Abdominal and Renal Trauma
While head injuries are responsible for the majority of pediatric trauma deaths, intra-abdominal and retroperitoneal injuries can still result in significant morbidity and mortality. Diagnostic uncertainty and delays in diagnosis can lead to long-term complications and adversely impact quality of life. Injuries to intra-abdominal organs occur in 10–15% of injured children.1 The spleen is the most frequently injured organ, and low velocity mechanisms, such as falls, is the most frequent mechanism of injury. The combination of the unique anatomic and physiologic features of children and differences in mechanism result in patterns of injury unique to the pediatric population.
As just mentioned, falls are the most frequent mechanism of injury in children. However, motor vehicle crashes (MVC) are the most deadly, and are the leading cause of death for all children after the age of 1 year.2 From the perspective of abdominal trauma, and using the spleen as a marker for intra-abdominal injury, pediatric injuries tend to be the result of lower-velocity mechanisms when compared to adults. In a study that compared splenic injuries at an adult level one trauma center and a pediatric level one trauma center, MVC accounted for 66.9% of adult injuries but only 23.7% of pediatric injuries.3 On the other hand, ‘sports mishaps’ resulted in only 2.3% of the adult injuries, but 17% of injuries involving children. Even in the MVC population, pediatric injuries tend to differ from those suffered by adults. Children are less likely to be in the driver’s seat (and hence less likely to suffer injuries to the thorax from the steering wheel), and are more likely to be victims of poorly fitted restraint systems. It is an important part of the initial history to ascertain whether the pediatric victims in an MVC were restrained, and the type and appropriateness of that restraint for the child’s age.
Anatomically, the smaller size of children, as compared to adults, results in a closer proximity of organs. The abdominal wall, rib cage, and pelvic girdle are underdeveloped and provide less protection to the abdominal contents. In addition, children have less body fat, and hence, less ‘padding’ to absorb and diffuse external force.4 From the physiologic perspective, children are generally healthy and have fewer underlying medical problems than adults. It is uncommon for children to be on medications, particularly those that potentially affect hemodynamics or hemostasis. Therefore, injured pediatric patients are better able to effectively compensate for physiologic insults such as acute blood loss. It is generally accepted that children can lose up to 45% of their circulating blood volume, and exhibit tachycardia as the only abnormal vital sign.4 Persistent hypotension is an ominous finding, suggesting the failure of compensatory mechanisms and the potential development of irreversible shock. Complicating the evaluation of injured children is the normal variability of vital signs depending on age.
Initial Evaluation and Diagnosis of Abdominal Injuries
As the number of children with significant abdominal injuries is relatively low, but the consequences of a missed injury are high, accurate diagnosis is important. Initial assessment begins before the child arrives at the hospital. Important information from the first responders includes mechanism of injury, use of protective or restraint devices, condition of the child in the field, and, in the case of MVC, damage to vehicle. Once in the emergency department (ED), a thorough history and physical examination is essential. In most statistical models regarding the diagnosis of intra-abdominal injury, an abnormal physical examination is the highest variable.5–8 While the examination can be challenging given the developmental level of the child, use of comfort strategies and distraction can calm an initially distraught child to a degree that he/she can reliably participate in the evaluation. Important physical findings include vital signs (particularly the presence of persistent tachycardia), abdominal contusions or abrasions, tenderness, or distention. Particular physical findings, such as the ‘seat belt sign’ and ‘handle bar mark,’ are suspicious for the presence of intra-abdominal injury (and potential spine fracture in the case of the seat belt mark) (Fig. 16-1).9
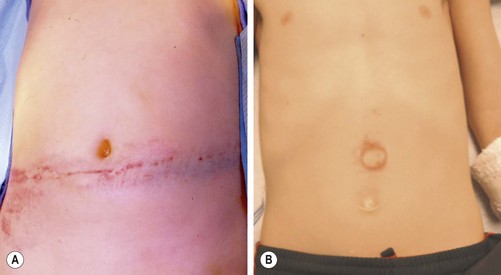
FIGURE 16-1 Important physical findings that might suggest intra-abdominal injuries include the (A) ‘seat belt sign’ and the (B) ‘handlebar mark.’ With patients exhibiting the seat belt sign, there is also potential for a spine fracture. The handlebar mark may herald an underlying duodenal hematoma or pancreatic injury.
Laboratory Testing
Laboratory testing for the purpose of diagnosing intra-abdominal injuries has generated considerable interest and conflicting results. One study reported that the combination of an abnormal physical examination and >50 red blood cells per high power field on urinalysis was a highly sensitive screen for the presence of intra-abdominal injury.5 The study, limited by the low number of children who actually had a documented injury (14 out of the total study population of 285), also concluded that laboratory abnormalities in this trauma population were relatively uncommon. The conclusion that routine laboratory studies add little to the evaluation has also been replicated in more recent studies.6,7 Conversely, studies using sophisticated regression analyses have demonstrated that elevations of aspartate aminotransferase (AST) and/or alamine aminotransferase (ALT), in combination with an abnormal physical examination, correlate with the presence of an intra-abdominal injury, although the tests are not diagnostic for a particular injured organ.8,10–12 A clinical prediction model using a combination of physical examination findings (hypotension and abnormal examination findings) and laboratory studies (AST, amylase, hematocrit, heme-positive urinalysis) successfully predicted the presence of intra-abdominal injury in a small, single center study.13 Interestingly, routine amylase and lipase determinations do not appear to be very reliable or cost effective screening tools.14 In the special population of children suspected of abuse, elevations in AST or ALT, or abnormal physical examination findings (such as bruising, distention, or tenderness), may indicate the need for further abdominal imaging looking for occult injury.15
Computed Tomography
Computed tomography (CT) with intravenous contrast (IV) is the preferred modality for the diagnosis of intra-abdominal injuries in hemodynamically stable children.4 Newer generation scanners have excellent sensitivity and specificity, especially for the evaluation of solid organ injuries. Upwards of 95% of liver, spleen, and renal injuries can be diagnosed and staged by CT (Fig. 16-2). Injuries to the intestine and pancreas are more difficult to definitively diagnose by CT. However, with the addition of coronal reconstructions, CT provides significant information to guide the clinician regarding these injuries. Similarly, the risk of a ‘missed’ intra-abdominal injury in a child with a completely negative CT is very low, leading some to advocate using CT as a means to decrease the need for in-patient observation after blunt abdominal trauma.16
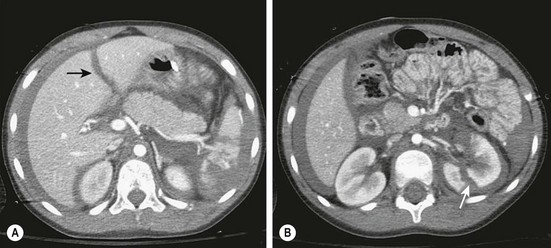
FIGURE 16-2 CT scans are highly accurate in demonstrating solid organ injuries. (A) Hemoperitoneum with a liver laceration (arrow) and a shattered spleen is seen. (B) Hemoperitoneum and a left renal laceration (arrow) is shown.
It has been suggested that in young children who lack visceral fat, the addition of oral contrast to the standard IV contrast may be helpful, especially in evaluating the duodenum and pancreatic head.17 The use of oral contrast, however, remains controversial due to concerns regarding aspiration, and may not provide significant additional information with current, multi-detector CT imaging. Intravenous contrast, however, is essential for the evaluation of traumatic injuries. If IV contrast is contraindicated, alternative methods of abdominal evaluation should be considered.
The radiation exposure during CT imaging has become an area of major concern in children. The use of CT has been rapidly increasing over the past decade, with over seven million scans performed on children, mostly for the evaluation of trauma and appendicitis.18 Using models extrapolated from radiation exposure from the atomic bomb explosions, a risk of one fatal cancer per 1000 CT scans performed in young children (above the baseline cancer risk of approximately one in four adults in the USA) has been estimated.19 A recently published longitudinal, population-based study in Great Britain demonstrated an increased incidence of leukemia and brain cancer after repeated CT scans in children.20 Infants and children are more sensitive than adults to the effects of radiation given their small size (larger absorbed dose per unit area) and growing organs.21 In response, the pediatric radiology community has developed an Image Gently® campaign to address the public’s concerns.21 In addition, two recent position papers, authored by the APSA Education Committee and the American Academy of Pediatrics (AAP) Radiology Committee, have addressed the issue of CT scans in children.22,23 Both endorse the ALARA principle (as low as reasonably achievable) and advocate for the use of scanners with pediatric dose reduction software, employing alternative imaging modalities (if available), limiting the number or phases of scans (for example with and without contrast or arterial and venous phases), and the use of limited scans. Other concepts include limiting the number of repeat scans and developing relationships with referring adult institutions to limit the number of scans performed on children prior to transfer.
Ultrasound
As concerns regarding CT have increased, there has been a renewed interest in the use of ultrasound (US) in the evaluation of pediatric abdominal trauma. The original descriptions about ultrasound in trauma centered on the rapid evaluation of the unstable adult trauma patient to determine the presence and source of life-threatening hemorrhage. The FAST (focused assessment with sonography in trauma) examination was developed to assess the presence of intra-abdominal free fluid (with examination of Morrison’s pouch, the pouch of Douglas, and the left flank) or fluid within the pericardial sac (subxiphoid view), and thus indicate the need for operative exploration. In multiple studies, the traditional FAST examination has been found to have a low sensitivity and specificity for the diagnosis of injury in children.24–27 A recently published large series directly comparing FAST examination in children to CT or laparotomy for the presence of free fluid concluded that a positive FAST suggested hemoperitoneum and associated abdominal injury, but a negative FAST adds little in decision making.28 In addition, since the majority of pediatric solid organ injuries, even those with significant free fluid (hemoperitoneum), can be managed nonoperatively, a positive FAST examination may not be very helpful in directing clinical care. On the other hand, the use of provider-performed ultrasound has increased dramatically over the past several years in the pediatric ED, and there is significant interest in developing algorithms that incorporate ultrasound into the evaluation of abdominal trauma.29,30 The ultimate goal is to limit the number of CT scans. In the less common scenario of the hemodynamically unstable child, a positive FAST examination supports the decision to rapidly proceed to the operating room.
Laparoscopy
Minimally invasive approaches are now well incorporated in pediatric surgical practice so it is not surprising that laparoscopy for the evaluation of abdominal trauma is being utilized. Despite the excellent anatomic definition provided by multi-detector CT, there remain areas of diagnostic uncertainty. The child with free fluid without evidence of solid organ injury, particularly with physical examination findings of a seat belt or handlebar mark, is one example. Another scenario is the child with significant abdominal tenderness with a nondiagnostic CT scan. If the findings at laparoscopy indicate the need for a formal laparotomy, an open approach can be targeted to the specific injury. In two relatively large reviews, laparoscopy was found to be safe and beneficial by avoiding laparotomy in a significant number of patients.31,32 Also, a number of injuries were amenable to laparoscopic repair. CT and laparoscopy now provide complementary information, with CT defining areas, such as the retroperitoneum, kidneys, and pancreas, which are difficult to assess using laparoscopy. On the other hand, laparoscopy allows for direct visualization of the bowel, mesentery, and diaphragmatic surfaces, regions that CT has traditionally not been as accurate (Fig. 16-3).
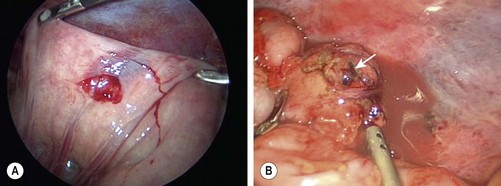
FIGURE 16-3 In some patients it is not always clear whether a significant intestinal injury has occurred from either blunt or penetrating trauma. Diagnostic laparoscopy is a useful technique in these patients. (A) Perforation of the bowel from penetrating trauma is seen at laparoscopy. This was closed primarily. (B) Full-thickness injury to the colon (arrow) in a patient with blunt trauma is shown. The laparoscopic approach was converted to an open operation for treatment of this injury.
Management
Liver and Spleen
Close to 90–95% of injuries to the liver and spleen in children can be managed nonoperatively. It is rare for isolated low grade injuries to these organs to require blood transfusion.33 Nonoperative management (NOM) is dependent upon the accurate diagnosis and staging of the injured organ, usually by CT imaging at present. Injuries are graded according to the American Association for the Surgery of Trauma (AAST) organ injury scale, with grade I injuries representing small lacerations or hematomas and grade V injuries indicating complete vascular disruption or massive parenchymal injury (Table 16-1).34 In order to be a candidate for NOM, the child should have normal hemodynamics, and be monitored closely for signs of ongoing hemorrhage. Most children who fail NOM do so within four hours of injury as a result of shock, peritonitis, or persistent bleeding.35 Late failures are often the result of peritonitis due to an evolving intestinal injury. There are published, evidence-based guidelines for NOM in a child with a liver or spleen injury.36,37 Essentially, these guidelines recommend hospitalization for ‘grade of injury plus one’ days, and note that children with higher grade injuries may benefit from intensive care unit observation. Routine follow-up imaging is not indicated, and children can return to regular activity after grade of injury plus two weeks from the time of injury. More recent work challenges these recommendations, finding that more abbreviated periods of bed rest and hospitalization does not result in delayed bleeding or return to the hospital.38 Fortunately, most splenic and hepatic injuries in children will resolve without the need for operative intervention with excellent long-term outcomes.
TABLE 16-1
Liver/Spleen Injury Grading Scale from the AAST
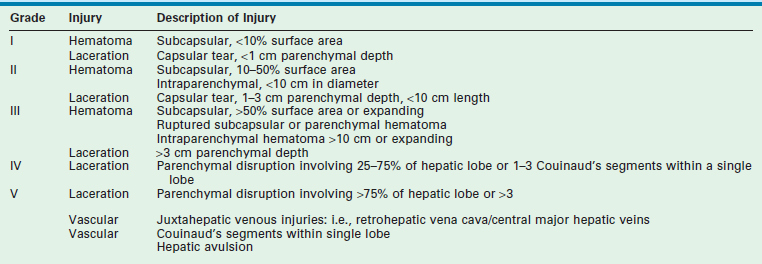
From Tinkoff G, Esposito TJ, Reed J, et al. American Association for the Surgery of Trauma Organ Injury Scale I: spleen, liver, and kidney, validation based on the National Trauma Data Bank. J Am Coll Surg 2008;207:646–55.
While bleeding from most solid organ injuries in children will stop, there are a small number in which the bleeding is significant. Tachycardia, not responsive to fluid resuscitation, is the initial sign of shock in these children. Hypotension is often a late finding and suggests significant hemorrhage. Evidence of ongoing bleeding with an abnormal abdominal examination or a positive abdominal FAST examination necessitates urgent operative exploration. Rapid transfusion protocols, while not formally validated in children, are utilized with the goal of 1 : 1 : 1 transfusion of packed red blood cells (PRBC), fresh frozen plasma (FFP), and platelets. In infants and children, this translates to 20 mL/kg of PRBC, FFP and platelets.39 In the operating room, a rapid transfusion device and cell saver should be available in the event of rapid blood loss. The patient is prepped from neck to knees to allow for entrance into either the chest or abdomen, and to have access to the femoral vessels. Upon entrance to the abdomen, the four quadrants are packed to tamponade the bleeding and allow the anesthesiologists to ‘catch-up.’ The peritoneal contents are then explored in a systematic fashion. The goal of initial operative exploration is to stop bleeding and control the fecal stream (damage control).
Splenectomy easily controls bleeding in the hemodynamically unstable patient with active exsanguination from a massively damaged spleen, although at the theoretical cost of a long-term risk of postsplenectomy sepsis. Children with splenic injuries who have ongoing bleeding, but are not in shock, are potential candidates for splenic sparing operations. Partial splenectomy and mesh splenorrhaphy are techniques that can successfully save splenic parenchyma, although they may be time consuming, and are therefore not appropriate in the unstable patient.40
Postsplenectomy sepsis is a rare, but potentially fatal consequence of splenectomy due to overwhelming infection by encapsulated organisms. The reported incidence is around 0.23% a year, with an increased incidence in children less than 2 years of age, and those that underwent splenectomy for hematologic reasons.41 Vaccination with the 23-valent pneumococcal vaccine, as well as vaccinations against Haemophilus influenzae type B and meningococcus, should be administered after splenectomy. With high grade splenic injuries managed nonoperatively, assessment of splenic function may also be indicated.
A major hepatic injury is considerably more difficult to control in the operating room. The segmental anatomy of the liver and the location of important arterial, venous, and portal structures is very important. Peitzman and Marsh recently reviewed operative techniques for the management of complex liver injury.42 Key components of operative control of hepatic parenchymal injury include adequate exposure, an experienced co-surgeon, good anesthesia support, and supradiaphragmatic intravenous access. They recommend initial management of deep parenchymal fractures with compression, followed by suture ligation of bleeding vessels, and the avoidance of deep liver sutures. The Pringle maneuver can help differentiate between hepatic arterial bleeding (decreases when the clamp is engaged) and hepatic venous bleeding. Ideally, intermittent clamping of the porta hepatis should be performed to decrease the degree of hepatic ischemia.
Abdominal Compartment Syndrome
Abdominal compartment syndrome (ACS) is defined as sustained intra-abdominal hypertension (IAH) that is associated with new onset organ dysfunction or failure.43,44 It is an uncommon, but potentially lethal condition that occurs when abdominal distension associated with IAH causes reduced perfusion to the intra-abdominal organs. The result is ischemia and refractory metabolic acidosis along with interference with cardiopulmonary function secondary to reduced preload from decreased central venous return to the heart, decreased respiratory compliance, and decreased functional residual capacity.45 ACS is associated with a 40–60% mortality in children.46–48
As IAH in children is different from adults, the current proposed working definition for ACS in children is an elevated intra-abdominal pressure (IAP) of 10 mmHg or greater with the development of new or worsening multiorgan failure.44 There are three different types of ACS: (1) primary ACS refers to ACS that occurs due to a primary intra-abdominal cause such as abdominal trauma; (2) secondary ACS or extra-abdominal compartment syndrome occurs as a result of massive bowel edema secondary to sepsis, capillary leak, and other conditions requiring massive fluid resuscitation; and (3) tertiary ACS or recurrent ACS in which ACS recurs after resolution of an earlier episode of either primary or secondary ACS.49 IAP can be measured by using the bladder pressure. If IAH is detected, serial IAP measurements are needed. It is important to note that clinical examination is an inaccurate predictor of IAP and should not be substituted for IAP measurement.50
Initial management strategies in the trauma patient include improving abdominal wall compliance via adequate sedation and paralysis, evacuation of intralumenal intestinal contents, evacuation of large abdominal fluid collections, optimization of fluid administration by goal-directed therapies and correcting positive fluid balance, and optimization of abdominal perfusion pressure.51 Over the last ten years, three major changes have led to significant reductions in the incidence and mortality from ACS in adult trauma patients. These are adoption of massive transfusion protocols and 1 : 1 blood to plasma transfusion strategies in trauma, the widespread use of damage control and open abdomen approaches to the polytraumatized abdominal cavity, and an increased use of plasma and colloids in the resuscitation of burn patients.51 Similar strategies and an increased awareness of ACS in pediatric trauma patients may also result in improved outcomes.
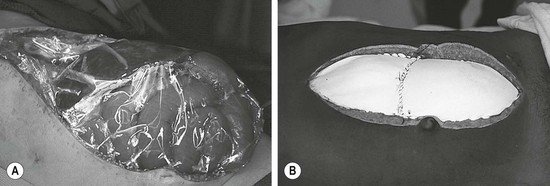
In the unstable trauma patient who requires an emergent laparotomy and massive fluid resuscitation, maintaining an open abdomen with planned staged closure may prevent the development of ACS but often needs to be performed prophylactically (Fig. 16-4A). In patients who develop ACS, early intervention via emergent decompressive laparotomy and some form of temporary abdominal wall closure, while awaiting resolution of IAH, can be lifesaving (Fig. 16-4B).46,48,52 The goals of operation are to decrease the elevated IAP to stop organ dysfunction, allow room for continued expansion of the viscera during ongoing resuscitation, provide temporary abdominal closure, prevent excessive fascial retraction, and allow a means for continued evacuation of fluid from the abdominal cavity.51 Application of a negative pressure wound dressing to the open abdomen is a useful temporary dressing as well as a modality to remove edema from the abdominal cavity and intestinal wall.53 Temporary patch abdominoplasty with a variety of materials and the placement of a silo have also been utilized in the setting of an open abdomen. However, these methods lack the capability to actively evacuate excess fluid. Subsequent staged abdominal closure with sutures or patch abdominoplasty is performed when IAH is corrected.
Role of Interventional Radiology
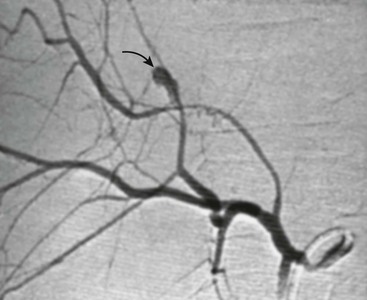
Angioembolization is a technique that is frequently utilized in adults with splenic or hepatic vascular injuries. The role of interventional radiology in children is less well defined. For example, embolization in adults is frequently employed for the management of a contrast blush demonstrated on CT scan. Multiple studies in children, however, demonstrate that a contrast blush is associated with the need for operative intervention in less than 20% of splenic injuries.54–56 Long-term follow-up of large cohorts of children with splenic and hepatic injuries also reveal a very low rate of late bleeding, suggesting that the rate of bleeding from an initially unrecognized arterial pseudoaneurysm is also very low.36,37 On the other hand, small single-center studies and case reports demonstrate that interventional radiological techniques are safe in children, and have been effective when utilized.57–60 The population that seems most amenable to this technique are children with evidence of ongoing bleeding but are hemodynamically stable, or those that develop bleeding later in their hospital course. In our institution, we have effectively utilized angioembolization in a few cases of hepatic arterial bleeding (Fig. 16-5).
Special Considerations with Liver Injuries
The initial concern with hepatic injury is control of bleeding, but injury to the biliary system can also occur. High grade liver injuries are associated with a small (4%) risk of a significant bile leak.61 If the patient has required operative management for the injury, it is prudent to place closed suction drains around the liver, particularly if a nonanatomic resection was performed. With nonoperative management, the development of a significant bile leak is often heralded by feeding intolerance, abdominal pain, elevations in hepatic enzymes, and fever.62 Abdominal imaging (either ultrasound or CT) reveals the presence of a fluid collection. Initial management involves the insertion of drains, usually performed percutaneously with image guidance.
Endoscopic retrograde cholangiopancreatography (ERCP) has been used to identify the location of the leak, and more importantly, with the addition of sphincterotomy, to decrease biliary pressure and promote internal drainage.63,64 Placement of biliary stents can also be performed, both to improve drainage and to treat the ductal injury. Therapeutic ERCP requires operator expertise, and heavy sedation or general anesthesia for the procedure to be safely performed in children. In the case of stent placement, a second endoscopic procedure to remove the stent is usually necessary. Complications of ERCP include bleeding, sepsis, and stent migration or clogging.
Pancreatic Injuries
Injury to the pancreas occurs in fewer than 5% of pediatric abdominal injuries, and can be difficult to diagnose. They are most frequently the result of blunt mechanisms, such as MVC and bicycle handlebar injuries. Patients usually present with significant epigastric pain and bilious emesis, particularly in the case of injuries that have a delayed presentation. CT scan with IV contrast is the preferred imaging study, although definitive identification of these injuries can be difficult (Fig. 16-6). In unusual cases, magnetic retrograde cholangiopancreatography (MRCP) can be helpful. ERCP, if available, may be helpful in determining whether there is a major ductal injury, and may have a potential therapeutic role, but is an invasive and a technically challenging procedure.65
Contusions, without evidence of pancreatic ductal injury, can be managed nonoperatively with nothing by mouth. The child is followed symptomatically as an oral diet is reintroduced. Trends in serum amylase and lipase may be helpful, although the absolute value of these tests does not correlate with outcome.66 Management of ductal transection is currently controversial. The standard approach for a distal ductal transaction is a spleen preserving distal pancreatectomy. This procedure, which can be performed via an open laparotomy or laparoscopically (Fig. 16-7),67,68 is generally well tolerated, and prevents pseudocyst formation. Concerns regarding late morbidity, particularly endocrine insufficiency, have led to other treatment approaches. One group has advocated for the use of Roux-en-Y distal pancreaticojejunostomy using a retrocolic jejunal limb to drain the distal pancreas.69 Others have advocated a nonoperative approach to pancreatic ductal injuries, with percutaneous or endoscopic drainage of subsequent pseudocysts.70,71 A recent APSA Trauma Committee retrospective review compared operative and nonoperative management, and demonstrated similar length of hospitalization, but a higher rate of pseudocyst formation and days on total parenteral nutritional (TPN) in the nonoperative group.72 Patients undergoing NOM often require ERCP to define the ductal anatomy, perform sphincterotomy, and potentially stent the pancreatic duct, as well as percutaneous or endoscopic drainage of pseudocysts. At this time, it is unclear which patient population will benefit from a nonoperative approach for a pancreatic ductal injury. In addition, the nonoperative approach requires the availability of advanced endoscopic techniques and ERCP.
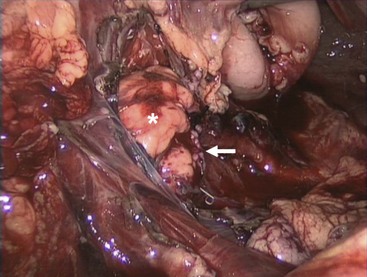
FIGURE 16-7 This patient presented with a pancreatic transection just lateral to the vertebral column. He underwent laparoscopic exploration followed by a laparoscopic distal pancreatectomy with preservation of the spleen. The remaining pancreas is noted with the asterisk and the staple line is marked with the arrow.
Diaphragmatic Injury
Blunt traumatic rupture of the diaphragm via massive compressive forces to the abdomen accounts for 80–90% of diaphragmatic injury in the pediatric population.73 This injury rarely occurs in isolation, but is often associated with multiple organ injury and a high index of severity scores.74 Abdominal contents may herniate into the thoracic cavity due to the pressure gradient between the pleura and peritoneal cavities. Right and left sided ruptures occur with equal frequency.75 Plain radiographs may suggest the diagnosis of traumatic diaphragm rupture via an obscured or elevated hemidiaphragm, gas in herniated viscus above the diaphragm (Fig. 16-8A), tip of nasogastric tube in the thorax, the presence of an atypical pneumothorax, and plate-like atelectasis adjacent to the diaphragm.73
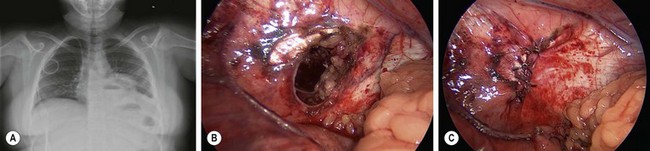
FIGURE 16-8 This teenager developed respiratory symptoms several weeks after a motor vehicle accident. (A) The chest radiograph shows air in either the stomach or the intestine in the left chest. (B) At laparoscopic exploration, the traumatic diaphragmatic hernia is seen after reduction of the stomach and several loops of small intestine. (C) The traumatic diaphragmatic hernia was repaired laparoscopically and the patient recovered uneventfully.
Emergent operative exploration in patients with diaphragm injury is indicated in the hemodynamically unstable patient with multiple organ injury. A thorough and systematic exploration of the entire abdomen and palpation of retroperitoneal structures is required due to the frequency of multiple organ injury. Repair of the diaphragmatic defect is typically possible after debridement of any compromised tissue. If large defects are found, a prosthetic patch may be needed to minimize the tension. Successful laparoscopic or thoracoscopic repair of diaphragmatic injuries can be performed in hemodynamically stable children and in cases with delayed diagnosis (Fig. 16-8B, C).76–78
Hollow Viscus Injury
Hollow viscus injury in children is typically caused by blunt trauma via crush injury from a focal or localized blow to the abdomen such as a bicycle handle bar, a punch to the abdomen, or from a seatbelt in an MVC. Distended hollow viscera are more prone to rupture with blunt trauma due to the increased intraluminal pressure.79 Areas of mesenteric fixation such as the proximal jejunum near the ligament of Trietz, the distal ileum near the ileocecal valve, and the rectosigmoid junction are particularly vulnerable to injury via acceleration/deceleration shearing forces. Seat belt signs may also be markers of severe deceleration injury to the abdomen with an associated intra-abdominal blunt hollow viscus injury.80 In this injury complex, the rapid deceleration from a high impact crash causes sudden flexion of the upper body around the fixed lap belt and consequent compression of the abdominal viscera between the lap belt and the spine. This injury is accentuated by using adult seat belts without booster seats in young children or using lap belts without shoulder straps. The use of age-appropriate child restraints in cars may prevent some of these injuries.81
Children with traumatic hollow viscus injury and perforation typically present with signs of peritoneal irritation due to the rapid contamination of the peritoneal cavity. In a neurologically intact patient with a perforated viscus, findings of abdominal tenderness, guarding, and rebound on initial and serial physical examinations are more specific for hollow viscus injury than abdominal ultrasound or CT findings for these injuries.82–84 Hemodynamically unstable patients with signs and symptoms of hollow viscus injury should undergo an emergent exploratory laparotomy. In stable patients, more time can be taken for evaluation and a CT scan of the abdomen and pelvis can be performed. CT findings suggestive of hollow viscus injury include bowel wall thickening and enhancement, mesenteric stranding, and free intraperitoneal fluid in the absence of solid organ injury.12 Despite currently available diagnostic studies, partial thickness lacerations, hematomas, or avulsions of mesenteric vessels may not initially appear significant, but can progress to full-thickness intestinal wall ischemia and perforation with leakage of intestinal content over hours to days. Some mesenteric injuries may result in an intestinal stricture or internal hernia diagnosed weeks after the accident. A recent multi-institutional retrospective review by the APSA Trauma Committee determined that delay in operative treatment up to 24 hours did not significantly affect outcome.85
In hemodynamically stable patients with evidence of bowel injury or in equivocal cases with concerning physical signs or symptoms, diagnostic laparoscopy is a very reasonable approach. In cases with penetrating trauma, local wound exploration to identify penetration of the anterior abdominal fascia is recommended as the initial diagnostic maneuver. If it is still unclear whether the peritoneum has been violated, then laparoscopy can be performed to determine whether there is penetration into the abdominal cavity and also to assist with the creation of a diverting stoma, if needed.32,86,87 Whether exploration is performed laparoscopically or via an open approach, a four quadrant inspection of the abdominal cavity with meticulous examination of both the intestinal tract and mesentery should be performed. The lesser sac should be opened to evaluate the posterior gastric wall, the pancreas, and diaphragm. Regardless of the approach, principles of management of hollow viscus injury include prompt resuscitation, complete removal of devitalized tissue, reconstruction or diversion of the intestinal tract, and perioperative antibiotic coverage.
Injury to the Stomach
Penetrating injury to the stomach is more common than blunt trauma, and results in a variable presentation of local tissue destruction. Despite its rarity, blunt injury to the stomach can occur and is typically seen in the patient who has just eaten, as the full stomach is more vulnerable to burst injury.88,89 When gastric rupture occurs, it is usually located along the greater curvature with a blow-out or stellate configuration. At exploration, the posterior wall of the stomach and the gastroesophageal junction should always be evaluated to avoid missed injuries.89 Debridement with repair of the injury is sufficient.
Duodenal Injuries
The majority of duodenal injuries in children result from blunt mechanisms.90,91 In younger patients, duodenal injury is often the result of nonaccidental trauma and isolated injury should raise suspicion if the history or mechanism is inconsistent with the injury.9,92 Due to its contiguous location to many other vital structures, associated injuries are common. Also, because of its location close to the vertebral column, blow-out injuries can occur.93 Diagnosis of a duodenal injury may be difficult due to the retroperitoneal nature of the duodenum, the poor sensitivity of plain radiographs, and the nonspecific nature of examination findings in these patients. Abdominal CT is the test of choice to evaluate for duodenal injury. Injuries to the duodenum as graded by the AAST range from hematomas involving only a portion of the duodenum (grade I) to devascularization of the duodenum or massive disruption of the duodenopancreatic complex (grade V) (Table 16-2).94
TABLE 16-2
Duodenal Injury Grading Scale from the AAST
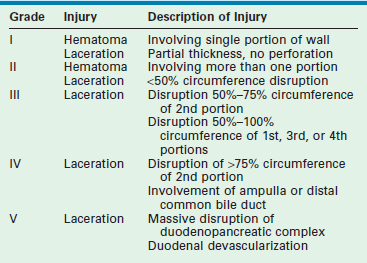
From Moore EE, Cogbill TH, Malangoni MA, et al. Organ injury scaling, II: Pancreas, duodenum, small bowel, colon, and rectum. J Trauma 1990;30:1427–9.
Duodenal hematomas may be found on CT or upper GI studies revealing transmural thickening with lumenal duodenal narrowing, or partial obstruction without evidence of extravasation of air or contrast (Fig. 16-9). They are typically managed nonoperatively with nasogastric decompression and TPN over one to three weeks.95 Operative evacuation of the hematoma may be required if obstructive signs and symptoms do not resolve. Evacuation of the hematoma may also be performed if the duodenal hematoma is found on laparotomy or laparoscopy for other injuries. CT scan findings of extravasation of air or contrast into the paraduodenal, pararenal, or retroperitoneal space is consistent with duodenal perforation.95 Early diagnosis can simplify management and minimize morbidity, but delay in diagnosis is not uncommon due to a delay in presentation or the paucity of findings on initial imaging.96–98 When high clinical suspicion for duodenal injury exists and initial radiographs and abdominal CT scans do not reveal significant injury, serial CT scans may be indicated to look for the delayed development of retroperitoneal air. Delay in diagnosis of greater than 24 hours is associated with established peritoneal inflammation, poor tissue integrity, and a higher leak rate following primary repair.91
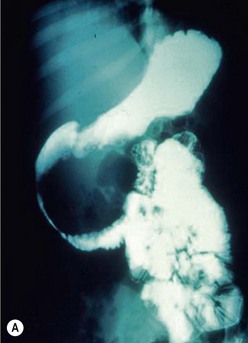
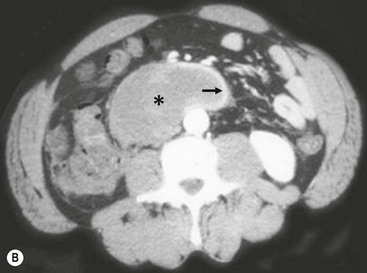
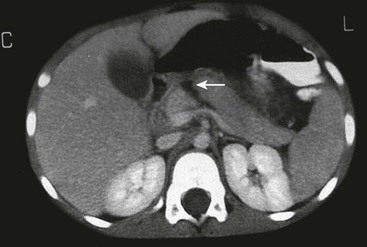
FIGURE 16-9 This patient developed emesis soon after a bicycle accident. The upper gastrointestinal contrast study (A) shows a very narrowed duodenum due to extrinsic compression from a large mass in either the pancreas or wall of the duodenum. The CT scan (B) in the same patient shows a very small rim of contrast material (arrow) and a very large intramural duodenal hematoma (asterisk).
Complications, such as fistula formation, are more common (2–14%) after repair of duodenal injuries than following operative repair for any other area of the gastrointestinal tract.91 Several operative techniques such as serosal patch repair, transverse primary repair, duodenal diverticularization, pyloric exclusion, and gastrojejunostomy have been applied to duodenal injuries to minimize potentially significant complications.93,95,98 Operative intervention for duodenal injuries should be made based on clinical judgment. Most full-thickness injuries with minimal tissue destruction not involving the drainage of the biliary or pancreatic ductal system can be repaired primarily.99 In patients with a complex duodenal injury, diversion and drainage may be needed (Fig. 16-10). In these cases, a duodenostomy tube and gastrostomy may be helpful for decompression. A feeding jejunostomy is recommended for early enteral nutrition, and drains should be placed near the repair. Earlier diagnosis of duodenal injuries may make the injury more amenable to primary repair while a significant delay in diagnosis (>24 hours), or those with a grade III or greater injury, may warrant proximal drainage via a gastrojejunostomy and pyloric exclusion.91,100
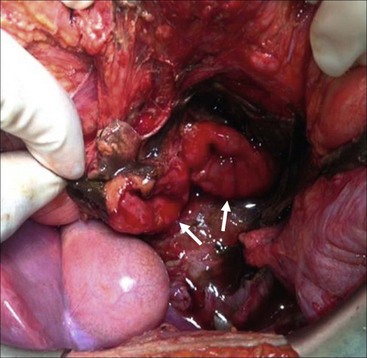
FIGURE 16-10 This patient was suspected of having a duodenal injury after a CT scan showed upper abdominal fluid and retroperitoneal air adjacent to the duodenum. At operation, this patient was found to have a complete duodenal transection following a handlebar injury. As the two segments of duodenum appeared viable (arrows), a primary repair was performed. The patient recovered uneventfully.
Injury to the Small Intestine and Colon
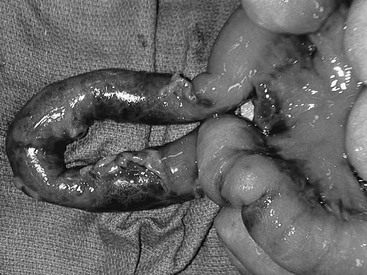
Injuries to the small intestine range from transmural hematomas, simple lacerations, complete transection, or mesenteric avulsions with segments of compromised bowel (Fig. 16-11). Even in cases of massive contamination, simple repair, debridement, or resection with primary anastomosis is usually appropriate.
Injury to the colon is infrequent but more often secondary to penetrating mechanisms than blunt injuries in pediatric patients.101 The infrequent nature of colonic injuries in children hinders the development of guidelines, and the management principles are extracted from the adult trauma experience. Historically, concerns over peritoneal contamination with colonic injury encouraged diversion to avoid anastomotic leaks and ongoing sepsis. Currently, primary repair of early diagnosed colonic injuries can be performed safely and is the preferred approach, even if there is peritoneal contamination.101,102 A Cochrane meta-analysis evaluating adult patients with colonic trauma summarizes outcomes with primary repair vs. diversion.102 This analysis significantly favored primary repair as the optimal treatment due to the reduction in morbidity and a decrease in procedure-related costs. In the setting of significant devitalizing colonic injury in a patient in shock, initial damage-control laparotomy is recommended with delayed colonic anastomosis at the time of abdominal wall closure. In this scenario, a higher complication rate has been found with delayed anastomosis if fascial closure occurs greater than 5 days after injury and in the case of a left colonic injury.103 A diverting colostomy rather than a delayed anastomosis should be performed at the time of abdominal wall closure in patients with recurrent intra-abdominal abscesses, severe bowel wall edema and inflammation, or persistent metabolic acidosis.104 A diverting colostomy may also be needed in patients with simultaneous extensive abdominal wall or perineal injury.
Injury to the Rectum
Pediatric anorectal injury is uncommon, but may occur through a variety of mechanisms, especially falls with straddle injuries, and sexual abuse.105 Accidental falls often cause injury to the perineal body, external genitalia, urethra and anus, but rectal injury is uncommon (Fig. 16-12). Impalement on fixed objects occurs, but is typically an isolated injury. Sexual abuse often causes isolated rectal or vaginal trauma, and should be considered as a possible etiology in patients with isolated perineal injuries.105 Blunt rectal injury secondary to MVC is often associated with multiple injuries, such as pelvic fractures and urinary tract injuries.106 Another notable mechanism in children occurs from the use of personal watercraft (jet skis, seadoos, and wave-runners).107–109 Passengers thrown from personal watercraft can experience significant hydrostatic force of water through the anal canal on landing, resulting in rectal injury and perforation. These hydrostatic rectal perforations, although rare, are occurring with increasing frequency, and are potentially devastating. In the USA, the National Transportation Safety Board has recommended wet suit bottoms for all children on personal watercraft as operators or passengers.109
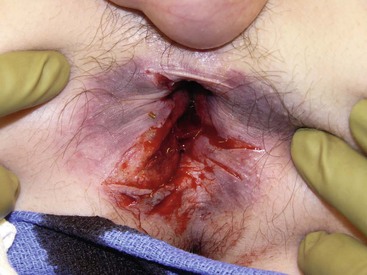
FIGURE 16-12 This teenager developed this full-thickness straddle rectal injury after falling off a trampoline. It was possible to close the injury over drains placed in the perirectal tissues. He has recovered uneventfully with full continence.
In the evaluation of rectal injury, digital rectal examination is unreliable.110 In the stable patient, abdominal and pelvic CT scans can be helpful.111 Diagnosis of the extent of injury often requires examination under anesthesia at which time, vaginoscopy, anoscopy, proctoscopy, and possible cystoscopy are performed as necessary.112 Laparoscopy may be needed to evaluate for intraperitoneal extension.106 Most vaginal, anal, and superficial perineal body injuries can be treated with primary repair. Historically, rectal trauma was managed with a diverting colostomy, drainage of the perineal wound, and rectal irrigation.113 Currently, selective diversion has been advocated for both pediatric and adult patients with good results.7,106
Gallbladder Injury
The gallbladder is rarely injured in children. However, associated injuries are common.114,115 Predisposing factors for gallbladder trauma are a thin-walled normal gallbladder, a distended gallbladder after a meal, and alcohol ingestion. If identified, a cholecystectomy is usually performed. This may be performed via laparoscopy or laparotomy.
Urinary Bladder
The bladder is the second most common genitourinary (GU) injury in children.116,117 Bladder injuries range from grade I contusions to grade V extraperitoneal or intraperitoneal ruptures involving the bladder neck or ureteral orifices.118 It is hypothesized that the bladder’s rostral location in relation to the pelvis increases the risk of injury in children.119 CT cystography is used to evaluate a suspected bladder injury. Prompt repair is required for intraperitoneal ruptures as incomplete drainage of intra-abdominal urine can lead to infection, peritonitis, and even death.120 Typically, a two layered closure with absorbable suture material is performed, and either transurethral or suprapubic drains are used for temporary decompression. Urethral catheter drainage is considered sufficient for uncomplicated extraperitoneal ruptures.120
Renal Trauma
With abdominal trauma in children, the kidney is injured in approximately 10% of patients, and is the most commonly injured GU organ.121 The susceptibility of children for major renal trauma compared to adults appears in part secondary to the fact that the kidney occupies a relatively larger amount of the retroperitoneal space, the thoracic cage is less well ossified, the abdominal musculature is weaker, and there is less cushioning from perirenal fat.122 Congenital renal anomalies such as hydronephrosis, tumors, or abnormal positions have been postulated to make the kidney more susceptible to trauma with relatively mild traumatic forces. However, recent studies do not support this concept.116 Congenital abnormalities are present in approximately 1–5% of renal injuries.
Blunt trauma accounts for 80–90% of renal injuries in children. The most common mechanisms are related to MVC, falls, bicycle, and all-terrain vehicle (ATV)-related injuries.123 In several series, the most severe grade of injury was related to dirt bikes, ATV rollovers, and bicycles.119,123 Most children who sustain renal injury in an MVC are unrestrained.124 ATV-related injuries suggest a unique injury mechanism such as a strike from the handlebars, collisions involving ejection, or ATV rollover. Increased awareness and use of proper safety equipment may contribute to decreased prevalence and severity, including abdominal or flank padding, to reduce blunt force and handlebar intrusion.119
Patients with renal trauma typically present with gross hematuria and flank pain. The diagnosis is confirmed by abdominal CT scan which is highly sensitive. Renal injuries have also been classified by the AAST (Table 16-3). This classification system has been useful in standardizing and validating treatment strategies.125 Management goals involve maximizing functional renal parenchyma while minimizing patient morbidity.126 Expectant NOM is widely accepted for hemodynamically stable grade I-III renal injuries which do not have urinary extravasation.127
TABLE 16-3
Renal Injury Grading Scale from the AAST
| Grade | Injury | Description of Injury |
| I | Contusion | Microscopic or gross hematuria, normal urologic studies |
| Hematoma | Subcapsular, nonexpanding without parenchymal laceration | |
| II | Hematoma | Nonexpanding perirenal hematoma confined to renal retroperitoneum |
| Laceration | <1.0 cm parenchymal depth of renal cortex without urinary extravasation | |
| III | Laceration | >1.0 cm parenchymal depth of renal cortex without collecting system rupture or urinary extravasation |
| IV | Laceration | Parenchymal laceration extending through renal cortex, medulla and collecting system |
| Vascular | Main renal artery or vein injury with contained hemorrhage | |
| V | Laceration | Completely shattered kidney |
| Vascular | Avulsion of renal hilum that devascularized kidney |
From Moore EE, Shackford SR, Pachter HL, et al. Organ injury scaling: spleen, liver, and kidney. J Trauma 1989;29:1664–6.
Treatment for children with high grade renal injury (grade IV and grade V) remains controversial (Fig. 16-13). Urinary extravasation and urinoma continue to be relative indications for exploration in some centers.128 Historically, patients with higher grade injury were also more likely to undergo endourologic interventions such as nephrostomies or ureteral stents.129,130 Most current pediatric series report successful nonoperative management for grade IV and V injuries.127 Endourologic interventions are reserved primarily for persistent extravasation or symptomatic urinomas rather than all injuries with disrupted collecting systems.127 Selective angioembolization of renal artery branches has been successful in nearly 80% of cases with delayed hemorrhage.131 Using individualized selective management, several studies have documented renal preservation in over 95% of children.127,132,133 The main indications for immediate exploration in a child with a renal injury are hemodynamic instability, penetrating mechanism, and associated nonrenal injuries.132
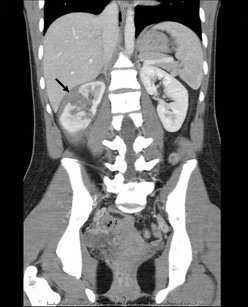
FIGURE 16-13 This child developed a grade III injury to the right kidney. There is a deep laceration through the central aspect of the kidney (arrow), but no evidence of urinary extravasation or development of a urinoma. This patient was managed nonoperatively and recovered uneventfully.
Stable patients with high grade injury are typically placed at bed rest with serial exams, blood counts, and close hemodynamic monitoring until the gross hematuria resolves. However, there are no evidence-based guidelines regarding length of activity restriction in these patients.134 A multi-institutional prospective study allowing for immediate ambulation and discharge based on standard criteria, rather than resolution of gross hematuria, is currently underway to address possible guidelines.
Penetrating Renal Injury
Penetrating renal injury in children is rare, but typically requires exploration for management. Selective observation for penetrating renal trauma, however, is also being applied and can be done safely.135,136 Hemodynamically unstable patients with penetrating injury or patients with an expanding retroperitoneal hematoma require renal exploration. During exploration, a one-shot IVP may be helpful to identify the injured area and confirm the presence of a functioning contralateral kidney.
Risk of Injury for Solitary Normal Kidney
It is not uncommon for patients with a single, normal kidney and their family to seek advice from physicians regarding the safety of participating in contact sports. As kidney injuries from sports are rare, the AAP recommends a ‘qualified yes’ for participation in contact or collision sports for young athletes with a single kidney.137 Still, many physicians are reluctant to give approval.138
A recent prospective study, analyzing more than 4.4 million high school ‘athlete exposures,’ discovered that sport-related renal injuries are very rare.139 Out of the 23,666 reported injuries in the study, only 18 involved a kidney, and none were catastrophic or required operation. This number of renal injuries is far fewer than injuries reported for other unpaired organs such as the head, neck, spine, or brain. Cycling, downhill skiing, and horseback riding (classically referred to as ‘limited contact sports’) are recreational activities that were found to have comparable or higher rates of renal injury in comparison with American football, yet are often not restricted by practitioners.140
Additionally, studies have shown that the risks of kidney injury from nonathletic pursuits are far more common than those from sport participation. MVCs alone account for two to ten times more kidney injury than sports.141 Likewise, ATV and motorcycle use has a much greater risk of serious renal injury compared with participation in contact sports.123
References
1. Gaines, BA. Intra-abdominal solid organ injury in children: Diagnosis and treatment. J Trauma. 2009; 67:S135–S139.
2. WISQARS. Centers for Disease Control and Prevention, 2008.
3. Powell, M, Courcoulas, A, Gardner, M, et al. Management of blunt splenic trauma: Significant differences between adults and children. Surgery. 1997; 122:654–660.
4. Trauma, AcoSCo, Advanced Trauma Life Support for Doctors, Student Course Manual Chicago. American College of Surgeons Committee on Trauma. 2004:251.
5. Isaacman, DJ, Scarfone, RJ, Kost, SI, et al. Utility of routine laboratory testing for detecting intra-abdominal injury in the pediatric trauma patient. Pediatrics. 1993; 92:691–694.
6. Capraro, AJ, Mooney, D, Waltzman, ML. The use of routine laboratory studies as screening tools in pediatric abdominal trauma. Pediatr Emerg Care. 2006; 22:480–484.
7. Haut, ER, Nance, ML, Keller, MS, et al. Management of penetrating colon and rectal injuries in the pediatric patient. Dis Colon Rectum. 2004; 47:1526–1532.
8. Holmes, JF, Sokolove, PE, Brant, WE, et al. Identification of children with intra-abdominal injuries after blunt trauma. Ann Emerg Med. 2002; 39:500–509.
9. Sokolove, PE, Kuppermann, N, Holmes, JF. Association between the ‘seat belt sign’ and intra-abdominal injury in children with blunt torso trauma. Acad Emerg Med. 2005; 12:808–813.
10. Holmes, JF, Sokolove, PE, Land, C, et al. Identification of intra-abdominal injuries in children hospitalized following blunt torso trauma. Acad Emerg Med. 1999; 6:799–806.
11. Cotton, BA, Beckert, BW, Smith, MK, et al. The utility of clinical and laboratory data for predicting intraabdominal injury among children. J Trauma. 2004; 56:1068–1074.
12. Flood, RG, Mooney, DP. Rate and prediction of traumatic injuries detected by abdominal computed tomography scan in intubated children. J Trauma. 2006; 61:340–345.
13. Streck, CJ, Jr., Jewett, BM, Wahlquist, AH, et al. Evaluation for intra-abdominal injury in children after blunt torso trauma: Can we reduce unnecessary abdominal computed tomography by utilizing a clinical prediction model? J. Trauma Acute Care Surg. 2012; 73:371–376.
14. Adamson, WT, Hebra, A, Thomas, PB, et al. Serum amylase and lipase alone are not cost-effective screening methods for pediatric pancreatic trauma. J Pediatr Surg. 2003; 38:354–357.
15. Lindberg, D, Makoroff, K, Harper, N, et al. Utility of hepatic transaminases to recognize abuse in children. Pediatrics. 2009; 124:509–516.
16. Hom, J. The risk of intra-abdominal injuries in pediatric patients with stable blunt abdominal trauma and negative abdominal computed tomography. Acad Emerg Med. 2010; 17:469–475.
17. Nastanski, F, Cohen, A, Lush, SP, et al. The role of oral contrast administration immediately prior to the computed tomographic evaluation of the blunt trauma victim. Injury. 2001; 32:545–549.
18. Brenner, DJ, Hall, EJ. Computed tomography–an increasing source of radiation exposure. N Engl J Med. 2007; 357:2277–2284.
19. Brenner, D, Elliston, C, Hall, E, et al. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol. 2001; 176:289–296.
20. Pearce, MS, Salotti, JA, Little, MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: A retrospective cohort study. Lancet. 2012; 380:499–505.
21. Image Gently. www.pedrad.org/associations/5364/1g.
22. Rice, HE, Frush, DP, Farmer, D, et al. Review of radiation risks from computed tomography: Essentials for the pediatric surgeon. J Pediatr Surg. 2007; 42:603–607.
23. Brody, AS, Frush, DP, Huda, W, et al. Radiation risk to children from computed tomography. Pediatrics. 2007; 120:677–682.
24. Benya, EC, Lim-Dunham, JE, Landrum, O, et al. Abdominal sonography in examination of children with blunt abdominal trauma. AJR Am J Roentgenol. 2000; 174:613–1616.
25. Coley, BD, Mutabagani, KH, Martin, LC, et al. Focused abdominal sonography for trauma (FAST) in children with blunt abdominal trauma. J Trauma. 2000; 48:902–906.
26. Emery, KH, McAneney, CM, Racadio, JM, et al. Absent peritoneal fluid on screening trauma ultrasonography in children: A prospective comparison with computed tomography. J Pediatr Surg. 2001; 36:565–569.
27. Holmes, JF, Gladman, A, Chang, CH. Performance of abdominal ultrasonography in pediatric blunt trauma patients: A meta-analysis. J Pediatr Surg. 2007; 42:1588–1594.
28. Fox, JC, Boysen, M, Gharahbaghian, L, et al. Test characteristics of focused assessment of sonography for trauma for clinically significant abdominal free fluid in pediatric blunt abdominal trauma. Acad Emerg Med. 2011; 18:477–482.
29. Cardamore, R, Nemeth, J, Meyers, C. Bedside emergency department ultrasonography availability and use for blunt abdominal trauma in Canadian pediatric centres. CJEM. 2012; 14:14–19.
30. Noble, VE, Blaivas, M, Blankenship, R, et al. Decision rule for imaging utilization in blunt abdominal trauma–where is ultrasound? Ann Emerg Med. 2010; 55:487–489.
31. Feliz, A, Shultz, B, McKenna, C, et al. Diagnostic and therapeutic laparoscopy in pediatric abdominal trauma. J Pediatr Surg. 2006; 41:72–77.
32. Marwan, A, Harmon, CM, Georgeson, KE, et al. Use of laparoscopy in the management of pediatric abdominal trauma. J Trauma. 2010; 69:761–764.
33. Gaines, BA, Ford, HR. Abdominal and pelvic trauma in children. Crit Care Med. 2002; 30:S416–S423.
34. Tinkoff, G, Esposito, TJ, Reed, J, et al. American Association for the Surgery of Trauma Organ Injury Scale I: spleen, liver, and kidney, validation based on the National Trauma Data Bank. J Am Coll Surg. 2008; 207:646–655.
35. Holmes, JH, 4th., Wiebe, DJ, Tataria, M, et al. The failure of nonoperative management in pediatric solid organ injury: A multi-institutional experience. J Trauma. 2005; 59:1309–1313.
36. Stylianos, S. Evidence-based guidelines for resource utilization in children with isolated spleen or liver injury. The APSA Trauma Committee. J Pediatr Surg. 2000; 35:164–169.
37. Stylianos, S. Compliance with evidence-based guidelines in children with isolated spleen or liver injury: A prospective study. J Pediatr Surg. 2002; 37:453–456.
38. St Peter, SD, Sharp, SW, Snyder, CL, et al. Prospective validation of an abbreviated bedrest protocol in the management of blunt spleen and liver injury in children. J Pediatr Surg. 2011; 46:173–177.
39. Dehmer, JJ, Adamson, WT. Massive transfusion and blood product use in the pediatric trauma patient. Semin Pediatr Surg. 2010; 19:286–291.
40. Jacobs, LM, Splenorrhaphy Woodbury, CTJacobs LM, ed. Advanced Trauma Operative Management. 2004:78–83.
41. Morgan, TL, Tomich, EB. Overwhelming post-splenectomy infection (OPSI): A case report and review of the literature. J Emerg Med. 2012; 43:758–763.
42. Peitzman, AB, Marsh, JW. Advanced operative techniques in the management of complex liver injury. J Trauma Acute Care Surg. 2012; 73:765–770.
43. Saggi, BH, Sugerman, HJ, Ivatury, RR, et al. Abdominal compartment syndrome. J Trauma. 1998; 45:597–609.
44. Ejike, JC, Newcombe, J, Baerg, J, et al. Understanding of abdominal compartment syndrome among pediatric healthcare providers. Crit Care Res Pract. 2010.
45. Vidal, MG, Ruiz Weisser, J, Gonzalez, F, et al. Incidence and clinical effects of intra-abdominal hypertension in critically ill patients. Crit Care Med. 2008; 36:1823–1831.
46. Beck, R, Halberthal, M, Zonis, Z, et al. Abdominal compartment syndrome in children. Pediatr Crit Care Med. 2001; 2:51–56.
47. Diaz, FJ, Fernandez Sein, A, Gotay, F. Identification and management of abdominal compartment syndrome in the pediatric intensive care unit. P R Health Sci J. 2006; 25:17–22.
48. Pearson, EG, Rollins, MD, Vogler, SA, et al. Decompressive laparotomy for abdominal compartment syndrome in children: Before it is too late. J Pediatr Surg. 2010; 45:1324–1329.
49. Cheatham, ML, Malbrain, ML, Kirkpatrick, A, et al. Results from the International Conference of Experts on intra-abdominal hypertension and abdominal compartment syndrome. II. Recommendations. Intensive Care Med. 2007; 33:951–962.
50. Sugrue, M, Bauman, A, Jones, F, et al. Clinical examination is an inaccurate predictor of intraabdominal pressure. World J Surg. 2002; 26:1428–1431.
51. Carr, JA. Abdominal compartment syndrome: A decade of progress. J Am Coll Surg. 2013; 216:135–146.
52. Gutierrez, IM, Gollin, G. Negative pressure wound therapy for children with an open abdomen. Langenbecks Arch Surg. 2012; 397:1353–1357.
53. Suliburk, JW, Ware, DN, Balogh, Z, et al. Vacuum-assisted wound closure achieves early fascial closure of open abdomens after severe trauma. J Trauma. 2003; 55:1155–1161.
54. Nwomeh, BC, Nadler, EP, Meza, MP, et al. Contrast extravasation predicts the need for operative intervention in children with blunt splenic trauma. J Trauma. 2004; 56:537–541.
55. Lutz, N, Mahboubi, S, Nance, ML, et al. The significance of contrast blush on computed tomography in children with splenic injuries. J Pediatr Surg. 2004; 39:491–494.
56. Davies, DA, Ein, SH, Pearl, R, et al. What is the significance of contrast ‘blush’ in pediatric blunt splenic trauma?. J Pediatr Surg. 2010; 45:916–920.
57. Puapong, D, Brown, CV, Katz, M, et al. Angiography and the pediatric trauma patient: A 10-year review. J Pediatr Surg. 2006; 41:1859–1863.
58. Kiankhooy, A, Sartorelli, KH, Vane, DW, et al. Angiographic embolization is safe and effective therapy for blunt abdominal solid organ injury in children. J Trauma. 2010; 68:526–531.
59. Graham, GP, Haan, JM. Splenic artery embolization in a 7-year-old with blunt traumatic splenic rupture. Am Surg. 2012; 78:E297–E298.
60. Yi, IK, Miao, FL, Wong, J, et al. Prophylactic embolization of hepatic artery pseudoaneurysm after blunt abdominal trauma in a child. J Pediatr Surg. 2010; 45:837–839.
61. Stylianos, S, Hicks, BA. Abdominal and Renal Trauma. Ashcraft’s Pediatric Surgery, 5th ed. Elsevier Inc.; 2010.
62. Giss, SR, Dobrilovic, N, Brown, RL, et al. Complications of nonoperative management of pediatric blunt hepatic injury: Diagnosis, management, and outcomes. J Trauma. 2006; 61:334–339.
63. Almaramhi, H, Al-Qahtani, AR. Traumatic pediatric bile duct injury: Nonoperative intervention as an alternative to surgical intervention. J Pediatr Surg. 2006; 41:943–945.
64. Ulitsky, A, Werlin, S, Dua, KS. Role of ERCP in the management of non-iatrogenic traumatic bile duct injuries in the pediatric population. Gastrointest Endosc. 2011; 73:823–827.
65. Wood, JH, Partrick, DA, Bruny, JL, et al. Operative vs nonoperative management of blunt pancreatic trauma in children. J Pediatr Surg. 2010; 45:401–406.
66. Herman, R, Guire, KE, Burd, RS, et al. Utility of amylase and lipase as predictors of grade of injury or outcomes in pediatric patients with pancreatic trauma. J Pediatr Surg. 2011; 46:923–926.
67. Rutkoski, JD, Segura, BJ, Kane, TD. Experience with totally laparoscopic distal pancreatectomy with splenic preservation for pediatric trauma–2 techniques. J Pediatr Surg. 2011; 46:588–593.
68. Iqbal, CW, Levy, SM, Tsao, K, et al. Laparoscopic versus open distal pancreatectomy in the management of traumatic pancreatic disruption. J Laparoendosc Adv Surg Tech A. 2012; 22:595–598.
69. Borkon, MJ, Morrow, SE, Koehler, EA, et al. Operative intervention for complete pancreatic transection in children sustaining blunt abdominal trauma: Revisiting an organ salvage technique. Am Surg. 2011; 77:612–620.
70. Ramesh, J, Bang, JY, Trevino, J, et al. Endoscopic ultrasound-guided drainage of pancreatic fluid collections in children. J Pediatr Gastroenterol Nutr. 2012.
71. de Blaauw, I, Winkelhorst, JT, Rieu, PN, et al. Pancreatic injury in children: Good outcome of nonoperative treatment. J Pediatr Surg. 2008; 43:1640–1643.
72. Paul, MD, Mooney, DP. The management of pancreatic injuries in children: Operate or observe. J Pediatr Surg. 2011; 46:1140–1143.
73. Simpson, J, Lobo, DN, Shah, AB, et al. Traumatic diaphragmatic rupture: Associated injuries and outcome. Ann R Coll Surg Engl. 2000; 82:97–100.
74. Koplewitz, BZ, Ramos, C, Manson, DE, et al. Traumatic diaphragmatic injuries in infants and children: Imaging findings. Pediatr Radiol. 2000; 30:471–479.
75. Ramos, CT, Koplewitz, BZ, Babyn, PS, et al. What have we learned about traumatic diaphragmatic hernias in children? J Pediatr Surg. 2000; 35:601–604.
76. Meyer, G, Hüttl, TP, Hatz, RA, et al. Laparoscopic repair of traumatic diaphragmatic hernias. Surg Endosc. 2000; 14:1010–1014.
77. Pitcher, G. Fiber-endoscopic thoracoscopy for diaphragmatic injury in children. Semin Pediatr Surg. 2001; 10:17–19.
78. Shehata, SM, Shabaan, BS. Diaphragmatic injuries in children after blunt abdominal trauma. J Pediatr Surg. 2006; 41:1727–1731.
79. Sharma, OP, Oswanski, MF, Kaminski, BP, et al. Clinical implications of the seat belt sign in blunt trauma. Am Surg. 2009; 75:822–827.
80. Chandler, CF, Lane, JS, Waxman, KS. Seatbelt sign following blunt trauma is associated with increased incidence of abdominal injury. Am Surg. 1997; 63:885–888.
81. Nance, ML, Lutz, N, Arbogast, KB, et al. Optimal restraint reduces the risk of abdominal injury in children involved in motor vehicle crashes. Ann Surg. 2004; 239:127–131.
82. Ciftci, AO, Tanyel, FC, Salman, AB, et al. Gastrointestinal tract perforation due to blunt abdominal trauma. Pediatr Surg Int. 1998; 13:259–264.
83. Moss, RL, Musemeche, CA. Clinical judgment is superior to diagnostic tests in the management of pediatric small bowel injury. J Pediatr Surg. 1996; 31:1178–1182.
84. Jerby, BL, Attorri, RJ, Morton, D, Jr. Blunt intestinal injury in children: The role of the physical examination. J Pediatr Surg. 1997; 32:580–584.
85. Letton, RW, Worrell, V. Delay in diagnosis and treatment of blunt intestinal injury does not adversely affect prognosis in the pediatric trauma patient. APSA Committee on Trauma Blunt Intestinal Injury Study Group. J Pediatr Surg. 2010; 45:161–166.
86. Gaines, BA, Rutkoski, JD. The role of laparoscopy in pediatric trauma. Semin Pediatr Surg. 2010; 19:300–303.
87. Garg, N, St Peter, SD, Tsao, K, et al. Minimally invasive management of thoracoabdominal penetrating trauma in a child. J Trauma. 2006; 61:211–212.
88. Tejerina Alvarez, EE, Holanda, MS, López-Espadas, F, et al. Gastric rupture from blunt abdominal trauma. Injury. 2004; 35:228–231.
89. Begossi, G, Danielson, PD, Hirsh, MP. Transection of the stomach after blunt injury in the pediatric population. J Pediatr Surg. 2007; 42:1604–1607.
90. Asensio, JA, Feliciano, DV, Britt, LD, et al. Management of duodenal injuries. Curr Probl Surg. 1993; 30:1023–1093.
91. Ladd, AP, West, KW, Rouse, TM, et al. Surgical management of duodenal injuries in children. Surgery. 2002; 132:748–753.
92. Gaines, BA, Shultz, BS, Morrison, K, et al. Duodenal injuries in children: Beware of child abuse. J Pediatr Surg. 2004; 39:600–602.
93. Vaughan, GD, 3rd., Frazier, OH, Graham, DY, et al. The use of pyloric exclusion in the management of severe duodenal injuries. Am J Surg. 1977; 134:785–790.
94. Moore, EE, Cogbill, TH, Malangoni, MA, et al. Organ injury scaling, II: Pancreas, duodenum, small bowel, colon, and rectum. J Trauma. 1990; 30:1427–1429.
95. Shilyansky, J, Pearl, RH, Kreller, M, et al. Diagnosis and management of duodenal injuries in children. J Pediatr Surg. 1997; 32:880–886.
96. Fang, JF, Chen, RJ, Lin, BC. Surgical treatment and outcome after delayed diagnosis of blunt duodenal injury. Eur J Surg. 1999; 165:133–139.
97. Cone, JB, Eidt, JF. Delayed diagnosis of duodenal rupture. Am J Surg. 1994; 168:676–679.
98. Cogbill, TH, Moore, EE, Feliciano, DV, et al. Conservative management of duodenal trauma: A multicenter perspective. J Trauma. 1990; 30:1469–1475.
99. Clendenon, JN, Meyers, RL, Nance, ML, et al. Management of duodenal injuries in children. J Pediatr Surg. 2004; 39:964–968.
100. Pokorny, WJ, Brandt, ML, Harberg, FJ. Major duodenal injuries in children: Diagnosis, operative management, and outcome. J Pediatr Surg. 1986; 21:613–616.
101. Dokucu, A, Oztürk, H, Ya mur, Y, et al. Colon injuries in children. J Pediatr Surg. 2000; 35:1799–1804.
mur, Y, et al. Colon injuries in children. J Pediatr Surg. 2000; 35:1799–1804.
102. Nelson, R, Singer, M. Primary repair for penetrating colon injuries. Cochrane Database Syst Rev. 2003.
103. Weinberg, JA, Griffin, RL, Vandromme, MJ, et al. Management of colon wounds in the setting of damage control laparotomy: A cautionary tale. J Trauma. 2009; 67:929–935.
104. Ordoñez, CA, Pino, LF, Badiel, M, et al. Safety of performing a delayed anastomosis during damage control laparotomy in patients with destructive colon injuries. J Trauma. 2011; 71:1512–1518.
105. Kadish, HA, Schunk, JE, Britton, H. Pediatric male rectal and genital trauma: Accidental and nonaccidental injuries. Pediatr Emerg Care. 1998; 14:95–98.
106. Bonnard, A, Zamakhshary, M, Wales, PW. Outcomes and management of rectal injuries in children. Pediatr Surg Int. 2007; 23:1071–1076.
107. Rubin, LE, Stein, PB, DiScala, C, et al. Pediatric trauma caused by personal watercraft: A ten-year retrospective. J Pediatr Surg. 2003; 38:1525–1529.
108. Kapur, SS, Frei, LW. Colorectal and vaginal injuries in personal watercraft passengers. J Trauma. 2007; 63:1161–1164.
109. Gill, RS, Mangat, H, Al-Adra, DP, et al. Hydrostatic rectosigmoid perforation: A rare personal watercraft injury. J Pediatr Surg. 2011; 46:402–404.
110. Esposito, TJ, Ingraham, A, Luchette, FA, et al. Reasons to omit digital rectal exam in trauma patients: No fingers, no rectum, no useful additional information. J Trauma. 2005; 59:1314–1319.
111. Leaphart, CL, Danko, M, Cassidy, L, et al. An analysis of proctoscopy vs computed tomography scanning in the diagnosis of rectal injuries in children: Which is better? J Pediatr Surg. 2006; 41:700–703.
112. Reinberg, O, Yazbeck, S. Major perineal trauma in children. J Pediatr Surg. 1989; 24:982–984.
113. Tuggle, D, Huber, PJ, Jr. Management of rectal trauma. Am J Surg. 1984; 148:806–808.
114. Sharma, O. Blunt gallbladder injuries: Presentation of twenty-two cases with review of the literature. J Trauma. 1995; 39:576–580.
115. Jaggard, MK, Johal, NS, Choudhry, M. Blunt abdominal trauma resulting in gallbladder injury: A review with emphasis on pediatrics. J Trauma. 2011; 70:1005–1010.
116. McAleer, IM, Kaplan, GW, Scherz, HC, et al. Genitourinary trauma in the pediatric patient. Urology. 1993; 42:563–568.
117. Deibert, CM, Spencer, BA. The association between operative repair of bladder injury and improved survival: Results from the National Trauma Data Bank. J Urol. 2011; 186:151–155.
118. Moore, EE, Cogbill, TH, Jurkovich, GJ, et al. Organ injury scaling. III: Chest wall, abdominal vascular, ureter, bladder, and urethra. J Trauma. 1992; 33:337–339.
119. Kluemper, C, Rogers, A, Fallat, M, et al. Genitourinary injuries in pediatric all-terrain vehicle trauma–a mechanistic relationship? Urology. 2010; 75:1162–1164.
120. Gomez, RG, Ceballos, L, Coburn, M, et al. Consensus statement on bladder injuries. BJU Int. 2004; 94:27–32.
121. Peclet, MH, Newman, KD, Eichelberger, MR, et al. Patterns of injury in children. J Pediatr Surg. 1990; 25:85–91.
122. Brown, SL, Elder, JS, Spirnak, JP. Are pediatric patients more susceptible to major renal injury from blunt trauma? A comparative study. J Urol. 1998; 160:138–140.
123. Wu, HY, Gaines, BA. Dirt bikes and all-terrain vehicles: The real threat to pediatric kidneys. J Urol. 2007; 178:1672–1674.
124. McAleer, IM, Kaplan, GW. Pediatric genitourinary trauma. Urol Clin North Am. 1995; 22:177–188.
125. Moore, EE, Shackford, SR, Pachter, HL, et al. Organ injury scaling: spleen, liver, and kidney. J Trauma. 1989; 29:1664–1666.
126. Santucci, RA, Fisher, MB. The literature increasingly supports expectant (conservative) management of renal trauma–a systematic review. J Trauma. 2005; 59:493–503.
127. Umbreit, EC, Routh, JC, Husmann, DA. Nonoperative management of nonvascular grade IV blunt renal trauma in children: Meta-analysis and systematic review. Urology. 2009; 74:579–582.
128. Wessel, LM, Scholz, S, Jester, I, et al. Management of kidney injuries in children with blunt abdominal trauma. J Pediatr Surg. 2000; 35:1326–1330.
129. Russell, RS, Gomelsky, A, McMahon, DR, et al. Management of grade IV renal injury in children. J Urol. 2001; 166:1049–1050.
130. Philpott, JM, Nance, ML, Carr, MC, et al. Ureteral stenting in the management of urinoma after severe blunt renal trauma in children. J Pediatr Surg. 2003; 38:1096–1098.
131. Goffette, PP, Laterre, PF. Traumatic injuries: Imaging and intervention in post-traumatic complications (delayed intervention). Eur Radiol. 2002; 12:994–1021.
132. Nerli, RB, Metgud, T, Patil, S, et al. Severe renal injuries in children following blunt abdominal trauma: Selective management and outcome. Pediatr Surg Int. 2011; 27:1213–1216.
133. Broghammer, JA, Fisher, MB, Santucci, RA. Conservative management of renal trauma: A review. Urology. 2007; 70:623–629.
134. Aguayo, P, Fraser, JD, Sharp, S, et al. Nonoperative management of blunt renal injury: A need for further study. J Pediatr Surg. 2010; 45:1311–1314.
135. Navsaria, PH, Nicol, AJ. Selective nonoperative management of kidney gunshot injuries. World J Surg. 2009; 33:553–557.
136. Wessells, H, McAninch, JW, Meyer, A, et al. Criteria for nonoperative treatment of significant penetrating renal lacerations. J Urol. 1997; 157:24–27.
137. Rice, SG. Medical conditions affecting sports participation. Pediatrics. 2008; 121:841–848.
138. Grinsell, MM, Showalter, S, Gordon, KA, et al. Single kidney and sports participation: Perception versus reality. Pediatrics. 2006; 118:1019–1027.
139. Grinsell, MM, Butz, K, Gurka, MJ, et al. Sport-related kidney injury among high school athletes. Pediatrics. 2012; 130:e40–e45.
140. Psooy, K. Sports and the solitary kidney: How to counsel parents. Can J Urol. 2006; 13:3120–3126.
141. Johnson, B, Christensen, C, Dirusso, S, et al. A need for reevaluation of sports participation recommendations for children with a solitary kidney. J Urol. 2005; 174:686–689.