CHAPTER 26 Abdominal Abscesses and Gastrointestinal Fistulas
ABDOMINAL ABSCESSES
PATHOPHYSIOLOGY
The development of an intra-abdominal abscess (IAA) occurs as a result of a host response to intra-abdominal bacterial contamination secondary to, or in conjunction with, various pathologic clinical entities. In 60% to 80% of cases, intra-abdominal abscess formation is associated with perforated hollow viscera, whether secondary to inflammatory disease such as appendicitis or diverticulitis or as a consequence of penetrating or blunt trauma to the abdomen.1–12 Other conditions associated with IAA formation include inflammatory bowel disease and complications of elective surgery (Table 26-1). Abscesses associated with solid organs such as the pancreas or liver are discussed in Chapters 58, 61, and 82.
Table 26-1 Causes of Intra-abdominal Abscesses
Clinical risk factors for the development of an IAA fall into two general categories: (1) factors related to the intra-abdominal source of infection found at the time of surgery for peritonitis and that can be considered local factors (see Chapter 37); and (2) factors that may have been present prior to surgery (e.g., preexisting comorbidities) or are related to generalized care of the patient during surgery, which can be considered systemic factors. Table 26-2 lists the local and systemic factors associated with increased risk of abscess formation postoperatively.
Table 26-2 Clinical Risk Factors for Intra-abdominal Abscess Formation
| Systemic Factors |
There is a delicate balance of opposing forces in the peritoneal cavity between bacterial factors and the host’s defense mechanisms, which attempt to clear bacterial contamination and localize infection (Table 26-3). These two opposing forces are often influenced by the presence of adjuvant factors (e.g., foreign material, fibrin) in the peritoneal cavity that often tip the balance toward bacterial infection with abscess formation (see Chapter 37).
Table 26-3 Factors Influencing Transition from Bacterial Contamination to Infection
| BACTERIAL FACTORS | ADJUVANT FACTORS | HOST DEFENSE FACTORS |
|---|---|---|
Adapted from Fartham EH, Schoffel U. Epidemiology and pathophysiology of intra-abdominal infections (IAI). Infection 1998; 26:329-34.
Once bacteria gain access to the peritoneal cavity through perforation of the intestinal wall, several factors come into play that determine whether an active infection is initiated. The typical bacteria that make up intra-abdominal infections have the ability to adhere to peritoneal surfaces and selectively grow and use host nutrients. These bacteria can undergo metabolic processes that are adapted to the host environment (e.g., obligate anaerobic metabolism). Furthermore, these bacteria have the capacity to resist antibiotic attack. Bacterial synergy plays an important role in the development of intra-abdominal infection (see later, “Bacteriology”).6
The peritoneum uses a number of host defenses to combat bacterial contamination.6,13 The balance of host defense factors in the setting of adjuvant factors determines whether contamination continues on to infection. Lymphatic clearance of bacteria is a major defense process that is so efficient that abscess formation occurs only when adjuvant substances such as hemoglobin, barium, or necrotic tissue are present.14 These adjuvant substances may block lymphatics (barium, fecal particulate matter), provide bacterial nutrients (iron from hemoglobin), or impair bacterial killing. Shortly after bacterial contamination, peritoneal macrophages are the predominant phagocytic cells. These cells are also cleared by the lymphatic system. As bacteria proliferate, polymorphonuclear leukocytes invade and become more numerous. The resultant peritoneal inflammation leads to an increase in splanchnic blood flow, with protein and fluid exudation into the peritoneal cavity. Procoagulatory effects of the inflammatory process and reduced levels of plasminogen activator activity enhance fibrin deposition and lead to entrapment of bacteria and localization of infection.13
These peritoneal defense mechanisms can have adverse effects. Lymphatic clearance of bacteria may be so brisk and effective that it results in a systemic response to bacteremia and sepsis. The exudation of fluid into the peritoneal cavity can lead to hypovolemia and shock; it can also dilute the opsonins needed in phagocytosis. Fibrin entrapment of bacteria can impair antimicrobial penetration and phagocytic migration with the potential to localize infection and lead to abscess formation.13 However, attempts to alter this balance of defense mechanisms are still not fully understood. In a study using a rodent intraperitoneal abscess model, recombinant tissue plasminogen activator (rt-PA) was used to increase intra-abdominal fibrinolytic activity. Rats treated with rt-PA had significantly fewer abscesses than controls, but they had significantly more bacteremic episodes and higher mortality rates.15 Further work in this area by the same group used similar rodent models of intraperitoneal infection to study the role of a hyaluronic acid solution in abdominal adhesion and abscess formation. In bacterial peritonitis, intraperitoneal hyaluronic acid solution in the presence of antibiotics reduced the development of adhesions and abscess formation without increasing mortality.16 Possible mechanisms of action include mechanical separation of wound surfaces, improvement of peritoneal healing, modulation of the inflammatory response, and enhanced fibrinolysis.17 The potential of hyaluronate-based agents to reduce intra-abdominal adhesions and abscesses in abdominal surgery and sepsis is a promising new concept.
Studies have suggested that the formation of adhesions is a complicated process that is not only dependent on surface apposition but is also under the tight control of positive and negative T cell costimulation.18 This was exemplified by a clinical trial19 of more than 1700 patients undergoing abdominopelvic surgery of the intestine, most for complications of inflammatory bowel disease. Patients were randomized to an adhesion barrier (Seprafilm [modified sodium hyaluronic acid and carboxymethyl cellulose], Genzyme, Cambridge, Mass) or control (no intervention) placed at the time of abdominal closing. Although abdominal and pelvic abscess rates were not different between the groups, there was a statistically higher rate of postoperative fistula formation and peritonitis in the Seprafilm group. This was particularly noted in patients who had the adhesion barrier wrapped around fresh intestinal anastomoses. Extensive experience has now accumulated with the use of Seprafilm. A recent meta-analysis20 of eight studies and over 4000 patients has shown that Seprafilm clearly decreases the severity and extent of postoperative adhesions. However, this came at the cost of increased rates of abdominal abscesses and anastomotic leaks. The effect was enhanced in the setting of surgery for inflammatory bowel disease. Newer preparations of hyaluronate-based membranes have also been associated with increased formation of postoperative intra-abdominal abscesses.21 Thus, the evidence for Seprafilm increasing postoperative abscess formation after surgery for acute peritonitis appears to be growing. The use of Seprafilm in this clinical situation to reduce postoperative abscess formation cannot be recommended.
BACTERIOLOGY
The typical abscess that forms as a complication of secondary bacterial peritonitis, defined as loss of integrity of the gastrointestinal (GI) tract, is a mixed aerobic and anaerobic infection. In studies of isolates from subphrenic,22 retroperitoneal,23 and diverticular abscesses,24 a range of 2.9 to 3.7 bacterial isolates per abscess was recovered. The most common aerobes were Escherichia coli and Enterococcus species (range, 1.3 to 1.6 isolates per specimen). The most common anaerobes were Bacteroides fragilis and Peptostreptococcus species, which accounted for 50% to 75% of all anaerobes isolated. Other Bacteroides species and Clostridium species made up the remainder of anaerobes isolated (range, 1.7 to 2.1 isolates per specimen). In all three studies, most abscesses contained mixed aerobic and anaerobic flora (60% to 75%); the minority contained aerobic isolates only (10% to 20%) or anaerobic isolates only (15% to 20%). The number of anaerobic isolates always was higher than the number of aerobic isolates.
Bacteroides species are important microbes in the formation of IAA. The existence of specific repeating negatively and positively charged cell wall polysaccharides on B. fragilis leads to a host response that results in the formation of an IAA. This host response is T cell–mediated and abscess formation can be experimentally prevented by vaccination with these repeating polysaccharide units. This vaccination does not appear to be antigen-specific in the traditional sense. Rather, the protective ability of these polysaccharides is conferred by, and perhaps specific for, a motif of oppositely charged groups. Vaccination with B. fragilis capsular polysaccharide complex significantly reduced the mortality rate and intra-abdominal abscess formation in a rat cecal ligation and puncture model.25 The cellular mechanism of IAA formation by B. fragilis has been elucidated.25 B. fragilis capsular polysaccharide complex adheres to peritoneal mesothelial cells and interacts with T cells and peritoneal macrophages to produce proinflammatory cytokines and chemokines, with subsequent expression of intercellular adhesion molecule-1 (ICAM-1) on host cells and recruitment of polymorphonuclear leukocytes to the abdominal cavity. Thus, the role of the capsular polysaccharide complex is to promote adhesion of B. fragilis to the peritoneal wall and coordinate the cellular events leading to the development of abscesses.
The bacteria associated with intra-abdominal infections and abscesses in patients in the ICU who have been subjected to broad-spectrum antimicrobial selection pressure are different from those in patients with abscesses that result from secondary bacterial peritonitis. Thus, the microbiologic agents that cause tertiary peritonitis, defined as persistent intra-abdominal sepsis with or without a discrete focus of infection, generally after an operation for secondary peritonitis, are no longer E. coli and B. fragilis (see Chapter 37). Rather, nosocomial infections with resistant gram-negative organisms, Enterococcus species, and/or yeast are more common.26,27 The microbiologic analysis of abscesses in severely ill patients (Acute Physiology and Chronic Health Evaluation [APACHE] II score > 15) revealed that 38% had monomicrobial infections. The most common organisms were Candida (41%), Enterococcus (31%), and Enterobacter (21%) spp. and Staphylococcus epidermidis (21%); E. coli and Bacteroides spp. accounted for only 17% and 7%, respectively.28
DIAGNOSIS AND TREATMENT
The optimal management of the patient with an IAA includes the following: (1) accurate diagnosis and localization of the collection; (2) removal or control of the source of peritoneal contamination; (3) drainage of any established collections; (4) elimination of residual contamination of the peritoneum through antimicrobial therapy; and (5) physiologic support of the patient.13 The symptoms and signs of IAA are nonspecific, and a high level of vigilance is needed to make the diagnosis. Fever and elevated leukocyte count are frequent but nonspecific findings. Abdominal pain, tenderness to palpation, distention, and a palpable mass are also common findings. Suspicion of the presence of an IAA warrants further diagnostic imaging.
Diagnostic Imaging
Computed Tomography
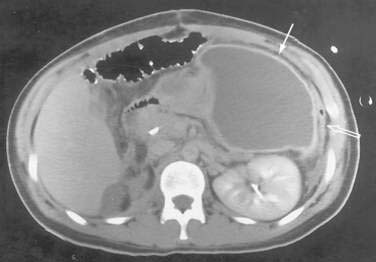
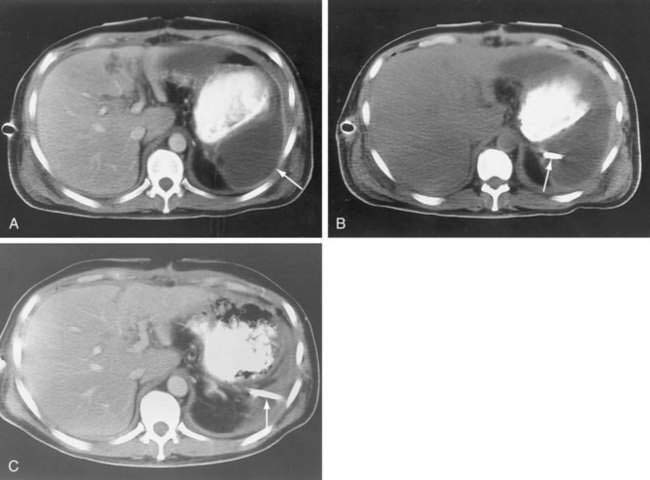
The CT diagnosis of abdominal abscess is suggested by identification of a loculated fluid density in an extraluminal location. Extraluminal gas within an abdominal mass is highly suggestive of an abscess, although necrotic tumors and resolving hematomas may occasionally exhibit this finding. Wall enhancement and adjacent inflammation favor the likelihood of infection in fluid collections (Fig. 26-1). Any fluid collection on CT should be clearly differentiated from nonopacified bowel. Delayed images are often necessary to allow bowel to opacify fully and to allow the investigator to distinguish an abscess from bowel confidently. The fluid in an abscess may occasionally be higher in density when proteinaceous material is present or when the collection represents an infected hematoma. Phlegmonous inflammatory tissue does not exhibit fluid density; rather, it is solid in appearance, often with inhomogeneous enhancement.
In some cases, the CT appearance can suggest the cause of the abscess. Periappendiceal abscesses commonly have a characteristic location in the right lower quadrant adjacent to the cecum and may demonstrate an appendicolith (Fig. 26-2). Peridiverticular abscesses are often associated with an inflamed adjacent colon demonstrating diverticula (Fig. 26-3). Abscesses associated with Crohn’s disease may demonstrate adjacent thickened small bowel.
Ultrasonography
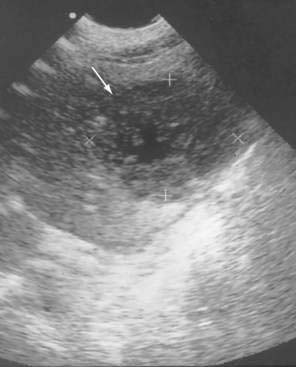
The classic ultrasonographic appearance of an abscess is a localized rounded or oval area of decreased echogenicity with internal debris and a thick irregular wall (Fig. 26-4). Most abscesses exhibit fluid characteristics on ultrasonography, but some may appear solid as a result of thick debris. Internal septations may be seen and are better identified by ultrasound than by CT. Gas within an abscess is suggested when areas of increased echogenicity are present, with posterior shadowing. The shadowing behind a gas collection tends to be less distinct than the more defined shadowing identified behind calculi on ultrasound. There is considerable overlap of the ultrasonographic appearance of infected and sterile fluid collections, and diagnostic aspiration is necessary to differentiate them. Ultrasound can be used for guidance during some percutaneous drainage procedures; however, poor visualization of intervening structures such as bowel in the midabdomen may limit its usefulness in some anatomic areas. Superficial and large abscesses tend to be more amenable to ultrasound guidance than smaller and deeper abscesses.
Mode of Drainage
Once an IAA is diagnosed and localized, a decision must be made regarding the optimal drainage technique and route. Percutaneous abscess drainage (PAD) has been shown to have equivalent success rates and less risk compared with surgery,29 although no randomized prospective studies are available. Assuming the availability of a safe route to the abscess, as occurs in 85% to 90% of cases, PAD should be the drainage procedure of choice.30–36 In the past, multiloculated, poorly organized, and multiple abscesses were not considered good candidates for PAD because of higher failure rates in these cases. Indications for PAD have now broadened to include these more challenging circumstances in many institutions, although longer drainage duration and multiple interventions may be necessary to obtain success.30–37 Although abscesses associated with enteric fistulae have lower success rates with PAD, successful PAD can be achieved in many cases.31,38 Rates of spontaneous closure up to 57% have been reported when aggressive catheter management has been combined with nutritional support.39 Interloop or intramesenteric collections are often not accessible percutaneously and surgery is often necessary. PAD is not appropriate for uncontained perforations or diffuse peritonitis.31,32,35 If surgery is chosen as the drainage mode of IAA (see later), an extraperitoneal approach is desirable to prevent contamination of the entire abdominal cavity.40 When feasible, some pancreatic abscesses or walled-off pancreatic necrosis (WOPN) can be drained endoscopically (see Chapter 61).
Percutaneous Abscess Drainage
Continuing advances in diagnostic imaging and percutaneous catheter development have allowed PAD of abdominal abscesses combined with systemic antibiotic therapy to become the standard initial treatment of abdominal abscesses.30–36,41,42 Success rates for PAD range from 70% to 93%.29,31,33,36–38,42 Most abdominal and pelvic abscesses can be safely accessed percutaneously. A safe route into the abscess should be chosen that avoids major vascular structures, bowel, and adjacent organs. In extreme circumstances, the liver and the stomach can be traversed.35 Small and large bowel should not be traversed with a catheter, making interloop abscesses often inaccessible percutaneously. With increasing experience of interventional radiologists, indications for PAD have expanded to include multiple abscesses, multiloculated abscesses, poorly defined collections, and more challenging access routes.30–36,38,43 Adjunctive thrombolytics can be used safely to increase success in septated abscesses or when thick debris is encountered.44
An inflammatory phlegmon without demonstrable fluid collection is not appropriate for percutaneous drainage. Some small fluid collections, typically less than 3 cm, also may not require a catheter and can be managed through percutaneous aspiration for diagnosis, followed by antibiotic therapy. Catheter management is generally preferred for larger collections in most institutions; however, one-step percutaneous needle aspiration of abdominal and pelvic abscesses combined with systemic antibiotics has also been advocated as an alternative to catheter placement in larger abscesses.45 Contraindications to PAD include lack of a safe access route and uncorrectable coagulopathy.32 Coagulation studies and correction of any coagulopathy are recommended before the procedure to reduce the risk of uncontrollable hemorrhage.
Guidance for PAD can be accomplished with a variety of imaging modalities including CT, ultrasonography, and fluoroscopy, or a combination of modalities. The imaging modality selected is dependent on the location and size of the abscess as well as operator preference. The most common imaging modality is CT because of its widespread use for the initial diagnosis of abdominal abscess and its superb visualization of bowel and vascular anatomy. Ultrasonography can provide more real-time visualization during catheter insertion and can be useful when extreme angling of the route is needed.34,35
After a safe percutaneous route is identified, the cavity is accessed using a trocar method or a needle and guidewire method. The tract is then dilated to a diameter approximating that of the planned catheter, and the catheter is advanced into the cavity. A sump-type double-lumen catheter is the most common catheter used. A 12- or 14-Fr catheter size is generally adequate to drain most abscesses, although a larger catheter size may be necessary for an abscess associated with a large amount of debris or hemorrhage.38
Postdrainage Management
PAD endpoints and decisions to obtain follow-up imaging studies depend on the clinical response, catheter drainage, and presence of suspected enteric communications. If the clinical response has been satisfactory and the catheter drainage has diminished to less than 20 mL/day, the catheter can be safely removed. If clinical response is inadequate, repeat imaging is warranted. Persistently high catheter output raises suspicion of a fistula. A catheter study performed by instilling water-soluble contrast medium through the catheter under fluoroscopy is the best method to assess for an internal fistula as the cause of high drainage output. If a fistula is located, the catheter can be repositioned adjacent to the opening into the bowel for better control of bowel effluent. Poor clinical response can also be caused by catheter dislodgment from the major abscess cavity, undrained loculations, multiple abscesses, or new abscesses. Repeat CT can evaluate for these possible causes of poor clinical response and guide additional percutaneous interventions when appropriate. Thick debris may occlude the catheter and inhibit daily flushing. In this case, the catheter can be exchanged for a larger catheter.41,46
Complications of Percutaneous Abscess Drainage
The complication rate of PAD ranges from 4% to 15%.29,38,41,47 Complications include transient sepsis, organ injury, hemorrhage, pneumothorax, peritonitis, empyema, and pain.
Recurrent Abscesses
Intra-abdominal abscess recurrence rates range from 1% to 9%.29,31,41,48 Even when an abscess recurs, repeat secondary PAD should be considered and can be curative. Success rates for secondary PAD up to 91% have been achieved in recurrent abscesses, although the mean duration of drainage to achieve success was significantly longer with the secondary procedure.31
Drainage of Specific Abscesses
Subphrenic Abscesses
Subphrenic abscesses can be drained percutaneously, with careful attention to technique (Fig. 26-5). Avoidance of the pleural space is optimal to prevent pneumothorax and seeding of infection to the chest. The pleural space typically extends to the level of the eighth thoracic vertebra (T8) anteriorly, T10 laterally, and T12 posteriorly. These guidelines can be used to prevent traversing of the pleural space. Some subphrenic fluid collections may not allow an extrapleural approach, in which case surgical risks should be weighed against the increased risk of empyema and pneumothorax posed by transpleural PAD. The safety of a transpleural approach has been debated.48,49
Pelvic Abscesses
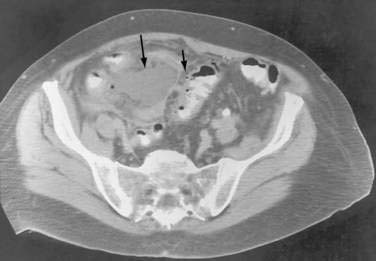
Anterior access to pelvic abscesses can be limited by intervening bowel, bladder, uterus, or vascular structures. A posterior transgluteal approach through the sciatic notch50 with the patient in the prone position has been used to drain deep pelvic fluid collections that are not accessible to an anterior approach (Fig. 26-6). Care must be taken to avoid the gluteal vasculature and the sciatic nerve. Ultrasound-guided transvaginal and transrectal drainage techniques have also been increasingly used for drainage of deep pelvic abscesses that are not accessible through other routes.51,52 A comparison of transrectal and transvaginal techniques has demonstrated better patient tolerance of the transrectal drainage route.53
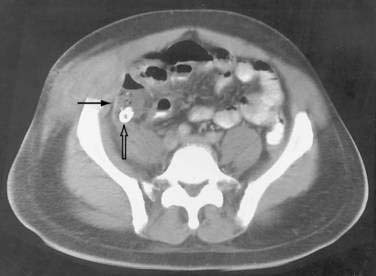
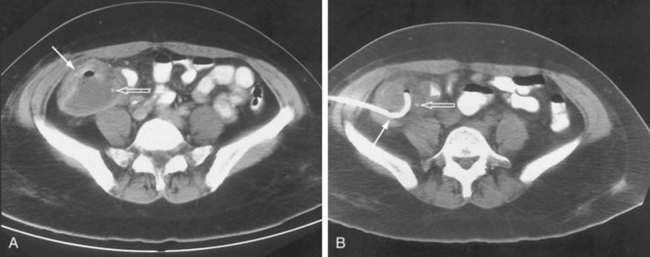
Appendiceal Abscesses
Periappendiceal abscesses can often be suggested by the CT appearance (Fig. 26-7A; see Fig. 26-2). Percutaneous abscess drainage has been increasingly accepted as the initial management of sepsis associated with a periappendiceal abscess, allowing the surgeon to perform a subsequent appendectomy, often laparoscopically, on an elective basis (see Fig. 26-7B).54,55
Surgical Management
Surgical management of an infected patient is indicated when the patient is not a candidate for PAD secondary to multiple or interloop abscesses, or inability to access the cavity. Alternatively, surgery may be indicated for failure of PAD to control the source of infection. In these cases, care must be taken to adequately plan the route for surgical abscess drainage adequately. If possible, an extraperitoneal approach, such as the posterior approach to a left subphrenic abscess with 12th rib resection,57 will allow dependent drainage of the collection without contaminating the remainder of the peritoneal cavity.
There are two groups of patients who pose difficult surgical challenges. These patients have an overwhelming intra-abdominal infection noted at their first operation with significant bowel inflammation and edema,58 or they have failed initial therapy aimed at controlling secondary peritonitis and are now being managed for tertiary peritonitis.59,60 In both cases, these patients will often be treated with an open abdomen. Simply defined, the term open abdomen refers to a surgical technique in which the midline fascia is purposely not closed as part of a planned approach to severe abdominal infection that requires multiple repeat surgical débridements and washouts. These patients tend to be older, more critically ill, as measured by APACHE scoring, and tend to have more significant organ dysfunction scores. The use of the open abdomen with repeated washout strategy has been facilitated by the development of the vacuum-assisted closure device (VAC; KCI, San Antonio, Tex). This device consists of a porous foam pad connected to subatmospheric suction under an occlusive dressing. The VAC can be applied directly to the open abdomen and allows for continuous suction therapy to clear abdominal exudative fluids, maintain tension on the abdominal wall, allow protection of the skin from repeated dressing changes, and decrease patient discomfort associated with multiple daily dressing changes. Although large well-designed trials are still lacking, use of the VAC strategy has allowed improved wound management that has led to earlier definitive closure of the abdomen after using an open abdomen technique with repeat abdominal washouts.12,61 Whether this technique will ultimately decrease complications and improve survival is yet to be defined.62
Antibiotic Treatment
Once an intra-abdominal infection has been diagnosed and source control has been obtained with percutaneous or surgical techniques, elimination of residual infection within the peritoneum is carried out by the use of antibiotics. In general, antibiotics are effective only after an abscess has been drained because of a number of factors, including poor penetration of antibiotics into abscess cavities,63 very high bacterial counts in the abscess cavity (>108 colony-forming units [CFUs]/mL) that may alter bactericidal activity,64 and the fact that pus has an acidic pH and low Po2 from necrotic tissue and a poor blood supply.65
After abscess drainage, the initial choice of antibiotic should be based on the clinical picture and Gram stain findings of the abscess fluid. In an otherwise healthy individual who has a secondary bacterial peritonitis or abscess, antibiotic selection should be directed to the common organisms isolated from that type of abscess, primarily coliforms such as E. coli and anaerobes such as B. fragilis. This could be a second-generation cephalosporin, β-lactamase inhibitor–extended-spectrum penicillin derivative combination, or classic combination therapy with an aminoglycoside and antianaerobe. Recent drug shortages and product discontinuation by major pharmaceutical companies have limited the role of second-generation cephalosporins as first-line therapy. Furthermore, two meta-analyses have suggested that aminoglycosides plus anti-anaerobe combinations are less efficacious than a variety of newer antibiotic comparators.66,67 Although there was no difference in all-cause mortality or infection-specific mortality, there were statistically significant decreases in the clinical and microbiologic success rates in the aminoglycoside group. Because of significant resistance of B. fragilis to clindamycin, the antianaerobe of choice is metronidazole.68 Studies have documented the equivalence of broad-spectrum single-agent regimens such as carbapenems, extended-spectrum penicillin–β-lactamase inhibitor combinations, and fluoroquinolone or third-generation cephalosporin-metronidazole combinations (Table 26-4).4,11,69–73 The Therapeutic Agents Committee of the Surgical Infection Society (SIS) has developed a position paper for the antibiotic treatment of intra-abdominal infection and noted that there was level 1 evidence to state that no regimen is superior to another.74 Newer agents that have been approved by the U.S. Food and Drug Administration (FDA) after showing noninferiority to standard antibiotic regimens in patients with intra-abdominal infections include a new carbapenem, ertapenem75,76; a fluoroquinolone, moxifloxacin77; and tigecycline, which is the first of a new class of antibiotics (glycylcyclines) based on tetracycline.78,79
Table 26-4 Antibiotic Choices in the Treatment of Intra-abdominal Infection
| Single-Agent Therapy |
| Second-Generation Cephalosporins, Bacteroides Species–Active |
| Cefoxitin |
| Carbapenems |
In a severely ill patient with postoperative tertiary peritonitis and an elevated APACHE II score, the choice of empirical therapy can be more difficult. As noted earlier, infections in this population are often monomicrobial. Therefore, the results of the Gram stain can be of great importance in choosing initial antibiotics. Because many of these patients have already been exposed to broad-spectrum antibiotics, antibiotic selection must be made with knowledge of previous prescriptions and information on the resistance patterns in the ICU in which the patient is housed. Attention must also be paid to the underlying organ dysfunction of any individual patient, which can also affect antibiotic selection (e.g., the use of aminoglycosides in the setting of renal dysfunction should be avoided). A broad-spectrum gram-negative coverage with a desirable sensitivity pattern should be considered. This would include a choice of carbapenems, extended-spectrum penicillins, or fluoroquinolones as appropriate therapy. Combination with a β-lactamase inhibitor–extended-spectrum penicillin is desirable under these circumstances. Data also suggest that the addition of an aminoglycoside does not add further activity in the treatment of intra-abdominal infections in this patient population.74 A recent Cochrane database analysis80 has shown that the addition of an aminoglycoside to broad-spectrum β-lactam therapy versus β-lactam monotherapy alone offered no survival benefit or increase in clinical cure. Furthermore, the addition of aminoglycoside therapy was at the expense of an increased risk of nephrotoxicity. If gram-positive organisms are found on Gram stain, vancomycin therapy should be strongly considered for the treatment of potential methicillin-resistant Staphylococcus or Enterococcus spp. (see later).
There is continued debate over the proper treatment of Enterococcus species isolated from IAA fluid. In an otherwise healthy population with minimal comorbid conditions, although no definitive conclusions are indicated by the literature on this topic, data support an antibiotic selection that does not specifically cover Enterococcus spp.81–83 This conclusion is based on the high success rate of regimens that do not have anti-Enterococcus spp. coverage, as high as that of regimens that do provide anti-Enterococcus coverage. For a critically ill patient in the ICU who has an elevated APACHE II score, the isolation of Enterococcus spp. takes on a different meaning, however. Several studies have shown that in a population of patients with an elevated APACHE II score, comorbid conditions, and early organ dysfunction, Enterococcus spp. isolation is an independent risk factor for treatment failure.74 Here, anti-Enterococcus spp. therapy is an important part of antibiotic selection. Combination therapy with a cell wall–specific antibiotic such as ampicillin in conjunction with an aminoglycoside has been shown to be synergistic as anti-Enterococcus spp. therapy.84 Recent development of resistance to β-lactam antibiotics as well as aminoglycosides has led to increasing use of vancomycin as anti-Enterococcus spp. therapy. Unfortunately, this has led to the development of new strains of Enterococcus spp. that carry plasmids encoding for vancomycin resistance (vancomycin-resistant enterococci, VRE).
Once the organism has been identified and its sensitivity pattern reported, antibiotic selection can be focused. It is important to follow the patient’s response to abscess drainage and antibiotics. Continued deterioration, with repeated fever and white blood cell count elevation, should prompt a search for an explanation. Repeat CT scanning is warranted in this situation to look for an area of undrained or new infection.37,43,85 A second cause of a poor response is microbial resistance to the antibiotic selection. Thought must be given to broadening the antibiotic selection further in this case. Another reason for poor response to therapy is the possibility of fungal superinfection. As noted, Candida spp. infections constitute approximately 20% to 40% of infections in the setting of postoperative tertiary peritonitis.27,28 Candida, like Enterococcus species, do not need to be treated in secondary peritonitis (see Chapter 37), but in high-risk surgical patients with intra-abdominal infections, data support the use of fluconazole prophylaxis, which prevented invasive intra-abdominal Candida spp. infections and resulting sepsis in this group of patients with complicated conditions.86 Candida spp. are notoriously difficult to culture from blood and deep tissues. When they are isolated in this high-risk population, they should be aggressively treated with a systemic antifungal agent—amphotericin B, fluconazole,87 or the newer class of antifungals, the echinocandins, which show increased activity to non-albicans Candida spp.
Duration of antibiotic therapy depends on the underlying patient condition and the adequacy of, and response to, invasive drainage techniques. A classic study has evaluated the risk of recurrent sepsis after the termination of antibiotics.88 In the group of patients who were afebrile with a persistent leukocytosis at the end of therapy, there was a 33% recurrence rate of IAA. When both fever and leukocytosis were present, recurrent IAA occurred in 57%. However, when the patient was afebrile and had a normal leukocyte count, there was no IAA recurrence. These data, which have been confirmed, suggest that antimicrobial treatment of IAA be continued until the patient has a normal leukocyte count and is afebrile.89 This is the present recommendation of the Surgical Infection Society.74 Although duration of antibiotic therapy may not be altered, the use of oral conversion strategies may shorten the length of hospital stay and reduce cost. Studies have suggested that appropriate oral conversion therapy is as effective as intravenous therapy in those able to tolerate oral intake and that length of hospital stay, although not significantly different, tends to be shorter in the oral conversion group.4,71
OUTCOMES
Outcomes after treatment of IAA is dependent on a number of factors. The mortality rate has been reported to range from less than 5% for simple secondary bacterial peritonitis to approximately 65% or higher for complicated tertiary peritonitis.26,27,62,90–92 Simple abscesses associated with perforated appendicitis that responds to surgical drainage and antibiotics have a low mortality rate. Higher mortality rates occur in older patients, in those who have complex abscesses, high APACHE II and multiple organ dysfunction (MOD) scores, or a therapeutic delay, male gender, and those who use glucocorticoids or are otherwise immunosuppressed.60 Other risk factors include multiple reoperations to control intra-abdominal sepsis, malnutrition, poor physiologic reserve, high New York Heart Association class, and MOD syndrome.93 Retrospective studies have suggested that planned reexploration of the abdomen may be more successful in controlling intra-abdominal sepsis for patients with complicated infections. Furthermore, patients treated with an open abdominal technique may also have improved outcomes. Prospective randomized trials on techniques to manage complicated intra-abdominal sepsis do not presently exist. As noted, patients who show evidence of MOD have a particularly poor outcome, which has been thought to be secondary to the inability to control intra-abdominal infection. It has been suggested that continued intra-abdominal infection is another manifestation of organ failure and not a cause74—that is, patients die with infection, not of infection. Aggressive surgical, antibiotic, and supportive care is required in this group of patients, and patients may benefit from defined clinical pathways that minimize variability in practice.8,89
Studies have suggested a beneficial role for drotrecogin alfa (activated; recombinant human activated protein C [rhAPC]) in the treatment of severe intra-abdominal infections. The data from the initial PROWESS trial94 was reviewed by a surgical evaluation committee to evaluate the outcomes of surgical patients, with special emphasis on those with severe sepsis of abdominal origin.95 There was an absolute risk reduction of 9.1% and a relative risk reduction in 28-day mortality of 30% in those undergoing abdominal procedures. Although this did not reach statistical significance because of relatively small numbers, the group with an APACHE II score higher than 25 had an absolute mortality risk reduction of 18.2%. The rate of serious bleeding complications was not different between groups. Subsequently, an analysis of the INDEPTH database,96 which is made up of five clinical trials of the efficacy of rhAPC, demonstrated a significant risk reduction in mortality (10.7% absolute, 34% relative) in surgical patients treated with rhAPC, over half of whom had intra-abdominal infections. This effect was only seen in those with an APACHE II score higher than 25. This finding was supported in a separate retrospective matched cohort study of patients with septic shock of abdominal origin.97 These data, taken together, are highly suggestive of a benefit of rhAPC in the treatment of critically ill patients with severe sepsis of abdominal origin.
GASTROINTESTINAL FISTULAS
DEFINITIONS AND CLASSIFICATIONS
A fistula is any abnormal anatomic connection between two epithelialized surfaces, a definition that includes many clinical entities. Because of this, fistulas are generally classified by anatomic and physiologic methods. Anatomic classifications rely on sites of fistula origin and drainage point. Inherent in this anatomic classification system is whether the fistula is internal or external. Physiologic classifications rely on fistula output in a 24-hour period (Table 26-5). Both fistula classifications are used clinically when describing a fistulous tract (e.g., a high-output enterocutaneous fistula).
| Anatomic |
Compared with fistulas connected to the skin that are obvious, internal fistulas may be difficult to diagnose, depending on the organs involved. This would be the case, for example, in a cholecystoduodenal fistula, which might first be manifested by gallstone ileus. In a colovesical fistula, the presenting signs are urinary tract infection, fecaluria, and pneumaturia. Fistulas arising in the abdomen can originate from any epithelialized surface of a hollow viscus or drainage duct in the GI or genitourinary (GU) tract, liver, or pancreas. This chapter focuses on GI fistulas; for specific discussions of fistulas arising from the pancreatic or biliary duct, see Chapters 58, 59, 61, and 70.
PATHOPHYSIOLOGY
GI fistulas can occur spontaneously or postoperatively. Spontaneous fistulas account for 15% to 25% of fistulas and arise in association with inflammatory processes, cancer, and radiation treatment.98–106 Inflammatory processes include diverticulitis, inflammatory bowel disease, peptic ulcer disease, and appendicitis. These fistulas can be internal or external and, depending on cause and anatomic variations, have different rates of spontaneous closure. The remaining 75% to 85% of fistulas are almost always postoperative, external, and iatrogenic in origin.107–112 These occur after cancer surgery, emergency surgery in which bowel cannot be adequately prepared and cleansed, trauma surgery in which injuries may be missed, and reoperative surgery in which extensive lysis of adhesions and partial-thickness bowel injury occur. Risk factors for the formation of spontaneous or postoperative fistulas include malnutrition, sepsis, shock or hypotension, vasopressor therapy, glucocorticoid therapy, associated disease states, and technical difficulties with a surgical anastomosis.113
It is important to determine the cause of fistula formation because it often determines therapy. Fistulas that arise in inflammatory bowel disease or from direct involvement of intestinal cancer are unlikely to close spontaneously and often require surgical correction. On the other hand, a postoperative low-output fistula arising from a partial anastomotic dehiscence frequently closes with appropriate conservative management. Conditions associated with the nonhealing of GI fistulas are listed in Table 26-6.
Table 26-6 Conditions Associated with Nonhealing Fistulas*
* The acronym FRIEND can be used to remember these conditions.
DIAGNOSIS
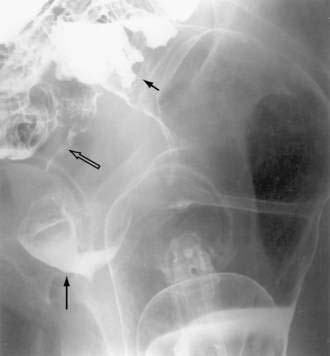
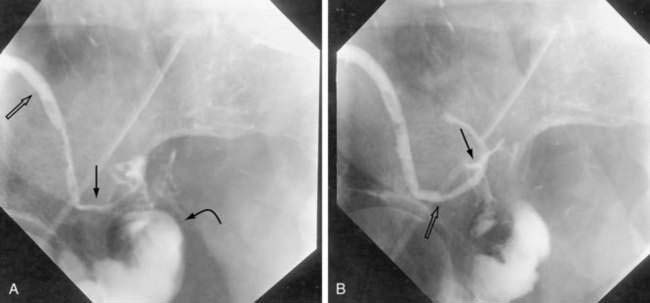
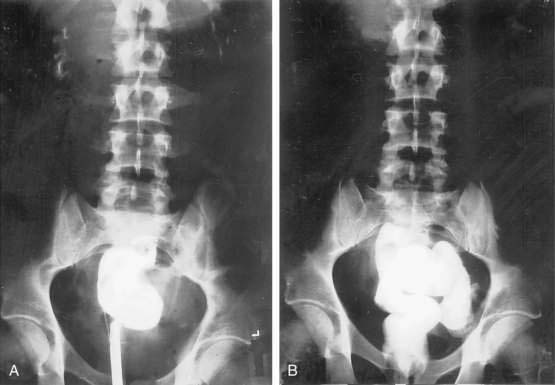
Once a fistula is suspected, early management should be directed to confirming the diagnosis. The anatomic site of origin and underlying cause can be determined when the patient’s condition is stabilized. One simple bedside maneuver to confirm the presence of an external fistula as the cause of suspicious postoperative wound drainage is to give the patient oral charcoal. The fistula can then be confirmed by the presence of charcoal in the suspicious drainage. Once confirmed, exact anatomic origins can be determined by radiographic contrast studies. These can include administration of contrast medium orally or rectally (depending on the site of suspicion) to define the site of origin via the bowel lumen (Fig. 26-8). Alternatively, contrast can be injected retrograde into the drainage site (fistulography) and followed to its site of origin in the bowel (Fig. 26-9). Internal fistulas can be diagnosed by injecting contrast medium into a hollow viscus (e.g., urinary bladder) with opacification of another viscus (e.g., rectosigmoid; Fig. 26-10).
TREATMENT
Treatment may be nonsurgical or surgical. Generally, nonsurgical treatment is the cornerstone of the early management strategy when treating GI fistulas. Once a diagnosis of an enterocutaneous fistula is confirmed (e.g., with enteral charcoal), early management is directed to fluid and electrolyte replacement. This can be a daunting task if the fistula has a high output (more than 500 mL/day; see Table 26-5). Output in excess of 1000 mL/day is not uncommon if the fistula originates in the proximal small bowel. To prevent intravascular volume depletion and electrolyte imbalance, fluid and electrolyte replacement must be a priority and should be addressed before more detailed diagnostic fistula studies are undertaken. Administration of replacement fluids should take into account the volume and electrolyte content lost through the fistula. Generally, fistula output is iso-osmotic and high in potassium. Therefore, output should be replaced milliliter for milliliter with a balanced salt solution that contains added potassium. If difficulties are encountered when managing electrolyte imbalances, a sample of fistula fluid can be sent to the laboratory for electrolyte determination. Subsequent electrolyte replacement can then be formulated on the basis of laboratory results.
A second cornerstone of the early management strategy in the treatment of enterocutaneous fistulas is establishment of adequate drainage of external fistulas. This may require minor surgical maneuvers, such as opening a recent surgical incision to allow adequate drainage. As noted, percutaneous catheters are often essential in controlling a fistula. This point requires early attention because if a fistula cannot be controlled, pooling of fistula contents within the abdominal cavity can lead to infection with abscess formation and sepsis. Because most enterocutaneous fistulas occur postoperatively, some ingenuity may be required when trying to protect the skin from the caustic effects of the fistula output. Most acute postoperative enterocutaneous fistulas decompress through the surgical incision. As the incision shows signs of infection and drainage, it must be opened. A reopened incision that is draining intestinal contents is not amenable to simple placement of an ostomy bag to collect the drainage. An experienced enterostomal therapist should be consulted when dealing with this difficult problem. A recent adjunct in the management of enterocutaneous fistulas has been local wound care with the VAC system, described earlier. The use of the VAC device has made management of these difficult wounds more standardized. By applying the VAC device, control of the effluent and the open wound can be managed simultaneously.107,114–116 The VAC system has been able to protect the skin and decrease the fistulous output by the use of nonporous plugs to prevent fistula drainage, despite subatmospheric pressure applied by suction on the wound to aid in wound healing. The use of the VAC system can effectively convert a high-output fistula into a low-output state. This wound care strategy has allowed easier nonoperative management of fistulas, thus restoring bowel continuity resulting in the initiation of enteral feeding (see Chapter 5).
Once the patient’s condition is stabilized in regard to fluids and electrolytes and the fistula is adequately drained, attention is turned to anatomic and diagnostic considerations to plan further therapy. Table 26-7 lists some prognostic factors important in determining whether the fistula has a high or low rate of spontaneous closure. Spontaneous fistula closure is more likely for low-output fistulas, fistulas secondary to surgical complications, and fistulas arising anatomically in the proximal small intestine. Fistulas that ultimately require surgical closure are more often associated with high output, jejunal origin, and ongoing sepsis. Well-nourished patients without infectious complications are also more likely to experience spontaneous closing.102,117–120 When spontaneous closure is likely, nutritional evaluation and support must be aggressively pursued (see Chapters 4 and 5). The causes of malnutrition in the patient with a GI fistula are multifactorial, including underlying disease states, lack of protein intake, protein losses through the fistula, and underlying sepsis with hypercatabolism.113
Table 26-7 Prognostic Indicators of Successful Spontaneous Fistula Closure
| PARAMETER | SPONTANEOUS CLOSURE LIKELY | SURGICAL CLOSURE MORE LIKELY TO BE NEEDED |
|---|---|---|
| Output (mL/day) | <500 | ≥500 |
| Age (yr) | <40 | >65 |
| Site | Proximal small bowel | Distal small bowel or colon |
| Nutritional status | Well nourished | Malnourished |
| Cause | Anastomotic breakdown | Malignancy, inflammatory or infectious disease, complete anastomotic dehiscence |
| Anatomic characteristics | Long fistulous tract | Eversion of mucosa |
| Duration | Acute | Chronic |
Adapted from Berry SM, Fischer JE. Enterocutaneous fistulas. Curr Probl Surg 1994; 31:469-566; and Rombeau JL, Rolandelli RH. Enteral and parenteral nutrition in patients with enteric fistulas and short bowel syndrome. Surg Clin North Am 1987; 67:551-71.
Nutrition
Total parenteral nutrition (TPN) seems to be the natural first choice for a patient with an enterocutaneous fistula. Soon after diagnosis, aggressive caloric support must be given. Once the anatomic origin of the fistula is determined, route of feeding is considered. Not all patients must be placed on TPN, however. In a study of 335 patients with external fistulas, 85% were managed solely with enteral feedings. In a subgroup of uncomplicated fistulas, 50% healed spontaneously with this mode of nutritional therapy alone.121 Enteral feeding has been shown to enhance mucosal proliferation and villous growth through direct and indirect mechanisms. Nutrients in contact with the bowel mucosa also provide direct stimulation to the enterocyte, and feedings high in glutamine may be particularly beneficial because glutamine is the main source of energy of the enterocyte.122 Furthermore, nutrients within the gut lumen release gut-derived hormones that have an indirect trophic effect on the intestinal mucosa. TPN, in contrast, has been shown to lead to gut mucosal atrophy. This may be the result, in part, of the fact that standard TPN solutions do not contain glutamine because it crystallizes out of solution. In a recent small study, 28 patients were fed an oral glutamine solution and the remainder of caloric intake was supplemented with TPN. Spontaneous resolution of fistula drainage was 13 times more likely in the oral glutamine-supplemented group.123 Despite the recent advances in enteral feeding of patients with GI fistulas, TPN remains the mainstay of nutritional support for most patients because they are unable to absorb sufficient calories enterally.124 In one study, Rose and associates reviewed 114 consecutive patients with GI fistulas, all treated with TPN and conservative therapy.125 They found that 61% of the fistulas closed spontaneously in an average of 26 days. The remainder continued on to surgical extirpation of the fistulous tract.
The decision to support the patient with a GI fistula with enteral or parenteral nutrition has to be based on anatomic and physiologic considerations. If the fistula has a low output and is anatomically distal in the intestine, a trial of enteral feedings should be pursued. If the fistula is in the proximal intestine and distal access to the intestine has been established, as in many postoperative fistulas in which a feeding jejunostomy has been placed at the time of surgery, enteral feeding into the distal bowel should be considered. Along with this, infusion of the proximal fistula drainage into the distal bowel should be considered. Reinfusion of succus entericus into the distal bowel has been shown to make fluid and electrolyte management easier, as well as decrease the output of the proximal fistula.105,126 It is not mandatory to provide full nutritional support via the enteral route to obtain the benefits of enteral feeding. Protein and caloric requirements can be supplemented by TPN.
Somatostatin Analogs
Another potential adjunct to TPN in the management of the patient with a GI fistula is the use of a long-acting somatostatin analog such as octreotide or lantreotide. Octreotide has been shown to decrease fistula output by three mechanisms. First, it inhibits the release of gastrin, cholecystokinin, secretin, motilin, and other GI hormones. This inhibition decreases secretion of bicarbonate, water, and pancreatic enzymes into the intestine, subsequently decreasing intestinal volume. Second, octreotide relaxes intestinal smooth muscle, thereby allowing for a greater intestinal capacity. Third, octreotide increases intestinal water and electrolyte absorption.127
Initial studies evaluating the effect of octreotide on spontaneous intestinal fistula closure were uncontrolled, used historical controls, or were unblinded. These studies suggested that octreotide decreases fistula output, leads to improved spontaneous fistula closure rates, decreases time to spontaneous closure, and reduces mortality rate. However, randomized, placebo-controlled, double-blind studies using strict entry criteria had less favorable findings for octreotide.128–130 These studies, which had relatively small group sizes, showed no significant effects of octreotide on fistula closure, complication, or mortality rate. One consistent finding in some studies has been an improvement in healing time with octreotide, perhaps by decreasing a high-output fistula to a low-output fistula.131–133 Currently, the role of octreotide is limited to occasional use for high-output fistulas.
Management of Crohn’s Disease
Historically, conservative management of fistulas associated with Crohn’s disease has been uniformly unrewarding, as most abdominal and perianal fistulas required surgical correction. The observation that tumor necrosis factor-α (TNF-α) production in the intestinal mucosa is increased in patients with Crohn’s disease134 has led to the development and clinical investigation of chimeric monoclonal antibodies against TNF-α (e.g., infliximab [Remicade]) for the treatment of Crohn’s disease. In a randomized, multicenter, double-blind, placebo-controlled trial of 94 Crohn’s disease patients with draining abdominal or perianal fistulas of at least three months’ duration, 68% of patients receiving infliximab (5 mg/kg) had at least a 50% reduction in draining fistulas compared with 26% of patients receiving placebo. Furthermore, 55% of patients receiving infliximab had closure of all fistulas as compared with 13% of patients assigned to the placebo group.135 Initial trials with short-term infusions revealed the salutary effects of infliximab to be transient in most patients.136 A subsequent prospective, randomized, double-blind, placebo-controlled study revealed the benefit of a maintenance infusion of infliximab given every eight weeks. The group that received maintenance therapy had significantly longer periods without fistula drainage compared with controls (more than 40 weeks vs. 14 weeks in controls) and at more than one year on maintenance infliximab infusions, 36% were fistula-free versus 19% of controls.137 This was associated with a decreased rate of subsequent hospitalization and surgical and nonsurgical procedures.138 Subsequent data analysis from these and other trials has shown that the use of infliximab is associated with better outcomes for perianal than for abdominal wall fistulas,139 for external than for internal fistulas140 and, when given as early fistula treatment, rather than late in the disease process.141 The use of infliximab has not been associated with increased infectious complications in fistula patients.142 For the initial management of fistulas in Crohn’s disease, a trial of an anti–TNF-α monoclonal antibody regimen should be considered and has been supported by a recent Cochrane database review.143 There are a number of small series that advocate for the use of aggressive immunosuppression of fistulizing Crohn’s patients with methotrexate144 or tacrolimus145 when other nonoperative therapy has failed. Fistula formation in Crohn’s disease is discussed in greater detail in Chapter 111.
Adjunctive Techniques
Another nonoperative approach to the management of refractory fistulas includes the percutaneous and endoscopic use of fibrin glue and other occlusive plugs. Although reports are limited to case series at this time, a variety of techniques, including fistuloscopy, fluoroscopy, and endoscopy, have been used to cannulate fistula tracts.146–149 Once cannulated, the tracts are débrided and then occluded with fibrin glue, collagen plugs, or gelatin sponges. Results have been encouraging in the small series evaluated, and the technique may serve as a useful adjunct for fistulas refractory to conservative management.
Surgical Therapy
Surgical therapy remains the mainstay of management of the complex fistula that is not a candidate for conservative management or has had a prolonged course of conservative management (longer than six weeks) without resolution of fistulous output.102,105 Indications for early surgery include inability to control the fistula without surgical drainage, sepsis or abscess formation, distal intestinal obstruction, bleeding, and persistence of fistulous output not responsive to conservative management. Some more complex fistulas may require surgery to remove mesh or other foreign bodies before closure can be undertaken. The goal of surgical therapy is to resect the involved bowel and restore intestinal continuity.150 This surgery allows the patient to start eating through normal routes. Minimally invasive surgery was shown to be an option in selected patients in the surgical management of intestinal fistulas.146,151
Fistula recurrence after surgery has been noted in 10% to 20% of postoperative patients. Factors associated with fistula recurrence after surgery include Crohn’s disease, poor nutritional status, complex fistula associated with an open abdomen or mesh implantation or infection, advanced underlying disease states, and oversewing the fistula instead of resection and reanastomosis.99–101,107,119,150
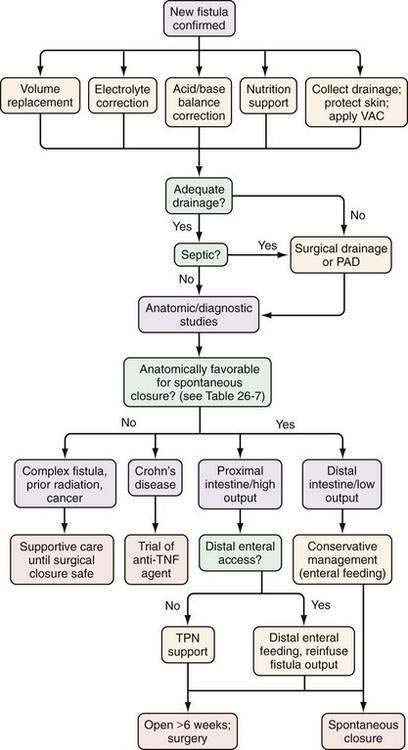
Although innovative therapy and supportive care have resulted in improving spontaneous closure rates, management of these difficult problems requires a multidisciplinary approach that includes a nutritional support service, enterostomal therapist, surgeon, invasive radiologist, and gastroenterologist. An algorithm to manage GI fistulas is presented in Figure 26-11.
OUTCOMES
Early morbidity and mortality in the management of external fistulas result from initial fluid and electrolyte derangements that go unchecked. However, the major cause of mortality in patients with GI fistulas is sepsis with multiple organ failure. The typical setting for septic complications is provided by complex fistulas for which there is inadequate or uncontrolled drainage. In this setting, pooling of enteric contents occurs within the abdominal cavity and acts as a nidus of infection. Therefore, as noted, aggressive attempts must be made to ensure that fistulous drainage is well controlled. The mortality rate from all causes in patients with fistulas ranges from 10% to 30%.99,100,102,105,107,118,119,152 Higher mortality rates are seen in those who are malnourished, have had previous irradiation therapy, or have complex fistulas associated with a postoperative abdominal wall dehiscence.111 A second major cause of mortality in patients with GI fistulas is severe underlying disease, most often cancer. Often, patients who are terminally ill secondary to malignancy forgo further operative procedures.153
Alivizatos V, Felekis D, Zorbalas A. Evaluation of the effectiveness of octreotide in the conservative treatment of postoperative enterocutaneous fistulas. Hepatogastroenterology. 2002;49:1010-2. (Ref 133.)
Beck DE, Cohen Z, Fleshman JW, et al. A prospective, randomized, multicenter, controlled study of the safety of Seprafilm adhesion barrier in abdominopelvic surgery of the intestine. Dis Colon Rectum. 2003;46:1310-19. (Ref 19.)
Behm BW, Bickston SJ. Tumor necrosis factor-alpha antibody for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev 2008; (1):CD006893. (Ref 143.)
Eggimann P, Francioli P, Bille J, et al. Fluconazole prophylaxis prevents intra-abdominal candidiasis in high-risk surgical patients. Crit Care Med. 1999;27:1066-72. (Ref 86.)
Farthmann EH, Schoffel U. Epidemiology and pathophysiology of intra-abdominal infections (IAI). Infection. 1998;26:329-34. (Ref 6.)
Haffejee AA. Surgical management of high output enterocutaneous fistulae: A 24-year experience. Curr Opin Clin Nutr Metab Care. 2004;7:309-16. (Ref 100.)
Lynch AC, Delaney CP, Senagore AJ, et al. Clinical outcome and factors predictive of recurrence after enterocutaneous fistula surgery. Ann Surg. 2004;240:825-31. (Ref 150.)
Martinez JL, Luque-de-Leon E, Mier J, et al. Systematic management of postoperative enterocutaneous fistulas: Factors related to outcomes. World J Surg. 2008;32:436-443. (Ref 118.)
Mazuski JE, Sawyer RG, Nathens AB, et al. The Surgical Infection Society Guidelines on Antimicrobial Therapy for Intra-Abdominal Infections: An Executive Summary. Surg Infect (Larchmt). 2002;3(3):161-73. (Ref 74.)
Paul M, Silbiger I, Grozinsky S, Soares-Weiser K, Leibovici L. Beta lactam antibiotic monotherapy versus beta lactam-aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst Rev 2006; (1):CD003344. (Ref 80.)
Payen D, Sablotzki A, Barie PS, et al. International integrated database for the evaluation of severe sepsis and drotrecogin alfa (activated) therapy: Analysis of efficacy and safety data in a large surgical cohort. Surgery. 2006;140:726-39. (Ref 96.)
Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med. 1999;340:1398-405. (Ref 135.)
Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876-85. (Ref 137.)
Wainstein DE, Fernandez E, Gonzalez D, et al. Treatment of high-output enterocutaneous fistulas with a vacuum-compaction device. A ten-year experience. World J Surg. 2008;32:430-5. (Ref 116.)
Wong PF, Gilliam AD, Kumar S, et al. Antibiotic regimens for secondary peritonitis of gastrointestinal origin in adults. Cochrane Database Syst Rev 2005; (2):CD004539. (Ref 67.)
1. Bahadursingh AM, Virgo KS, Kaminski DL, Longo WE. Spectrum of disease and outcome of complicated diverticular disease. Am J Surg. 2003;186:696-701.
2. Bulger EM, McMahon K, Jurkovich GJ. The morbidity of penetrating colon injury. Injury. 2003;34:41-6.
3. Capitan MC, Tejido SA, Piedra Lara JD, et al. Retroperitoneal abscesses—analysis of a series of 66 cases. Scand J Urol Nephrol. 2003;37:139-44.
4. Cohn SM, Lipsett PA, Buchman TG, et al. Comparison of intravenous/oral ciprofloxacin plus metronidazole versus piperacillin/tazobactam in the treatment of complicated intraabdominal infections. Ann Surg. 2000;232:254-62.
5. Dente CJ, Tyburski J, Wilson RF, et al. Ostomy as a risk factor for posttraumatic infection in penetrating colonic injuries: Univariate and multivariate analyses. J Trauma. 2000;49:628-34.
6. Farthmann EH, Schoffel U. Epidemiology and pathophysiology of intraabdominal infections (IAI). Infection. 1998;26:329-34.
7. Guller U, Jain N, Peterson ED, et al. Laparoscopic appendectomy in the elderly. Surgery. 2004;135:479-88.
8. Miller PR, Fabian TC, Croce MA, et al. Improving outcomes following penetrating colon wounds: Application of a clinical pathway. Ann Surg. 2002;235:775-81.
9. Montravers P, Chalfine A, Gauzit R, et al. Clinical and therapeutic features of nonpostoperative nosocomial intra-abdominal infections. Ann Surg. 2004;239:409-16.
10. Pessaux P, Msika S, Atalla D, et al. Risk factors for postoperative infectious complications in noncolorectal abdominal surgery: A multivariate analysis based on a prospective multicenter study of 4718 patients. Arch Surg. 2003;138:314-24.
11. Solomkin JS, Yellin AE, Rotstein OD, et al. Ertapenem versus piperacillin/tazobactam in the treatment of complicated intraabdominal infections: Results of a double-blind, randomized comparative phase III trial. Ann Surg. 2003;237:235-45.
12. Suliburk JW, Ware DN, Balogh Z, et al. Vacuum-assisted wound closure achieves early fascial closure of open abdomens after severe trauma. J Trauma. 2003;55:1155-60.
13. McClean KL, Sheehan GJ, Harding GK. Intraabdominal infection: A review. Clin Infect Dis. 1994;19:100-16.
14. Dunn DL, Barke RA, Ahrenholz DH, et al. The adjuvant effect of peritoneal fluid in experimental peritonitis. Mechanism and clinical implications. Ann Surg. 1984;199:37-43.
15. van Goor H, de Graaf JS, Kooi K, et al. Effect of recombinant tissue plasminogen activator on intra-abdominal abscess formation in rats with generalized peritonitis. J Am Coll Surg. 1994;179:407-11.
16. Reijnen MM, Meis JF, Postma VA, van Goor H. Prevention of intra-abdominal abscesses and adhesions using a hyaluronic acid solution in a rat peritonitis model. Arch Surg. 1999;134:997-1001.
17. Reijnen MM, Bleichrodt RP, van Goor H. Pathophysiology of intra-abdominal adhesion and abscess formation, and the effect of hyaluronan. Br J Surg. 2003;90:533-41.
18. Holsti MA, Chitnis T, Panzo RJ, et al. Regulation of postsurgical fibrosis by the programmed death-1 inhibitory pathway. J Immunol. 2004;172:5774-781.
19. Beck DE, Cohen Z, Fleshman JW, et al. A prospective, randomized, multicenter, controlled study of the safety of Seprafilm adhesion barrier in abdominopelvic surgery of the intestine. Dis Colon Rectum. 2003;46:1310-19.
20. Zeng Q, Yu Z, You J, Zhang Q. Efficacy and safety of Seprafilm for preventing postoperative abdominal adhesion: Systematic review and meta-analysis. World J Surg. 2007;31:2125-31.
21. Cohen Z, Senagore AJ, Dayton MT, et al. Prevention of postoperative abdominal adhesions by a novel, glycerol/sodium hyaluronate/carboxymethylcellulose-based bioresorbable membrane: A prospective, randomized, evaluator-blinded multicenter study. Dis Colon Rectum. 2005;48:1130-39.
22. Brook I, Frazier EH. Microbiology of subphrenic abscesses: A 14-year experience. Am Surg. 1999;65:1049-53.
23. Brook I, Frazier EH. Aerobic and anaerobic microbiology of retroperitoneal abscesses. Clin Infect Dis. 1998;26:938-41.
24. Brook I, Frazier EH. Aerobic and anaerobic microbiology in intra-abdominal infections associated with diverticulitis. J Med Microbiol. 2000;49:827-30.
25. Tzianabos AO, Kasper DL. Role of T cells in abscess formation. Curr Opin Microbiol. 2002;5:92-6.
26. Malangoni MA. Evaluation and management of tertiary peritonitis. Am Surg. 2000;66:157-61.
27. Nathens AB, Rotstein OD, Marshall JC. Tertiary peritonitis: Clinical features of a complex nosocomial infection. World J Surg. 1998;22:158-63.
28. Sawyer RG, Rosenlof LK, Adams RB, et al. Peritonitis into the 1990s: Changing pathogens and changing strategies in the critically ill. Am Surg. 1992;58:82-7.
29. Golfieri R, Cappelli A. Computed tomography-guided percutaneous abscess drainage in coloproctology: Review of the literature. Tech Coloproctol. 2007;11:197-208.
30. Gerzof SG, Johnson WC, Robbins AH, Nabseth DC. Expanded criteria for percutaneous abscess drainage. Arch Surg. 1985;120:227-32.
31. Gervais DA, Ho CH, O’Neill MJ, et al. Recurrent abdominal and pelvic abscesses: Incidence, results of repeated percutaneous drainage, and underlying causes in 956 drainages. AJR Am J Roentgenol. 2004;182:463-66.
32. Gervais DA, Brown SD, Connolly SA, et al. Percutaneous imaging-guided abdominal and pelvic abscess drainage in children. Radiographics. 2004;24:737-54.
33. Khurrum BM, Hua ZR, Batista O, et al. Percutaneous postoperative intra-abdominal abscess drainage after elective colorectal surgery. Tech Coloproctol. 2002;6:159-64.
34. Lee MJ. Non-traumatic abdominal emergencies: imaging and intervention in sepsis. Eur Radiol. 2002;12:2172-9.
35. Maher MM, Gervais DA, Kalra MK, et al. The inaccessible or undrainable abscess: How to drain it. Radiographics. 2004;24:717-35.
36. vanSonnenberg E, Wittich GR, Goodacre BW, et al. Percutaneous abscess drainage: update. World J Surg. 2001;25:362-9.
37. Cinat ME, Wilson SE, Din AM. Determinants for successful percutaneous image-guided drainage of intra-abdominal abscess. Arch Surg. 2002;137:845-9.
38. van Sonnenberg E, Mueller PR, Ferrucci JTJr. Percutaneous drainage of 250 abdominal abscesses and fluid collections. Part I: Results, failures, and complications. Radiology. 1984;151:337-41.
39. Schuster MR, Crummy AB, Wojtowycz MM, McDermott JC. Abdominal abscesses associated with enteric fistulas: percutaneous management. J Vasc Interv Radiol. 1992;3:359-63.
40. Fang JF, Chen RJ, Lin BC, et al. Retroperitoneal laparostomy: An effective treatment of extensive intractable retroperitoneal abscess after blunt duodenal trauma. J Trauma. 1999;46:652-5.
41. Lambiase RE, Deyoe L, Cronan JJ, Dorfman GS. Percutaneous drainage of 335 consecutive abscesses: Results of primary drainage with 1-year follow-up. Radiology. 1992;184:167-79.
42. Akinci D, Akhan O, Ozmen MN, et al. Percutaneous drainage of 300 intraperitoneal abscesses with long-term follow-up. Cardiovasc Intervent Radiol. 2005;28:744-50.
43. Benoist S, Panis Y, Pannegeon V, et al. Can failure of percutaneous drainage of postoperative abdominal abscesses be predicted? Am J Surg. 2002;184:148-53.
44. Beland MD, Gervais DA, Levis DA, et al. Complex abdominal and pelvic abscesses: Efficacy of adjunctive tissue-type plasminogen activator for drainage. Radiology. 2008;247:567-3.
45. Wroblicka JT, Kuligowska E. One-step needle aspiration and lavage for the treatment of abdominal and pelvic abscesses. AJR Am J Roentgenol. 1998;170:1197-203.
46. van Sonnenberg E, D’Agostino HB, Casola G, et al. Percutaneous abscess drainage: Current concepts. Radiology. 1991;181:617-26.
47. Betsch A, Wiskirchen J, Trubenbach J, et al. CT-guided percutaneous drainage of intra-abdominal abscesses: APACHE III score stratification of 1-year results. Acute physiology, age, chronic health evaluation. Eur Radiol. 2002;12:2883-9.
48. Neff CC, Mueller PR, Ferrucci JTJr, et al. Serious complications following transgression of the pleural space in drainage procedures. Radiology. 1984;152:335-41.
49. McNicholas MM, Mueller PR, Lee MJ, et al. Percutaneous drainage of subphrenic fluid collections that occur after splenectomy: Efficacy and safety of transpleural versus extrapleural approach. AJR Am J Roentgenol. 1995;165:355-9.
50. Harisinghani MG, Gervais DA, Maher MM, et al. Transgluteal approach for percutaneous drainage of deep pelvic abscesses: 154 cases. Radiology. 2003;228:701-5.
51. Alexander AA, Eschelman DJ, Nazarian LN, Bonn J. Transrectal sonographically guided drainage of deep pelvic abscesses. AJR Am J Roentgenol. 1994;162:1227-30.
52. Feld R, Eschelman DJ, Sagerman JE, et al. Treatment of pelvic abscesses and other fluid collections: Efficacy of transvaginal sonographically guided aspiration and drainage. AJR Am J Roentgenol. 1994;163:1141-5.
53. Hovsepian DM, Steele JR, Skinner CS, Malden ES. Transrectal versus transvaginal abscess drainage: Survey of patient tolerance and effect on activities of daily living. Radiology. 1999;212:159-63.
54. Lasson A, Lundagards J, Loren I, Nilsson PE. Appendiceal abscesses: Primary percutaneous drainage and selective interval appendicectomy. Eur J Surg. 2002;168:264-9.
55. Brown CV, Abrishami M, Muller M, Velmahos GC. Appendiceal abscess: Immediate operation or percutaneous drainage? Am Surg. 2003;69:829-32.
56. Siewert B, Tye G, Kruskal J, et al. Impact of CT-guided drainage in the treatment of diverticular abscesses: Size matters. AJR Am J Roentgenol. 2006;186:680-6.
57. Bosscha K, Roukema AJ, van Vroonhoven TJ, van der WC. Twelfth rib resection: A direct posterior surgical approach for subphrenic abscesses. Eur J Surg. 2000;166:119-22.
58. Ozguc H, Yilmazlar T, Gurluler E, et al. Staged abdominal repair in the treatment of intra-abdominal infection: Analysis of 102 patients. J Gastrointest Surg. 2003;7:646-51.
59. Paugam-Burtz C, Dupont H, Marmuse JP, et al. Daily organ-system failure for diagnosis of persistent intra-abdominal sepsis after postoperative peritonitis. Intensive Care Med. 2002;28:594-8.
60. Koperna T, Schulz F. Relaparotomy in peritonitis: Prognosis and treatment of patients with persisting intra-abdominal infection. World J Surg. 2000;24:32-7.
61. Cedidi C, Berger A, Ingianni G. The two-stage concept with temporary subcutaneous implantation of a vacuum sealing system: An alternative surgical approach in infected partial abdominal defects after lapratomy or abdominoplasty. Eur J Med Res. 2002;7:399-403.
62. Adkins AL, Robbins J, Villalba M, et al. Open abdomen management of intra-abdominal sepsis. Am Surg. 2004;70:137-40.
63. Galandiuk S, Lamos J, Montgomery W, et al. Antibiotic penetration of experimental intra-abdominal abscesses. Am Surg. 1995;61:521-5.
64. Konig C, Simmen HP, Blaser J. Bacterial concentrations in pus and infected peritoneal fluid—implications for bactericidal activity of antibiotics. J Antimicrob Chemother. 1998;42:227-32.
65. Simmen HP, Blaser J. Analysis of pH and pO2 in abscesses, peritoneal fluid, and drainage fluid in the presence or absence of bacterial infection during and after abdominal surgery. Am J Surg. 1993;166:24-7.
66. Bailey JA, Virgo KS, DiPiro JT, et al. Aminoglycosides for intra-abdominal infection: Equal to the challenge? Surg Infect (Larchmt). 2002;3:315-35.
67. Wong PF, Gilliam AD, Kumar S, et al. Antibiotic regimens for secondary peritonitis of gastrointestinal origin in adults. Cochrane Database Syst Rev 2005; (2):CD004539.
68. Betriu C, Campos E, Cabronero C, et al. Susceptibilities of species of the Bacteroides fragilis group to 10 antimicrobial agents. Antimicrob Agents Chemother. 1990;34:671-3.
69. Barie PS, Vogel SB, Dellinger EP, et al. A randomized, double-blind clinical trial comparing cefepime plus metronidazole with imipenem-cilastatin in the treatment of complicated intra-abdominal infections. Cefepime Intra-abdominal Infection Study Group. Arch Surg. 1997;132:1294-302.
70. Ohlin B, Cederberg A, Forssell H, et al. Piperacillin/tazobactam compared with cefuroxime/metronidazole in the treatment of intra-abdominal infections. Eur J Surg. 1999;165:875-84.
71. Starakis I, Karravias D, Asimakopoulos C, et al. Results of a prospective, randomized, double blind comparison of the efficacy and the safety of sequential ciprofloxacin (intravenous/oral) + metronidazole (intravenous/oral) with ceftriaxone (intravenous) + metronidazole (intravenous/oral) for the treatment of intra-abdominal infections. Int J Antimicrob Agents. 2003;21:49-57.
72. Wilson SE. Results of a randomized, multicenter trial of meropenem versus clindamycin/tobramycin for the treatment of intra-abdominal infections. Clin Infect Dis. 1997;24(Suppl 2):S197-206.
73. Yellin AE, Hassett JM, Fernandez A, et al. Ertapenem monotherapy versus combination therapy with ceftriaxone plus metronidazole for treatment of complicated intra-abdominal infections in adults. Int J Antimicrob Agents. 2002;20:165-73.
74. Mazuski JE, Sawyer RG, Nathens AB, et al. The Surgical Infection Society guidelines on antimicrobial therapy for intra-abdominal infections: An executive summary. Surg Infect (Larchmt). 2002;3:161-73.
75. Dela Pena AS, Asperger W, Kockerling F, et al. Efficacy and safety of ertapenem versus piperacillin-tazobactam for the treatment of intra-abdominal infections requiring surgical intervention. J Gastrointest Surg. 2006;10:567-74.
76. Namias N, Solomkin JS, Jensen EH, et al. Randomized, multicenter, double-blind study of efficacy, safety, and tolerability of intravenous ertapenem versus piperacillin/tazobactam in treatment of complicated intra-abdominal infections in hospitalized adults. Surg Infect (Larchmt). 2007;8:15-28.
77. Malangoni MA, Song J, Herrington J, et al. Randomized controlled trial of moxifloxacin compared with piperacillin-tazobactam and amoxicillin-clavulanate for the treatment of complicated intra-abdominal infections. Ann Surg. 2006;244:204-11.
78. Babinchak T, Ellis-Grosse E, Dartois N, et al. The efficacy and safety of tigecycline for the treatment of complicated intra-abdominal infections: analysis of pooled clinical trial data. Clin Infect Dis. 2005;41(Suppl 5):S354-67.
79. Oliva ME, Rekha A, Yellin A, et al. 301 Study Group. A multicenter trial of the efficacy and safety of tigecycline versus imipenem/cilastatin in patients with complicated intra-abdominal infections [Study ID Numbers: 3074A1-301-WW; ClinicalTrials.gov Identifier: NCT00081744]. BMC Infect Dis. 2005;5:88.
80. Paul M, Silbiger I, Grozinsky S, et al. Beta lactam antibiotic monotherapy versus beta lactam-aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst Rev 2006; (1):CD003344.
81. Burnett RJ, Haverstock DC, Dellinger EP, et al. Definition of the role of enterococcus in intraabdominal infection: Analysis of a prospective randomized trial. Surgery. 1995;118:716-21.
82. Sitges-Serra A, Lopez MJ, Girvent M, et al. Postoperative enterococcal infection after treatment of complicated intra-abdominal sepsis. Br J Surg. 2002;89:361-7.
83. Teppler H, McCarroll K, Gesser RM, Woods GL. Surgical infections with enterococcus: Outcome in patients treated with ertapenem versus piperacillin-tazobactam. Surg Infect (Larchmt). 2002;3:337-49.
84. Willey SH, Hindes RG, Eliopoulos GM, Moellering RCJr. Effects of clindamycin and gentamicin and other antimicrobial combinations against enterococci in an experimental model of intra-abdominal abscess. Surg Gynecol Obstet. 1989;169:199-202.
85. Velmahos GC, Kamel E, Berne TV, et al. Abdominal computed tomography for the diagnosis of intra-abdominal sepsis in critically injured patients: Fishing in murky waters. Arch Surg. 1999;134:831-6.
86. Eggimann P, Francioli P, Bille J, et al. Fluconazole prophylaxis prevents intra-abdominal candidiasis in high-risk surgical patients. Crit Care Med. 1999;27:1066-72.
87. Jacobs S, Price Evans DA, Tariq M, Al Omar NF. Fluconazole improves survival in septic shock: a randomized double-blind prospective study. Crit Care Med. 2003;31:1938-1946.
88. Lennard ES, Dellinger EP, Wertz MJ, Minshew BH. Implications of leukocytosis and fever at conclusion of antibiotic therapy for intra-abdominal sepsis. Ann Surg. 1982;195:19-24.
89. Helmer KS, Robinson EK, Lally KP, et al. Standardized patient care guidelines reduce infectious morbidity in appendectomy patients. Am J Surg. 2002;183:608-13.
90. Visser MR, Bosscha K, Olsman J, et al. Predictors of recurrence of fulminant bacterial peritonitis after discontinuation of antibiotics in open management of the abdomen. Eur J Surg. 1998;164:825-9.
91. Gleason TG, Crabtree TD, Pelletier SJ, et al. Prediction of poorer prognosis by infection with antibiotic-resistant gram-positive cocci than by infection with antibiotic-sensitive strains. Arch Surg. 1999;134:1033-40.
92. Mulier S, Penninckx F, Verwaest C, et al. Factors affecting mortality in generalized postoperative peritonitis: multivariate analysis in 96 patients. World J Surg. 2003;27:379-84.
93. Wickel DJ, Cheadle WG, Mercer-Jones MA, Garrison RN. Poor outcome from peritonitis is caused by disease acuity and organ failure, not recurrent peritoneal infection. Ann Surg. 1997;225:744-53.
94. Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699-709.
95. Barie PS, Williams MD, McCollam JS, et al. Benefit/risk profile of drotrecogin alfa (activated) in surgical patients with severe sepsis. Am J Surg. 2004;188:212-20.
96. Payen D, Sablotzki A, Barie PS, et al. International integrated database for the evaluation of severe sepsis and drotrecogin alfa (activated) therapy: Analysis of efficacy and safety data in a large surgical cohort. Surgery. 2006;140:726-39.
97. Barie PS, Hydo LJ, Shou J, Eachempati SR. Efficacy and safety of drotrecogin alfa (activated) for the therapy of surgical patients with severe sepsis. Surg Infect (Larchmt). 2006;7(Suppl 2):S77-80.
98. Awe JA, Soliman MA, Gourdie RW. Appendico-cutaneous fistula presenting clinically as right loin necrotizing fasciitis: A case report. Int Surg. 2003;88:121-5.
99. Hollington P, Mawdsley J, Lim W, Gabe SM, et al. An 11-year experience of enterocutaneous fistula. Br J Surg. 2004;91:1646-51.
100. Haffejee AA. Surgical management of high output enterocutaneous fistulae: A 24-year experience. Curr Opin Clin Nutr Metab Care. 2004;7:309-16.
101. Poritz LS, Gagliano GA, McLeod RS, et al. Surgical management of entero and colocutaneous fistulae in Crohn’s disease: 17 years’ experience. Int J Colorectal Dis. 2004;19:481-5.
102. Bissett IP. Postoperative small bowel fistula: Back to basics. Trop Doct. 2000;30:138-40.
103. Chebli JM, Gaburri PD, Pinto JR. Enterovesical fistula in Crohn’s disease. Lancet. 2004;364:68.
104. Grunshaw ND, Ball CS. Palliative treatment of an enterorectal fistula with a covered metallic stent. Cardiovasc Intervent Radiol. 2001;24:438-40.
105. Kaur N, Minocha VR. Review of a hospital experience of enterocutaneous fistula. Trop Gastroenterol. 2000;21:197-200.
106. Present DH. Crohn’s fistula: Current concepts in management. Gastroenterology. 2003;124:1629-35.
107. Dionigi G, Dionigi R, Rovera F, et al. Treatment of high output entero-cutaneous fistulae associated with large abdominal wall defects: Single center experience. Int J Surg. 2008;6:51-6.
108. Chew DK, Choi LH, Rogers AM. Enterocutaneous fistula 14 years after prosthetic mesh repair of a ventral incisional hernia: A life-long risk? Surgery. 2000;127:352-3.
109. Costa D, Tomas A, Lacueva J, et al. Late enterocutaneous fistula as a complication after umbilical hernioplasty. Hernia. 2004;8:271-2.
110. Hyon SH, Martinez-Garbino JA, Benati ML, et al. Management of a high-output postoperative enterocutaneous fistula with a vacuum sealing method and continuous enteral nutrition. ASAIO J. 2000;46:511-14.
111. Mayberry JC, Burgess EA, Goldman RK, et al. Enterocutaneous fistula and ventral hernia after absorbable mesh prosthesis closure for trauma: The plain truth. J Trauma. 2004;57:157-62.
112. Patwardhan N, McHugh K, Drake D, Spitz L. Gastroenteric fistula complicating percutaneous endoscopic gastrostomy. J Pediatr Surg. 2004;39:561-4.
113. Wang XB, Ren JA, Li JS. Sequential changes of body composition in patients with enterocutaneous fistula during the 10 days after admission. World J Gastroenterol. 2002;8:1149-52.
114. Medeiros AC, Aires-Neto T, Marchini JS, et al. Treatment of postoperative enterocutaneous fistulas by high-pressure vacuum with a normal oral diet. Dig Surg. 2004;21:401-5.
115. Gunn LA, Follmar KE, Wong MS, et al. Management of enterocutaneous fistulas using negative-pressure dressings. Ann Plast Surg. 2006;57:621-5.
116. Wainstein DE, Fernandez E, Gonzalez D, et al. Treatment of high-output enterocutaneous fistulas with a vacuum-compaction device. A ten-year experience. World J Surg. 2008;32:430-5.
117. Berry SM, Fischer JE. Enterocutaneous fistulas. Curr Probl Surg. 1994;31:469-566.
118. Martinez JL, Luque-de-Leon E, Mier J, et al. Systematic management of postoperative enterocutaneous fistulas: factors related to outcomes. World J Surg. 2008;32:436-43.
119. Visschers RG, Olde Damink SW, Winkens B, et al. Treatment strategies in 135 consecutive patients with enterocutaneous fistulas. World J Surg. 2008;32:445-53.
120. Rombeau JL, Rolandelli RH. Enteral and parenteral nutrition in patients with enteric fistulas and short bowel syndrome. Surg Clin North Am. 1987;67:551-71.
121. Levy E, Frileux P, Cugnenc PH, Honiger J, Ollivier JM, Parc R. High-output external fistulae of the small bowel: Management with continuous enteral nutrition. Br J Surg. 1989;76:676-9.
122. Heys SD, Walker LG, Smith I, Eremin O. Enteral nutritional supplementation with key nutrients in patients with critical illness and cancer: A meta-analysis of randomized controlled clinical trials. Ann Surg. 1999;229:467-77.
123. de Aguilar-Nascimento JE, Caporossi C, Dock-Nascimento DB, et al. Oral glutamine in addition to parenteral nutrition improves mortality and the healing of high-output intestinal fistulas. Nutr Hosp. 2007;22:672-6.
124. Dudrick SJ, Maharaj AR, McKelvey AA. Artificial nutritional support in patients with gastrointestinal fistulas. World J Surg. 1999;23:570-6.
125. Rose D, Yarborough MF, Canizaro PC, Lowry SF. One hundred and fourteen fistulas of the gastrointestinal tract treated with total parenteral nutrition. Surg Gynecol Obstet. 1986;163:345-50.
126. Bissett IP. Succus entericus reinfusion to treat postoperative small-bowel fistula. Arch Surg. 2002;137:1446-47.
127. Dorta G. Role of octreotide and somatostatin in the treatment of intestinal fistulae. Digestion. 1999;60(Suppl 2):53-6.
128. Hernandez-Aranda JC, Gallo-Chico B, Flores-Ramirez LA, et al. [Treatment of enterocutaneous fistula with or without octreotide and parenteral nutrition. Nutr Hosp. 1996;11:226-9.
129. Sancho JJ, di Costanzo J, Nubiola P, et al. Randomized double-blind placebo-controlled trial of early octreotide in patients with postoperative enterocutaneous fistula. Br J Surg. 1995;82:638-41.
130. Scott NA, Finnegan S, Irving MH. Octreotide and postoperative enterocutaneous fistulae: A controlled prospective study. Acta Gastroenterol Belg. 1993;56:266-70.
131. Jamil M, Ahmed U, Sobia H. Role of somatostatin analogues in the management of enterocutaneous fistulae. J Coll Physicians Surg Pak. 2004;14:237-40.
132. Memon AS, Siddiqui FG. Causes and management of postoperative enterocutaneous fistulas. J Coll Physicians Surg Pak. 2004;14:25-8.
133. Alivizatos V, Felekis D, Zorbalas A. Evaluation of the effectiveness of octreotide in the conservative treatment of postoperative enterocutaneous fistulas. Hepatogastroenterology. 2002;49:1010-12.
134. Reimund JM, Wittersheim C, Dumont S, et al. Mucosal inflammatory cytokine production by intestinal biopsies in patients with ulcerative colitis and Crohn’s disease. J Clin Immunol. 1996;16:144-50.
135. Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med. 1999;340:1398-405.
136. Nikolaus S, Raedler A, Kuhbacker T, et al. Mechanisms in failure of infliximab for Crohn’s disease. Lancet. 2000;356:1475-9.
137. Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876-85.
138. Lichtenstein GR, Yan S, Bala M, et al. Infliximab maintenance treatment reduces hospitalizations, surgeries, and procedures in fistulizing Crohn’s disease. Gastroenterology. 2005;128:862-9.
139. Parsi MA, Lashner BA, Achkar JP, et al. Type of fistula determines response to infliximab in patients with fistulous Crohn’s disease. Am J Gastroenterol. 2004;99:445-9.
140. Uza N, Nakase H, Ueno S, et al. The effect of medical treatment on patients with fistulizing Crohn’s disease: A retrospective study. Intern Med. 2008;47:193-99.
141. Matsumoto T, Iida M, Motoya S, et al. Therapeutic efficacy of infliximab on patients with short duration of Crohn’s disease: A Japanese multicenter survey. Dis Colon Rectum. 2008;51:916-23.
142. Sands BE, Blank MA, Diamond RH, et al. Maintenance infliximab does not result in increased abscess development in fistulizing Crohn’s disease: Results from the ACCENT II study. Aliment Pharmacol Ther. 2006;23:1127-36.
143. Behm BW, Bickston SJ. Tumor necrosis factor-alpha antibody for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev 2008; (1):CD006893.
144. Schroder O, Blumenstein I, Schulte-Bockholt A, Stein J. Combining infliximab and methotrexate in fistulizing Crohn’s disease resistant or intolerant to azathioprine. Aliment Pharmacol Ther. 2004;19:295-301.
145. Gonzalez-Lama Y, Abreu L, Vera MI, et al. Long-term oral tacrolimus therapy in refractory to infliximab fistulizing Crohn’s disease: A pilot study. Inflamm Bowel Dis. 2005;11:8-15.
146. Garcia GD, Freeman IH, Zagorski SM, Chung MH. A laparoscopic approach to the surgical management of enterocutaneous fistula in a wound healing by secondary intention. Surg Endosc. 2004;18:554-6.
147. Gonzalez-Ojeda A, Avalos-Gonzalez J, Mucino-Hernandez MI, et al. Fibrin glue as adjuvant treatment for gastrocutaneous fistula after gastrostomy tube removal. Endoscopy. 2004;36:337-41.
148. Khairy GE, al Saigh A, Trincano NS, et al. Percutaneous obliteration of duodenal fistula. J R Coll Surg Edinb. 2000;45:342-4.
149. Okamoto K, Watanabe Y, Nakachi T, et al. The use of autologous fibrin glue for the treatment of postoperative fecal fistula following an appendectomy: Report of a case. Surg Today. 2003;33:550-2.
150. Lynch AC, Delaney CP, Senagore AJ, et al. Clinical outcome and factors predictive of recurrence after enterocutaneous fistula surgery. Ann Surg. 2004;240:825-31.
151. Kazantsev GB, Balli JE, Franklin ME. Laparoscopic management of enterocutaneous fistula. Surg Endosc. 2000;14:87.
152. Campos AC, Andrade DF, Campos GM, et al. A multivariate model to determine prognostic factors in gastrointestinal fistulas. J Am Coll Surg. 1999;188:483-90.
153. Chamberlain RS, Kaufman HL, Danforth DN. Enterocutaneous fistula in cancer patients: Etiology, management, outcome, and impact on further treatment. Am Surg. 1998;64:1204-11.