damage control has created new challenges for clinicians, including recognition and management of ACS, management of the open abdomen, and early multiple organ failure (MOF).
 Historical Perspective
Historical Perspective
After 2 decades of re-recognition, ACS is still a heavily investigated critical care topic. Before the most recent description, IAP measurement, intraabdominal hypertension and ACS-related pathophysiology were investigated and published more than 150 years ago in both animal and human studies.8,9 Initially, IAP was thought to be negative (subatmospheric), but by the beginning of the 20th century, animal studies verified that IAP is generally positive and if significantly increased can cause cardiac failure.10 These laboratory observations had little impact on clinical practice until the 1950s, when pediatric surgeons recognized the catastrophic consequences of acutely closing large congenital abdominal defects. Silo closure with gradual reduction of the abdominal defect was recommended to prevent fulminant organ failures.11 In the 1980s, vascular surgeons described ACS after abdominal aortic aneurysm surgery. Additionally, they described the present technique of IAP measurement and used high IAP as a criterion for re-exploration.1 However, it was not until the 1990s, when trauma surgeons adopted the liberal use of the damage-control strategy, that sufficient numbers of patients were available to define the epidemiology and pathophysiology of this previously rare and elusive complication.12–15 Early observational case descriptions and retrospective series allowed for development of appropriate prospective epidemiologic characterization. These clinical observations stimulated laboratory investigations which have revealed some surprising and potentially important immunologic consequences of decompressive laparotomy of ACS after traumatic shock resuscitation (i.e., it may serve as a “second hit” in the systemic inflammatory response that causes early MOF).15 Parallel with these advances in understanding postinjury ACS is the recognition that ACS occurs in a variety of clinical scenarios such as extreme constipation,16 ovarian hyperstimulation,17 noninvasive ventilation,18 pancreatitis,19 and severe burns.20 Since 2004, the World Society of the Abdominal Compartment Syndrome has offered leadership in consensus definitions, regular conferences, educational material, and organization of clinical trials.
 Intraabdominal Pressure Measurement
Intraabdominal Pressure Measurement
Clinical examination of the abdomen is inaccurate for determining the presence of intraabdominal hypertension.21,22 A standardized measurement of IAP is fundamental to the definition of intraabdominal hypertension and ACS.1,2 IAP has been measured in virtually all parts of the abdominal cavity. The intravesical technique using a standard urinary catheter seems to be the most reliable and least invasive method. The rationale is that IAP is transmitted to the urinary bladder, which serves as a pressure transducer when filled with normal saline. Traditionally, a larger volume of saline was recommended, but recent studies showed that as little as 20 mL of instilled normal saline is enough for accurate measurement. Pressure is conducted by the fluid in the bladder to fluid in the urinary catheter, which is clamped during the interval when pressure is being measured. Pressure in the catheter tubing can be measured by inserting a sterile needle into the sample port of the catheter tube. Alternatively, a T-piece with three-way stopcock can be inserted into the catheter tube, connecting one limb to a strain-gauge pressure transducer.23 The intravesical technique has been shown to correlate well with IAP measured directly using a laparoscopic insufflator.24 The vesical route is more accurate than the use of rectal and gastric probes, which tend to provide different readouts, depending on the position of the patient.24 Animal studies have shown that the pressure in the inferior vena cava correlates well with the vesical pressure,25 but the inferior vena caval and direct peritoneal routes are more invasive. The urinary bladder pressure technique for IAP measurement was originally described by Kron et al.3 and validated by Iberti et al.26 The technique was simplified by Sugrue et al., who described the insertion of a T-connector into the drainage tubing.23 This modification eliminated the need for multiple needle insertions into the sample port and minimized the risk of needlestick injury and microbial contamination of the bladder. This technique is relatively simple and can be performed in any ICU where a pressure transducer is available. Several proprietary devices are available for clinicians. Unfortunately, obtaining an accurate measurement requires about 7 minutes of nursing time, limiting the frequency with which measurements can be obtained. Even when personnel are highly aware of the possible consequences of ACS, screening measurements of IAP are rarely obtained more often than every 4 hours. ACS can develop 4 to 6 hours after ICU admission in patients who are at high risk.5 The standard protocol for intermittent measurements of IAP does not provide information about the duration of intraabdominal hypertension. To address these shortcomings (labor intensity, intermittent nature), a continuous IAP measurement technique was developed and is currently being validated. The IAP can be continuously measured without clamping the tubing and instilling fluid into the bladder. For this new method, a standard three-way catheter is inserted, and the pressure transducer is connected to the saline-filled irrigation port. Once the setup is zeroed, the continuous IAP trace can be monitored without any further intervention or interference with the urine flow or tubing; this is the Balogh-Sugrue technique.27
 Pathophysiology
Pathophysiology
The pathophysiologic effects of increased pressure in a closed body compartment are well described in other regions (e.g., tension pneumothorax, pericardial tamponade, increased intracranial pressure, extremity compartment syndromes) and are taught in the basic medical curriculum. The abdominal cavity is a “neglected” compartment (see Historical Perspective). The volume of the abdominal cavity is limited by its least tensile component, the fascia. Increased pressure can be due to an increase in the volume of the abdominal contents or to a decrease in the volume of the “container” (Table 201-1). After IAP increases to greater than 20 mm Hg, the abdominal cavity is on the steep portion of its pressure-volume curve, and as a result, small increases in content volume or decreases in cavity volume can cause dramatic increases in IAP. This is when close monitoring of IAP (preferably continuously) and organ function is essential for timely intervention.
TABLE 201-1 Causes of Intraabdominal Hypertension and Abdominal Compartment Syndrome
| Increased Abdominal Contents | Decreased Abdominal Volume |
|---|---|
| Ascites | Reduction of large long-standing hernia |
| Hemoperitoneum | Direct closure of large, long-standing abdominal wall defect |
| Visceral edema | Circumferential abdominal-wall burnContinuous positive-pressure ventilation |
| Abdominal packs | |
| Peritonitis | |
| Retroperitoneal edema (pancreatitis) | Retroperitoneal edema (pancreatitis) |
| Large pelvic, retroperitoneal hematoma | Large pelvic, retroperitoneal hematoma |
| Intestinal obstruction | |
| Ileus | |
| Gastric distention (esophageal ventilation) | |
| Abdominal aortic aneurysm | |
| Severe constipation | |
| Large abdominal tumor (chronic) | |
| Morbid obesity (chronic) | |
| Pregnancy (chronic) |
 Pathophysiologic Response of Specific Organs
Pathophysiologic Response of Specific Organs
Cerebral Perfusion
Increased IAP forces the diaphragm cephalad, thus decreasing the size of the thoracic cavity and causing intrathoracic pressure to increase. High intrathoracic pressure increases jugular venous pressure and impedes venous return from the brain. This effect can increase intracranial pressure and consequently decrease cerebral blood flow.28–30 The effect of intraabdominal hypertension on intracranial pressure is especially relevant in severe blunt trauma, because head and abdominal injuries frequently coexist.
Cardiac Function
Increased IAP impedes venous return to the heart, causing sequestration of blood in the lower extremities. High intrathoracic pressure increases central venous pressure and pulmonary capillary wedge pressure but does not increase right or left ventricular end-diastolic volume. In other words, when intrathoracic pressure is increased, central venous and pulmonary capillary wedge pressures are not reliable indices for assessing the adequacy of preload. Simultaneously, left ventricular afterload increases owing to increased systemic vascular resistance. Increased intrathoracic pressure can increase right ventricular afterload, potentially leading to right ventricular failure and dilation, with consequent leftward displacement of the ventricular septum and impairment of left ventricular filling.31–34 Cardiac failure with elevated pulmonary capillary wedge pressure, increased systemic vascular resistance, and decreased cardiac index is a typical finding in profound intraabdominal hypertension and defines ACS. The cardiac index usually does not respond to fluid challenges, which can be detrimental if the underlying cause (ACS) is not treated. The cardiac index’s response to decompression is predictive of outcome; patients who survive have a significantly greater increase in cardiac index after decompression than those who subsequently die.5
Respiratory Function
Increased IAP pushes the diaphragm into the thoracic cavity. Thoracic compliance decreases, and increased airway pressure is required for mechanical ventilation. Additionally, functional residual capacity decreases, and ventilation/perfusion mismatching increases, leading to impaired oxygenation.34,35 In the setting of massive resuscitation, these changes can be misinterpreted as being caused by acute lung injury. Historically, ACS was diagnosed by the presence of a firm abdomen in the setting of oliguria and increased airway pressures. Although airway pressure promptly decreases in response to abdominal decompression, this finding does not differentiate survivors from nonsurvivors.5 The peak airway pressure is an important parameter to monitor during attempted primary fascial closure after laparotomy when ACS is a possible complication.
Renal Function
Oliguria or anuria despite aggressive fluid resuscitation is a typical sign of ACS. Mechanisms responsible for decreased renal function include direct compression of the renal parenchyma, decreased perfusion of the kidneys due to decreased cardiac index, and increased water and sodium retention due to activation of the renin-angiotensin system.36–38 The usual threshold for defining acute oliguria—urinary output less than 0.5 mL/kg/h—should be used cautiously and considered in the context of the magnitude of the resuscitation. Among patients who require massive resuscitation, the index of suspicion for ACS should be high when urinary output is less than 1 mL/kg/h.5
Gut Function
Increased IAP impairs splanchnic perfusion by decreasing the cardiac index and increasing splanchnic vascular resistance. When severe, tissue ischemia can result.39–42 Intestinal perfusion can be assessed objectively using gastric tonometry. Decreased gastric intramural pH (pHi), increased gastric regional partial pressure of carbon dioxide (PCO2), and a wide gap between gastric regional PCO2 and end-tidal PCO2 are all indicators of impaired abdominal visceral perfusion. Combined with urinary bladder pressure measurements, the newer semicontinuous tonometers are an excellent adjunct for the early identification of impending ACS.3 Moreover, the physiologic response to decompression can be evaluated by assessing changes in pHi and related parameters using gastric tonometry.5
Extremity Perfusion
Increased IAP increases femoral venous pressure, increases peripheral vascular resistance, and reduces femoral artery blood flow by as much as 65%.43
Microcirculation
Laboratory studies have shown that decompression of ACS causes circulating neutrophils to increase CD11b adhesion receptor expression.44 Decompression of ACS is also associated with the release of cytokines into the portal circulation and increased lung permeability, similar in degree to that seen after hemorrhagic shock and resuscitation.44,45 Moreover, when ACS decompression is appropriately sequenced with hemorrhagic shock, it can serve as a “second hit” (i.e., ACS decompression 8 hours after hemorrhagic shock causes more intense acute lung injury than does ACS decompression 2 or 18 hours after shock).44–46
 Classification
Classification
ACS can be classified based on the duration of the syndrome, the presence or absence of intraperitoneal pathology, and the cause of the raised IAP (Table 201-2).
TABLE 201-2 Classification of Abdominal Compartment Syndrome
| Basis of Classification | Subcategories |
|---|---|
| Time frame | Acute |
| Chronic | |
| Relation to peritoneal cavity | Primary |
| Secondary | |
| Etiology | Trauma |
| Burn | |
| Postoperative | |
| Pancreatitis | |
| Bowel obstruction | |
| Ileus | |
| Abdominal aortic aneurysm | |
| Oncologic | |
| Gynecologic |
Acute Versus Chronic
The pathophysiologic responses described earlier are usually acute phenomena in critically ill or injured patients. However, the organ dysfunctions characterizing ACS can be present for long periods (chronic intraabdominal hypertension or ACS) in certain clinical conditions such as morbid obesity, chronic constipation, and pregnancy. In morbid obesity, chronic headaches and tinnitus are features of persistently increased intracranial pressure. The symptoms markedly improve when a special device is used to apply negative pressure to the abdomen to decrease IAP.47
Primary Versus Secondary
Irrespective of cause, the presence of intraperitoneal pathology defines primary ACS. A typical case is one in which the damage-control paradigm was followed and perihepatic packing, combined with temporary closure of the abdominal wall, was used to tamponade bleeding from the liver.48 As time progressed, intraabdominal bleeding and bowel edema (secondary to resuscitation) caused the volume of the intraabdominal contents to increase, precipitating ACS. Recognition of this problem has prompted trauma surgeons to leave the abdominal incision open after many damage-control procedures, reducing but not eliminating the risk of ACS. Primary ACS can also occur in patients who fail nonoperative management of abdominal organ injuries because of ongoing bleeding.49
Secondary ACS typically occurs in the setting of severe shock requiring massive resuscitation (whole body ischemia-reperfusion injury) in the absence of intraperitoneal pathology or injury.5 Because there is no abdominal cause, secondary ACS is a more elusive diagnosis, and recognition is often delayed.50 Typical causes are hypovolemic shock related to multiple open extremity fractures, unstable pelvic fractures, penetrating chest injuries,51 and severe burns.52 Secondary ACS can also develop during resuscitation for septic shock.53
 Epidemiology
Epidemiology
Incidence
Because of different definitions and different study populations, the reported incidence of ACS is inconsistent. In the trauma literature of the mid-1990s, the reported incidence among high-risk patients undergoing laparotomy varied from 3% to 36%.15 Fietsam and colleagues reported a 4% incidence of ACS in patients undergoing operation with primary fascial closure for ruptured abdominal aortic aneurysms.54 Malbrain prospectively investigated medical ICU patients and documented the incidence of ACS at 2%.55
Another issue is that the epidemiology of ACS changes as treatment strategies evolve. For example, Meldrum et al.56 and Balogh et al.3 studied similar traumatic shock populations, and both reported that the incidence of ACS was 14%. These two studies, however, were performed 6 years apart. In the earlier series reported by Meldrum, only primary ACS was considered, and liberal use of the open abdomen was just starting. In contrast, in the series described by Balogh 6 years later, the abdomen was initially left open in virtually all cases of damage-control laparotomy (Bogota bag closure), and this strategy was associated with a decreased incidence of primary ACS. However, the previously unrecognized problem of secondary ACS was now an equally prevalent clinical entity.
If intraabdominal hypertension is used as a surrogate for ACS, the incidence is higher but similarly inconsistent. Sugrue and colleagues reported that the incidence of intraabdominal hypertension among general surgical patients undergoing laparotomy was 33% to 81%, depending on the definition (20 mm Hg or 18 mm Hg).23,38 In a study of medical patients, Malbrain reported that the incidence of intraabdominal hypertension was only 18%, despite using a liberal cutoff value (12 mm Hg).55 Using a cutoff value of 20 mm Hg, Balogh and coworkers reported a 39% incidence of intraabdominal hypertension in a cohort of patients with severe traumatic shock.57 Ivatury et al. reported that the incidence of intraabdominal hypertension was 32% among patients with life-threatening penetrating abdominal trauma.42
Outcome
Full-blown ACS with organ dysfunction was once uniformly fatal. With more timely diagnosis and treatment, more than half (depending on etiology) of afflicted patients are now surviving. With decompressive laparotomy, organ dysfunction typically improves transiently, but most patients who survive more than 48 hours progress into MOF.3,53 A fundamental problem is differentiating incomplete resuscitation from early organ failure. ACS and MOF appear to be closely linked. In our series, ACS was a surprisingly early event (occurring, on average, 12 hours after hospital admission) and was shown to be a strong independent predictor for subsequent MOF and death.
 Prediction and Diagnosis
Prediction and Diagnosis
Epidemiologic studies carried out during the 1990s clearly documented that ACS is a significant clinical problem.15 Additionally, more recent studies indicate that despite early recognition and decompression, the outcome remains poor for patients with ACS. Thus, early and accurate prediction is important because it allows us to recognize the population at risk and concentrate our preventive efforts on decreasing the incidence of ACS.5,51 The urinary bladder pressure measurement is a widely accepted, inexpensive, and simple monitoring tool for ACS. However, organ dysfunction associated with ACS can occur when IAP is less than 20 mm Hg, and some patients with IAP greater than 30 mm Hg do not develop any symptoms. Not surprisingly, surgeons are reluctant to make decisions regarding decompression based only on measurements of IAP.58 Potential risk factors for ACS include severe hemorrhagic shock, damage-control laparotomy, fascial closure after damage-control laparotomy, high abdominal trauma index, high injury severity score, and decreased pHi.42,59 Studies of secondary ACS have identified resuscitation fluid volume thresholds that warrant monitoring urinary bladder pressure. Maxwell et al. recommended monitoring when the resuscitation volume exceeds 10 L of crystalloid fluid or 10 units of packed red blood cells.60 Ivy et al. suggested that the trigger to initiate urinary bladder pressure monitoring should be greater than 0.25 L/kg of crystalloid resuscitation.20,52 Biffl and coworkers reported that both these cutoffs are ineffective and recommended the following thresholds: 6 L or more of crystalloid resuscitation or 6 units or more of packed red blood cells in a 6-hour period in patients with a base deficit greater than 10 mEq/L, especially if a vasopressor agent is required.53
More recent studies from general surgical, burn, and trauma populations have tried to identify the independent risk factors for ACS. For example, McNelis and coworkers performed a case-control study of 22 patients with ACS (diagnosed by elevated IAP and peak airway pressure) and 22 general surgical patients without ACS and created a predictive equation61:
where z = −18.6763 + 0.1671 (peak airway pressure) + 0.0009 (24-hour fluid balance).
In our experience, postinjury ACS occurs most frequently during the first 12 hours after injury, and waiting for a 24-hour fluid balance entails too much delay. By this time, most susceptible patients already exhibit the full-blown syndrome.5,51 Postinjury ACS recognized after 24 hours is lethal.5,50 Additionally, two prospective studies of trauma patients failed to identify predictors for ACS, possibly because the study populations were either too heterogeneous or too homogeneous. In a study of unselected trauma patients requiring ICU admission (mean injury severity score 18), Hong and colleagues found that only 2% of the patients developed intraabdominal hypertension and only 1% developed ACS.62 In a review of patients undergoing damage-control laparotomy (mean injury severity score 29), Raeburn and associates found that the incidence of ACS was 36%.63 Both of these groups failed to identify independent predictors of ACS.
From a prediction modeling perspective, patients requiring traumatic shock resuscitation are an ideal group to study. They are at substantial risk for ACS, the time of insult is defined, and the subsequent treatment (resuscitation) can be standardized. We therefore performed a multiple logistic regression analysis on a prospective database of major torso trauma patients who required shock resuscitation.5 Given the early occurrence of postinjury ACS, we focused our prediction models on the first 6 hours after hospital admission. We developed two prediction models: emergency department (ED) model (0-3 hours; i.e., all patients had an initial diagnostic workup and clinical laboratory results and were discharged from the ED) and ICU model (0-6 hours; i.e., all patients were admitted to the ICU, and their first physiologic monitor and clinical laboratory measurements on a standardized resuscitation protocol were available). Our goals were to identify the independent risk factors that may be causative and to build prediction models that could identify high-risk patients early during resuscitation so that standard care could be modified to prevent or improve the outcome of patients at risk for ACS.
The variables used in the multivariate prediction models included demographic parameters, shock severity, injury severity, interventions, hospital times, crystalloid and blood volumes, and vital signs. In the ICU, they also included initial pulmonary artery catheter readings, mechanical ventilator settings and response parameters, gastric tonometry data, and blood gas, clinical chemistry, and coagulation results. Among these variables, those listed in Table 201-3 were found to be independent risk factors for ACS. The primary ACS predictors at ICU admission (low temperature, low hemoglobin concentration, high base deficit) are all indicators of the so-called vicious circle physiology, the reason damage-control surgery is elected. The secondary ACS predictors (high crystalloid infusion volume, impaired renal function) suggest that the process is strongly related to the standard of care in the United States during the late 1990s (i.e., crystalloid resuscitation). The receiver operator characteristic analysis showed that ACS can be predicted with 0.88 accuracy at the time of ED discharge and, surprisingly, with 0.99 accuracy 1 hour after ICU admission with adequate monitoring. Use of these predictors together (even without urinary bladder pressure measurements) permits very early detection of the impaired physiologic findings characteristic of ACS. Because the predictors of ACS include both physiologic measurements and resuscitative interventions, this model should perform better in clinical situations during ongoing resuscitation than arbitrary urinary bladder pressure and organ dysfunction thresholds.56 The ED model (≈3 hours after admission) is very sensitive (overinclusive), which minimizes the chance of missing ACS patients; the ICU model (≈6 hours after hospital admission) is very specific and can pinpoint individuals at highest risk.
TABLE 201-3 Independent Predictors of Postinjury Primary and Secondary Abdominal Compartment Syndrome
| ED Model | ICU Model | |
|---|---|---|
| INDEPENDENT PREDICTORS | INDEPENDENT PREDICTORS | |
| Primary ACS | To OR < 75 min | Temp ≤ 34°C |
| Crystalloids ≥ 3 L | GAPCO2 ≥ 16 | |
| Hb ≤ 8/dL | ||
| BD ≥ 12 mEq/L | ||
| Secondary ACS | Crystalloids ≥ 3 L | GAPCO2 ≥ 16 |
| No urgent surgery | Crystalloids ≥ 7.5 L | |
| PRBC ≥ 3 units | UO ≤ 150 mL |
ACS, abdominal compartment syndrome; BD, arterial base deficit; CI, confidence interval; ED, emergency department; GAPCO2, carbon dioxide gap; Hb, hemoglobin concentration; ICU, intensive care unit; OR, operating room; PRBC, packed red blood cells; Temp, temperature; UO, urine output.
 Treatment
Treatment
Nonsurgical Methods
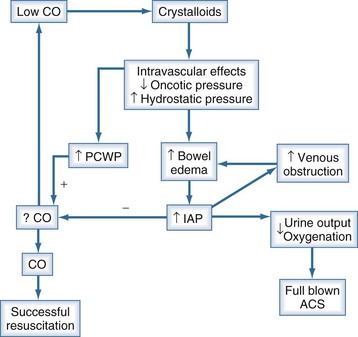
Support of early organ dysfunction by traditional ICU interventions is often necessary in patients with impending ACS but may aggravate the underlying pathophysiology. For example, ventilator strategies to increase mean airway pressure to improve oxygenation (e.g., high levels of positive end-expiratory pressure) directly increase intraabdominal hypertension by pushing down on the diaphragm. Additionally, increased mean airway pressure increases intrathoracic pressure, impeding venous outflow from the abdominal cavity. This promotes more gut edema with ongoing crystalloid resuscitation, another intervention often used in patients with impending ACS. Seminal papers in the mid-1990s advocated hypervolemic resuscitation to ameliorate cardiac and renal dysfunction. The concept was that increased IAP elevates pulmonary capillary wedge pressure but not preload, and fluid should be administered to increase left ventricular end-diastolic volume to improve the cardiac index.64 This approach seems harmful according to the most recent evidence.57,65 Patients with similar demographic characteristics, injuries, and shock severity without impending ACS responded very well to preload-directed resuscitation and increased the cardiac index appropriately.65 However, patients with impending ACS did not respond with increased cardiac index, despite vigorous crystalloid infusion. Vigorous attempts to increase preload (especially with crystalloid infusions) in patients with intraabdominal hypertension have a detrimental effect on outcome (futile crystalloid cycle; Figure 201-1).
Theoretically, other nonsurgical interventions may have beneficial effects, but their efficacy is unproven.66 Colloids and albumin could mobilize interstitial fluids into the vascular space, and muscle relaxants might have a salutary effect by decreasing tension in the abdominal wall.52,67 Continuous external application of negative abdominal pressure with a suction device showed some promise in morbidly obese patients with cerebral symptoms secondary to chronic ACS.47
Percutaneous Methods
If intraabdominal hypertension or ACS is a result of acute or chronic fluid collection, symptoms can be relieved by percutaneous drainage. Case reports described successful drainage of abdominal fluid in burn patients with secondary ACS and the drainage of blood in nonoperatively managed liver injuries.67–69 The major limitation of the technique is that it is applicable only when a significant amount of fluid is causing increased IAP. This technique will not work and might be dangerous when extensive bowel edema or retroperitoneal hematoma is the dominant contributing factor.
Surgical Decompression
Surgical decompression remains the primary recommended intervention. Decompression is achieved by opening the midline fascia (avascular plane) along its full length. Virtually all reports describe a very good physiologic response to decompression, but this does not necessarily translate into better outcomes. The best predictors of survival are post-decompression improvement in cardiac index and urine output.5,51 The decision to undertake surgical decompression is a difficult one, because it results in a chronically open abdomen that is associated with numerous hazards. Several case series have shown that early decompression is associated with better outcomes. However, in those studies, “late” decompression was often carried out days after the initial signs of ACS. If decompression is carried out within 12 hours of hospital admission, timing has no significant effect on outcome.5,51 Patients with ACS are in critical condition and require mechanical ventilation and other forms of organ support. Any unnecessary intrahospital transportation of these patients can be detrimental. Thus, if no other intraabdominal surgical intervention is needed, decompression can be performed at the bedside in the ICU. More recently, alternatives to midline laparotomy (transverse laparotomy and linea alba fasciotomy) were described. These approaches were popularized in cases of severe acute pancreatitis, where transverse laparotomy can be the surgical access of choice.70 The (subcutaneous) linea alba fasciotomy can prevent peritoneal contamination in selected pancreatitis cases where laparotomy is not required, only reduction of intraabdominal pressure.71,72
 Management of the Open Abdomen
Management of the Open Abdomen
Decompressive laparotomy results in an open abdomen, because the incision should not be closed until the risk of recreating ACS by closing the fascia diminishes. After abdominal decompression, temporary abdominal closure is applied to the wound to keep the fascia open. Several methods (towel clips, Bogota bag, synthetic mesh, vacuum-assisted closure, Velcro patch, zipper) are available. It is advantageous for the ICU specialist to understand each of these methods and discuss them with the surgical team. The key goals of temporary abdominal closure are as follows: prevent evisceration, allow enough room for swelling of the abdominal contents, control peritoneal fluids, prevent contamination, and preserve the fascia and skin for possible later closure or reconstruction. During the last 15 years, the morbidity and mortality of open abdomen management significantly decreased, but the strategy still carries considerable complications and potential long-term morbidity.73,74 Fistulas, abdominal infections, and intraabdominal collections were common, and the end result was usually a large abdominal wall defect. Early experience with a vacuum-assisted closure technique was very promising, and use of this approach may improve management of the open abdomen.75,76 A growing body of evidence is available about techniques which are successfully minimizing the morbidity and mortality of open abdomen management and improve long-term outcomes.74
 Prevention, Surveillance, and Future Directions
Prevention, Surveillance, and Future Directions
Prospective data suggest that the mortality rate for ACS, even with early decompression and resuscitation, is very high. In addition, early favorable physiologic responses to decompression do not necessarily translate into improved outcomes.5 Accordingly, prevention of ACS is paramount. Avoidance of fascial closure after high-risk laparotomy reduces the incidence of MOF and mortality.59 In the operating room, monitoring for increases in peak airway pressures during the attempted fascial closure is valuable in the absence of IAP measurement. In the ICU, all patients with severe shock and subsequent resuscitation (whole body ischemia-reperfusion injury), regardless of the cause (burn, trauma, sepsis, or hypovolemia), benefit from IAP monitoring, which is a simple, noninvasive tool.
ACS is strongly associated with the magnitude and quality of resuscitation.5,50–53,57,60,65 Uncontrolled goal-oriented resuscitation of trauma victims, chasing supranormal values for oxygen delivery, is harmful.57 To eliminate uncontrolled resuscitation, treatment of the underlying cause of shock is crucial. Timely hemorrhage control and elimination of septic foci should happen simultaneously. There is increasing evidence that Ringer’s lactate solution is proinflammatory, and use of this agent is an independent predictor of postinjury ACS.77 During burn and trauma resuscitation, crystalloid limits should be implemented, and after reaching them, alternative resuscitation fluids should be used. The best resuscitation fluid during impending ACS has yet to be determined.
In postinjury primary ACS, correction of the vicious circle of coagulopathy, acidosis, and hypothermia should be an early goal. Abbreviated laparotomy saves lives, but the tight abdominal packing increases the risk of ACS. Use of topical hemorrhage control techniques (e.g., fibrin sealants) offers a workable solution.78 When abnormalities in respiratory and renal function are identified, ACS should be included in the differential diagnosis and is an easily excludable cause if IAP measurements are performed. A direct effect of ACS is impaired abdominal visceral perfusion. Gastric tonometry is a relatively noninvasive monitor for intraabdominal hypertension. A high gastric regional PCO2 (>60 mm Hg) and a wide gap between gastric and end-tidal PCO2 (>16 mm Hg) are important indicators and predictors of ACS. With the availability of continuous IAP measurement, abdominal perfusion pressure (mean arterial pressure minus IAP) can be easily monitored at the bedside. The value of this variable has yet to be prospectively validated.
Key Points
Balogh Z, McKinley BA, Cox CSJr, et al. Abdominal compartment syndrome: the cause or effect of postinjury multiple organ failure. Shock. 2003;20:483-492.
Balogh Z, McKinley BA, Holcomb JB, et al. Both primary and secondary abdominal compartment syndrome can be predicted early and are harbingers of multiple organ failure. J Trauma. 2003;54:848-861.
Ivy ME, Atweh NA, Palmer J, et al. Intra-abdominal hypertension and abdominal compartment syndrome in burn patients. J Trauma. 2000;49:387-391.
Malbrain ML. Abdominal pressure in the critically ill: measurement and clinical relevance. Intensive Care Med. 1999;25:1453-1458.
Sugrue M, Bauman A, Jones F, et al. Clinical examination is an inaccurate predictor of intraabdominal pressure. World J Surg. 2002;26:1428-1431.
1 Malbrain ML, Cheatham ML, Kirkpatrick A, et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med. 2006;32:1722-1732.
2 Cheatham ML, Malbrain ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, et al. Results from the conference of experts on intra-abdominal hypertension and abdominal compartment syndrome. Part II: Recommendations. Intensive Care Med. 2007;33:951-962.
3 Kron IL, Harman PK, Nolan SP. The measurement of intra-abdominal pressure as a criterion for exploration. Ann Surg. 1984;199:28-30.
4 Sanchez NC, Tenofsky PL, Dort JM, et al. What is normal intra-abdominal pressure? Am Surg. 2001;67:243-248.
5 Balogh Z, McKinley BA, Holcomb JB, et al. Both primary and secondary abdominal compartment syndrome can be predicted early and are harbingers of multiple organ failure. J Trauma. 2003;54:848-861.
6 Burch JM, Ortiz VB, Richardson RJ, et al. Abbreviated laparotomy and planned reoperation for critically injured patients. Ann Surg. 1992;215:476-484.
7 Moore EE, Thomas G. Orr Memorial Lecture: Staged laparotomy for the hypothermia, acidosis and coagulopathy syndrome. Am J Surg. 1996;172:405-410.
8 Wendt E. Ueber den Einfluss des intraabdominalen Druckes auf die Absonderungsgeschwindigkeit des Harnes. Arch Physiol Heilkunde. 1867;8:527-575.
9 Heinricius G. Ueber den Einfluss der Bauchfulling auf Circulation und Respiration. Zeitschr Biol. 1890;26:113-202.
10 Emerson H. Intra-abdominal pressures. Arch Intern Med. 1911;7:754-784.
11 Gross R. A new method for surgical treatment of large omphalocoeles. Surgery. 1948;24:277-292.
12 Rotondo MF, Schwab CW, McGonigal MD, et al. “Damage control”: An approach for improved survival in exsanguinating penetrating abdominal injury. J Trauma. 1993;35:375-382.
13 Morris JAJr, Eddy VA, Blinman TA, et al. The staged celiotomy for trauma: Issues in unpacking and reconstruction. Ann Surg. 1993;217:576-584.
14 Ivatury RR, Diebel L, Porter JM, Simon RJ. Intra-abdominal hypertension and the abdominal compartment syndrome. Surg Clin North Am. 1997;77:783-800.
15 Balogh Z, McKinley BA, Cox CSJr, et al. Abdominal compartment syndrome: The cause or effect of postinjury multiple organ failure. Shock. 2003;20:483-492.
16 Gorecki PJ, Kessler E, Schein M. Abdominal compartment syndrome from intractable constipation. J Am Coll Surg. 2000;190:371.
17 Cil T, Tummon IS, House AA, et al. A tale of two syndromes: Ovarian hyperstimulation and abdominal compartment. Hum Reprod. 2000;15:1058-1060.
18 De Keulenaer BL, De Backer A, Schepens DR, et al. Abdominal compartment syndrome related to noninvasive ventilation. Intensive Care Med. 2003;29:1177-1181.
19 Gecelter G, Fahoum B, Gardezi S, Schein M. Abdominal compartment syndrome in severe acute pancreatitis: An indication for a decompressing laparotomy? Dig Surg. 2002;19:402-404.
20 Ivy ME, Possenti PP, Kepros J, et al. Abdominal compartment syndrome in patients with burns. J Burn Care Rehabil. 1999;20:351-353.
21 Kirkpatrick AW, Brenneman FD, McLean RF, et al. Is clinical examination an accurate indicator of raised intra-abdominal pressure in critically injured patients? Can J Surg. 2000;43:207-211.
22 Sugrue M, Bauman A, Jones F, et al. Clinical examination is an inaccurate predictor of intraabdominal pressure. World J Surg. 2002;26:1428-1431.
23 Sugrue M, Jones F, Janjua KJ, et al. Temporary abdominal closure: A prospective evaluation of its effects on renal and respiratory physiology. J Trauma. 1998;45:914-921.
24 Obeid F, Saba A, Fath J, et al. Increases in intra-abdominal pressure affect pulmonary compliance. Arch Surg. 1995;130:544-547.
25 Lacey SR, Bruce J, Brooks SP, et al. The relative merits of various methods of indirect measurement of intraabdominal pressure as a guide to closure of abdominal wall defects. J Pediatr Surg. 1987;22:1207-1211.
26 Iberti TJ, Lieber CE, Benjamin E. Determination of intra-abdominal pressure using a transurethral bladder catheter: Clinical validation of the technique. Anaesthesiology. 1989;70:40-45.
27 Balogh Z, Jones F, D’Amours SK, et al. Continuous intra-abdominal pressure measurement technique—a new gold standard. Am J Surg. 2004;188:679-684.
28 Bloomfield GL, Ridings PC, Blocher CR, et al. A proposed relationship between increased intraabdominal, intrathoracic and intracranial pressure. Crit Care Med. 1997;25:496-503.
29 Josephs L, McDonald J, Birkett D, et al. Diagnostic laparoscopy increases intracranial pressure. J Trauma. 1994;36:815-819.
30 Bloomfield GL, Ridings PC, Blocher CR, et al. Effects of increased intraabdominal pressure upon intracranial and cerebral perfusion pressure before and after volume expansion. J Trauma. 1996;40:936-940.
31 Richardson JD, Trinkle JK. Hemodynamic and respiratory alterations with increased intra-abdominal pressure. J Surg Res. 1976;20:401-404.
32 Kasthan J, Green JF, Parsons EQ, et al. Hemodynamic effects of increased abdominal pressure. J Surg Res. 1981;30:249-255.
33 Robotham JL, Wise RA, Bomberger-Barnea B. Effects of changes in abdominal pressure on left ventricular performance and regional blood flow. Crit Care Med. 1985;12:803-808.
34 Ridings PC, Blocher CR, Sugerman HJ. Cardiopulmonary effects of raised intra-abdominal pressure before and after intravascular volume expansion. J Trauma. 1995;39:1071-1075.
35 Cullen DJ, Coyle JP, Teplick R, Long MC. Cardiovascular, pulmonary and renal effects of massively increased intra-abdominal pressure in critically ill patients. Crit Care Med. 1989;17:118-121.
36 Richards WO, Scovill W, Shin B, et al. Acute renal failure associated with increased intra-abdominal pressure. Ann Surg. 1983;197:183-187.
37 Harmann PK, Kron IL, McLachlan HD, et al. Elevated intra-abdominal pressure and renal function. Ann Surg. 1982;196:594-597.
38 Sugrue M, Buist MD, Hourihan F, et al. Prospective study of intraabdominal hypertension and renal function after laparotomy. Br J Surg. 1995;82:235-238.
39 Diebel LN, Dulchavsky SA, Wilson RF. Effects of increased intraabdominal pressure on mesenteric arterial and intestinal mucosal blood flow. J Trauma. 1992;33:45-49.
40 Rasmussen IB, Berggren U, Arvidsson D, et al. Effects of pneumoperitoneum on splanchnic hemodynamics: An experimental study in pigs. Eur J Surg. 1995;161:819-824.
41 Sugrue M, Jones F, Lee A, et al. Intraabdominal pressure and gastric intramucosal pH: Is there an association? World J Surg. 1996;20:988-991.
42 Ivatury RR, Porter JM, Simon RJ, et al. Intra-abdominal hypertension after life-threatening penetrating abdominal trauma: Prophylaxis, incidence, and clinical relevance of gastric mucosal pH and abdominal compartment syndrome. J Trauma. 1998;44:1016-1023.
43 Biffl WL, Moore EE, Burch JM. Femoral arterial graft failure caused by the secondary abdominal compartment syndrome. J Trauma. 2001;50:740-742.
44 Rezende-Neto JB, Moore EE, Masuno T, et al. The abdominal compartment syndrome as a second insult during systemic neutrophil priming provokes multiple organ injury. Shock. 2003;20:303-308.
45 Oda J, Ivatury RR, Blocher CR, et al. Amplified cytokine response and lung injury by sequential hemorrhagic shock and abdominal compartment syndrome in a laboratory model of ischemia-reperfusion. J Trauma. 2002;52:625-631.
46 Rezende-Neto J, Moore EE, Melo de Andrade MV, et al. Systemic inflammatory response secondary to abdominal compartment syndrome: Stage for multiple organ failure. J Trauma. 2001;53:1121-1128.
47 Sugerman HJ, Felton WLIII, Sismanis A, et al. Continuous negative abdominal pressure device to treat pseudotumor cerebri. Int J Obes Relat Metab Disord. 2001;25:486-490.
48 Meldrum DR, Moore FA, Moore EE, et al. Cardiopulmonary hazards of perihepatic packing for major liver injuries. Am J Surg. 1995;170:537-542.
49 Chen RJ, Fang JF, Chen MF. Intra-abdominal pressure monitoring as a guideline in the nonoperative management of blunt hepatic trauma. J Trauma. 2001;51:44-50.
50 Kopelman T, Harris C, Miller R, Arrillaga A. Abdominal compartment syndrome in patients with isolated extraperitoneal injuries. J Trauma. 2000;49:744-749.
51 Balogh Z, McKinley BA, Cocanour CS, et al. Secondary abdominal compartment syndrome: An elusive complication of traumatic shock resuscitation. Am J Surg. 2002;184:538-543.
52 Ivy ME, Atweh NA, Palmer J, et al. Intra-abdominal hypertension and abdominal compartment syndrome in burn patients. J Trauma. 2000;49:387-391.
53 Biffl WL, Moore EE, Burch JM, et al. Secondary abdominal compartment syndrome is a highly lethal event. Am J Surg. 2001;182:645-648.
54 Fietsam R, Villalba M, Glover JL, et al. Intra-abdominal compartment syndrome as a complication of ruptured abdominal aortic aneurysm repair. Am Surg. 1989;55:396-402.
55 Malbrain ML. Abdominal pressure in the critically ill: Measurement and clinical relevance. Intensive Care Med. 1999;25:1453-1458.
56 Meldrum DR, Moore FA, Moore EE, et al. Prospective characterization and selective management of the abdominal compartment syndrome. Am J Surg. 1997;174:667-672.
57 Balogh Z, McKinley BA, Cocanour CS, et al. Supra-normal trauma resuscitation causes more cases of abdominal compartment syndrome. Arch Surg. 2003;138:637-643.
58 Mayberry JC, Goldman RK, Mullins RJ, et al. Surveyed opinion of American trauma surgeons on the prevention of the abdominal compartment syndrome. J Trauma. 1999;47:509-513.
59 Offner PJ, de Souza AL, Moore EE, et al. Avoidance of abdominal compartment syndrome in damage-control laparotomy after trauma. Arch Surg. 2001;136:676-681.
60 Maxwell RA, Fabian TC, Croce MA, Davis KA. Secondary abdominal compartment syndrome: An underappreciated manifestation of severe hemorrhagic shock. J Trauma. 1999;47:995-999.
61 McNelis J, Soffer S, Marini CP, et al. Abdominal compartment syndrome in the surgical intensive care unit. Am Surg. 2002;68:18-23.
62 Hong JJ, Cohn SM, Perez JM, et al. Prospective study of the incidence and outcome of the abdominal compartment syndrome. Br J Surg. 2002;89:591-596.
63 Raeburn CD, Moore EE, Biffl WL, et al. The abdominal compartment syndrome is a morbid complication of postinjury damage control surgery. Am J Surg. 2001;182:542-546.
64 Burch JM, Moore EE, Moore FA, Franciose R. The abdominal compartment syndrome. Surg Clin North Am. 1996;76:833-842.
65 Balogh Z, McKinley BA, Kozar RA, et al. Patients with impending abdominal compartment syndrome do not respond to early volume loading. Am J Surg. 2003;182:602-607.
66 Cheatham ML. Nonoperative management of intraabdominal hypertension and abdominal compartment syndrome. World J Surg. 2009;33:1116-1122.
67 Greenhalgh DG, Warden GD. The importance of intra-abdominal pressure measurements in burned children. J Trauma. 1994;36:685-690.
68 Yang EY, Marder SR, Hastings G, Knudson MM. The abdominal compartment syndrome complicating nonoperative management of major blunt liver injuries: Recognition and treatment using multimodality therapy. J Trauma. 2002;52:982-986.
69 Corcos AC, Sherman HF. Percutaneous treatment of secondary abdominal compartment syndrome. J Trauma. 2001;51:1062-1064.
70 Leppäniemi A, Mentula P, Hienonen P, Kemppainen E. Transverse laparostomy is feasible and effective in the treatment of abdominal compartment syndrome in severe acute pancreatitis. World J Emerg Surg. 2008;30:6.
71 Leppäniemi A. Surgical management of abdominal compartment syndrome; indications and techniques. Scand J Trauma Resusc Emerg Med. 2009;14:17.
72 Cheatham ML, Fowler J, Pappas P. Subcutaneous linea alba fasciotomy: a less morbid treatment for abdominal compartment syndrome. Am Surg. 2008;74:746-749.
73 Cothren CC, Moore EE, Johnson JL, Moore JB, Burch JM. One hundred percent fascial approximation with sequential abdominal closure of the open abdomen. Am J Surg. 2006;192:238-242.
74 Cheatham ML, Safcsak K. Longterm impact of abdominal decompression: a prospective comparative analysis. J Am Coll Surg. 2008;207:573-579.
75 Garner GB, Ware DN, Cocanour CS, et al. Vacuum-assisted wound closure provides early fascial reapproximation in trauma patients with open abdomens. Am J Surg. 2001;182:630-638.
76 Suliburk JW, Ware DN, Balogh Z, et al. Vacuum assisted wound closure allows for early abdominal fascial closure in severely injured trauma patients after damage control laparotomy. J Trauma. 2003;55:1155-1160.
77 Rhee P, Wang D, Paul R, et al. Human neutrophil activation and increased adhesion by various resuscitation fluids. Crit Care Med. 2000;38:74-78.
78 Holcomb JB, Pusateri AE, Harris RA, et al. Dry fibrin sealant dressings reduce blood loss, resuscitation volume, and improve survival in hypothermic coagulopathic swine with grade V liver injuries. J Trauma. 1999;47:233-240.